Abstract
Background
Although screening with 12-lead electrocardiography and transthoracic echocardiography for cardiac involvement has been recommended for patients with biopsy-proven extracardiac sarcoidosis, cardiac sarcoidosis has been reported even in patients with normal electrocardiography and echocardiography findings. We investigated the prevalence and characteristics of these patient cohorts.
Methods
We studied 112 consecutive patients (age, 55±17 years, 64% females) with biopsy-proven extracardiac sarcoidosis who had undergone 18F-fluorodeoxyglucose positron emission tomography and cardiac magnetic resonance imaging for cardiac sarcoidosis evaluation. The patients were categorised as those showing normal findings both in electrocardiography and transthoracic echocardiography (normal group) and those showing abnormal findings in one or both examinations (abnormal group).
Results
33 (29%) and 79 (71%) patients were categorised into the normal and abnormal groups, respectively, of which 6 (18%) and 43 (54%) patients, respectively, were diagnosed with cardiac sarcoidosis (p<0.01). Of these six patients in the normal group, two with multiple-organ sarcoidosis showed clinical deterioration of cardiac involvement and required steroid therapy; three with small cardiac involvement showed natural remission over follow-up assessments; and one underwent steroid therapy and showed an improvement in the left ventricular ejection fraction to within normal limits.
Conclusions
The prevalence of cardiac sarcoidosis in patients with biopsy-proven extracardiac sarcoidosis and normal electrocardiography and transthoracic echocardiography findings was ∼20%. Electrocardiography and transthoracic echocardiography may not detect cardiac sarcoidosis in patients without conduction and morphological abnormalities. However, some of these patients may subsequently show clinically manifested cardiac sarcoidosis. Physicians should be mindful of this population.
Short abstract
ECG and transthoracic echocardiography may not detect cardiac sarcoidosis in patients without conduction and morphological abnormalities. Some of these patients may subsequently develop clinically manifested cardiac sarcoidosis. https://bit.ly/3qeQuff
Introduction
Sarcoidosis is a systemic disease of unknown aetiology that can affect multiple organs, including the lymph nodes, lungs, eyes, skin, liver and heart. Of these, cardiac involvement is associated with life-threatening complications. Patients with cardiac involvement may complain of syncope, presyncope, palpitations, dyspnoea on exertion due to advanced atrioventricular block, ventricular arrhythmias and heart failure secondary to cardiac sarcoidosis (CS). After the onset of the aforementioned clinical symptoms, the diagnosis of CS is delayed. Therefore, effective screening in patients with extracardiac sarcoidosis (extra-CS) is desirable for early detection, with the goal of reducing the mortality from CS.
The Heart Rhythm Society (HRS) consensus statement recommends that patients with biopsy-proven extra-CS should be screened for cardiac involvement with 12-lead electrocardiography (ECG) (class I) and transthoracic echocardiography (TTE) (class IIa) [1]. However, early-stage CS may not be detected by ECG and TTE in patients without conduction and morphological abnormalities. The prevalence of CS in extra-CS patients with normal ECG and TTE findings has not been elucidated to date. Thus, the aims of this study were to investigate the prevalence of cardiac involvement and explore the characteristics of biopsy-proven extra-CS in patients with normal ECG and TTE findings.
Materials and methods
Study population
We included 112 consecutive patients with biopsy-proven extra-CS who presented to the First Department of Medicine at Hokkaido University Hospital from June 2003 to January 2021. All patients underwent screening ECG and TTE. They were categorised into two groups: the normal group comprising patients with normal ECG and TTE findings, and the abnormal group of patients with abnormal ECG and/or TTE findings. Abnormal ECG findings were defined as ventricular arrhythmias (non-sustained ventricular tachycardia, multifocal or frequent premature ventricular contractions), bundle branch blocks, axis deviation or abnormal Q waves [2]. Abnormal TTE findings were defined as basal thinning of the interventricular septum, abnormal ventricular wall anatomy, regional ventricular wall-motion abnormalities or left ventricular contractile dysfunction (left ventricular ejection fraction <50%) [2]. All patients underwent 18F-fluoro-deoxyglucose positron emission tomography (18F-FDG PET) and cardiac MRI (CMR) for further assessment of CS. The updated guidelines for the diagnosis and treatment of CS by the Japanese Circulation Society were used as the standard for the diagnosis of CS [2]. This study was approved by the Research Ethics Committee of Hokkaido University. All patients provided informed consent.
Study protocol
All patients underwent 18F-FDG PET and CMR on admission. They were instructed to fast for at least 12 h and intravenously administered low-dose unfractionated heparin (UFH) (50 IU kg−1) before 18F-FDG administration if they did not have any contraindications [3–6]. Since August 2009, all patients were provided with a low-carbohydrate, high-fat and protein-permitted diet as dinner the day before 18F-FDG PET scans for further suppression of the background 18F-FDG uptake. Since October 2015, we have changed the pretest preparation for 18F-FDG PET. We stopped the pretest administration of UFH. Instead, all patients were instructed to fast for at least 18 h and provided with a low-carbohydrate, high-fat and protein-permitted diet three times the day before the 18F-FDG PET scan.18F-FDG PET and CMR scans were performed within 1 week of each other.
18F-FDG. PET imaging
18F-FDG PET was performed using a whole-body PET camera (Siemens EACT EXACT 47; Siemens Medical Systems, Knoxville, TN, USA). Intravenous injection of 18F-FDG (185 MBq) was followed by an uptake phase of 45–60 min, after which transmission scanning was performed for attenuation correction (AC) using 68Ge line sources. Data were acquired in 3D mode. The AC was reconstructed using ordered subset expectation maximisation. These images were resliced into a series of short-axis, vertical long-axis and horizontal long-axis images. Since February 2009, PET studies have been performed using PET/computed tomography (CT) imaging with a Biograph 64 TruePoint TrueV scanner (Siemens, Tokyo, Japan). The acquired datasets were corrected for attenuation by low-dose CT images and were reconstructed using a point spread function-based iterative algorithm (TrueX; Siemens) with two iterations per 21 subsets, a matrix size of 168×168, a voxel size of 4.1×4.1×2.0 mm and a Gaussian filter at 4.0 mm full-width at half-maximum. The transaxial and axial fields of view were 58.5 and 21.6 cm, respectively. Approximately 4.5 MBq·kg−1 (body weight) of 18F-FDG was administered intravenously. Patients were positioned supine in the PET/CT scanner at either 60 min (first scan; 3 min·bed−1 for whole-body imaging) or 75 min (second scan; 5 min·bed−1 for thoracic region) after tracer injection. Focal 18F-FDG uptake patterns, such as focal or focal-on-uptake, were defined as positive findings [7–10].
Cardiac MRI imaging
CMR studies were performed using a 1.5-T scanner (Symphony; Siemens Medical System, Erlangen, Germany or Philips Medical Systems, Best, The Netherlands) with a previously described protocol [6]. Late gadolinium enhancement (LGE)-CMR imaging was performed 10–15 min after administration of Gd-DTPA (0.1 mmol·kg−1, Magnevist; Berlex Laboratories, Wayne, NJ, USA) with an inversion recovery prepared three-dimensional fast field echo pulse sequence. Myocardial LGE was assessed visually by a board-certified radiologist with CMR experience of more than 20 years (N.O.M.) and considered to show positive results if the findings were confirmed in both short- and long-axis orientations, as in previous studies.
Statistical analysis
Descriptive statistics are expressed as number (percentage) for categorical variables and mean±sd for continuous variables. A chi-squared test was used to determine significant differences in positive tests and prevalence rates between the normal and abnormal groups. For all analyses, a p-value less than 0.05 was considered statistically significant. All analyses were performed using SAS software (version 9.3; SAS Institute).
Results
Patient characteristics
The patient characteristics are shown in table 1. Among the 112 patients, 99 (88%) had lung biopsy-proven sarcoidosis and 13 (12%) had extra-lung biopsy-proven sarcoidosis. Among these patients, 33 were categorised into the normal group, which showed both normal ECG and TTE findings, and 79 patients were categorised into the abnormal group, which showed abnormalities on ECG and/or TTE. The mean±sd patient age was 55±17 years, and both groups predominantly included women (64% females). Lymph nodes were the most frequently involved organs, followed by the lung, eye, skin and liver. The number of involved organs, except for the heart, was 2.70 in the normal group and 2.68 in the abnormal group.
TABLE 1.
Patient characteristics
| All | Normal | Abnormal | p-value | |
| Subjects n | 112 | 33 | 79 | |
| Age years, mean±sd | 55.2±17.2 | 52.6±16.5 | 56.3±17.5 | 0.30 |
| Women | 72 (64) | 25 (76) | 47 (59) | 0.10 |
| Biopsy site n (lung/extra-lung) | 99/13 | 30/3 | 69/10 | 0.58 |
| Steroid n (yes/no) | 11/101 | 3/30 | 8/71 | 0.87 |
| Involved organ(s) | ||||
| Eye | 73 (65) | 21 (64) | 52 (66) | 0.82 |
| LN | 105 (94) | 31 (94) | 74 (94) | 0.96 |
| Lung | 77 (69) | 23 (70) | 54 (68) | 0.89 |
| Skin | 22 (20) | 5 (15) | 17 (22) | 0.44 |
| Liver | 5 (4) | 2 (6) | 3 (4) | 0.60 |
| Number of involved organ(s) except for heart, mean±sd | 2.69±0.95 | 2.70±0.95 | 2.68±0.95 | 0.95 |
| Positive FDG PET consistent with CS | 51 (46) | 9 (27) | 42 (53) | 0.012 |
| Positive LGE-CMR consistent with CS | 70 (63) | 14 (42) | 56 (71) | 0.005 |
| Diagnosis of CS | 49 (44) | 6 (18) | 43 (54) | 0.0004 |
Data presented as n (%) unless otherwise stated. LN: lymph node; FDG PET: 18F-fluorodeoxyglucose positron emission tomography; CS: cardiac sarcoidosis; LGE-CMR: late gadolinium-enhanced cardiac magnetic resonance imaging.
Frequency of positive scans
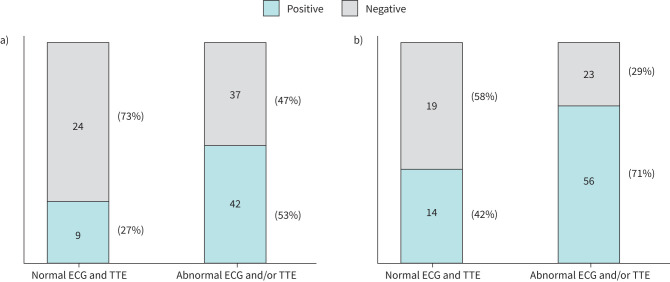
Nine of the 33 patients (27%) in the normal group and 42 of the 79 patients (53%) in the abnormal group showed positive findings consistent with active CS on 18F-FDG PET. The frequency of positive scans was higher in the abnormal group (p=0.012) (figure 1a). In contrast, 14 of the 33 patients (42%) in the normal group and 56 of 79 patients (71%) in the abnormal group had positive findings consistent with CS on CMR, with a significantly higher frequency in the abnormal group (p=0.005) (figure 1b).
FIGURE 1.
Frequency of positive scans. a) Positive 18F-fluoro-deoxyglucose positron emission tomography (18F-FDG PET) findings consistent with cardiac sarcoidosis (CS) were recorded in 27% of the patients in the normal group and 53% of those in the abnormal group, with the frequency of positive findings being significantly higher in the abnormal group (p=0.012). b) Positive cardiac MRI (CMR) findings consistent with CS were recorded in 42% of the patients in the normal group and 71% of those in the abnormal group, with the frequency of positive findings being significantly higher in the abnormal group (p=0.005). ECG: electrocardiography; TTE: transthoracic echocardiography.
Prevalence of cardiac sarcoidosis
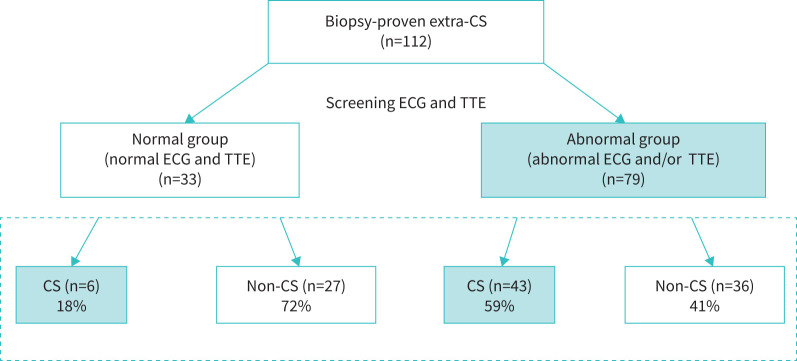
Six of the 33 patients (18%) in the normal group and 43 of the 79 patients (54%) in the abnormal group were diagnosed with CS on the basis of the updated Japanese guidelines, with a significantly higher frequency in the abnormal group (p=0.0004) (figure 2).
FIGURE 2.
Study flow and prevalence of cardiac sarcoidosis. A flow diagram illustrating the classification of normal and abnormal groups based on the ECG and TTE results. Six of the 33 patients (18%) in the normal group and 43 of the 79 patients (59%) in the abnormal group were diagnosed with CS on the basis of Japanese guidelines, with a significantly higher frequency in the abnormal group (p=0.0004). CS: cardiac sarcoidosis; ECG: electrocardiography; TTE: transthoracic echocardiography.
Detailed results for the six patients with CS in the normal group
Table 2 summarises the findings for the six patients who were diagnosed with CS in the normal group. Among these six patients, three showed small cardiac involvement (one in the right ventricular papillary muscle, one in the lateral wall of the left ventricle (LV), and one in the inferoseptal wall of the LV), two showed multiorgan involvement (five organs involved), and one showed a reduced LV ejection fraction (LVEF) (49%). For the three patients with small cardiac involvement, follow-up studies showed disappearance of focal myocardial 18F-FDG uptake and remnant LGE, which suggested natural remission of active CS. Among the two patients with multiorgan involvement, one developed syncope with a transient second-degree atrioventricular block type 2 (AVB type II). The patient was administered oral corticosteroids (prednisolone 30 mg·day−1 orally), and syncope did not recur subsequently. The other patient with multiorgan involvement underwent a follow-up study, which showed significantly increased focal 18F-FDG uptake and enlarged distribution of LGE, consistent with deterioration of active CS. Corticosteroid therapy was initiated, and a follow-up TTE examination performed after 4 weeks revealed thinning and hypokinesis of the basal segment of the interventricular septum. The LGE persisted, but the focal myocardial 18F-FDG uptake decreased significantly. The sixth patient, who showed a relatively reduced LVEF, underwent corticosteroid treatment (prednisolone 30 mg·day−1 orally) at the time of diagnosis. The patient's LVEF improved to the normal range. Figures 3–5 show the representative cases.
TABLE 2.
Detailed results for the six patients with cardiac sarcoidosis (CS) in the normal group
| No. | Sex | Age years | Involved organ(s) except for heart | 18F-FDG PET | Cardiac MRI | ACE IU L −1 | Comments | Follow-up timings of PET and MRI | Outcome |
| 1 | F | 51 | LN#, skin# | FDG uptake in the RV papillary muscle | LGE in the RV papillary muscle | 21.4 | Small cardiac lesion in the RV | 6 M, 12 M, 24 M, 42 M | Natural remission after 3.5 years; FDG uptake disappeared and LGE remained at 42 M |
| 2 | F | 22 | Eye, LN#, lung# | FDG uptake in the mid-segment of the lateral wall of the LV | LGE in the mid-segment of the lateral (epicardial and endocardial layers) wall of the LV | 28.9 | Small cardiac lesion in the lateral wall of the LV | 6 M | Natural remission after 6 M; FDG uptake disappeared and LGE remained at 6 M |
| 3 | M | 35 | Bone, LN, skin#, stomach# | FDG uptake in the basal to mid-segments of the anteroseptal walls of the LV | LGE in the basal to mid-segment of the anteroseptal walls (subepicardial layer) of the LV | 23.5 | Multiorgan involvement (bone, LN, skin, stomach and heart) | 1 M, 6 M | Syncope with transient 2nd degree AVB and initiate steroid therapy. AVB improved; FDG uptake disappeared and LGE remained at 1 M and 6 M |
| 4 | F | 71 | Eye, LN#, lung | FDG uptake in the basal segments of the anterolateral and inferoseptal walls of the LV | LGE in the basal segment of the anterolateral (subepicardial layer) and inferoseptal walls (mid-layer) of the LV | 14.9 | Small cardiac lesions in the anterolateral and inferoseptal walls of the LV | 24 M | Natural remission after 24 M; FDG uptake disappeared and LGE remained at 24 M |
| 5 | F | 25 | Eye, LN#, lung, spleen | FDG uptake in the mid to apical segments of the anteroseptal and inferior walls | LGE in the mid-segments of the anteroseptal (subendocardial layer) and inferior walls (subepicardial layer) | 18.4 | Multiorgan involvement (eye, LN, lung, spleen and heart) | 3 M, 4 M | Follow-up after 3 M revealed progression of active cardiac and extracardiac involvements; degree and extent of FDG uptake and LGE enhanced at 3 M. Start steroid therapy. FDG uptake reduced and LGE remained at 4 M |
| 6 | M | 35 | LN, lung# | FDG uptake in the basal to mid-segments of the anterior wall and mid to apical segments of the septum | LGE in the basal to mid-segments of the anterior to anterolateral walls (subepicardial layer) and mid-segment of the septum (RV side) | 7.9 | Mildly reduced LVEF (49%) | 1 M, 3 M, 12 M | Start steroid therapy to preserve LVEF; no FDG uptake and LGE remained at 1 M, 3 M and 12 M; LVEF recovered to the normal range |
FDG: 18F-fluorodeoxy glucose; PET: positron emission tomography; MRI: magnetic resonance imaging; ACE: angiotensin-converting-enzyme; M: months; LN: lymph nodes; LV: left ventricle; RV: right ventricle; LGE: late gadolinium enhancement; AVB: atrioventricular block; LVEF: left ventricular ejection fraction. #: organ with diagnostic histology consistent with sarcoidosis.
FIGURE 3.
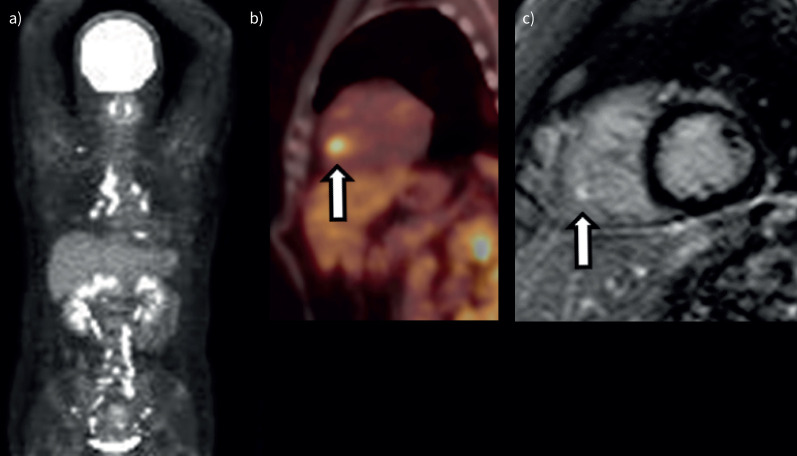
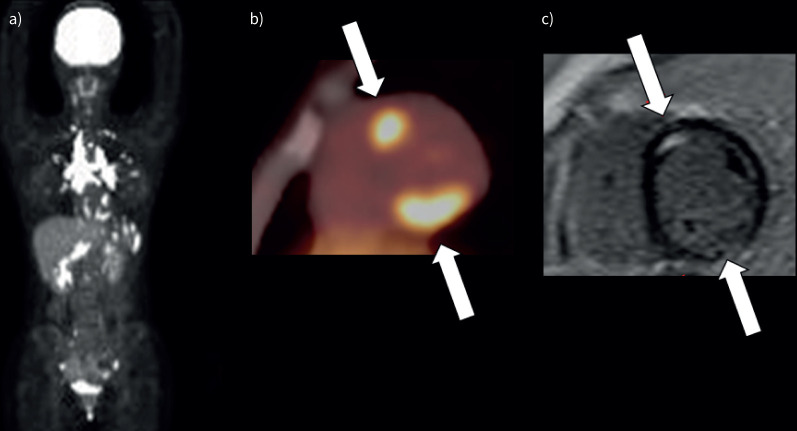
Representative images (small cardiac lesion) (patient 1 in table 2). a). Maximum intensity projection 18F-fluoro-deoxyglucose positron emission tomography (18F-FDG PET) image shows 18F-FDG uptake in the mediastinal, bilateral hilar and abdominal lymph nodes and heart. b) 18F-FDG PET image shows focal myocardial 18F-FDG uptake in the right ventricular papillary muscle (arrow). c) Late gadolinium-enhanced magnitude inversion recovery image in the short axis shows hyperenhancement in the right ventricular papillary muscle (arrow).
FIGURE 5.
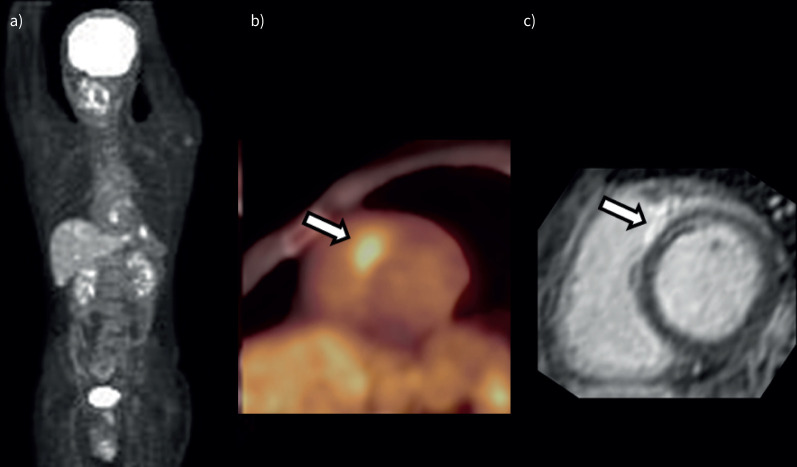
A case with reduced LV ejection fraction (LVEF) (patient 6 in table 2). a) Maximum intensity projection 18F-fluoro-deoxyglucose positron emission tomography (18F-FDG PET) image shows 18F-FDG uptake in the mediastinal lymph nodes and heart. b) 18F-FDG PET image shows focal myocardial 18F-FDG uptake in the basal segment of the anteroseptal wall (arrow). c) Late gadolinium-enhanced magnitude inversion recovery image in the short axis shows hyperenhancements in the basal segment of the anteroseptal wall (arrow).
FIGURE 4.
Representative images (multiorgan involvement) (patient 5 in table 2). a) Maximum intensity projection 18F-fluoro-deoxyglucose positron emission tomography (18F-FDG PET) image shows 18F-FDG uptake in the mediastinal, bilateral hilar and abdominal lymph nodes, spleen and heart. b) 18F-FDG PET image shows focal myocardial 18F-FDG uptake in the mid-segments of the anteroseptal and inferior walls (arrows). c) Late gadolinium-enhanced magnitude inversion recovery image in the short axis shows hyperenhancements in the mid-segments of the anteroseptal and inferior walls (arrows).
Discussion
We investigated the prevalence of cardiac involvement and explored the characteristics of biopsy-proven extra-CS in patients showing normal ECG and/or TTE findings. Among the 112 patients with biopsy-proven extra-CS, 33 (29%) showed normal ECG and TTE findings, while 79 (71%) showed abnormalities on ECG and/or TTE. CS was observed in six of the 33 patients (18%) in the normal group and 43 of the 79 patients (54%) in the abnormal group. All six CS patients with normal ECG and TTE findings had a clinically silent condition at baseline. Among these patients, three showed natural remission, two showed clinical deterioration and one patient showed a clinically stable condition. Our data demonstrated that ∼20% of extra-CS patients with normal ECG and TTE findings have clinically silent CS and half of them subsequently show spontaneous remission, but some patients could clinically manifest CS subsequently.
The HRS consensus statement recommends that patients with biopsy-proven extra-CS should be asked about unexplained syncope, presyncope or significant palpitations and screened for cardiac involvement with ECG (class I) and TTE (class IIa). In contrast, advanced cardiac imaging procedures, such as 18F-FDG PET or CMR, are not recommended for patients without abnormalities on initial screening by symptoms, ECG or TTE [1].
Previous studies have indicated that most patients with extra-CS show subclinical cardiac involvement. Autopsy studies have estimated that the prevalence of cardiac involvement was 25% to 75%, with the highest prevalence reported in Japanese cohorts [11–15]. Several studies have shown that clinically silent CS is not negligible in patients with extra-CS (4%–55%) [16–20]. Smedema et al. evaluated 82 patients with pulmonary sarcoidosis who showed no cardiac-related symptoms. They were screened by ECG, TTE, 201Tl myocardial perfusion imaging or CMR. Three of the 82 (3.7%) patients were diagnosed with CS on the basis of the modified Japanese guidelines proposed in 1993. None of these patients showed significant cardiovascular complications or died over the mean follow-up period of 1.7 years [16]. Patel et al. screened for cardiac involvement in 81 patients with extra-CS. 10 of the 81 (12.2%) patients were diagnosed with CS on the basis of the 1993 modified Japanese guidelines. Among these patients, eight had major adverse events: five died, two had ventricular tachycardia and one required permanent pacemaker implantation due to an advanced atrioventricular block over a follow-up period of 21±8 months [17]. Mehta et al. interviewed extra-CS patients to determine whether they experienced cardiac-related symptoms and performed a baseline evaluation with ECG and TTE [18]. Patients with symptoms or abnormal findings were studied using 18F-FDG PET or CMR. Of the 62 patients, 24 (39%) were diagnosed with CS on the basis of the 1993 modified Japanese criteria (including hypermetabolism on 18F-FDG PET). Among the patients with CS, symptoms, ECG and TTE showed abnormalities in 11 (46%), two (8%), and six patients (25%), respectively. None of the patients died over a 2-year follow-up period, but three required implantable cardioverter-defibrillator implantation. Kouranos [21] et al. investigated the complementary role of CMR to ECG and TTE. In their report, they demonstrated that for biopsy-proven sarcoidosis patients with normal ECG and TTE, CMR detected cardiac involvement in ∼20% of the patients. They used HRS consensus criteria as a gold standard for diagnosis of CS. However, their findings were similar to ours. Two other studies reported the prevalence of asymptomatic CS [19, 20]. The prevalence varied due to differences in patient backgrounds and diagnostic criteria.
In 1993, the Japanese Ministry of Health and Welfare proposed guidelines for the diagnosis of CS [22]. These guidelines were subsequently updated in 2006 [23] and 2015 [2]. Positive findings on 18F-FDG PET and CMR were included as major criteria in the latest version. Diagnoses of extra-CS should be based on histological or clinical findings, and patients meeting two or more of the five major criteria are diagnosed with CS. These major criteria are as follows: 1) advanced AV block or sustained VT, 2) basal thinning of the interventricular septum or morphological abnormalities, 3) positive 18F-FDG uptake in the heart, 4) depressed LVEF less than 50% or regional wall-motion abnormality and 5) the presence of LGE on cardiac MRI. Thus, even if patients have normal ECG and TTE findings, the third and fifth criteria are still not assessed. Thus, these patients should show positive findings on 18F-FDG PET and CMR to be diagnosed with CS in accordance with the guidelines.
ECG is an appropriate examination for all patients with extra-CS [24, 25]. Previous studies have reported that ECG abnormalities, such as conduction disturbances, arrhythmias, nonspecific ST-T abnormalities or abnormal Q waves, were present in 20%–30% of patients with extra-CS [15, 26–28]. One autopsy study reported that 75% of patients with severe CS, which indicates gross evidence of cardiac granulomas or infiltration at necropsy, had arrhythmia or conduction disturbances; however, only 42% of patients with mild CS, which refers to microscopically evident granulomas, had these findings [15]. Thus, ECG is not sensitive in detecting cardiac involvement. As in our cases, patients may show no ECG abnormalities despite the presence and spread of cardiac involvement.
TTE is another useful screening test for CS that can evaluate chamber size, cardiac function and structural changes. Burstow et al. [29] reported echocardiographic abnormalities in 14% of patients with extra-CS, while other studies reported that echocardiographic abnormalities were detected in 14% to 56% of patients with extra-CS [28, 30–33]. TTE is a cost-effective and noninvasive test for assessing cardiac involvement; however, it shows low sensitivity for many aspects of CS, especially in patients with mild cardiac involvement, obesity or pulmonary disease who do not have good echocardiographic window.
Limitations
The present study has several limitations. First, the study was performed at a single centre. Second, the study was retrospective in nature. Third, the pretest preparations for the 18F-FDG PET scans differed before and after 2015. Pretest preparation is performed with the aim of reducing physiological 18F-FDG uptake in the normal myocardium, but the best methods have evolved over time. However, we believe that the differences in the pretest preparation did not affect the 18F-FDG PET scan results.
Conclusion
The prevalence of CS diagnosed by the latest Japanese guidelines in biopsy-proven extra-CS patients with normal ECG and TTE findings is ∼20%. ECG or TTE may not detect CS in patients without conduction and morphological abnormalities, although some of these patients may subsequently develop clinically manifested CS. Physicians should be mindful of the presence of such an unrecognised population and the risk of underdiagnosis of CS. Patients with extra-CS should be followed up carefully.
Acknowledgements
The authors wish to thank the late Dr Keiichiro Yoshinaga (Diagnostic and Therapeutic Nuclear Medicine, National Institutes for Quantum and Radiological Science and Technology, National Institute of Radiological Sciences, Chiba, Japan) for his advice on the research design and analysis. We would like to thank Editage (www.editage.com) for English language editing.
Provenance: Submitted article, peer reviewed.
Conflict of interest: None declared.
Support statement: This study was supported by Japan Society for the Promotion of Science grant 20K08042. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014; 11: 1305–1323. doi: 10.1016/j.hrthm.2014.03.043 [DOI] [PubMed] [Google Scholar]
- 2.Terasaki F, Yoshinaga K. New guidelines for diagnosis of cardiac sarcoidosis. Ann Nucl Cardiol 2017; 3: 42–45. doi: 10.17996/anc.17-00042 [DOI] [Google Scholar]
- 3.Ishimaru S, Tsujino I, Takei T, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J 2005; 26: 1538–1543. doi: 10.1093/eurheartj/ehi180 [DOI] [PubMed] [Google Scholar]
- 4.Youssef G, Leung E, Mylonas I, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med 2012; 53: 241–248. doi: 10.2967/jnumed.111.090662 [DOI] [PubMed] [Google Scholar]
- 5.Mc Ardle BA, Birnie DH, Klein R, et al. Is there an association between clinical presentation and the location and extent of myocardial involvement of cardiac sarcoidosis as assessed by 1⁸F-fluorodeoxyglucose positron emission tomography. Circ Cardiovasc Imaging 2013; 6: 617–626. doi: 10.1161/CIRCIMAGING.112.000289 [DOI] [PubMed] [Google Scholar]
- 6.Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging 2008; 35: 933–941. doi: 10.1007/s00259-007-0650-8 [DOI] [PubMed] [Google Scholar]
- 7.Gropler RJ, Siegel BA, Lee KJ, et al. Nonuniformity in myocardial accumulation of fluorine-18-fluorodeoxyglucose in normal fasted humans. J Nucl Med 1990; 31: 1749–1756. [PubMed] [Google Scholar]
- 8.Bartlett ML, Bacharach SL, Voipio-Pulkki LM, et al. Artifactual inhomogeneities in myocardial PET and SPECT scans in normal subjects. J Nucl Med 1995; 36: 188–195. [PubMed] [Google Scholar]
- 9.Morooka M, Moroi M, Uno K, et al. Long fasting is effective in inhibiting physiological myocardial 18F-FDG uptake and for evaluating active lesions of cardiac sarcoidosis. EJNMMI Res 2014; 4: 1. doi: 10.1186/2191-219X-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohira H, Ardle BM, deKemp RA, et al. Inter- and intraobserver agreement of 18F-FDG PET/CT image interpretation in patients referred for assessment of cardiac sarcoidosis. J Nucl Med 2017; 58: 1324–1329. doi: 10.2967/jnumed.116.187203 [DOI] [PubMed] [Google Scholar]
- 11.Iwai K, Sekiguti M, Hosoda Y, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis 1994; 11: 26–31. [PubMed] [Google Scholar]
- 12.Perry A, Vuitch F. Causes of death in patients with sarcoidosis. a morphologic study of 38 autopsies with clinicopathologic correlations. Arch Pathol Lab Med 1995; 119: 167–172. [PubMed] [Google Scholar]
- 13.Matsui Y, Iwai K, Tachibana T, et al. Clinicopathological study of fatal myocardial sarcoidosis. Ann NY Acad Sci 1976; 278: 455–469. doi: 10.1111/j.1749-6632.1976.tb47058.x [DOI] [PubMed] [Google Scholar]
- 14.Bargout R, Kelly RF. Sarcoid heart disease: clinical course and treatment. Int J Cardiol 2004; 97: 173–182. doi: 10.1016/j.ijcard.2003.07.024 [DOI] [PubMed] [Google Scholar]
- 15.Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978; 58: 1204–1211. doi: 10.1161/01.CIR.58.6.1204 [DOI] [PubMed] [Google Scholar]
- 16.Smedema JP, Snoep G, van Kroonenburgh MP, et al. Cardiac involvement in patients with pulmonary sarcoidosis assessed at two university medical centers in the Netherlands. Chest 2005; 128: 30–35. doi: 10.1378/chest.128.1.30 [DOI] [PubMed] [Google Scholar]
- 17.Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation 2009; 120: 1969–1977. doi: 10.1161/CIRCULATIONAHA.109.851352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta D, Lubitz SA, Frankel Z, et al. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest 2008; 133: 1426–1435. doi: 10.1378/chest.07-2784 [DOI] [PubMed] [Google Scholar]
- 19.Dhôte R, Vignaux O, Blanche P, et al. [Value of MRI for the diagnosis of cardiac involvement in sarcoidosis]. Rev Med Interne 2003; 24: 151–157. doi: 10.1016/S0248-8663(02)00808-1 [DOI] [PubMed] [Google Scholar]
- 20.Vignaux O, Dhote R, Duboc D, et al. Detection of myocardial involvement in patients with sarcoidosis applying T2-weighted, contrast-enhanced, and cine magnetic resonance imaging: initial results of a prospective study. J Comput Assist Tomogr 2002; 26: 762–767. doi: 10.1097/00004728-200209000-00017 [DOI] [PubMed] [Google Scholar]
- 21.Kouranos V, Tzelepis GE, Rapti A, et al. Complementary role of CMR to conventional screening in the diagnosis and prognosis of cardiac sarcoidosis. JACC Cardiovasc Imaging 2017; 10: 1437–1447. doi: 10.1016/j.jcmg.2016.11.019 [DOI] [PubMed] [Google Scholar]
- 22.Hiraga H, Hiroe M, Iwai K. Guidelines for diagnosis of cardiac sarcoidosis: study report on diffuse pulmonary diseases (in Japanese). Tokyo, The Japanese Ministry of Health and Welfare, 1993: pp. 23–24. [Google Scholar]
- 23.Soejima K, Yada H. The work-up and management of patients with apparent or subclinical cardiac sarcoidosis: with emphasis on the associated heart rhythm abnormalities. J Cardiovasc Electrophysiol 2009; 20: 578–583. doi: 10.1111/j.1540-8167.2008.01417.x [DOI] [PubMed] [Google Scholar]
- 24.Statement on Sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999; 160: 736–755. doi: 10.1164/ajrccm.160.2.ats4-99 [DOI] [PubMed] [Google Scholar]
- 25.Nunes H, Freynet O, Naggara N, et al. Cardiac sarcoidosis. Semin Respir Crit Care Med 2010; 31: 428–441. doi: 10.1055/s-0030-1262211 [DOI] [PubMed] [Google Scholar]
- 26.Roberts WC, McAllister HA, Ferrans VJ. Sarcoidosis of the heart. a clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med 1977; 63: 86–108. doi: 10.1016/0002-9343(77)90121-8 [DOI] [PubMed] [Google Scholar]
- 27.Thunéll M, Bjerle P, Stjernberg N. ECG abnormalities in patients with sarcoidosis. Acta Med Scand 1983; 213: 115–118. doi: 10.1111/j.0954-6820.1983.tb03701.x [DOI] [PubMed] [Google Scholar]
- 28.Gibbons WJ, Levy RD, Nava S, et al. Subclinical cardiac dysfunction in sarcoidosis. Chest 1991; 100: 44–50. doi: 10.1378/chest.100.1.44 [DOI] [PubMed] [Google Scholar]
- 29.Burstow DJ, Tajik AJ, Bailey KR, et al. Two-dimensional echocardiographic findings in systemic sarcoidosis. Am J Cardiol 1989; 63: 478–482. doi: 10.1016/0002-9149(89)90323-8 [DOI] [PubMed] [Google Scholar]
- 30.Lewin RF, Mor R, Spitzer S, et al. Echocardiographic evaluation of patients with systemic sarcoidosis. Am Heart J 1985; 110: 116–122. doi: 10.1016/0002-8703(85)90524-1 [DOI] [PubMed] [Google Scholar]
- 31.Moiseyev SV, Kornev BM, Shatkovsky NP, et al. Non-invasive diagnosis of cardiac sarcoidosis. Lancet 1987; 2: 739–740. doi: 10.1016/S0140-6736(87)91095-6 [DOI] [PubMed] [Google Scholar]
- 32.Fahy GJ, Marwick T, McCreery CJ, et al. Doppler echocardiographic detection of left ventricular diastolic dysfunction in patients with pulmonary sarcoidosis. Chest 1996; 109: 62–66. doi: 10.1378/chest.109.1.62 [DOI] [PubMed] [Google Scholar]
- 33.Kinney EL, Jackson GL, Reeves WC, et al. Thallium-scan myocardial defects and echocardiographic abnormalities in patients with sarcoidosis without clinical cardiac dysfunction. An analysis of 44 patients. Am J Med 1980; 68: 497–503. doi: 10.1016/0002-9343(80)90292-2 [DOI] [PubMed] [Google Scholar]