Abstract
Background:
Acute severe organophosphorus pesticide poisoning (ASOPP) is one of the major diseases that endanger human life and health. However, the effects of conventional therapy including gastric lavages, mechanical ventilation, muscarinic antagonist drugs, and cholinesterase reactivators were uncertain. This meta-analysis aims to investigate the safety and efficacy of hemoperfusion combined with hemofiltration besides routine therapy for ASOPP.
Materials and Methods:
A comprehensive search for candidate publications was performed through PubMed, Medline, Cochrane Library, WanFang, Chinese Biomedical Literature, and China National Knowledge Infrastructure from database inception to May 12, 2020. The retrieved studies were screened by the predefined inclusion and exclusion criteria. The data of important end points were extracted. The risk ratio (RR) and weighted mean difference (WMD) were pooled for categorical variables and continuous variables, respectively. Meta-analyses and publication bias were conducted by using STATA software version 15.1.
Results:
A total of 11 randomized controlled trials with 811 patients were included. Compared to conventional therapy group, patients in the hemoperfusion plus hemofiltration group were significantly superior with regard to mortality (RR 0.38, 95% confidence interval [CI] [0.25, 0.57], P < 0.001), total atropine dosing (WMD −147.34 mg, 95% CI [−199.49, −95.18], P < 0.001), duration of mechanical ventilation (WMD −2.34 days, 95% CI [−3.77, −0.92], P < 0.001), cholinesterase recovery time (WMD −2.49 days, 95% CI [−3.14, −1.83], P < 0.001), and length of stay (WMD −4.52 days, 95% CI [−5.31, −3.73], P < 0.001).
Conclusion:
Combined hemoperfusion and hemofiltration was a very safe and effective treatment protocol for ASOPP, not only resulting in significantly decreased mortality but also resulting in reduced total atropine dosing, duration of mechanical ventilation, cholinesterase recovery time, and length of stay.
Keywords: Hemofiltration, hemoperfusion, meta-analysis, mortality, organophosphorus poisoning
INTRODUCTION
Organophosphorus pesticide self-poisoning is a major clinical and public health problem all over the world, especially in China, South Asia, and Western Pacific. It is conservatively estimated that the total global burden of pesticide suicide is a plausible range of 9,859,667–17,303,333 deaths from 1960 to 2018.[1] Organophosphorus pesticides inhibit acetyl cholinesterase, resulting in excessive accumulation of acetylcholine at synapses, which causes muscarinic, nicotinic, and central nervous system symptoms, simultaneously with changes in the peripheral nervous system.[2] The conventional therapy mainly includes gastric lavages, wash of skin surface, intubation and mechanical ventilation, muscarinic antagonist drugs, and cholinesterase reactivators.[3] Nevertheless, there is currently no high-quality evidence to support the clinical effectiveness of gastric lavages;[4] atropine cannot counter nicotinic symptoms and excessive use will induce atropine poisoning, and oximes are less effective in the management of some organophosphorus insecticides poisoning such as malathion.[5]
It has been extensively recognized that hemoperfusion could effectively adsorb and remove the poisons from extracorporeal circulation since the 1970s.[6] However, hemoperfusion is unable to correct the pathophysiological changes caused by organophosphorus pesticides,[7] and there are some complications related to the procedures such as thrombocytopenia, hypocalcemia, decrease of immunoglobulins, and coagulation factors.[8] Conversely, hemofiltration, the most common modality, is continuous venovenous hemofiltration, not only help eliminate the toxins from the blood and regulate the body fluid balance but also clear the large number of inflammatory mediators and inflammatory factors.[9]
Thus, combined hemoperfusion and hemofiltration may theoretically have compensated for the respective deficiencies.[10] Whether this combined therapy could improve the clinical outcomes of patients with acute severe organophosphorus pesticide poisoning (ASOPP) in practice remains controversial. We aim to perform a meta-analysis evaluating the safety and efficacy among patients with ASOPP receiving hemoperfusion plus hemofiltration versus those not receiving.
MATERIALS AND METHODS
Literature search strategy
A comprehensive search for relevant publications was performed through PubMed, Medline, Cochrane Library, WanFang, Chinese Biomedical Literature, and China National Knowledge Infrastructure from database inception to May 12, 2020. The predefined search terms included hemoperfusion AND hemofiltration AND (organophosphorus OR organophosphate poisoning) AND (random OR “randomized controlled trial” OR RCT). No restrictions in language were implemented. The reference lists of included studies were also manually screened to identify the additional trials.
Inclusion and exclusion criteria
The following inclusion criteria were considered in this meta-analysis. (1) Randomized controlled trials (RCTs) investigating the benefits of hemoperfusion in combination with hemofiltration for patients. (2) All patients were certainly diagnosed as ASOPP by the exposure history of known organophosphorus pesticides and decreased cholinesterase activity (less than 30%). (3) Patients who received conventional therapy were included into control group, whereas those received hemoperfusion plus hemofiltration besides were included into trial group. (4) Mortality is the primary outcome, and the secondary outcomes include at least one or more as follows: atropine dosing, duration of mechanical ventilation, restoration time of cholinesterase, and length of stay. Articles were excluded if any of the following were present: (1) with other adjuvant treatments, (2) study data unavailable, (3) duplicate publications of the same study population, and (4) case reports, review articles, and letters to editor.
Data extraction and quality assessment
Two researchers independently screened titles and abstracts of all trial reports we identified above. Discrepancies were resolved through discussion with a third reviewer, as required. Then, another two authors independently extracted the following information from the included trials: first author's name, publication year, sample size, mean age of patients, intervention, and main outcome measures.
Two reviewers independently assessed the risk of bias of the included studies using the tool described in the Cochrane Handbook.[11] When the assessors disagreed, the final rating was decided through discussion or with the involvement of another member of the review group, if necessary. Quality assessment was assessed by the Review Manager software (version 5.4, the Cochrane's collaboration, Oxford, UK).
Statistical analysis
The outcomes were described as risk ratio (RR) with 95% confidence interval (CI) for dichotomous data (mortality) and the weighted mean difference (WMD) with 95% CI for continuous data (atropine, ventilation, cholinesterase, and length of stay). Heterogeneity among the studies was quantified by using the Cochrane test (Q) and I-square (I2) test.[12] The fixed-effect model (Mantel–Haenszel method) would be selected when there were no heterogeneity (P > 0.10 and I2 < 50%), whereas the random-effect model (DerSimonian–Laird method) was used when there were obvious heterogeneity (P ≤ 0.10 and I2 ≥ 50%).[13] To evaluate the influence of an individual study on the pooled estimate, we performed sensitivity analysis by excluding studies one by one. To determine the potential publication bias, both the Begg's and Egger's test, and the funnel plots were performed.[14] Statistical significance for all analyses was set at P < 0.05. All the statistical analyses were performed by using the STATA software (version 15.1, Stata Corporation, College Station, TX, USA).
RESULTS
Characteristics of eligible studies
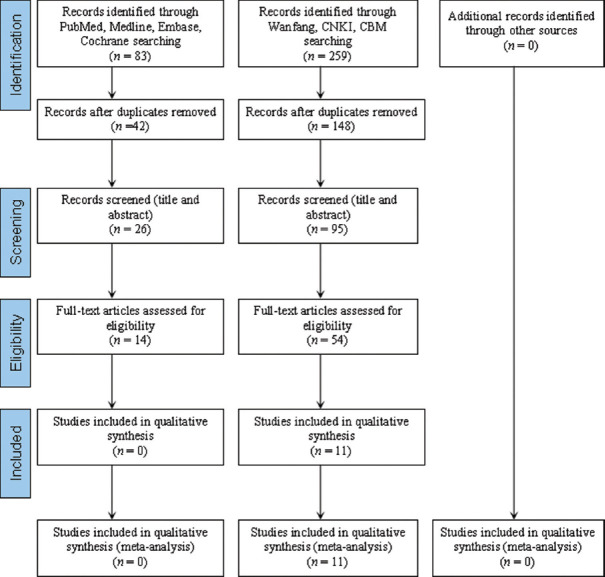
An initial search identified 342 articles from searching databases including 54 from PubMed, 27 from Medline, 2 from Cochrane Library, 158 from WanFang, 30 from CNKI, and 71 from CBM. The flow diagram that detailed the process is shown in Figure 1. A total of 11 studies (including 811 patients) were eventually identified after abstract and full-text screening, which were all carried out in China.[15,16,17,18,19,20,21,22,23,24,25] Main characteristics of the patients enrolled as trial group and control group are shown in Table 1.
Figure 1.
Flowchart for the procedure of the literature search
Table 1.
Main characteristics of included articles
| First author | Year | Trial group | Control group | Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Therapy | n | Male/female | Age | Therapy | n | Male/female | Age | |||
| Han[15] | 2017 | HP+HF+Con | 11 | 6/5 | 38.7±4.3 | HP+Con | 13 | 7/6 | 38.1±4.2 | ➀➁➂➃➄ |
| Jiao[16] | 2016 | HP+HF+Con | 37 | 14/23 | 41.9±5.1 | HP+Con | 37 | 17/20 | 42.1±5.2 | ➀➃➄ |
| Liu[17] | 2016 | HP+HF+Con | 17 | 9/8 | 43.1±8.0 | HP+Con | 17 | 10/7 | 42.9±8.3 | ➀➁➂➃➄ |
| Wang[18] | 2016 | HP+HF+Con | 60 | 21/39 | 68.5±5.3 | HP+Con | 60 | 21/39 | 68.5±5.3 | ➀➂➃ |
| Xia[19] | 2013 | HP+HF+Con | 34 | 12/22 | 45.5±9.1 | Con | 32 | 12/20 | 46.5±8.2 | ➀➁➄ |
| Xie[20] | 2019 | HP+HF+Con | 55 | 30/25 | 44.4±4.7 | HP+Con | 55 | 32/23 | 44.5±4.8 | ➀➁➃➄ |
| Xu[21] | 2014 | HP+HF+Con | 34 | 12/22 | 36.2±2.3 | Con | 32 | 11/21 | 35.6±7.4 | ➀➂➃➄ |
| Xue[22] | 2019 | HP+HF+Con | 31 | 10/21 | 66.7±6.2 | HF+Con | 31 | 12/19 | 67.5±5.3 | ➀➃➄ |
| Yang[23] | 2014 | HP+HF+Con | 41 | 19/22 | 38.5±6.2 | HP+Con | 40 | 17/23 | 37.8±3.6 | ➀➃ |
| Yang[24] | 2016 | HP+HF+Con | 32 | 24/8 | 40.5±3.2 | Con | 30 | 25/5 | 40.4±3.3 | ➀➃➄ |
| Yue[25] | 2015 | HP+HF+Con | 56 | 24/32 | 43.2±8.4 | Con | 56 | 28/28 | 44.3±8.2 | ➂➃➄ |
Outcomes: ➀=mortality; ➁=atropine dosing; ➂=duration of mechanical ventilation; ➃=restoration time of cholinesterase; ➄=length of stay. HP=Hemoperfusion; HF=Hemofiltration; Con=Conventional
All of the included studies suggested randomization, but only six studies reported the method of random sequence generation, while all of the studies failed to report details about allocation concealment, only one article applied blinding. There were low risk of incomplete outcome data, selective reporting bias, and other bias. Therefore, all studies were judged to be of a relatively poor methodological quality [Figures 2 and 3].
Figure 2.
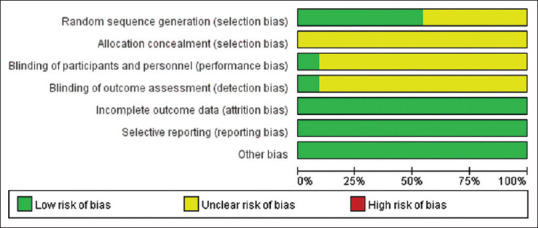
Risk of bias graph
Figure 3.
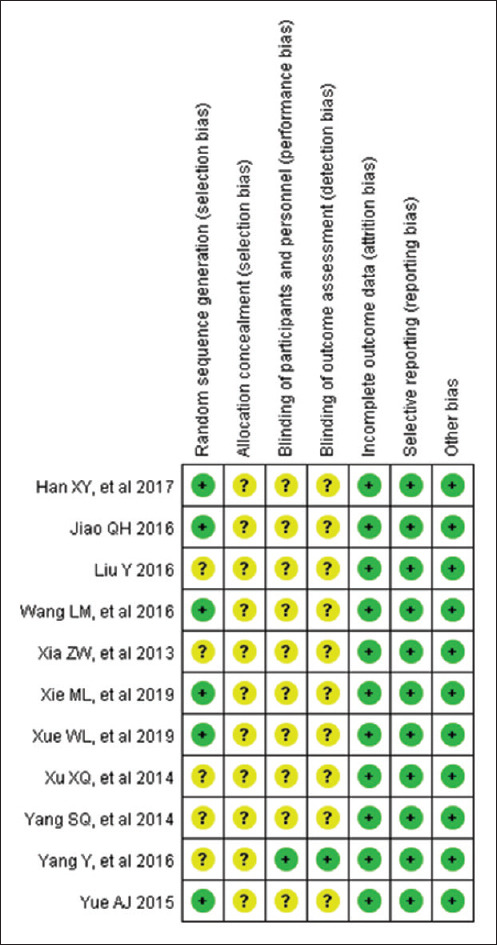
Risk of bias summary
Meta-analysis
Mortality
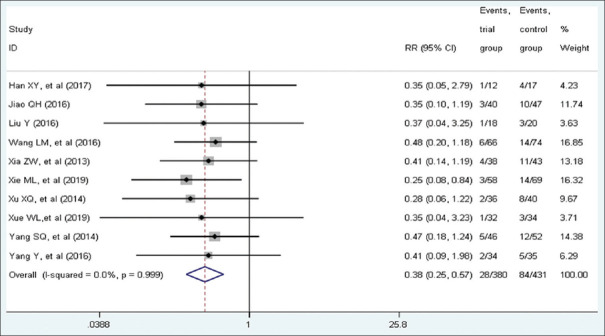
Ten studies with a total of 699 patients included eligible data on mortality. About 352 patients were in the trial group, of which 28 died (7.95%), while 347 cases were in the control group, of which 84 died (24.21%). A fixed-effect model was conducted because of low heterogeneity among studies (I2 = 0.00%, P = 0.99). Pooled statistics demonstrated that patients in the trial group was associated with a lower mortality than the control group (RR = 0.38, 95% CI [0.25, 0.57], P < 0.001). Forest plot is shown in Figure 4.
Figure 4.
Forest plot of mortality
Atropine dosing
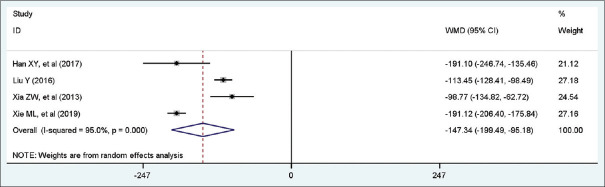
Four studies with a total of 234 patients included eligible data on atropine. A random-effect model was conducted because of high heterogeneity among studies (I2 = 95.0%, P = 0.00). Pooled statistics demonstrated that patients in the trial group was significantly associated with less atropine dosing than control group (WMD = −147.34 mg, 95% CI [−199.49, −95.18], P < 0.001). Forest plot is shown in Figure 5.
Figure 5.
Forest plot of atropine dosing
Duration of mechanical ventilation
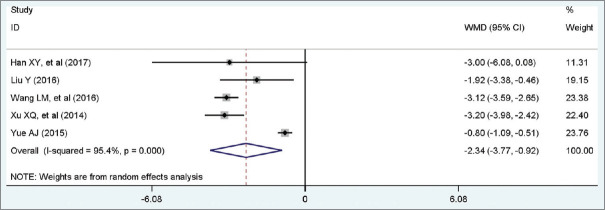
Five studies with a total of 234 patients included eligible data on ventilation. A random-effect model was conducted because of high heterogeneity among studies (I2 = 95.4%, P = 0.00). Pooled statistics demonstrated that patients in the trial group was significantly associated with shorter duration of mechanical ventilation than control group (WMD = −2.34 days, 95% CI [−3.77, −0.92], P < 0.001). Forest plot is shown in Figure 6.
Figure 6.
Forest plot of duration of mechanical ventilation
Restoration time of cholinesterase
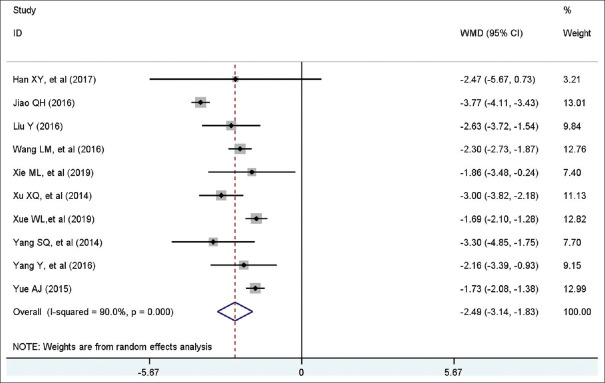
Ten studies with a total of 745 patients included eligible data on cholinesterase. A random-effect model was conducted because of high heterogeneity among studies (I2 = 90.0%, P = 0.00). Pooled statistics demonstrated that patients in the trial group was significantly associated with shorter cholinesterase than control group (WMD = −2.49 days, 95% CI [−3.14, −1.83], P < 0.001). Forest plot is shown in Figure 7.
Figure 7.
Forest plot of restoration time of cholinesterase
Length of stay
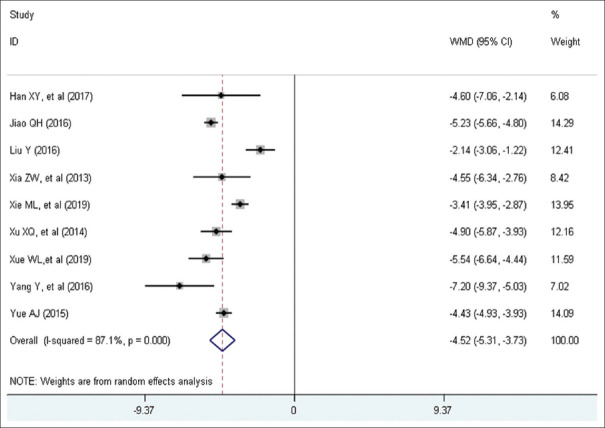
Nine studies with a total of 610 patients included eligible data on length of stay. A randomized-effect model was conducted because of high heterogeneity among studies (I2 = 87.1%, P = 0.00). Pooled statistics demonstrated that patients in the trial group was significantly associated with shorter length of stay than control group (WMD = −4.52 days, 95% CI [−5.31, −3.73], P < 0.001). Forest plot is shown in Figure 8.
Figure 8.
Forest plot of length of stay
Sensitivity analysis
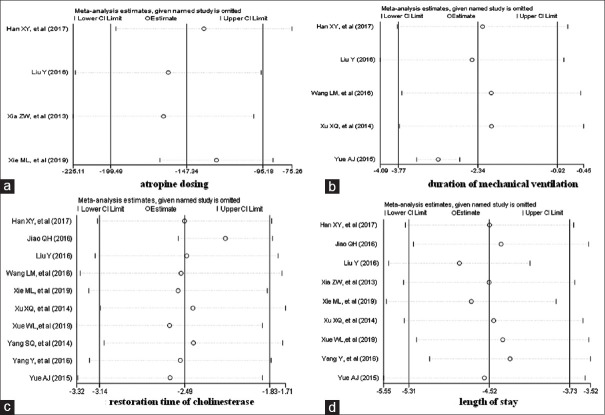
The heterogeneity among the included studies was tested by using the sensitivity analysis. The pooled RR for mortality was not changed when any individual study was omitted [Figure 9]. The pooled WMDs for atropine, ventilation, cholinesterase, and length of stay were not changed when any individual study was omitted [Figure 10].
Figure 9.
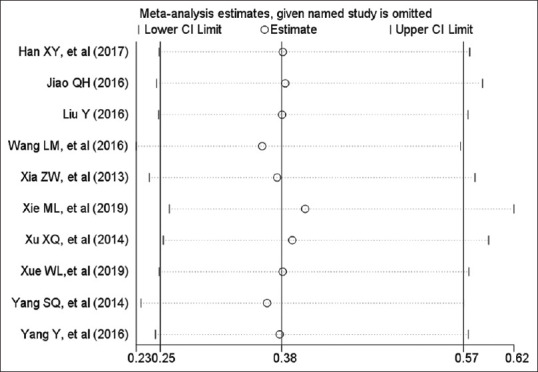
Influence plot. The graph demonstrates the sensitivity analysis for mortality
Figure 10.
Influence plot. The graph demonstrates the sensitivity analysis for atropine, ventilation, cholinesterase, and length of stay
Publication bias
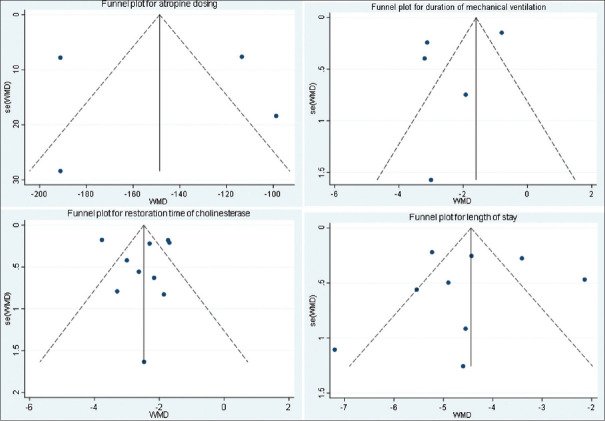
The publication bias was explored via funnel plot with the Begg's and Egger's tests. The funnel plots showed no obviously asymmetry among studies [Figures 11 and 12]. No evidence of publication bias was found for mortality (Begg's test: P = 0.283, Egger's test: P = 0.205). In addition, there was no publication bias for atropine (Begg's test: P = 0.734, Egger's test: P = 0.945), ventilation (Begg's test: P = 1.00, Egger's test: P = 0.379), cholinesterase (Begg's test: P = 0.721, Egger's test: P = 0.988), and length of stay (Begg's test: P = 0.754, Egger's test: P = 0.906).
Figure 11.
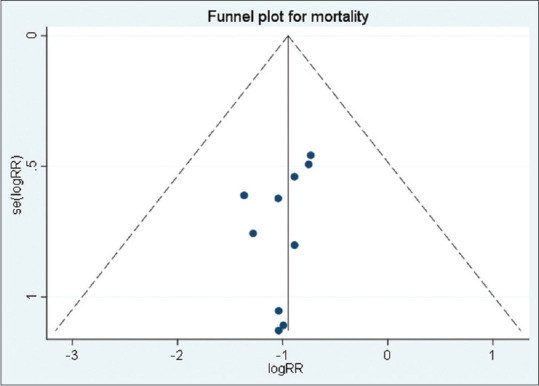
Funnel plot to show publication bias (mortality)
Figure 12.
Funnel plot to show publication bias (atropine, ventilation, cholinesterase, and length of stay)
DISCUSSION
It is the first meta-analysis to systematically evaluate the beneficial effects in patients who had undergone hemoperfusion in combination with hemofiltration besides conventional therapy. Our meta-analysis demonstrated that mortality was significantly lower in patients of the trail group compared with the control group. On the other hand, combined hemoperfusion and hemofiltration could more effectively decrease the length of stay, total atropine dosing, duration of mechanical ventilation, and restoration time of cholinesterase than those received classic treatments alone.
Organophosphorus pesticides are mostly lipophilic and low-molecular-weight poisons (e.g., chlorpyrifos 350 Da, malathion 330 Da, dichlorvos 221 Da), which are rapidly absorbed to the blood and distributed to target tissues and organs after ingestion, and then display a high degree of protein binding.[26] The toxicial mechanism mainly involves the combination with cholinesterase to form a stable phosphorylated cholinesterase which could hardly be broken down by acetyl cholinesterase and results in accumulation of large amounts of acetylcholine in synapses of the autonomic nervous system, central nervous system, and neuromuscular junction.[27]
Blood purification has been recommended as a therapeutic modality by extracorporeal circulation to remove organophosphorus pesticide from blood in recent decades. With the improved efficacy of hemodialysis, hemoperfusion is now less frequently performed in the United States but still remains popular in China.[28] Hemoperfusion is favored to eliminate poisons with middle-molecular-weight (less than 5000 Da), low volume of distribution, and high protein binding.[8] Previous studies have reported hemoperfusion could effectively decrease poisoning severity by removing significant amounts of organophosphate pesticides and increasing serum cholinesterase level.[29] Compared to activated charcoal column, the synthetic resin column is exhibit enhanced adsorption and clearance of organophosphorus pesticide and has less of a tendency to serious complications including thrombocytopenia, leukocytopenia, and decrease in platelet count.[30] However, we could not compare the effects and complications between these two columns due to the absence of information. In addition, although there is currently insufficient evidence to support the routine use of polymyxin B-immobilized hemoperfusion to treat patients with sepsis, this specific technique has been clinically used in many countries.[31]
Hemofiltration not only helps maintain stable hemodynamics, resulting in improved cardiovascular tolerability, but also protect a favorable balance of water and salt metabolism, resulting in better homeostasis.[32] It is reported that the poison and solvent of organophosphorus pesticide are simultaneously removed by convection and replaced by a physiological solution during hemofiltration.[33] On the other hand, hemofiltration can effectively decrease in serum interleukin 1 (IL-1), IL-6, IL-8, and tumor necrosis factor alpha, increase in serum IL-10 levels, reduce the chain reaction initiated by inflammatory cytokines, and limit damage resulting from ASOPP, which may be beneficial for critical patients with multiple organ dysfunction syndrome.[34]
In the present study, the whole descriptions of organophosphorus pesticides in all included articles were not available, which may contribute a lot to the heterogeneity among trials. There is substantial variability in clinical course, response to oximes, and mortality with different organophosphorus insecticides in human self-poisoning.[35] Acikalin et al. reported that long hospital stay, low initial serum cholinesterase level, respiratory depression necessitating intubation, and mechanical ventilation are independently associated with poor prognostic factors for ASOPP.[36] However, the usefulness of the serum cholinesterase level remains controversial,[37] and some organophosphate pesticides inhibit butyryl cholinesterase more effectively than they inhibit acetyl cholinesterase. Although chlorpyrifos inhibits acetyl cholinesterase and initial serum cholinesterase level is generally low, it is least mortality in patients of acute chlorpyrifos poisoning.[38]
Limitations
There are still some limitations as follows. First of all, the identified articles included were all carried out in China and published in Chinese. Furthermore, the methodological quality of these included studies was generally low, especially none considered the procedures of allocation concealment. Only six studies carefully descripted the random sequence generation method, and only one used blinding methods during their implementations. Last but not least, we were unable to obtain the unpublished data based on current conditions. Those may have some effects on the pooled results.
CONCLUSION
ASOPP still has a high incidence and mortality despite the banning of rang poison and improved therapy. This systematic review indicated that combined hemoperfusion and hemofiltration may be beneficial adjuncts to patients with ASOPP. However, this evidence is inadequate to recommend their use in clinical practice. Larger high-quality multicenter RCTs are required to establish effectiveness.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Karunarathne A, Gunnell D, Konradsen F, Eddleston M. How many premature deaths from pesticide suicide have occurred since the agricultural Green Revolution? Clin Toxicol (Phila) 2020;58:227–32. doi: 10.1080/15563650.2019.1662433. [DOI] [PubMed] [Google Scholar]
- 2.Čadež T, Kolić D, Šinko G, Kovarik Z. Assessment of four organophosphorus pesticides as inhibitors of human acetylcholinesterase and butyrylcholinesterase. Sci Rep. 2021;11:21486. doi: 10.1038/s41598-021-00953-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajracharya SR, Prasad PN, Ghimire R. Management of organophosphorus poisoning. J Nepal Health Res Counc. 2016;14:131–8. [PubMed] [Google Scholar]
- 4.Li Y, Tse ML, Gawarammana I, Buckley N, Eddleston M. Systematic review of controlled clinical trials of gastric lavage in acute organophosphorus pesticide poisoning. Clin Toxicol (Phila) 2009;47:179–92. doi: 10.1080/15563650701846262. [DOI] [PubMed] [Google Scholar]
- 5.Eddleston M. Are oximes still indicated for acute organophosphorus insecticide self-poisoning? J Med Toxicol. 2018;14:1–2. doi: 10.1007/s13181-018-0651-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gil HW, Kim SJ, Yang JO, Lee EY, Hong SY. Clinical outcome of hemoperfusion in poisoned patients. Blood Purif. 2010;30:84–8. doi: 10.1159/000318585. [DOI] [PubMed] [Google Scholar]
- 7.Altintop L, Aygun D, Sahin H, Doganay Z, Guven H, Bek Y, et al. In acute organophosphate poisoning, the efficacy of hemoperfusion on clinical status and mortality. J Intensive Care Med. 2005;20:346–50. doi: 10.1177/0885066605279834. [DOI] [PubMed] [Google Scholar]
- 8.Ghannoum M, Bouchard J, Nolin TD, Ouellet G, Roberts DM. Hemoperfusion for the treatment of poisoning: Technology, determinants of poison clearance, and application in clinical practice. Semin Dial. 2014;27:350–61. doi: 10.1111/sdi.12246. [DOI] [PubMed] [Google Scholar]
- 9.Puzio TJ, Chrobak D, Jawed Y, Tripathy P, Carlos W., 3rd Severe accidental hypothermia managed with continuous venovenous hemofiltration. Am Surg. 2020;86:73–5. [PubMed] [Google Scholar]
- 10.Nasr Isfahani S, Farajzadegan Z, Sabzghabaee AM, Rahimi A, Samasamshariat S, Eizadi-Mood N. Does hemoperfusion in combination with other treatments reduce the mortality of patients with paraquat poisoning more than hemoperfusion alone: A systematic review with meta-analysis. J Res Med Sci. 2019;24:2. doi: 10.4103/jrms.JRMS_478_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Propadalo I, Tranfic M, Vuka I, Barcot O, Pericic TP, Puljak L. In Cochrane reviews, risk of bias assessments for allocation concealment were frequently not in line with Cochrane's Handbook guidance. J Clin Epidemiol. 2019;106:10–7. doi: 10.1016/j.jclinepi.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Delgado-Rodríguez M, Sillero-Arenas M. Systematic review and meta-analysis. Med Intensiva (Engl Ed) 2018;42:444–53. doi: 10.1016/j.medin.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 14.Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74:785–94. doi: 10.1111/biom.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han XY, Li MX, Qu CQ, Huang YP. Cardioprotective effects of blood perfusion combined with continuous veno-venous hemofiltration in patients with acute organophosphorus pesticide poisoning. J Nanchang Univ (Medical Sciences) 2017;57:46–9. [Google Scholar]
- 16.Jiao QH. Effect of continuous blood purification treatment of acute organophosphorus poisoning. Clin Misdiagnosis Mistreatment. 2016;29:88–91. [Google Scholar]
- 17.Liu Y. Effect of continuous veno-venous hemofiltration combined with hemoperfusion in the treatment of acute organophosphorus pesticide poisoning complicated with respiratory failure. Med Innov China. 2016;13:112–4. [Google Scholar]
- 18.Wang LM, Rong GC, Xie ZY, Xing YH. Protective effect of hemoperfusion combined with continous renal replacement therapy on heart and kidney injury of elderly patients with severe organophosphorus poisoning. Chin Pharmac. 2016;25:57–60. [Google Scholar]
- 19.Xia ZW, Ding HT, Li YS, Liu WQ, Xiong J, Li SS. Clinical outcomes of continuous bedside hemofiltration and hemoperfusion for severe organophosphorus pestcide poisoning. Med J Wuhan Univ. 2013;34:290–2. [Google Scholar]
- 20.Xie ML, Du YF, Yang XY, Jiang HL. Effect of sequential blood purification on acute severe organophosphorus poisoning and myocardial fatty acid binding protein. Chin J Clin. 2019;47:184–7. [Google Scholar]
- 21.Xu XQ, Luo QX, Chen ZM, Huang SB. Clinical research of patients with acute severe organic phosphorus pesticide poisoning treated by hemoperfusion combined with continuous veno-venous hemofiltration. Lingnan J Emerg Med. 2014;19:39–40, 50. [Google Scholar]
- 22.Xue WL, Zhang L. Effect of hemoperfusion on serum levels of CHE, DA, TGF-β1 and TNF-α in elderly patients with severe organophosphorus pesticide poisoning. Chin J Gerontol. 2019;39:351–4. [Google Scholar]
- 23.Yang SQ, Liu Z, Wang B, Yang WB, Liu JG, Yuan JY, et al. Study of hemoperfusion combined with continuous veno-venous hemofiltration on heart protection in pantients with acute organophosphorus pesticide poisoning. Chin J Clin (Electronic Edition) 2014;8:62–6. [Google Scholar]
- 24.Yang Y. Emergency rescue for acute organic phosphorus pesticide poisoning complicated with respiratory failure. Int Med Health Guide Newsp. 2016;22:1103–5. [Google Scholar]
- 25.Yue AJ. Study the clinical effect of hemoperfusion combined with continuous veno-venous hemofiltration for organophosphate poisoning. Med Equip. 2015;28:24–5. [Google Scholar]
- 26.Marquart K, Prokopchuk O, Worek F, Thiermann H, Martignoni ME, Wille T. Human small bowel as a useful tool to investigate smooth muscle effects of potential therapeutics in organophosphate poisoning. Toxicol Lett. 2018;293:235–40. doi: 10.1016/j.toxlet.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Job L, Köhler A, Escher B, Worek F, Skerra A. A catalytic bioscavenger with improved stability and reduced susceptibility to oxidation for treatment of acute poisoning with neurotoxic organophosphorus compounds. Toxicol Lett. 2020;321:138–45. doi: 10.1016/j.toxlet.2019.12.030. [DOI] [PubMed] [Google Scholar]
- 28.Holubek WJ, Hoffman RS, Goldfarb DS, Nelson LS. Use of hemodialysis and hemoperfusion in poisoned patients. Kidney Int. 2008;74:1327–34. doi: 10.1038/ki.2008.462. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Wang G, Zhen G, Zhang Y, Liu J, Liu S. Application of hemoperfusion in severe acute organophosphorus pesticide poisoning. Turk J Med Sci. 2017;47:1277–81. doi: 10.3906/sag-1611-40. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Ding G. Effects of different blood purification methods on serum cytokine levels and prognosis in patients with acute severe organophosphorus pesticide poisoning. Ther Apher Dial. 2015;19:185–90. doi: 10.1111/1744-9987.12233. [DOI] [PubMed] [Google Scholar]
- 31.Fujii T, Ganeko R, Kataoka Y, Furukawa T, Featherstone R, Doi K, et al. Polymyxin B-immobilized hemoperfusion and mortality in critically ill adult patients with sepsis/septic shock: A systematic review with meta-analysis and trial sequential analysis. Int Care Med. 2017;44:167–78. doi: 10.1007/s00134-017-5004-9. [DOI] [PubMed] [Google Scholar]
- 32.Baird JS. Sieving and extraction of peptides and proteins during hemofiltration: A systematic review. Clin Nephrol. 2017;87:271–7. doi: 10.5414/CN108841. [DOI] [PubMed] [Google Scholar]
- 33.Ghannoum M, Hoffman RS, Gosselin S, Nolin TD, Lavergne V, Roberts DM. Use of extracorporeal treatments in the management of poisonings. Kidney Int. 2018;94:682–8. doi: 10.1016/j.kint.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 34.Si X, Li J, Bi X, Wu L, Wu X. Clinical evaluation of high-volume hemofiltration with hemoperfusion followed by intermittent hemodialysis in the treatment of acute wasp stings complicated by multiple organ dysfunction syndrome. PLoS One. 2015;10:e0132708. doi: 10.1371/journal.pone.0132708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eddleston M, Eyer P, Worek F, Mohamed F, Senarathna L, von Meyer L, et al. Differences between organophosphorus insecticides in human self-poisoning: A prospective cohort study. Lancet. 2005;366:1452–9. doi: 10.1016/S0140-6736(05)67598-8. [DOI] [PubMed] [Google Scholar]
- 36.Acikalin A, Dişel NR, Matyar S, Sebe A, Kekec Z, Gokel Y, et al. Prognostic factors determining morbidity and mortality in organophosphate poisoning. Pak J Med Sci. 2017;33:534–9. doi: 10.12669/pjms.333.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang EJ, Seok SJ, Lee KH, Gil HW, Yang JO, Lee EY, et al. Factors for determining survival in acute organophosphate poisoning. Korean J Intern Med. 2009;24:362–7. doi: 10.3904/kjim.2009.24.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mojsak P, Łozowicka B, Kaczyński P. Estimating acute and chronic exposure of children and adults to chlorpyrifos in fruit and vegetables based on the new, lower toxicology data. Ecotoxicol Environ Saf. 2018;159:182–9. doi: 10.1016/j.ecoenv.2018.05.006. [DOI] [PubMed] [Google Scholar]