Abstract
SMAP29, an ovine cathelicidin, was systematically altered to create a family of 23 related peptides for MIC and minimum bactericidal concentration determinations. SMAP28, SMAP29, and a derivative of SMAP29 called ovispirin were all antimicrobial. However, many congeners of SMAP29 and ovispirin were not as active as the parent molecules. With immunoelectron microscopy, SMAP29 was seen on membranes and within the cytoplasm of Pseudomonas aeruginosa PAO1.
Sheep myeloid antimicrobial peptides (SMAPs) are cathelicidins with broad-spectrum antimicrobial activity against gram-negative and gram-positive bacteria and fungi (1, 4, 8, 12, 14). One cathelicidin, SMAP29, has been proposed elsewhere as a potent candidate for further research in the therapeutic treatment of acute and chronic respiratory infections including Pseudomonas aeruginosa associated with chronic respiratory inflammation in cystic fibrosis (4, 12, 14). The composition of SMAP29 (also known as SC5) was first deduced from sheep myeloid DNA (1, 8), and SMAP29 was later synthesized to assess its antimicrobial activity (4, 12, 14). SMAP29 is a broad-spectrum antibiotic (4, 8, 12, 14), is active in both low- and high-ionic-strength conditions (14), and induces significant morphological alterations in bacterial surfaces (12).
The activity of cathelicidins varies depending upon the peptide composition (4, 14), and even small alterations in the molecule can dramatically alter its properties. For example, SMAP29 shows little hemolytic activity towards human or sheep erythrocytes (14), while SMAP28, with an N-terminal amine, causes hemolysis of human but not sheep erythrocytes (12). In this study, we altered SMAP29 to create a family of 23 related peptides and determined their MICs and minimum bactericidal concentrations (MBCs) for nine ovine pathogens and Aspergillus fumigatus. Polyclonal goat antiserum against SMAP29 and protein G-colloidal gold (PG-CG) was then used to detect SMAP29 on membranes and in the cytoplasm by immunoelectron microscopy.
SMAPs (Table 1) and CAP18 were synthesized as previously described (4, 14). For the broth microdilution assay (4, 15, 16), peptides were diluted in 0.4% bovine serum albumin containing 0.02% acetic acid (0.16 to 80.00 μg/ml) and added to polypropylene microtiter plates (Sigma, St. Louis, Mo.). Sodium phosphate buffer (10 mM; pH 7.2) with 140 mM NaCl (phosphate-buffered saline [PBS]) was added to control wells. Mueller-Hinton broth containing 1.0 × 105 CFU of nine ovine pathogens; P. aeruginosa PAO1, as a susceptible control (4, 14); or A. fumigatus NADC 0073 (Tables 2 and 3) per ml was added. Mueller-Hinton broth was added to wells containing PBS and used as the plate blank. After 24 and 48 h at 37°C, the optical density of bacterial growth was determined (Spectromax microplate reader; Molecular Devices Corp., Sunnyvale, Calif.). The MIC (i.e., the lowest concentration of peptide that reduced visible growth) and the MBC were determined. The hemolytic activity of the peptides was assayed with a 1.0% suspension of washed ovine erythrocytes as previously described (14).
TABLE 1.
Amino acid sequences of SMAP28, SMAP29, ovispirin, and their congeners
TABLE 2.
Antimicrobial activities of SMAP28, SMAP29, and their congenersa
| SMAP peptide |
Mannheimia haemolytica serovar:
|
Pasteurella trehalosi serovar 4
|
Salmonella enterica subsp. arizonae
|
Pasteurella multocida
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1
|
2
|
6
|
||||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| 28 | 0.9 ± 0.3 | 0.5 ± 0.1 | 1.7 ± 0.4 | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 | 1.3 ± 0.0 | 0.8 ± 0.2 | 1.3 ± 0.0 | 2.1 ± 0.4 | 0.3 ± 0.0 | 0.3 ± 0.0 |
| 29 | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.8 ± 0.2 | 3.8 ± 3.1 | 1.3 ± 0.0 | 1.3 ± 0.0 | 2.5 ± 0.0 | 3.3 ± 0.8 | 0.8 ± 0.2 | 1.5 ± 0.6 | 0.6 ± 0.0 | 0.6 ± 0.0 |
| 29-18 | >20.0 | >20.0 | 0.9 ± 0.4 | 0.7 ± 0.3 | >20.0 | >20.0 | ≥20.0 | ≥20.0 | >20.0 | >20.0 | ≥20.0 | ≥20.0 |
| 29-20 | >20.0 | >20.0 | 10.8 ± 5.1 | 2.1 ± 0.4 | ≥20.0 | ≥20.0 | 20.0 ± 0.0 | 11.7 ± 4.4 | >20.0 | >20.0 | >20.0 | >20.0 |
| 29-21 | >20.0 | >20.0 | 2.1 ± 0.4 | 5.0 ± 2.5 | >20.0 | >20.0 | ≥20.0 | 10.0 ± 0.0 | >20.0 | >20.0 | >20.0 | >20.0 |
| 29-18AA | 10.0 ± 0.0 | ≥20.0 | 8.3 ± 1.7 | 8.3 ± 1.7 | >20.0 | >20.0 | >20.0 | >20.0 | >20.0 | >20.0 | ≥20.0 | ≥20.0 |
|
Klebsiella pneumoniae
|
P. aeuruginosa PAO1
|
Corynebacterium pseudotuberculosis ATCC 19410
|
Staphylococcus avreus
|
A. fumigatus
|
Hemolytic activity (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| 0.4 ± 0.2 | 0.4 ± 0.2 | 0.6 ± 0.0 | 1.7 ± 0.4 | 20.0 ± 0.0 | ≥20.0 | 1.3 ± 0.0 | 1.0 ± 0.2 | 5.0 ± 0.0 | 11.7 ± 4.4 | ND |
| 0.6 ± 0.0 | 0.6 ± 0.0 | 0.8 ± 0.2 | 0.8 ± 0.2 | >20.0 | >20.0 | 2.5 ± 0.0 | 2.5 ± 0.0 | ND | ND | 7.9 ± 0.01 |
| ≥20.0 | >20.0 | 1.3 ± 0.0 | 1.3 ± 0.0 | >20.0 | >20.0 | >20.0 | >20.0 | >40.0 | >40.0 | 3.6 ± 0.01 |
| 20.0 ± 0.0 | 20.0 ± 0.0 | >20.0 | >20.0 | >20.0 | >20.0 | >20.0 | >20.0 | >40.0 | >40.0 | 4.0 ± 0.01 |
| 8.3 ± 1.7 | 8.3 ± 1.7 | 20.0 ± 0.0 | ≥20.0 | >20.0 | >20.0 | >20.0 | >20.0 | >40.0 | >40.0 | 3.8 ± 0.01 |
| >20.0 | >20.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | >20.0 | >20.0 | >20.0 | >20.0 | >40.0 | >40.0 | ND |
Amino acid sequences are shown in Table 1. MICs and MBCs are shown in micrograms per milliliter as means ± standard errors of the means of three replications. ND, not determined.
TABLE 3.
Antimicrobial activities of ovispirin and congenersa
| Ovispirin peptide |
Mannheimia haemolytica serovar:
|
Pasteurella trehalosi serovar 4
|
Salmonella enterica subsp. arizonae
|
Pasteurella multocida
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1
|
2
|
6
|
||||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| OV | 3.8 ± 1.0 | ND | ND | ND | 2.1 ± 0.4 | 2.1 ± 0.4 | 10.0 ± 0.0 | 10.0 ± 0.00 | 6.7 ± 1.7 | 8.3 ± 1.7 | 8.3 ± 1.7 | 8.3 ± 1.7 |
| OV-1 | 1.5 ± 0.6 | 0.8 ± 0.2 | 0.6 ± 0.0 | 0.5 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.0 | 2.3 ± 1.4 | 1.0 ± 0.2 | 6.7 ± 1.7 | 6.7 ± 1.7 | 0.6 ± 0.0 | 0.8 ± 0.2 |
| OV-2 | 5.0 ± 0.0 | 5.0 ± 0.0 | 1.8 ± 0.7 | 0.2 ± 0.1 | >20.0 | >20.0 | ≥20.0 | ≥20.0 | >20.0 | >20.0 | >20.0 | >20.0 |
| OV-3 | 1.7 ± 0.4 | 1.7 ± 0.4 | 0.8 ± 0.2 | 0.8 ± 0.2 | 5.0 ± 0.0 | 5.0 ± 0.0 | 3.3 ± 0.8 | 4.2 ± 0.8 | 3.3 ± 0.8 | 3.3 ± 0.8 | 1.3 ± 0.0 | 2.5 ± 1.3 |
| OV-4 | 1.3 ± 0.0 | 1.3 ± 0.0 | 1.7 ± 0.4 | 1.3 ± 0.0 | 3.3 ± 0.8 | 2.5 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| OV-5 | 0.8 ± 0.2 | 1.0 ± 0.2 | 8.3 ± 5.8 | 2.5 ± 0.0 | 2.5 ± 0.0 | 2.5 ± 0.0 | 11.7 ± 4.4 | 2.5 ± 0.0 | 2.1 ± 0.4 | 2.1 ± 0.4 | 1.3 ± 0.0 | 1.3 ± 0.0 |
| OV-6 | 1.3 ± 0.0 | 1.3 ± 0.0 | 5.0 ± 2.5 | 1.9 ± 0.6 | 2.5 ± 0.0 | 2.5 ± 0.0 | 2.1 ± 0.4 | 1.3 ± 0.0 | 5.0 ± 0.0 | 6.7 ± 1.7 | 2.1 ± 0.4 | 2.1 ± 0.4 |
| OV-7 | 1.5 ± 0.6 | 1.3 ± 0.0 | 3.3 ± 0.8 | 2.7 ± 1.3 | 3.3 ± 0.8 | 3.3 ± 0.8 | 7.5 ± 2.5 | 2.5 ± 0.0 | 5.0 ± 0.0 | 5.0 ± 0.0 | 1.3 ± 0.0 | 2.5 ± 1.3 |
| OV-8 | 5.0 ± 0.0 | >20.0 | 6.7 ± 1.7 | 5.0 ± 0.0 | 16.7 ± 3.3 | 16.7 ± 3.3 | ≥20.0 | >20.0 | >20.0 | ≥20.0 | 10.0 ± 5.0 | 15.0 ± 4.1 |
| OV-9 | 2.5 ± 0.0 | 5.0 ± 0.0 | 2.5 ± 0.0 | 4.2 ± 0.8 | 1.3 ± 0.0 | 1.3 ± 0.0 | 5.8 ± 2.2 | 5.8 ± 2.2 | >20.0 | ≥20.0 | 1.3 ± 0.0 | 1.7 ± 0.4 |
| OV-10 | 10.0 ± 0.0 | >20.0 | 4.2 ± 0.8 | 4.2 ± 0.8 | 6.7 ± 1.7 | 6.7 ± 1.7 | >20.0 | ≥20.0 | >20.0 | >20.0 | 15.0 ± 4.1 | 15.0 ± 4.1 |
| OV-11 | 3.3 ± 0.8 | 5.0 ± 0.0 | 1.7 ± 0.4 | 1.7 ± 0.4 | 4.2 ± 0.8 | 4.2 ± 0.8 | 5.0 ± 2.5 | 5.0 ± 2.5 | 20.0 ± 0.0 | 20.0 ± 0.0 | 5.0 ± 2.5 | 5.0 ± 2.5 |
| OV-12 | 8.3 ± 1.7 | >20.0 | 4.6 ± 2.7 | 4.6 ± 2.7 | 4.2 ± 0.8 | 4.2 ± 0.8 | 20.0 ± 0.0 | 20.0 ± 0.0 | >20.0 | >20.0 | ≥20.0 | ≥20.0 |
| OV-13 | >20.0 | >20.0 | 10.4 ± 5.4 | 7.1 ± 2.9 | 6.7 ± 1.7 | 6.7 ± 1.7 | >20.0 | >20.0 | >20.0 | >20.0 | >20.0 | >20.0 |
| OV-14 | 16.7 ± 3.3 | >20.0 | 8.3 ± 5.8 | 9.2 ± 5.5 | 8.3 ± 1.7 | 8.3 ± 1.7 | >20.0 | >20.0 | >20.0 | >20.0 | >20.0 | >20.0 |
| OV-15 | >20.0 | >20.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | >20.0 | >20.0 | >20.0 | >20.0 | ≥20.0 | >20.0 |
| OV-16 | ≥20.0 | >20.0 | 13.3 ± 3.3 | 13.3 ± 3.3 | 20.0 ± 0.0 | 20.0 ± 0.0 | >20.0 | >20.0 | >20.0 | >20.0 | >20.0 | >20.0 |
|
Klebsiella pneumoniae
|
P. aeruginosa PAO1
|
Corynebacterium pseudotuberculosis ATCC 19410
|
Staphylococcus aureus
|
A. fumigatus
|
Hemolytic activity (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| 1.3 ± 0.0 | 1.3 ± 0.0 | 4.2 ± 0.8 | 5.0 ± 2.5 | >20.0 | >20.0 | 5.0 ± 0.0 | 4.2 ± 0.8 | ND | ND | 100.0 ± 0.01 |
| 0.8 ± 0.2 | 0.8 ± 0.2 | 2.1 ± 0.4 | 2.5 ± 0.0 | >20.0 | >20.0 | 15.0 ± 5.0 | >20.0 | 10.0 ± 5.0 | 20.0 ± 0.0 | 8.1 ± 0.01 |
| >20.0 | >20.0 | >20.0 | >20.0 | >20.0 | >20.0 | >20.0 | >20.0 | 6.7 ± 1.7 | 10.0 ± 0.0 | 6.2 ± 0.01 |
| 2.5 ± 0.0 | 2.5 ± 0.0 | 2.5 ± 0.0 | 5.0 ± 2.5 | >20.0 | >20.0 | 2.5 ± 0.0 | 2.5 ± 0.0 | 8.3 ± 1.7 | 16.7 ± 3.3 | 9.4 ± 0.01 |
| 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | >20.0 | >20.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 5.0 ± 0.0 | 10.0 ± 0.0 | ND |
| 1.7 ± 0.4 | 1.3 ± 0.0 | 2.1 ± 0.4 | 3.3 ± 0.8 | ≥20.0 | ≥20.0 | 4.2 ± 0.8 | 5.0 ± 0.0 | 6.7 ± 1.7 | 10.0 ± 0.0 | 8.0 ± 0.01 |
| 1.3 ± 0.0 | 1.3 ± 0.0 | 2.5 ± 0.0 | 2.5 ± 0.0 | ≥20.0 | >20.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 13.3 ± 3.3 | 16.7 ± 3.3 | ND |
| 1.7 ± 0.4 | 4.6 ± 2.7 | 4.2 ± 0.8 | 4.2 ± 0.8 | ≥20.0 | >20.0 | 10.0 ± 0.0 | 10.0 ± 0.0 | 8.3 ± 1.7 | 13.3 ± 3.3 | ND |
| ≥20.0 | ≥20.0 | 3.3 ± 0.8 | 5.0 ± 0.0 | >20.0 | >20.0 | >20.0 | >20.0 | 33.3 ± 6.7 | 40.0 ± 0.0 | 3.3 ± 0.01 |
| 10.0 ± 0.0 | 20.0 ± 0.0 | 2.5 ± 0.0 | 2.5 ± 0.0 | ≥20.0 | ≥20.0 | 10.0 ± 0.0 | ≥20.0 | 10.0 ± 0.0 | 23.3 ± 8.8 | ND |
| >20.0 | >20.0 | 5.0 ± 0.0 | 10.0 ± 0.0 | ≥20.0 | >20.0 | >20.0 | >20.0 | 40.0 ± 0.0 | >40.0 | 3.7 ± 0.01 |
| 6.7 ± 1.7 | 8.3 ± 1.7 | 1.7 ± 0.4 | 2.5 ± 0.0 | ≥20.0 | ≥20.0 | 10.0 ± 0.0 | ≥20.0 | 8.3 ± 1.7 | 8.3 ± 1.7 | 7.6 ± 0.01 |
| >20.0 | >20.0 | 0.6 ± 0.0 | 1.3 ± 0.0 | >20.0 | >20.0 | >20.0 | >20.0 | 5.8 ± 2.2 | 16.7 ± 3.3 | 2.4 ± 0.01 |
| ≥20.0 | ≥20.0 | 1.7 ± 0.4 | 2.5 ± 0.0 | >20.0 | >20.0 | >20.0 | >20.0 | 26.7 ± 6.7 | 26.7 ± 6.7 | 2.5 ± 0.01 |
| 15.0 ± 5.0 | 16.7 ± 3.3 | 20.0 ± 0.0 | 20.0 ± 0.0 | >20.0 | >20.0 | >20.0 | >20.0 | 20.0 ± 0.0 | 20.0 ± 0.0 | 2.5 ± 0.01 |
| 13.3 ± 3.3 | ≥20.0 | 5.0 ± 0.0 | 10.0 ± 0.0 | >20.0 | >20.0 | >20.0 | >20.0 | 40.0 ± 0.0 | >40.0 | 2.5 ± 0.01 |
| 11.7 ± 4.4 | 13.3 ± 3.3 | 10.0 ± 0.0 | 13.3 ± 3.3 | >20.0 | >20.0 | >20.0 | >20.0 | 40.0 ± 0.0 | ≥40.0 | 2.9 ± 0.01 |
Amino acid sequences are shown in Table 1. MICs and MBCs are shown in micrograms per milliliter as means ± standard errors of the means of three replications. OV, ovispirin; ND, not determined.
The α-amino groups of SMAP29 and CAP18 (5.0 mg) were coupled to 5.0 mg of keyhole limpet hemocyanin with glutaraldehyde and used as antigens to immunize goats. The conjugate was suspended in PBS (3.1 mg/ml), emulsified in Freund's complete adjuvant (50% emulsion; total volume of 0.6 ml), and injected into four subcutaneous dorsal sites. Subsequent immunizations (days 14, 42, and 56 post-initial immunization) utilized the same conjugates. Antisera were collected on day 70.
All goats had antibody titers to P. aeruginosa (mean titer, 1:256), indicating previous natural exposure. As these antibodies would interfere with the specificity of the immunoelectron microscopy, sera were incubated for 1 h at 37°C with glutaraldehyde-fixed whole PAO1 cells. The cells were removed by centrifugation, and this procedure was repeated three times. After absorption, enzyme-linked immunosorbent assay titers, determined as previously described (3), were substantially reduced (mean titer, 1:8).
A dot blot assay was used to titrate the preimmune and immune serum titers. Absorbed preimmune serum had a titer of 1:2, and absorbed immune serum to SMAP29 had a titer of 1:256. Absorbed preimmune serum had a titer of 1:2, and absorbed immune serum to CAP18 had a titer of 1:2,048.
A suspension of P. aeruginosa (1.1 × 108 CFU/ml) in 0.01 M phosphate buffer, pH 7.2, containing 1% Luria-Bertani broth was split among three groups. Acetic acid (0.02%; control solution), SMAP29 (50 μg/ml, final concentration), and CAP18 (50 μg/ml, final concentration) were added and briefly mixed. Samples were removed at time zero and 0.25, 0.5, 1, 2, 4, 8, and 16 h for quantitative plate counts (Table 4), and immunoelectron microscopy was performed as previously described (2).
TABLE 4.
Rapid killing of P. aeruginosa PAO1 incubated with SMAP29 or CAP18
| CFU/ml
| |||
|---|---|---|---|
| Time (h) | PAO1 control | PAO1 + SMAP29 (50 μg/ml) | PAO1 + CAP18 (50 μg/ml) |
| 0.0 | 1.1 × 108 | 3.5 × 104 | 3.3 × 101 |
| 0.25 | 8.9 × 107 | 0 | 0 |
| 0.5 | 8.8 × 107 | 0 | 0 |
| 1.0 | 8.6 × 107 | 0 | 0 |
| 2.0 | 1.2 × 108 | 0 | 0 |
| 4.0 | 2.3 × 108 | 0 | 0 |
| 8.0 | 2.6 × 108 | 0 | 0 |
| 16.0 | 2.5 × 108 | 0 | 0 |
Antimicrobial activity.
We altered SMAP29 to identify peptides with potent antimicrobial activity that may have applications in the treatment of acute and chronic respiratory infections. An alteration in SMAP29 to form SMAP28 was effective (MIC range, 0.3 to 1.7 μg/ml for SMAP28 versus 0.6 to 2.5 μg/ml for SMAP29) (Table 2). SMAP28 is thought to be the native form of the peptide (8, 12). However, further modifications in SMAP29 were not effective, and SMAP29-18, SMAP29-20, SMAP29-21, and SMAP29-18AA were less active (Table 2). SMAP29 was slightly hemolytic, and SMAP29-18, SMAP29-20, and SMAP29-21 were not (Table 2).
A derivation in SMAP29, converting residues 1 and 2 to K and N and residues 6, 7, 11, 13, 14, and 28 to I, and adding an amine to the C-terminal G, resulted in a peptide called OV. This peptide was effective against ovine pathogens (MIC ranges, 1.3 to 10.0 μg/ml) (Table 3). Modifications in OV peptides (e.g., OV-3, OV-4, OV-5, OV-6, and OV-7) did not substantially increase their activities. However, one congener, OV-1, had increased activity. As OV was shortened from both ends, activity declined (e.g., OV-13 to OV-16). In some cases, substituting residues in these peptides or adding an N-terminal amine could restore activity. OV had the highest hemolytic activity, and OV-12 had the lowest hemolytic activity (Table 3).
Immunoelectron microscopy.
Like other cationic antimicrobial peptides, SMAP29 induced ultrastructural damage in bacterial cells (Fig. 1 and 2) characterized by rough surfaces containing extracellular debris and outer membranous blebs (5, 7, 9, 10, 13), thickened cell walls (5, 7), and electron-dense cytoplasmic material (7). Interestingly, the ultrastructural changes induced by SMAP29 were different from those induced by CAP18 (Fig. 1 and 2). Although membrane damage induced in bacteria by cationic antimicrobial peptides has been reported, ultrastructural localization of peptide has never been shown, and we expected SMAP29 to localize in the outer and inner membranes. However, we found that SMAP29 (and CAP18) rapidly penetrated the outer and inner membranes and entered into the bacterial cytoplasm as early as time zero (Fig. 1 and 2). Whether this is a result of a mechanism associated with peptide activity or a result of the presence of antimicrobial peptide transporters is not known. The latter is possible, as transport of antimicrobial peptides into cells can occur via the ATP-binding cassette transporter (6, 11).
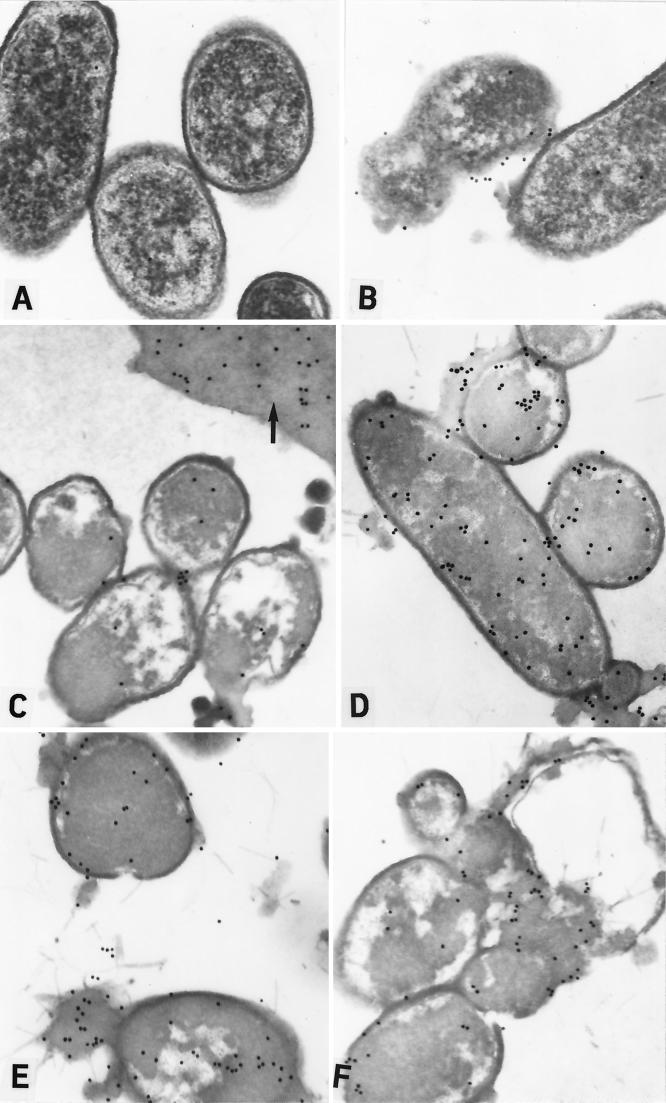
FIG. 1.
Immunoelectron microscopy of P. aeruginosa PAO1 incubated with SMAP29 (50 μg/ml) and detected with goat antiserum to SMAP29 and PG-CG. PAO1 incubated with SMAP29 (50 μg/ml) and then with preimmune goat serum and PG-CG did not contain any label (A). At time zero, PAO1 was morphologically normal although many cells were dead upon culture (Table 4). PG-CG labeling patterns indicated that SMAP29 was already attached to the outer membrane (B), and some outer membranous material and debris could be seen attached to many cells. By 0.5 h, most cells contained a very dense cytoplasm and large intracellular vacuolar spaces (C). Vast sheets of extracellular debris or cytoplasmic contents containing label could be seen (arrow). At 2 h, PG-CG label was seen attached to the extracellular debris and bacterial surface and throughout the cytoplasm (D). At 4 h, the outer envelope was very thick and the cytoplasm became more electron dense in the dead cells (E). At 8 h, bacterial cells were coalescing among extensive amounts of extracellular debris (F).
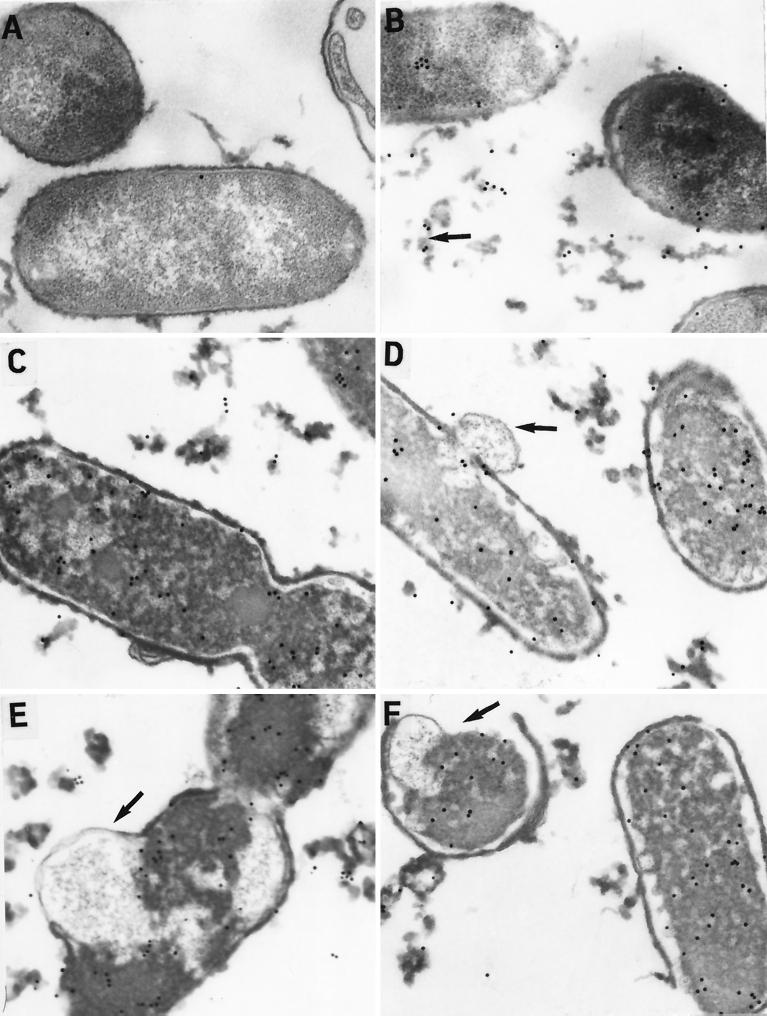
FIG. 2.
Immunoelectron microscopy of P. aeruginosa PAO1 incubated with CAP18 (50 μg/ml) and detected with goat antiserum to CAP18 and PG-CG. PAO1 incubated with CAP18 (50 μg/ml) and preimmune goat serum and PG-CG did not contain any label (A). At time zero, PAO1 was morphologically normal and there was PG-CG labeling on the outer membrane, in the periplasm, and already throughout the cytoplasm (B). Extensive amounts of labeled outer membrane material were already sloughing from the bacterial cell (arrow). At 0.5 h, the amounts of extracellular debris increased and the integrity of the inner and outer membranes (arrow) began to deteriorate (C and D). At 4 h, many cells showed evidence of membrane damage (arrow) and extensive labeling throughout the cytoplasm (E). At 8 h, most bacterial cells were lysing (F) and the outer envelope was missing (arrow).
In conclusion, derivatives and congeners of SMAP29, with potent antimicrobial activity, may have applications in the treatment or prevention of infection, including P. aeruginosa associated with chronic respiratory inflammation in cystic fibrosis patients.
Acknowledgments
We thank Gwen Laird, Abby Lozano, and Shawn Brogden for technical assistance and graphic design.
REFERENCES
- 1.Bagella L, Scocchi M, Zanetti M. cDNA sequences of three sheep myeloid cathelicidins. FEBS Lett. 1995;376:225–228. doi: 10.1016/0014-5793(95)01285-3. [DOI] [PubMed] [Google Scholar]
- 2.Brogden K A. Ovine pulmonary surfactant induces killing of Pasteurella haemolytica, Escherichia coli, and Klebsiella pneumoniae by normal serum. Infect Immun. 1992;60:5182–5189. doi: 10.1128/iai.60.12.5182-5189.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brogden K A, Ackermann M R, DeBey B M. Pasteurella haemolytica lipopolysaccharide-associated protein induces pulmonary inflammation after bronchoscopic deposition in calves and sheep. Infect Immun. 1995;63:3595–3599. doi: 10.1128/iai.63.9.3595-3599.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brogden K A, Kalfa V C, Ackermann M R, Palmquist D E, McCray P B, Jr, Tack B F. The ovine cathelicidin SMAP29 kills ovine respiratory pathogens in vitro and in an ovine model of pulmonary infection. Antimicrob Agents Chemother. 2001;45:331–334. doi: 10.1128/AAC.45.1.331-334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freer E, Pizarro-Cerda J, Weintraub A, Bengoechea J A, Moriyon I, Hultenby K, Gorvel J P, Moreno E. The outer membrane of Brucella ovis shows increased permeability to hydrophobic probes and is more susceptible to cationic peptides than are the outer membranes of mutant rough Brucella abortus strains. Infect Immun. 1999;67:6181–6186. doi: 10.1128/iai.67.11.6181-6186.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groisman E A. How bacteria resist killing by host-defense peptides. Trends Microbiol. 1994;2:444–448. doi: 10.1016/0966-842x(94)90802-8. [DOI] [PubMed] [Google Scholar]
- 7.Henk W G, Todd W J, Enright F M, Mitchell P S. The morphological effects of two antimicrobial peptides, hecate-1 and melittin, on Escherichia coli. Scanning Microsc. 1995;9:501–507. [PubMed] [Google Scholar]
- 8.Mahoney M M, Lee A Y, Brezinski-Caliguri D J, Huttner K M. Molecular analysis of the sheep cathelin family reveals a novel antimicrobial peptide. FEBS Lett. 1995;377:519–522. doi: 10.1016/0014-5793(95)01390-3. [DOI] [PubMed] [Google Scholar]
- 9.Oren Z, Hong J, Shai Y. A comparative study on the structure and function of a cytolytic alpha-helical peptide and its antimicrobial beta-sheet diastereomer. Eur J Biochem. 1999;259:360–369. doi: 10.1046/j.1432-1327.1999.00047.x. [DOI] [PubMed] [Google Scholar]
- 10.Oren Z, Lerman J C, Gudmundsson G H, Agerberth B, Shai Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem J. 1999;341:501–513. [PMC free article] [PubMed] [Google Scholar]
- 11.Parra-Lopez C, Baer M T, Groisman E A. Molecular genetic analysis of a locus required for resistance to antimicrobial peptides in Salmonella typhimurium. EMBO J. 1993;12:4053–4062. doi: 10.1002/j.1460-2075.1993.tb06089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skerlavaj B, Benincasa M, Risso A, Zanetti M, Gennaro R. SMAP-29: a potent antibacterial and antifungal peptide from sheep leukocytes. FEBS Lett. 1999;463:58–62. doi: 10.1016/s0014-5793(99)01600-2. [DOI] [PubMed] [Google Scholar]
- 13.Tiozzo E, Rocco G, Tossi A, Romeo D. Wide-spectrum antibiotic activity of synthetic, amphipathic peptides. Biochem Biophys Res Commun. 1998;249:202–206. doi: 10.1006/bbrc.1998.9114. [DOI] [PubMed] [Google Scholar]
- 14.Travis S M, Anderson N N, Forsyth W R, Espiritu C, Conway B D, Greenberg E P, McCray P B, Jr, Lehrer R I, Welsh M J, Tack B F. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect Immun. 2000;68:2748–2755. doi: 10.1128/iai.68.5.2748-2755.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner J, Cho Y, Dinh N N, Waring A J, Lehrer R I. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chem. 1998;42:2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu M, Hancock R E. Interaction of the cyclic antimicrobial cationic peptide bactenecin with the outer and cytoplasmic membrane. J Biol Chem. 1999;274:29–35. doi: 10.1074/jbc.274.1.29. [DOI] [PubMed] [Google Scholar]