Abstract
To determine whether rifampin reduces concentrations of trimethoprim (TMP) and sulfamethoxazole (SMX) in serum of human immunodeficiency virus (HIV)-infected persons, levels of these agents were determined by high-performance liquid chromatography before and after more than 12 days of standard antituberculosis treatment for 10 patients who had been taking one double-strength tablet of co-trimoxazole once daily for more than 1 month. Statistically significant, 47 and 23% decreases in TMP and SMX mean areas under the concentration-time curve from 0 to 24 h (AUC0–24), respectively, were observed after administration of rifampin. N-Acetyl-SMX profiles without and with rifampin were similar. The steady-state AUC0–24 metabolite/parent drug ratio increased by 32% with rifampin administration. Our study shows that rifampin reduces profiles of TMP and SMX in serum of HIV-infected patients.
Trimethoprim (TMP)-sulfamethoxazole (SMX) (co-trimoxazole) is the drug of choice for dual prevention of Pneumocystis carinii pneumonia and toxoplasmosis and is the most widely used prophylaxis for human immunodeficiency virus (HIV)-infected patients (2). These patients show a high incidence of tuberculosis and often receive concomitant co-trimoxazole and antituberculosis treatment. Rifampin is a potent inducer of the hepatic microsomal system and produces a considerable decrease in concentrations in serum of drugs with extensive metabolism by the microsomal enzymes (1, 7). The effect of rifampin on the pharmacokinetics of TMP and SMX has not been evaluated.
In a previous clinical study, we found that rifampin can reduce the efficacy of co-trimoxazole for prophylaxis against toxoplasmosis in HIV-infected patients (8). Therefore, we initiated a drug-drug interaction study to determine if coadministration of co-trimoxazole and rifampin leads to a decrease in co-trimoxazole components in serum.
Ten adult HIV-infected patients undergoing co-trimoxazole prophylaxis admitted to our hospital with tuberculosis from November 1997 and February 1999 were included in the study. A pharmacokinetic study was carried out before beginning antituberculosis treatment and after more than 12 days of treatment. All subjects had taken one double-strength tablet of co-trimoxazole (containing 160 mg of TMP and 800 mg of SMX) once daily for more than 1 month. Patients could receive any other treatment for their illness (benzodiazepins, three patients; omeprazol, two patients; methadone, one patient; and nucleoside analogues, seven patients) as long as there was no change in therapy between the two pharmacokinetic studies, except for the start of antituberculosis treatment. This consisted of once-daily rifampin (600 mg/day), isoniazid (300 mg/day), pyrazinamide (30 mg/kg of body weight/day), and ethambutol (25 mg/kg/day). No patients received protease inhibitors.
Blood samples (8 ml) were collected for determination of serum drug concentrations at the following times relative to dose administration: prior to dosing (0 h) and at 0.5, 1, 1.5, 2, 4, 6, and 12 h postdosing. All samples were centrifuged at 900 × g for 10 min, and serum was stored at −70°C until assay.
Concentrations in serum of TMP, SMX, and N-acetyl-SMX were determined by high-performance liquid chromatography using a modification of the method reported by DeAngelis et al. (4). Chromatographic separation was achieved with a short column (30 mm by 4.6 mm inside diameter) packed with Perkin-Elmer C18, with a particle size of 3 μm. TMP, SMX and acetyl-SMX were determined simultaneously in the same chromatogram. The retention times were 1.1 min (TMP), 3.2 min (SMX), and 4.3 min (N-acetyl-SMX). Between-day coefficients of variation ranged from 6.4 to 9.6%. The sensitivity of the assay was as follows: TMP, 0.12 μg/ml; SMX, 1.25 μg/ml; and N-acetyl-SMX, 0.25 μg/ml.
Serum rifampin concentrations were determined by high-performance liquid chromatography using a modification of the method reported by Le Guellec et al. (5). As for co-trimoxazole compound, chromatographic separation was achieved with a short column (30 mm by 4.6 mm inside diameter) packed with Perkin-Elmer C18, with a particle size of 3 μm. The retention time for rifampin was 2.1 min. The batch-to-batch precision and sensitivity of the assay were 7.5% and 0.1 μg/ml, respectively.
The highest serum drug concentration over the dose interval at steady state (Cmax) and the time at which it occurred (Tmax) were obtained directly from individual concentration-time profiles. The area under the serum concentration-time curve (AUC) was calculated by using the trapezoidal rule for 0 to 24 h in the Abbottbase Pharmacokinetic Systems (Abbott Laboratories, Abbott Park, Ill.).
Descriptive statistics were summarized as median and range interval. Using the Wilcoxon test, a pairwise comparison was performed on changes in serum drug concentrations and pharmacokinetic parameters before and during rifampin administration. Statistical significance was defined as a two-sided P value of <0.05.
A total of 10 patients (9 men and 1 woman), with a median age of 33 (range, 22 to 42) years, a median weight of 56 (range, 40 to 75) kg, and a median height of 172 (range, 150 to 185) cm were enrolled in and completed the study. The mean CD4 lymphocyte count was 81 × 106 (range, 27 × 106 to 432 × 106) cells/liter.
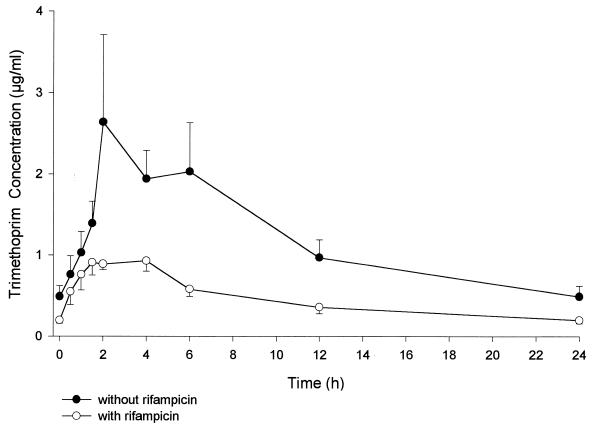
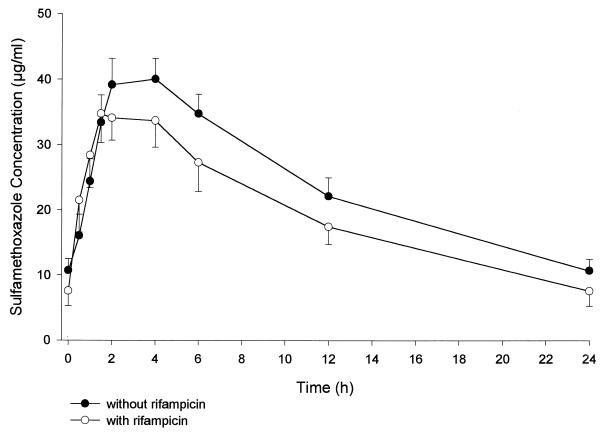
As can be seen in Table 1 and Fig. 1 and 2, serum TMP and SMX concentrations were lower in the presence of rifampin than when co-trimoxazole was given alone. TMP Cmax values were 2.0 and 1.1 μg/ml before and during rifampin administration, respectively, yielding nonsignificant differences. There was a statistically significant 47% decrease in TMP AUC0–24 with concomitant rifampin administration. The decrease in the TMP concentration during rifampin dosing was significant from 2 h postdosing on. SMX Cmax was about 42 μg/ml, with no differences before and during rifampin administration. There was a statistically significant 23% decrease in SMX AUC0–24 with concomitant rifampin administration. The decrease in serum SMX concentration with rifampin was statistically significant from 4 h postdosing on. N-Acetyl-SMX serum concentrations without and with rifampin were similar, with a nonsignificant 12% increase in AUC0–24 after rifampin dosing. The steady-state AUC0–24 metabolite/parent drug ratio increased a statistically significant 32%.
TABLE 1.
Steady-state pharmacokinetic parameters for TMP, SMX, and N-acetyl-SMX after administration of 160/800 mg of co-trimoxazole alone or with 600 mg of rifampina
| Drug and administration | Cmax (μg/ml) | Tmax (h) | AUC (μg · h/ml) | ΔAUC (%) |
|---|---|---|---|---|
| TMP | ||||
| Alone | 2.01 (1.07 to 12.99) | 2 (1.5 to 6) | 22.4 (13.5 to 65.2) | |
| With rifampin | 1.13 (0.70 to 1.94) | 2 (1 to 4) | 9.8 (7.0 to 17.7) | −46.7 (−84.9 to +5.1) |
| P | 0.17 | 0.088 | 0.013 | |
| SMX | ||||
| Alone | 42.1 (30.2 to 55.7) | 2 (1.5 to 4) | 574.2 (342.6 to 796.3) | |
| With rifampin | 41.5 (24.8 to 66.4) | 2.5 (1 to 4) | 412.4 (273.0 to 930.2) | −22.7 (−29.3 to +22.9) |
| P | 0.72 | 0.67 | 0.047 | |
| N-Acetyl-SMX | ||||
| Alone | 8.7 (5.8 to 25.4) | 4 (1 to 6) | 146.3 (63.3 to 485.2) | |
| With rifampin | 8.4 (4.2 to 21.4) | 5 (1.5 to 12) | 152.8 (57.8 to 390.5) | +12.2 (−48.9 to +80.1) |
| P | 0.57 | 0.14 | 0.79 | |
| Metabolite/parent ratio | ||||
| Alone | 0.29 (0.09 to 1.15) | |||
| With rifampin | 0.38 (0.14 to 1.18) | +31.6 (−14.1 to +164.0) | ||
| P |
The values shown are medians (ranges) (n = 10).
FIG. 1.
Mean steady-state concentrations (± standard errors of the means) of TMP in serum after administration of 160/800 mg of co-trimoxazole alone or with 600 mg of rifampin.
FIG. 2.
Mean steady-state concentrations (± standard errors of the means) of SMX in serum after administration of 160/800 mg of co-trimoxazole alone or with 600 mg of rifampin.
Median pharmacokinetic parameters (range) of rifampin were as follows: AUC0–24, 76.3 (13.1 to 113.0) μg · h/ml; Cmax, 11.2 (4.3 to 20.9) μg/ml; and Tmax, 1.75 (1 to 8) h. Cmin was <0.1 μg/ml in 7 out of 10 patients.
Our findings demonstrate that concurrent administration of rifampin resulted in lower serum TMP and SMX concentrations than when co-trimoxazole was given alone. The TMP and SMX AUC0–24 decreased a significant 47 and 23%, respectively, when administered with rifampin.
Lee et al. (B. L. Lee, H. Lampiris, D. C. Colborn, R. C. Lewis, P. K. Narang, and P. Sullam, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A36, p. 7, 1995) examined the pharmacokinetic interactions between rifabutin and co-trimoxazole in 12 HIV-infected patients. Rifabutin significantly decreased the TMP concentration and did not influence the disposition of SMX. The decreases that we found in serum TMP and SMX concentrations in the presence of rifampin were greater than those found for rifabutin. These results are in keeping with the fact that rifampin is a more potent inducer of the hepatic microsomal system than is rifabutin (6).
The oxidative drug metabolism of TMP is mediated by hepatic cytochrome P450 (CYP). Only 10 to 20% of this drug is metabolized via CYP enzymes to inactive metabolites (9). The individual isozymes involved in TMP metabolism are not well defined, though it is likely that CYP2C9 plays an important role, and CYP3A4 may also be implicated. Both isozymes are known to be induced by rifampin. More than 80% of SMX is metabolized in the liver, mainly by N-acetylation but also by glucuronidation and by hydroxylation (10). Only a small percentage of SMX is metabolized to hydroxylamine, and CYP2C9 is the primary enzyme responsible for this metabolism (3). Rifampin also induces uridine-diphosphate-glucuronosyltransferase and, therefore, might increase the glucuronidation of SMX. However, the 23% decrease in the SMX AUC0–24 and the 32% increase in the metabolite/parent ratio (N-acetyl-SMX/SMX) that we found indicate that rifampin could very likely be a mild inducer of SMX hepatic acetylation as well.
The concentrations of TMP and SMX in serum required for prophylaxis of P. carinii pneumonia and toxoplasmosis have not been established. Thus, we cannot deduce whether the moderate decrease in serum co-trimoxazole levels caused by rifampin has clinical implications. In a previous study, we found that rifampin reduced the efficacy of co-trimoxazole for the prevention of toxoplasmic encephalitis (8). This reduction in prophylactic efficacy was much more important for patients receiving low doses of co-trimoxazole than for those receiving high doses. It is reasonable to think that, if co-trimoxazole concentrations are reduced because of drug-drug interaction with rifampin, the efficacy of prophylaxis will be diminished, particularly when low doses, closer to the minimum effective dose, are given.
There are no works studying the effect of rifampin on co-trimoxazole efficacy in the prevention of P. carinii pneumonia. It is likely that the clinical repercussions of the decrease in concentrations of TMP and SMX in serum are more important in the prevention of toxoplasmic encephalitis than in that of P. carinii pneumonia, since toxoplasmosis prophylaxis requires adequate levels of the drugs in the central nervous system.
In summary, rifampin-containing antituberculosis regimens produced a significant, though moderate, decrease in TMP and SMX serum concentrations when administered concurrently with co-trimoxazole in HIV patients. The results of this study are in keeping with those from our previous clinical work, in which the lower doses of co-trimoxazole used for primary prophylaxis of toxoplasmic encephalitis showed reduced efficacy when given together with rifampin.
Acknowledgments
We thank Rosa Garos, Pilar Gil, Montse Llinas, Pilar Mateo, M. Angeles Chamorro, and Carme Profitos and the other members of the nursing staff for technical advice.
REFERENCES
- 1.Burman W J, Gallicano K, Peloquin C. Therapeutic implications of drug interactions in the treatment of human immunodeficiency virus-related tuberculosis. Clin Infect Dis. 1999;28:419–430. doi: 10.1086/515174. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1999 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. Morb Mortal Wkly Rep. 1999;48:1–67. [Google Scholar]
- 3.Cribb A E, Spielberg S P, Griffin G P. N4-hydroxylation of sulfamethoxazole by cytochrome P450 of the P4502C subfamily and reduction of sulfamethoxazole hydroxylamine in human and rat hepatic microsomes. Drug Metab Dispos. 1995;23:406–414. [PubMed] [Google Scholar]
- 4.DeAngelis D V, Woolley J L, Sigel C W. High-performance liquid chromatographic assay for the simultaneous measurement of trimethoprim and sulfamethoxazole in plasma or urine. Ther Drug Monit. 1990;12:382–392. doi: 10.1097/00007691-199007000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Le Guellec C, Gaudet M L, Lamanetre S, Breteau M. Stability of rifampin in plasma: consequences for therapeutic monitoring and pharmacokinetic studies. Ther Drug Monit. 1997;19:669–674. doi: 10.1097/00007691-199712000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Li A P, Reith M K, Rasmussen A, Gorski J C, Hall S D, Xu L, Kaminski D L, Cheng L K. Primary human hepatocytes as a tool for the evaluation of structure-activity relationship in cytochrome P450 induction potential of xenobiotics: evaluation of rifampicin, rifapentine and rifabutin. Chem-Biol Interact. 1997;107:17–30. doi: 10.1016/s0009-2797(97)00071-9. [DOI] [PubMed] [Google Scholar]
- 7.Pozniak A L, Miller R, Ormerod P. The treatment of tuberculosis in HIV-infected persons. AIDS. 1999;13:435–445. doi: 10.1097/00002030-199903110-00001. [DOI] [PubMed] [Google Scholar]
- 8.Ribera E, Fernandez-Sola A, Juste C, Rovira A, Romero F J, Armadans-Gil L, Ruiz I, Ocaña I, Pahissa A. Comparison of high and low doses of trimethoprim-sulfamethoxazole for primary prevention of toxoplasmic encephalitis in HIV-infected patients. Clin Infect Dis. 1999;29:1461–1466. doi: 10.1086/313515. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Pharmacopeia. USP-D1 drug information for the health care provider. 2nd ed. Rockville, Md: U.S. Pharmacopeia; 2000. pp. 3059–3062. [Google Scholar]
- 10.Van der Ven A J A M, Vree T B, Van Ewijk-Beneken E W J, Koopmans P P, Van der Meer J W M. Urinary recovery and kinetics of sulphamethoxazole and its metabolites in HIV-seropositive patients and healthy volunteers after a single oral dose of sulphamethoxazole. Br J Clin Pharmacol. 1995;39:621–625. doi: 10.1111/j.1365-2125.1995.tb05721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]