Abstract
Background
With the emergence of the COVID-19 pandemic, increasing numbers of cases of the multisystem inflammatory syndrome in children (MIS-C) have been reported worldwide; however, it is unclear whether this syndrome has a differential pattern in children from Latin America and the Caribbean (LAC). We conducted a systematic review and meta-analysis to analyze the epidemiological, clinical, and outcome characteristics of patients with MIS-C in LAC countries.
Methods
A systematic literature search was conducted in the main electronic databases and scientific meetings from March 1, 2020, to June 30, 2021. Available reports on epidemiological surveillance of countries in the region during the same period were analyzed.
Results
Of the 464 relevant studies identified, 23 were included with 592 patients with MIS-C from LAC. Mean age was 6.6 years (IQR, 6–7.4 years); 60% were male. The most common clinical manifestations were fever, rash, and conjunctival injection; 59% showed Kawasaki disease. Pool proportion of shock was 52%. A total of 47% of patients were admitted to the pediatric intensive care unit (PICU), 23% required mechanical ventilation, and 74% required vasoactive drugs. Intravenous gamma globulin alone was administered in 87% of patients, and in combination with steroids in 60% of cases. Length of hospital stay was 10 days (IQR, 9–10) and PICU stay 5.75 (IQR, 5–6). Overall case fatality ratio was 4% and for those hospitalized in the PICU it was 7%.
Conclusion
Limited information was available on the clinical outcomes. Improvements in the surveillance system are required to obtain a better epidemiologic overview in the region.
Keywords: MIS-C, COVID-19, SARS-CoV2, children and adolescents, prevalence, prognosis, use of resources
Introduction
COVID-19 is usually mild in pediatric patients (2). Children presenting with severe shock-like syndrome, incomplete Kawasaki disease (KD), or toxic shock syndrome (3) were reported in the United Kingdom and Italy in April 2020. Subsequently, children with a similar clinical presentation were also reported in the rest of Europe, America, and South Africa (4). The entity was identified as multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 infection with a spectrum of manifestations, such as KD, toxic shock syndrome, sepsis, and macrophage activation syndrome.
The World Health Organization (WHO) (5), the Center for Disease Prevention and Control (CDC) (6), and the Royal College of Pediatrics and Child Health of the United Kingdom (RCPCH) (7) issued definitions for case identification. Systematic reviews based on case reports, case series, and observational studies have been published; however, these include children from different backgrounds and ethnicities, mainly from Europe and North America. MIS-C appears to vary between regions, with few cases reported in children from Asia (8). Poor accessibility to the health system and delay in diagnosis and treatment in countries with limited resources may lead to a poor prognosis in children with MIS-C in Latin America and the Caribbean region (LAC). Some research has been conducted in LAC, but the topic is largely unexplored. Existing data come from specific collaborative networks of intensive therapists, cardiologists, rheumatologists, and pediatric infectious disease practitioners in the region, but these findings have not been routinely collected or analyzed. To our knowledge, there are no published systematic reviews that include surveillance data and epidemiological records about MIS-C in Latin American children.
The current study aimed to describe the clinical course, laboratory findings, epidemiology, treatment, and use of resources of MIS-C in children from LAC through a systematic review and a meta-analysis incorporating data from regional surveillance systems.
Materials and Methods
For this systematic review and meta-analysis, we followed the Cochrane methods and the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (9) statement for reporting results. The protocol for the present systematic review was registered (10) in the University of York’s PROSPERO database (CRD42021242505).
Risk of bias was assessed by two reviewers. Disagreements were resolved by consensus of the entire team. If consensus could not be reached, the conflict was resolved by a third reviewer.
For cohort and cross-sectional studies, the NIH instrument was used to score 14 items. Six studies were assessed as fair quality and three as good quality, with a score as having a moderate-to-low risk of bias. The best scores were for research questions and population selection. On the other hand, the included case series had a quality assessment as poor in five, fair in seven and good quality in one, with a high risk of bias. The only included case-control study received a fair quality score with a moderate-to-high risk of bias. In summary, most of the included studies were rated as being of low-to-moderate methodological quality with a moderate-to-high risk of bias. Risk of bias is shown in Supplementary Tables 2–4.
We applied an arc-sine transformation to stabilize the variance of proportions following the Freeman-Tukey variant of the arc-sine square root of transformed proportions method (11).
Eligibility Criteria
Comparative and non-comparative study designs were included regardless of publication status, publication year, or language. Studies without a clear denominator, narrative and case series reviews, and articles with unavailable full text and systematic reviews (SRs) were excluded; however, the reference lists of SRs on the subject were examined for relevant studies.
We searched the Latin-American and Caribbean Health Sciences Literature (LILACS), Medline, Embase, SciELO, Cochrane Library database, and WHO Database publications on MIS-C and SARS-CoV-2 and CRD York Prospero and preprint databases (ArXiv, BiorXiv, medRxiv, search.bioPreprint). We also searched the proceedings of international, national, or regional (LAC) scientific meetings from March 1st, 2020, to June 30th, 2021. The search strategies utilized can be found in Supplementary Material.
Outcomes of Interest
We explored epidemiological outcomes (prevalence, incidence, mortality), use of resources, clinical outcomes and complications, laboratory findings, and overlap with KD.
Study Selection, Data Extraction, and Assessment of the Risk of Bias in Included Studies
Pairs of reviewers independently screened articles for selection, evaluating titles and abstracts of studies. Discrepancies were solved by consensus of the whole team. We used COVIDENCE Software (12) for the initial phases of the systematic review. We also explored the reports of passive surveillance report systems from Pan American Health Organization (PAHO) and LAC countries.
Potentially eligible studies were retrieved in full text, and two reviewers independently extracted and assessed the risk of bias. Disagreements were also resolved by discussion among the review team members. For data extraction an online spreadsheet was used. This was piloted initially on ten papers to refine the process. The research team extracted study characteristics (type of publication, year published, authors, geographic location, study design including risk of bias method), population characteristics, and outcomes (incidence rate, specific mortality, and fatality rate). Authors of articles were contacted when necessary for Supplementary Information.
The risk of bias of observational studies and the control arm of trials was assessed using a checklist developed by the United States National Heart, Lung, and Blood Institute (13), which classifies studies as high (Poor), moderate (Fair), and low (Good) risk of bias. Cross-sectional and case series require 14 items, while cohort studies require nine items and case-control studies require 12 items.
Data Synthesis
To analyze the data, proportion meta-analyses were conducted. We applied arc-sine transformation to stabilize the variance of proportions (Freeman-Tukey variant of the arc-sine square-root of transformed proportions method), y = arcsine[√(r/(n + 1))] + arcsine[√(r/(n + 1)/(n + 1)], with variance of 1/(n + 1), where n is the population size. Pooled proportions were calculated as the back-transformation of the weighted mean of the transformed proportions, using inverse arcsine variance weights for the fixed and random-effects model. We applied DerSimonian-Laird weights for the random-effects model where heterogeneity between studies was found. We calculated the I2 statistic to measure the proportion of the overall variation attributable to between-study heterogeneity. The R software package meta and its functions metamean, metaprop, and forest.meta, and STATA 14.0 were used. Extracted data were synthesized using both descriptive and meta-analytic approaches by outcome measures (RRs or Mantel-Haenszel ORs) or (Peto ORs) for dichotomous data. Mean difference (MD) and 95% confidence interval (CI) were reported for all outcomes for continuous data. We used simple descriptive statistics whenever impossible to calculate association measurements.
Results
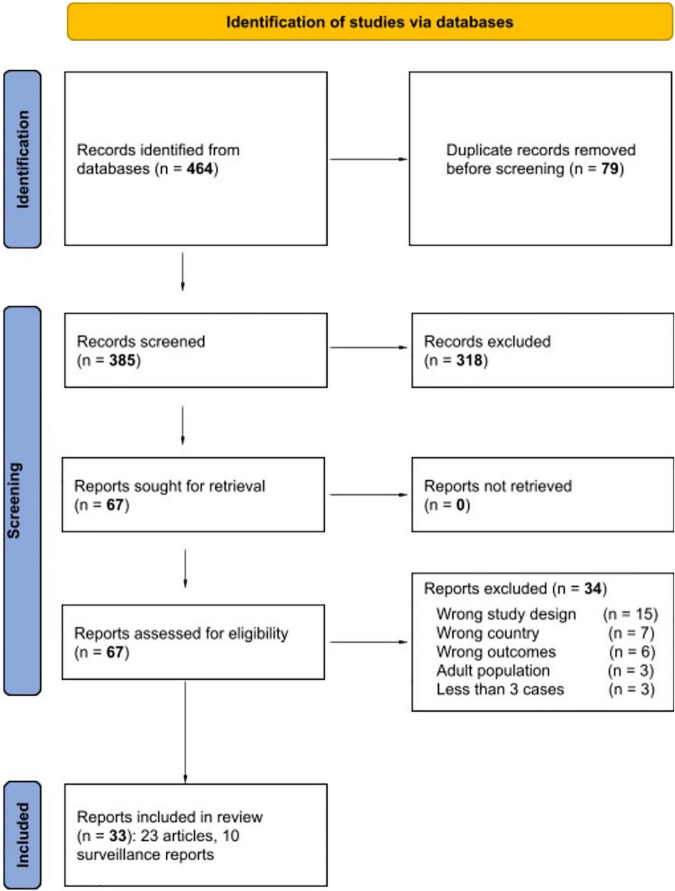
The search strategy yielded 464 potentially relevant studies in the databases. Figure 1 shows a diagram of the study selection process. Twenty-three full-text studies that met the inclusion criteria for data synthesis were included. All were observational studies with 13 case series, seven cohort studies, two cross-sectional studies, and one case-control study. In addition, surveillance system reports were included for separate analysis. The most frequent reasons for exclusion were wrong study design (N = 15), wrong country (N = 7), wrong outcomes (N = 6), adult population (N = 3), and three studies were left out because they reported fewer than three cases. The included studies collected data from 592 patients from Peru, Brazil, Argentina, Chile, Costa Rica, Colombia, Mexico, Venezuela, and other countries. The main characteristics of clinical studies of MIS-C included are described in Table 1. The mean age reported was 6.6 years (IQR, 6–7.4 years); 355 (60%) were male (95% CI, 55–65). A list of excluded studies during the full text assessment can be found in Supplementary Table 5 (65–67).
FIGURE 1.
PRISMA 2020 study flow diagram. The PRISMA 2020 checklist and the evidence quality scores of the articles included (risk of bias) can be found in Supplementary Table 1.
TABLE 1.
Characteristics of the clinical studies of MIS-C identified in Latin America and the Caribbean (N = 23).
| References | Country | Outcomes | Number of patients | Study design | Time period | Age (years) Mean (SD) | Male/ Female |
| Coronado Muñoz et al. (14) | Peru | Clinical manifestations (mortality during hospitalization) | 21 | Cohort | March-August, 2020 | 7 (5.4) | 15/6 |
| Seery et al. (15) | Argentina | Laboratory features | 21 | Cohort | May-Oct, 2020 | 6 (5.9) | 16/5 |
| Coila Paricahua et al. (16) | Peru | Use of resources | 13 | Case series | April-Oct, 2020 | 8 (2.6) | 7/6 |
| Torres et al. (17) | Chile | Clinical manifestations, laboratory features, complementary studies, and use of resources | 27 | Cohort | May-June, 2020 | 6* | 14/13 |
| De Coll-Vela et al. (18) | Peru | Clinical manifestations, laboratory features, complementary studies, and treatment | 8 | Case series | May-June, 2020 | 5.5* | 5/3 |
| Sandoval et al. (19) | Chile | Clinical manifestations (neurologic manifestations) | 17 | Case series | April–July, 2020 | 6.5* | NR |
| Fontes (20) | Brazil | Clinical manifestations | 42 | Case series | July–Dec, 2020 | 8* | 25/17 |
| Lima-Setta et al. (21) | Brazil | Clinical manifestations, laboratory features, complementary studies, and treatment | 56 | Cohort | March–July, 2020 | 6.2 (5.9) | 39/17 |
| del Aguila et al. (22) | Peru | Clinical manifestations (Kawasaki, shock), use of resources (ICU, ventilation) | 37 | Case series | Apri–August, 2020 | 8 (4.4) | 25/12 |
| Álvarez et al. (23) | Chile | Clinical manifestations (Kawasaki, shock), use of resources (ICU) | 23 | Case series | May-July, 2020 | 6.2* | 14/9 |
| Niño-Taravilla et al. (24) | Chile, Colombia, Peru, Others | Use of resources (mechanical ventilation, inotropic support, complementary studies) | 26 | Case series | May-August, 2020 | 6.5 (6.3) | 15/11 |
| Ivankovich-Escoto et al. (25) | Costa Rica | Clinical manifestations | 11 | Case series | NR | 5.4 (2.1) | 7/4 |
| Brenes-Chacón et al. (26) | Costa Rica | Clinical manifestations | 26 | Case series | March 2020–January 2021 | 6.2* | 12/14 |
| Gutiérrez-Hidalgo et al. (27) | Costa Rica | Clinical manifestations, use of resources | 4 | Case series | NR | 7 (4.9) | 2/2 |
| Luna-Muñoz et al. (28) | Peru | Clinical manifestations, use of resources | 10 | Case series | June-August, 2020 | 7 (2.4) | 7/3 |
| de Farias et al. (29) | Brazil | Clinical manifestations, laboratory features, complementary studies, and use of resources | 11 | Case series | April–June, 2020 | 4.9 (3) | 9/2 |
| Duarte-Neto et al. (30) | Brazil | Clinical manifestations, laboratory features, complementary studies, and treatment | 3 | Case series | March–August, 2020 | 8 (2.2) | 1/2 |
| Antúnez-Montes et al. (31) | Mexico, Colombia, Peru, Costa Rica, Brazil | Clinical manifestations, complementary studies, treatment, and use of resources | 95 | Cohort | June–August, 2020 | 7* | 52/43 |
| Rosanova et al. (32) | Argentina | Clinical manifestations, laboratory features, complementary studies and treatment | 25 | Case control | April–October, 2020 | 8.7* | 9/16 |
| Prata-Barbosa et al. (33) | Brazil | Clinical manifestations, laboratory features, complementary studies | 10 | Cohort | March–May, 2020 | 5.2 (5.1) | 8/2 |
| Yock-Corrales et al. (34) | Argentina, Colombia, Costa Rica, Mexico, Peru | Use of resources (antibiotic use) | 69 | Cohort | April–October, 2020 | 6 (3.5) | 45/24 |
| Pereira et al. (35) | Brazil | Clinical manifestations, laboratory features and use of resources | 6 | Cross-sectional | April–June, 2020 | 7.8* | 5/1 |
| Domínguez Rojas et al. (36) | Peru | Clinical manifestations and treatment | 31 | Cross-sectional | March–August, 2020 | 5.4* | 18/13 |
*SD not reported; NR, Not reported.
Clinical Characteristics of the Study Population
A total of 23 studies with 592 patients with confirmed MIS-C associated with SARS-CoV-2 infection were included. Of the 23 studies, 11 mentioned following the CDC criteria for MIS-C definition in at least some of the included patients (n = 291), six followed the WHO criteria (110 patients), and one the RCPCH criteria (37 patients). One study with a total of 26 patients used the criteria by the Public Health Ministry of Chile. Most studies were case series (Table 1).
The principal outcomes analyzed were laboratory features, clinical manifestations and outcome, complementary studies, treatment, and use of resources. In most of the studies included, the male sex was predominant (Table 1).
Clinical Manifestations
Most patients reported were previously healthy, while 32% had a history of one or more comorbidities, most frequently obesity, diabetes, chronic pulmonary disease, heart disease, immunocompromised, neurologic disease, and liver or kidney disease. Near half of the patients had contact with a confirmed SARS-CoV-2-infection case. One-third of the patients analyzed had positive RT-PCR SARS-CoV-2 tests, and 74% had positive SARS-CoV-2 serology tests (Table 2). The most frequent clinical manifestations were fever, rash, and conjunctival injection. KD was reported in 12 studies with a total of 161 of 273 patients (pool proportion 61%; 95% CI, 48–79%). Shock was present in 142 of 331 with a pool proportion of 52% (Table 2 and Supplementary Table 6).
TABLE 2.
Clinical characteristics, laboratory features, and use of resources: meta-analyses.
| Variables | Studies (*), n/N | Cases/Total, n/N | Pooled proportion [95% CI] from meta-analyses |
| Age, years (median, IQR) | 23/23 | 592/592 | 6.5 years (6–7.4 years) |
| Male sex | 23/23 | 355/592 | 0.60 [0.55–0.65] |
| Previously healthy | 16/23 | 250/433 | 0.71 [0.52–0.84] |
| Obesity | 9/23 | 18/179 | 0.11 [0.06–0.20] |
| Close contact | 14/23 | 153/317 | 0.59 [0.39–0.76] |
| Positive PCR | 18/23 | 139/449 | 0.32 [0.22–0.44] |
| Positive serology | 20/23 | 302/472 | 0.74 [0.58–0.86] |
| Fever | 18/23 | 378/423 | 0.99 [0.9–1] |
| Rash | 11/23 | 167/258 | 0.74 [0.51–0.89] |
| Conjunctival injection | 11/23 | 149/264 | 0.67 [0.42–0.86] |
| Kawasaki disease (KD) | 12/23 | 161/273 | 0.61 [0.48–0.79] |
| Shock | 16/23 | 142/331 | 0.52 [0.34–0.70] |
Fifteen studies reported patients with one or more gastrointestinal symptoms; diarrhea was the most common (221 patients, 60%), followed by abdominal pain (175 patients, 47%), and vomiting (144 patients, 38%). Respiratory symptoms were reported in 16 studies with 372 patients; cough was the most common symptom (94 patients, 25%), followed by dyspnea (42 patients, 11%).
In 11/23 studies neurological manifestations were reported, of which headache was the most common (82 patients, 23%). Eleven studies reported mucocutaneous symptoms, most commonly rash and conjunctival injections, followed by edema (45 patients, 17%) and lymphadenopathy (15 patients, 6%).
Inflammatory status was confirmed by laboratory tests in most studies (Table 3 and Supplementary Figure 1). CRP and D-dimer were elevated. Lymphopenia was observed in most patients. A slight decrease of albumin level was also reported.
TABLE 3.
Laboratory findings, pooled results: meta-analyses.
| Studies (*), n/N | Random effects (Mean [95% CI]) | |
| C-reactive protein (CRP) | 12/23 | 19.8 [14.27–27.54] mg/dL |
| Ferritin | 11/23 | 394.46 [294.6–528] ng/mL |
| D-dimer | 11/23 | 3,275 [2,504–4,285] ng/mL |
| Lymphocyte count | 5/23 | 1,196 [1,087–1,316] cell/mm3 |
| Platelets | 9/23 | 182,745 [172,931–193,116] cell/mm3 |
| Albumin | 7/23 | 2.83 [2.64–3.02] g/dL |
Cardiac Involvement in Patients With and Without Kawasaki Disease
Of the total 273 patients included, 59% (N = 161) were patients meeting KD criteria. In these reports, 32% (N = 87 patients) had abnormal echocardiograms, 21% (N = 59 patients) had pericarditis or pericardial effusion, 12.8% (N = 35) ventricular dysfunction, 14% (N = 38) coronary aneurysm/dilatation/abnormality, 8% (N = 23) myocarditis, 2,5% (N = 7) valvular dysfunction, 4% (N = 12) abnormal ECG, while 15% (N = 41) of the patients had elevated BNP and troponin levels.
A total of 11 reports analyzed cardiac involvement in 319 patients without KD; 8% (N = 28) had abnormal echocardiograms, 3% (N = 11) pericarditis or pericardial effusion, 2.5% (N = 8) myocarditis, 2% (N = 7) ventricular dysfunction, 4% (N = 13) coronary aneurysm/dilatation/abnormality, without valvular dysfunction or abnormal ECG, 1.6% (N = 5) had elevated BNP levels and 1.2% (N = 4) had elevated troponin levels (Supplementary Table 7).
Treatment and Use of Resource
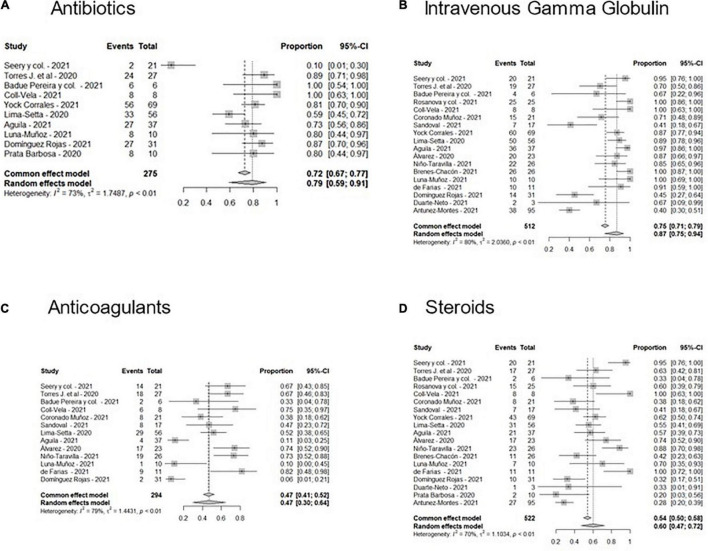
Figure 2 shows the Forest plots with meta-analyses of the different treatments administered. Most patients received antibiotics and gamma globulin alone or in combination with corticosteroids; only seven patients received tocilizumab or siltuximab. A pool of 47% of the patients received anticoagulants.
FIGURE 2.
Forest plots of the frequencies of treatments administered in MIS-C patients in Latin America. (A) Antibiotics, (B) Intravenous Gamma Globulin, (C) Anticoagulants, and (D) Steroids.
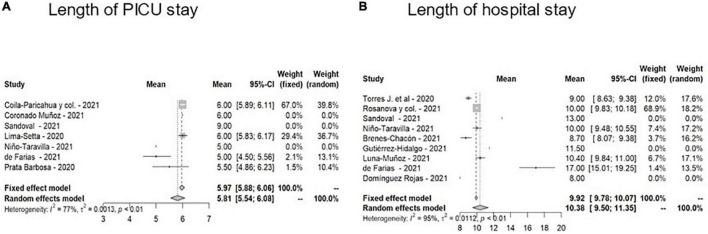
As regards the use of resources (Figure 3) in 7/23 studies, median PICU stay reported was 5.75 (IQR, 5–6 days), and in 9/23 studies, median hospital stay was 10 days (IQR 9–10). Overall case fatality rate observed was 4% (25/592).
FIGURE 3.
Forest plots of length of stay of patients in the PICU (A) or on the general ward (B).
Clinical, Laboratory, and Outcome of Multisystem Inflammatory Syndrome in Children in the Subgroup of Patients Admitted to the Pediatric Intensive Care Unit
In fifteen studies with a total of 415 patients, need for PICU admission was reported in 47% (195 patients). Six studies described severe MIS-C requiring intensive care; four studies were from Brazil, one from Peru, and the remaining one from Chile. Severe MIS-C occurred in males in 71% of cases and 34% had one or more comorbidities. Fever was observed in 98% of children and cardiovascular impairment in 85%. Mechanical ventilation was required in 23%. In only four studies, vasoactive drugs were used in 74% of the children. Most patients were treated with IVIG (88%), and 70% received steroids, 68% aspirin, and 58% anticoagulation. The overall mortality rate in patients with MIS-C admitted to the PICU was 7%. Clinical features, laboratory characteristics, and resource utilization in patients requiring PICU are described in Table 4 and in Supplementary Tables 8–10.
TABLE 4.
Pooled meta-analyses in MIS-C patients admitted to the PICU.
| Studies, n/N (*) | Random effects (Mean [95% CI]) | |
| Age | 5/5 | 5.15 [5.01–6.61] years |
| Male | 5/5 | 73% [63–82%] |
| Platelets | 4/5 | 145,344 [115,027–183,652] cell/mm3 |
| C-reactive protein (CRP) | 5/5 | 25.9 [10.76–62.37] mg/dL |
| Ferritin | 5/5 | 469.99 [372.52–592.97] ng/mL |
| D-dimer | 5/5 | 3465.55 [3,031–3,961] ng/mL |
| Non-invasive ventilation | 3/5 | 12% [4–33%] |
| Mechanical ventilation | 5/5 | 36% [11–70%] |
| Inotropes | 3/5 | 73% [55–85%] |
| IVIG | 4/5 | 88% [79–93%] |
| Steroids | 5/5 | 64% [28–89%] |
Data Analysis From Regional Surveillance System Reports
Among the countries from the Region of the Americas, Brazil was one of the most severely affected, registering a total of 11,439,558 cumulative confirmed cases of COVID-19 in the general population and 277,102 deaths by March 13, 2021 (37). Data from the epidemiological surveillance system showed that in Brazil, among 14 epidemiological week reports in 2020 and 10 in 2021, a total of 813 cases of MIS-C and 51 deaths were recorded in children and adolescents aged 0–19 years, with a mortality rate of 6.3%.
Most of the cases belonged to patients under 10 years of age, with 41% in the age group 0–4 years, followed by 34% in the group 5–9 years; 56.7% were male.
When analyzing Pan American Health Organization (PAHO) epidemiological reports, between May 2020 to March 2021, Brazil notified 769 confirmed cases of MIS-C and 47 deaths, a relatively lower number than the cases registered in the country’s surveillance system for the same period analyzed (38, 39).
COVID-19 was reported in 19,433 cases in Chile during the period analyzed, 10.2% younger than 19 years old. Similarly, between the 15th weekly report in 2020 and the 12th in 2021, 174 MIS-C cases and 3 deaths were reported in children and adolescents under 19 years of age, with a mortality of 1.7%.
Regarding the temporal distribution of both events, the highest number of COVID-19 cases in children and adolescents was registered between the 23rd and 25th weekly epidemiological reports in 2020 and the 9th to 10th of 2021, while the highest number of MIS-C cases was reported a few weeks later, between the 25th and 28th weekly epidemiological reports in 2020 and the 10th of 2021, although in the latter period the absolute number of cases was lower. The mean age of MIS-C cases was 6 years (IQR 7–9 years) and more than half of the patients, 99 (57%), were male (40). Between May 2020 and March 10th, 2021, Chile reported to PAHO 157 cases of MIS-C and two deaths (39).
As of November 28, 2020, Ecuador recorded 190,909 confirmed cases of COVID-19 and 13,371 deaths (41). Until epidemiological week 48 in 2020, 128 suspected cases of MIS-C in children and adolescents were registered, with the highest number during epidemiological week 24–25. Distribution by age group, most of the cases belonged to the 5–9-year-old group (n = 45; 35.2%) and the 10–14-year-old group (n = 33; 25.8%), 61.7% were male (42, 43). Among the 127 reported cases, three required critical care. By October 8, 2020, Ecuador reported to PAHO 7 confirmed cases, 16 probable and 90 cases with suspected MIS-C, and no deaths.
On September 2, 2020, the Dominican Republic recorded 113,962 confirmed cases of COVID-19 in the general population and 2,128 deaths 0.41 in 40 weekly epidemiological reports during 2020, the same country reported 65 cases of MIS-C and two deaths in children and adolescents aged 0–15 years. Distribution by age group was like Brazil, with the 0–4 age group being the most affected followed by the 5–9 age group.
Discussion
In our systematic review of MIS-C in LAC and analysis of data from regional surveillance systems performed we found significant heterogeneity. Most studies were case series. Countries included in our study were Argentina, Brazil, Chile, Colombia, Costa Rica, Mexico, Peru, and Venezuela. Health-Ministry data on MIS-C are very patchy and even the PAHO reports are deficient because limited information is available for Latin America and the Caribbean countries, related probably to the lack of mandatory notification of MIS-C in some countries.
Data from the epidemiological surveillance system show that during 2020, the highest number of MIS-C cases was observed around 3 weeks after the highest number of COVID-19 cases in children and adolescents in Chile. A 2–5-week time lag has been observed between the peak of COVID-19 cases within communities and the increase in MIS-C cases. This suggests that acquired immunity may play a role in its development.
Likewise, it has been observed that the number of MIS-C cases reported by PAHO is lower than that reported by the epidemiological reports of the countries within similar temporal parameters. Nevertheless, this may be related to under-registration of cases due to differences in epidemiological records. Also, the reporting of data from the central level may not reflect the behavior of the surveillance system at the subnational level since delays in reports could occur due to the registration of a large number of suspected cases of COVID-19. MIS-C case fatality rate for the same period was lower in Chile than in Brazil according to country surveillance reports. Chilean MIS-C cases and mortality reported by PAHO were slightly lower than those recorded by the country’s surveillance system for the same period. The pooled case fatality rate from 23 reports of 4% was slightly higher than the 1–2% overall reported rate from other regions (44).
Similar to other regions, in LAC countries more than half the patients were male (45). The median age of the included patients was 6.6 years, slightly lower than 8–9 years described in other populations. Apparently, the affected population in LAC is younger than in other regions, such as Europe and North America. It would be interesting to investigate possible causes, such as sociodemographic, ethnic, or other variables that could determine this difference (44, 46–49).
In general terms, the distribution of cases by sex was similar with a frequency of male sex between 51 and 58%. Gender was not related to severity of the clinical course; however, we observed that 73% of patients requiring PICU admission were male. In adults, male sex is a risk factor for severe COVID-19. Specifically, in a Latin-American study including data from patients with COVID-19 and MIS-C, girls had a lower frequency of hospitalization without differences in mortality among patients who developed MIS-C (44, 46–50).
In 59% of patients with MIS-C, close contact with a person with COVID-19 was described. This finding was more frequent than in other reviews reporting only 15–20% (44). The high percentage of household infections in Latin-America may be related to overcrowded housing conditions and other socio-demographic factors. In most epidemiological surveillance registries from Chile and other Latin American countries, most cases were confirmed by clinical or epidemiological criteria with a low frequency of concomitant positive SARS-CoV-2 tests.
Around one-third of the patients had one or more comorbidities. This has also been described in other reviews, with a frequency between 20 and 25%. The most observed comorbidities were obesity and/or overweight, chronic respiratory diseases, onco-hematological diseases, and neurological disorders (64). This finding confirms previous reports showing that MIS-C predominantly affects healthy individuals (44, 49, 51, 52). In most reviews, MIS-C diagnosis was made by serology in 60% to almost 90% of cases, while direct diagnostic methods such as PCR for SARS-CoV-2, were positive in swabs in less than of 40% of cases, similar to our findings. This would support the fact that MIS-C occurs as a post-infection phenomena (47, 48, 51, 53, 54).
Regarding the presenting signs and symptoms, fever was the most frequent followed by gastrointestinal manifestations, rash, and conjunctival injection. Neurological, respiratory, and cardiological manifestations were also reported. In most reports, fever and abdominal or gastrointestinal symptoms (abdominal pain, diarrhea, and vomiting) are described as the most frequent (46, 48, 53). On the other hand, the presenting symptoms of MIS-C may mimic an acute abdomen, including acute appendicitis, as shown in a multicenter Latin-American study, in which 34/1,010 (3.3%) patients with COVID-19 and MIS-C had an intraoperative diagnosis of appendicitis (55). Furthermore, a systematic review of abdominal pain in MIS-C found an incidence of acute abdomen of 19%, which was non-surgical in most cases. Patients who required surgery due to appendicitis or obstruction were 17/72 (23.6%) patients with acute abdomen (55, 56).
Mucocutaneous manifestations, such as rash were reported between 40 and 60%; conjunctivitis in 50%, similar to our study (52). Neurological involvement was less frequent, with headache, neck stiffness, and visual disturbances reported in 30% of cases (50). MIS-C patients generally have less respiratory symptoms than COVID-19 as reported in large series and reviews. In a systematic review, around 50% of the patients showed respiratory symptoms, including cough or dyspnea with radiological findings (51–54). A variable proportion of patients with MIS-C develop hypotension and shock because of acute myocardial dysfunction or hyperinflammatory state. In our study, hemodynamic involvement was less frequent compared to other reviews (48, 54, 57).
In the current review, 59% of MIS-C cases had Kawasaki-like syndrome. Kaushik et al. reported cardiac involvement in MIS-C patients with Kawasaki-like syndrome, of whom 20% had coronary artery dilatation/aneurysms and hypotension and 28% shock, like our findings. Despite some similar phenotypic characteristics between MIS-C and KD, there are other differential parameters, such as age less than 5 years in children with KD and the fact that approximately 7% of KD patients present with cardiovascular collapse (KDSS). In our study, 49% of the patients evolved to shock (58).
Inflammatory markers (CRP, D-dimer, and ferritin) were elevated and so was the incidence of lymphopenia. Feldstein et al. reported similar findings in patients with MIS-C compared to those with severe acute COVID-19 (8). In addition, in patients with MIS-C who required PICU admission we observed elevated inflammatory markers and lower lymphocyte and platelet counts compared to patients with MIS-C in general. These findings are consistent with other systematic reviews (53).
When comparing the overall analysis of patients included in our study with that of the subgroup of those with severe MIS-C, an increased male predominance (71% vs. 59%), a higher frequency of comorbidities, higher CRP levels, and lower lymphopenia counts are found. Different series of patients with severe MIS-C reported similar features (59, 60). Tripathi et al. reported a comorbidity rate (33%) similar to our study (61). Mortality was higher when compared to that of developed countries (7% vs. 3–4.7%), but lower than that observed in the Colombian MISCO study (9%) (61). It is likely that the delay in access to health care, diagnosis, and patient care explains, at least in part, this higher mortality and heterogeneity between countries in the region. In a report by Farias et al. a high frequency of pre-existing diseases and immunosuppression was found, which may have contributed to the high mortality rate.
IVIG and/or steroids are proposed as first-line treatment in patients with MIS-C, although to date there are no controlled studies comparing IVIG and corticosteroids alone or in combination. Some observational studies have found combination therapy to be beneficial. A French study including 111 patients with MIS-C found a lower rate of treatment failure in those who received combination therapy vs. IVIG as monotherapy (62). A US study in 518 MIS-C patients observed that combination therapy was associated with a lower risk of new or persistent cardiovascular dysfunction compared to IVIG alone. Both studies found a lower requirement for second-line therapy and hemodynamic support within 24–48 h after initial treatment (63). On the other hand, a large study carried out in multiple countries comparing three treatment regimens (combination therapy, IVIG, or corticosteroids alone) found no differences in morbidity and mortality. Nevertheless, the selection and outcome criteria were different in the three studies (45). In our study, most patients received IVIG, in combination with corticosteroids in more than half of the patients.
In agreement with a study by Pereira et al. (35) we observed an increased use of antibiotics, related to the main differential diagnoses of MIS-C, septic shock, and toxic shock syndrome. Antibiotic prescriptions among MIS-C patients may not have been appropriate in most cases and antimicrobial stewardship should be explored in MIS-C patients. Tocilizumab or siltuximab were used only in a small number of patients.
Other frequently used medications as part of treatment were aspirin and anticoagulants. The wide variety of treatments used may have been due to the lack of knowledge of MIS-C, especially in the first months after described in Europe. In addition, many treatments are unavailable in the region due to their high costs. Most importantly, there is still no clear consensus on the treatments to be administered beyond the recommended use of IVIG and corticosteroids.
PICU admissions were less frequent than in other reports (44, 48). One possible explanation is that at the moment that MIS-C started to be reported, no guidelines or alerts to detect and treat children with MIS-C were available. Use of resources in terms of length of hospital and PICU stay were like other reports (48).
Information available from LAC was found to be limited, probably related to the lack of mandatory notification of MIS-C locally. One of the key limitations of the study is that most of the data reported were from case series and were highly heterogeneous. To our knowledge this is the first systematic review with meta-analysis incorporating data from regional surveillance systems from LAC.
Conclusion
The results of the present study provide important information to help understanding MIS -C in children and adolescents from LAC. Epidemiological information in the region was very limited. Optimization of case registration and surveillance is needed. Knowledge about this syndrome and the impact of the recent introduction of COVID-19 vaccination schedules in several Latin American countries is still insufficient.
Author Contributions
SRu and AB conceived the study. CV, MR, AF, NV, MB, SRo, and RU-G collected and analyzed the studies included. SRu, CV, MR, AF, NV, MB, SRo, RU-G, and AB drafted the manuscript. All authors contributed and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Agustín Ciapponi, epidemiologist from IECS for general guidance, and Daniel Comandé, librarian at IECS and María Andrea Lerda, librarian at Hospital de Pediatría Garrahan for their help with the search strategies.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.881765/full#supplementary-material
References
- 1.Götzinger F, Santiago-García B, Noguera-Julián A. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study - the lancet child & adolescent health. Lancet Child Adolesc Heal. (2020) 4:653–61. 10.1016/S2352-4642(20)30177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. (2020) 395:1607–8. 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). World Health Organization Scientif Brief: Multisystem inflammatory syndrome in children and adolescents with COVID-19. Geneva: WHO; (2020). [Google Scholar]
- 4.World Health Organization (WHO). Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19: Scientific Brief 15 May 2020. WHO. (2020) 10:1–9. [Google Scholar]
- 5.Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, et al. Information for healthcare providers about multisystem inflammatory syndrome in children (MIS-C). Arthritis Rheumatol. (2020) 72:1791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Royal College of Paediatrics and Child Health. Royal College of Paediatrics and Child Health. Guidance - Paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS). London: Royal College of Paediatrics and Child Health; (2020). [Google Scholar]
- 7.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. (2020) 383:334–46. 10.1056/nejmoa2021680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page MJ, McKenzie JE, Bossuyt P, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. Med Flum. (2021) 57:444–65. 10.21860/medflum2021_264903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: John Wiley & Sons; (2019). 10.1002/9781119536604 [DOI] [Google Scholar]
- 10.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. (1950) 21:607–11. 10.1214/aoms/1177729756 [DOI] [Google Scholar]
- 11.Veritas Health Innovation. Covidence Systematic Review Software Melbourne, Australia. Melbourne, VIC: Veritas Health Innovation; (2021). [Google Scholar]
- 12.NIH. Study Quality Assessment Tools | NHLBI, NIH. Bethesda, ML: National Heart, Lung, and Blood Institute; (2014). [Google Scholar]
- 13.Coronado Munoz A, Tasayco J, Morales W, Moreno L, Zorrilla D, Stapleton A, et al. High incidence of stroke and mortality in pediatric critical care patients with COVID-19 in Peru. Pediatr Res. (2021): [Epub ahead of print]. 10.1038/s41390-021-01547-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seery V, Raiden SC, Algieri SC, Grisolía NA, Filippo D, De Carli N, et al. Blood neutrophils from children with COVID-19 exhibit both inflammatory and anti-inflammatory markers. EBioMedicine. (2021) 67:103357. 10.1016/j.ebiom.2021.103357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coila Paricahua E, Rodriguez Portilla R, Cieza Yamunaqué L, Baique Sánchez P, Guerra Ríos C. Presentaciones clínicas asociadas al SARS-CoV-2 en una unidad de cuidados intensivos pediátricos Covid de un hospital nacional de Lima. Rev Fac Med Hum. (2021) 21:221–3. 10.25176/rfmh.v21i1.3595 [DOI] [Google Scholar]
- 16.Torres JP, Izquierdo G, Acuña M, Pavez D, Reyes F, Fritis A, et al. Multisystem inflammatory syndrome in children (MIS-C): report of the clinical and epidemiological characteristics of cases in Santiago de Chile during the SARS-CoV-2 pandemic. Int J Infect Dis. (2020) 100:75–81. 10.1016/j.ijid.2020.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Coll-Vela LE, Zamudio-Aquise MK, Nuñez-Paucar H, Bernal-Mancilla RR, Schult-Montoya SC, Ccorahua-De La Paz M. Síndrome inflamatorio multisistémico asociado a COVID-19 en niños: serie de casos en un hospital pediátrico de Perú. Rev Peru Med Exp Salud Publica. (2020) 37:559–65. 10.17843/rpmesp.2020.373.6126 [DOI] [PubMed] [Google Scholar]
- 18.Sandoval F, Julio K, Méndez G, Valderas C, Echeverría AC, Perinetti MJ, et al. Neurologic features associated with SARS-CoV-2 infection in children: a case series report. J Child Neurol. (2021) 36:853–66. 10.1177/0883073821989164 [DOI] [PubMed] [Google Scholar]
- 19.Fontes LGM, da Costa Saavedra R, Melo do Amaral Carvalho J, Barbosa VBVR, Azevedo de Araújo F, Oliveira Manezes G. Síndrome inflamatória multissistêmica pediátrica (Sim-P) Na Bahia, Em 2020. Rev Baiana Saúde Pública. (2021) 2021:46–61. 10.22278/2318-2660.2021.v45.NEspecial [DOI] [Google Scholar]
- 20.Lima-Setta F, Magalhães-Barbosa MC, de Rodrigues-Santos G, Figueiredo EA, Jacques M, Zeitel R, et al. Multisystem inflammatory syndrome in children (MIS-C) during SARS-CoV-2 pandemic in Brazil: a multicenter, prospective cohort study. J Pediatr. (2021) 97:354–61. 10.1016/j.jped.2020.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Aguila O, Domínguez-Rojas J, Garcés-Ghilardi R, Estupiñan-Vigil M, Alvarado-Gamarra G. Síndrome inflamatorio multisistémico pediátrico asociado a COVID-19: reporte preliminar de un hospital del Perú. Rev Peru Med Exp Salud Publica. (2021) 38:180–2. 10.17843/rpmesp.2021.381.6460 [DOI] [PubMed] [Google Scholar]
- 22.Álvarez P, Acevedo V, Valenzuela ML, Montes V, Aroca P, García C, et al. Compromiso cardiovascular en pacientes con Síndrome Inflamatorio Pediátrico Multisistémico, asociado a infección por SARS-CoV-2. Rev Chil Cardiol. (2020) 39:208–15. 10.4067/s0718-85602020000300208 27315006 [DOI] [Google Scholar]
- 23.Niño-Taravilla C, Otaola-Arca H, Lara-Aguilera N, Zuleta-Morales Y, Ortiz-Fritz P. Multisystem infl ammatory syndrome in children, chile, may-august 2020. Emerg Infect Dis. (2021) 27:1457–61. 10.3201/eid2705.204591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivankovich-Escoto G, Ávila-Morales S, Oconitrillo-Chaves M, Hidalgo-Matlock B, Brenes-Chacón H, Montenegro-Villalobos J, et al. Eritema de párpados como un hallazgo llamativo en pacientes con diagnóstico de Síndrome Inflamatorio Multisistémico en niños y adolescentes asociado a COVID-19 (MIS-C). In: XXIV Congreso Interamericano de Pediatría, XV Congreso Internacional De Otorrinolaringología De La IAPO. Ciudad de México: IAPO; (2021). [Google Scholar]
- 25.Brenes-Chacón H, Montenegro-Villalobos J, Ivankovich-Escoto G, Yock-Corrales A, Ávila-Agüero ML, Camacho-Badilla K, et al. Características clínicas de paciente con Síndrome Inflamatorio Multisistémico asociado a COVID-19 (MIS-C) en un hospital pediátrico. In: XXIV Congreso Interamericano de Pediatría, XV Congreso Internacional De Otorrinolaringología De La IAPO. Ciudad de México: IAPO; (2021). [Google Scholar]
- 26.Gutiérrez-Hidalgo M, Hidalgo-Matlock B, Ivankovich-Escoto G, Brenes-Chacón H, Ulloa-Gutiérrez R. Líneas de Beau posterior a Síndrome Inflamatorio Multisistémico en niños y adolescentes asociado a COVID-19 (MIS-C). In: XXIV Congreso Interamericano de Pediatría, XV Congreso Internacional De Otorrinolaringología De La IAPO. Ciudad de México: IAPO; (2021). [Google Scholar]
- 27.Luna-Muñoz C, Reyes-Florian G, Seminario-Aliaga M, Stapleton-Herbozo A, Correa-López LE, Quiñones-Laveriano DM. Pediatric inflammatory multisystem syndrome associated with COVID-19: A report of 10 cases in a Peruvian hospital. Medwave. (2021) 21:e8142–8142. 10.5867/medwave.2021.02.8142 [DOI] [PubMed] [Google Scholar]
- 28.de Farias ECFM, Pedro Piva JP, de Mello MLFMFB, do Nascimento LMPPB, Costa CC, Machado MMMB, et al. Multisystem inflammatory syndrome associated with coronavirus disease in children a multi-centered study in Belém, Pará, Brazil. Pediatr Infect Dis J. (2020) 39:e374–6. 10.1097/INF.0000000000002865 [DOI] [PubMed] [Google Scholar]
- 29.Duarte-Neto AN, Caldini EG, Gomes-Gouvêa MS, Kanamura CT, de Almeida Monteiro RA, Ferranti JF, et al. An autopsy study of the spectrum of severe COVID-19 in children: From SARS to different phenotypes of MIS-C. EClinicalMedicine. (2021) 35:100850. 10.1016/j.eclinm.2021.100850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antúnez-Montes OY, Escamilla MI, Figueroa-Uribe AF, Arteaga-Menchaca E, Lavariega-Saráchaga M, Salcedo-Lozada P, et al. COVID-19 and multisystem inflammatory syndrome in Latin American Children: a multinational study. Pediatr Infect Dis J. (2020) 40:e1—-e6. 10.1097/INF.0000000000002949 [DOI] [PubMed] [Google Scholar]
- 31.Rosanova MT, Perez G, Katsicas MM, Arias AP, Picollo M, Palladino M, et al. Pediatric inflammatory multisystem syndrome associated with SARS-CoV-2: a retrospective cohort study from Argentina. Ind Pediatr. (2021) 58:639–42. 10.1007/s13312-021-2259-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prata-Barbosa A, Lima-Setta F, Santos GR, dos Lanziotti VS, de Castro REV, de Souza DC, et al. Pediatric patients with COVID-19 admitted to intensive care units in Brazil: a prospective multicenter study. J Pediatr. (2020) 96:582–92. 10.1016/j.jped.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yock-Corrales A, Lenzi J, Ulloa-Gutiérrez R, Gómez-Vargas J, Antúnez-Montes OY, Rios Aida JA, et al. High rates of antibiotic prescriptions in children with COVID-19 or multisystem inflammatory syndrome: a multinational experience in 990 cases from Latin America. Acta Paediatr. (2021) 110:1902–10. 10.1111/apa.15847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira MFB, Litvinov N, Farhat SCL, Eisencraft AP, Gibelli MABC, de Carvalho WB, et al. Severe clinical spectrum with high mortality in pediatric patients with covid-19 and multisystem inflammatory syndrome. Clinics. (2020) 75:1–7. 10.6061/clinics/2020/e2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Domínguez Rojas J, Estupiñan Vigil M, Garcés-Ghilardi R, Alvarado-Gamarra G, del Águila O, Lope Tenorio AF, et al. Cross-sectional study of the clinical characteristics and outcomes of children hospitalized with COVID-19 in Lima, Peru. Medwave. (2021) 21:e8107–8107. 10.5867/medwave.2021.01.8107 [DOI] [PubMed] [Google Scholar]
- 36.Ministério da Saúde. Boletim Epidemiologico Especial 54. Doença pelo Coronavírus COVID-19. Brazil: Ministério da Saúde; (2021). [Google Scholar]
- 37.Ministério da Saúde. Boletim Epidemiológico. Brazil: Ministério da Saúde; (2021). [Google Scholar]
- 38.Actualización Epidemiológica. Enfermedad por Coronavirus (COVID-19). Brazil: Ministério da Saúde; (2021). [Google Scholar]
- 39.Ministerio de Salud de Chile. Descripción Epidemiológica de Niños, Niñas y Adolescentes con COVID -19. Santiago: Ministerio de Salud de Chile; (2021). [Google Scholar]
- 40.Ministerio de Salud Pública de Ecuador. Situación Nacional por COVID-19 Infografía n°275. Inicio 29/02/2020 - corte 28/11/2020. Quito: Ministerio de Salud Pública de Ecuador; (2020). [Google Scholar]
- 41.World Health Organization (WHO). PAHO Daily COVID-19 Update. Geneva: WHO; (2021). [Google Scholar]
- 42.Ministerio de Salud Pública. Dirección General de Epidemiología. Sistema Nacional de Vigilancia Epidemiológica. Semana Epidemiológica (SE) No. 40. Santo Domingo: Gobierno de Republica Dominicana; (2020). [Google Scholar]
- 43.Kaushik A, Gupta S, Sood M, Sharma S, Verma S. A systematic review of multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection. Pediatr Infect Dis J. (2020) 39:e340–334. 10.1097/INF.0000000000002888 [DOI] [PubMed] [Google Scholar]
- 44.McArdle AJ, Vito O, Patel H, Seaby EG, Shah P, Wilson C, et al. Treatment of multisystem inflammatory syndrome in children. N Engl J Med. (2021) 385:1. 10.1056/nejmoa2102968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haghighi Aski B, Manafi Anari A, Abolhasan Choobdar F, Zareh Mahmoudabadi R, Sakhaei M. Cardiac abnormalities due to multisystem inflammatory syndrome temporally associated with Covid-19 among children: A systematic review and meta-analysis. IJC Hear Vasc. (2021) 33:100764. 10.1016/j.ijcha.2021.100764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aronoff SC, Hall A, Del Vecchio MT. The natural history of severe acute respiratory syndrome coronavirus 2-related multisystem inflammatory syndrome in children: a systematic review. J Pediatric Infect Dis Soc. (2020) 9:746–51. 10.1093/jpids/piaa112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bustos BR, Jaramillo-Bustamante JC, Vasquez-Hoyos P, Cruces P, Díaz F. Pediatric inflammatory multisystem syndrome associated with SARS-CoV-2: a case series quantitative systematic review. Pediatr Emerg Care. (2021) 37:44–7. 10.1097/PEC.0000000000002306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radia T, Williams N, Agrawal P, Harman K, Weale J, Cook J, et al. Multi-system inflammatory syndrome in children & adolescents (MIS-C): a systematic review of clinical features and presentation. Paediatr Respir Rev. (2021) 38:51–7. 10.1016/j.prrv.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brizuela M, Lenzi J, Ulloa-Gutiérrez R, Antúnez-Montes OY, Aida JAR, del Aguila O, et al. Influence of sex on disease severity in children with multisystem inflammatory syndrome and covid-19 in latin america. Ital J Gender Specific Med. (2021) 7:128–33. 10.1723/3673.36590 [DOI] [Google Scholar]
- 50.Baradaran A, Malek A, Moazzen N, Shaye ZA. COVID-19 associated multisystem inflammatory syndrome: A systematic review and meta-analysis. Iran J Allergy Asthma Immunol. (2020) 19:570–88. 10.18502/ijaai.v19i6.4927 [DOI] [PubMed] [Google Scholar]
- 51.Dhar D, Dey T, Samim MM, Padmanabha H, Chatterjee A, Naznin P, et al. Systemic inflammatory syndrome in COVID-19–SISCoV study: systematic review and meta-analysis. Pediatr Res. (2021): [Epub ahead of print]. 10.1038/s41390-021-01545-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang Y, Li W, Baskota M, Zhou Q, Fu Z, Luo Z, et al. Multisystem inflammatory syndrome in children during the coronavirus disease 2019 (COVID-19) pandemic: a systematic review of published case studies. Transl Pediatr. (2021) 10:121–35. 10.21037/tp-20-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoste L, Van Paemel R, Haerynck F. Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur J Pediatr. (2021) 180:2019–34. 10.1007/s00431-021-03993-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yock-Corrales A, Lenzi J, Ulloa-Gutiérrez R, Gómez-Vargas J, Antúnez-Montes OY, Rios Aida JA, et al. Acute Abdomen and Appendicitis in 1010 Pediatric Patients with COVID-19 or MIS-C: A Multinational Experience from Latin America. Pediatr Infect Dis J. (2021) 40:E364–9. 10.1097/INF.0000000000003240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rouva G, Vergadi E, Galanakis E. Acute abdomen in multisystem inflammatory syndrome in children: a systematic review. Acta Paediatr. (2021) 2021:16178. 10.1111/apa.16178 [DOI] [PubMed] [Google Scholar]
- 56.Zou H, Lu J, Liu J, Wong JH, Cheng S, Li Q, et al. Characteristics of pediatric multi-system inflammatory syndrome (PMIS) associated with COVID-19: a meta-analysis and insights into pathogenesis. Int J Infect Dis. (2021) 102:319–26. 10.1016/j.ijid.2020.11.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanegaye JT, Wilder MS, Molkara D, Frazer JR, Pancheri J, Tremoulet AH, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. (2009) 123:1–13. 10.1542/peds.2008-1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davies P, Evans C, Kanthimathinathan HK, Lillie J, Brierley J, Waters G, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Heal. (2020) 4:669–77. 10.1016/S2352-4642(20)30215-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Acevedo L, Piñeres-Olave BE, Niño-Serna LF, Vega LM, Gomez IJA, Chacón S, et al. Mortality and clinical characteristics of multisystem inflammatory syndrome in children (MIS-C) associated with covid-19 in critically ill patients: an observational multicenter study (MISCO study). BMC Pediatr. (2021) 21:516. 10.1186/s12887-021-02974-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tripathi S, Gist KM, Bjornstad EC, Kashyap R, Boman K, Chiotos K, et al. Society of critical care medicine discovery viral infection and respiratory illness universal study (VIRUS): COVID-19 Registry Investigator Group. Coronavirus Dis 2019-Associated PICU Admissions A Rep From Soc Crit Care Med Discov Netw Viral Infect Respir Illn Univers Study Regist Pediatr Crit care Med a J Soc Cri. (2021) 22:603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ouldali N, Toubiana J, Antona D, Javouhey E, Madhi F, Lorrot M, et al. Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA. (2021) 325:855–64. 10.1001/jama.2021.0694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Son MBF, Murray N, Friedman K, Young CC, Newhams MM, Feldstein LR, et al. Multisystem inflammatory syndrome in children — initial therapy and outcomes. N Engl J Med. (2021) 385:23–34. 10.1056/NEJMoa2102605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moragas M, Gomez S, Fernández MF, Golemba MD, Palladino M, Borgnia D, et al. Case report: hyperinflammatory status in an immunocompromised child with a highly sustained viral load of SARS-CoV-2. Front Med. (2021) 8:675282. 10.3389/fmed.2021.675282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caro-Domínguez P, Navallas M, Riaza-Martin L, Ghadimi Mahani M, Ugas Charcape CF, Valverde I, et al. Imaging findings of multisystem inflammatory syndrome in children associated with COVID-19. Pediatr Radiol. (2021) 51:1608–20. 10.1007/s00247-021-05065-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lindan CE, Mankad K, Ram D, Kociolek LK, Silvera VM, Boddaert N, et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Heal. (2021) 5:167–77. 10.1016/S2352-4642(20)30362-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kehar M, Ebel NH, Ng VL, Baquero JER, Leung DH, Slowik V, et al. Severe acute respiratory syndrome coronavirus-2 infection in children with liver transplant and native liver disease: an international observational registry study. J Pediatr Gastroenterol Nutr. (2021) 72:807–14. 10.1097/MPG.0000000000003077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bautista-Rodriguez C, Sanchez-de-Toledo J, Clark BC, Herberg J, Bajolle F, Randanne PC, et al. Multisystem inflammatory syndrome in children: an international survey. Pediatrics. (2021) 147:24554. 10.1542/peds.2020-024554 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.