Abstract
Background
Cognitive aging is a complex phenomenon, which comprises various cognitive skills, broadly categorized into fluid and crystallized intelligence. Crystallized intelligence (gc) tends to be maintained, as opposed to fluid intelligence (gf), which tends to decline rapidly with age. The association of the two with cognitive decline remains a matter of conjecture requiring further research.
Aim
The aim of the study was to identify the variables of gc and gf from a population data of Longitudinal Aging Study in India-Diagnostic Assessment of Dementia (LASI-DAD) study and investigate its relationship with the onset of cognitive impairment using discrepancy analysis against neuropsychological tests.
Methods
This analysis of data from LASI-DAD study was carried out on a sample of 3,223 participants. They were assessed on extensive thirteen cognitive tests and one subjective test of cognition. Standardized score was used for discrepancy analysis. Fluid ability minus crystallized ability was used to assess the cognitive impairment. Any statistical significance with the score difference >0.99 SD was defined as a presence of cognitive decline. Hindi Mental Status Examination (HMSE) and the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) were used as gold standard.
Results
With increased discrepancy score, each cognitive parameter score declined which was found to be statistically significant. In HMSE (Normal = 25.81 ± 3.39; Impaired = 23.17 ± 3.54; p = <0.001), there was a drop of 2 point scores in identifying cognitive impairment in the population sample as per the gold standard. A similar trend was evident in other neurocognitive domains as well.
Conclusion
Crystallized-fluid intelligence discrepancy analysis has a strong potential in predicting the onset of cognitive decline ahead of time, facilitating early intervention.
Keywords: Fluid intelligence, Crystallized intelligence, Cognitive impairment, LASI-DAD, Discrepancy analysis
Introduction
Aging of the population is a significant demographic trend of twenty-first century that is affecting high as well as low- and middle-income countries [1]. A key element of aging is decline of cognitive function [2].
Cognitive aging, a complex phenomenon, involves several specific cognitive domains including attention, memory, executive functions, language, and visuospatial abilities [3]. Cognitive performance follows a bell-shaped profile over the lifespan [4]. Each of these domains has measurable decline with age in later life [3]. Two main types of cognitive skills: fluid intelligence and crystallized intelligence have been described in published literature [5, 6]. The former is concerned with abstract thinking and logical concept which appears to be chiefly susceptible to aging [7]. On the other hand, culturally acquired rules, factual knowledge comprise crystallized intelligence which tends to remain stable in the normal aging process [7, 8].
Growing old is associated with diminished peripheral sensory functions and central nervous system which leads to decline in overall intellectual aptitude [9]. Existing literature offers several possible mechanisms for the age-related decline in fluid intelligence [10]. The most accepted evidence suggests that age-related atrophic changes take place prominently in frontal brain structures which undermine the functioning of executive abilities, thereby, resulting in gradual decline of fluid intelligence [11].
Further, in light of dementia, it is well known that patients with Alzheimer's disease (AD) tend to show more decline in fluid ability than crystallized ability; such decline is predictive of AD progression rate [12]. This is supported by a recent mendelian randomization study wherein negative correlation between fluid intelligence and high polygenic risk score for AD was also demonstrated [13]. Such evidence suggests that the underlying pathological process of dementia has already taken place, even before the clinical diagnosis is made [14]. A couple of research studies show that cognitive assessment can provide adequate information about the dementia prognosis [15, 16]. Only a few have investigated prediction over a longer period, wherein cognitive test results have indicated cognitive impairment, even before the disease is clinically manifested [13]. From the cognitive perspective, it has been recently suggested that fluid intelligence decline with increasing age can be a plausible early predictor of future cognitive impairment or dementia, even before manifestations of forgetfulness [16]. Thus, cognitive testing plays a very crucial role. In spite of these substantial literatures about the fluid intelligence and aging, the link among them has not yet been established.
The rationale of the present study was to identify the variables of fluid and crystallized intelligence from a population data of Longitudinal Aging Study in India-Diagnostic Assessment of Dementia (LASI-DAD) study and investigate its relationship with aging. Consistent with this idea, we investigated discrepancy analysis (crystallized intelligence minus fluid intelligence) using widely acceptable neuropsychological tests. This may help to distinguish normal from pathological aging. It is hypothesized that larger discrepancy score in healthy adults would be associated with preclinical cognitive decline as evidenced by Hindi Mental State Examination (HMSE) [17] and Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) [18]. This study may help to predict the onset of cognitive decline a priori and planning the early intervention.
Methodology
Data from LASI-DAD were included in the analysis. It is an extensive study with detailed cognitive assessment, based on Harmonized Cognitive Assessment Protocol (HCAP) [19]. It includes a subsample of 3,300 adults aged 60 years and older in 14 States of India. The data are validated for the illiterate populations as well. Further, it has rich data on risk factors of cognitive decline and dementia which help to understand the determinants of cognitive impairment and dementia [20].
Participants
Three thousand two hundred twenty-four participants completed a full set of cognitive tests. There were 77 participants whose data were either missing as per the LASI-DAD protocol or were inconsistent with the selected tests for the present study.
Variables
Eight cognitive tests were categorized into two domains, fluid intelligence and crystallized intelligence following three-stratum theory of cognitive abilities [21, 22]. For assessing fluid intelligence, we used Raven's Progressive Matrices, Symbol Cancellation test, Go-No-Go test, and Hand Sequencing Test. While for crystallized intelligence, Community Screening Interview for Dementia (CSI-D), Retrieval fluency test, Health and Retirement Study-Telephone Interview for Cognitive Status (HRS-TICS), and Token test were conducted. The informant interview scale was also used to capture the cognitive and memory functioning of the participants. The details of each test are elaborated in LASI-DAD protocol [20]. Hindi Mental State Examination (HMSE) and Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) were used as the standard parameter for classifying cognitive decline objectively and subjectively, respectively.
Statistical Analysis
All statistical analyses were performed using strata 14 and are presented in mean (SD) and frequency (%). For deriving measures of fluid and crystallized intelligence, correlation analysis was conducted. Eight cognitive measures were z-transformed and then entered into a factor analysis using principal component extraction method following varimax rotation. For determining the number of factors, the criteria of eigenvalues and scree plot were used. Sampling adequacy was confirmed using Kaiser-Meyer Olkin and Bartlett's test of sphericity. For deriving a “pure normal” sample, a standard cut off with no literacy bias was followed wherein HMSE score ≥17 and IQCODE score <3.35 were taken as inclusion criteria. A sample of 1,621 was retained as pure normal and yielded 5 true measures (3 measures of fluid intelligence and 2 measures for crystallized intelligence) as the best fit for the overall dataset.
For calculating discrepancy score (z score fluid minus z score crystallized), we used the results from factor analyses. Further, the association of discrepancy scores was analyzed with specific neuropsychological tests using the Pearson coefficient. The continuous variables were compared between the groups by independent t test or Mann-Whitney as appropriate. Further, the categorical variables were compared by χ2. A p value less than 0.05 was considered as statistically significant.
Results
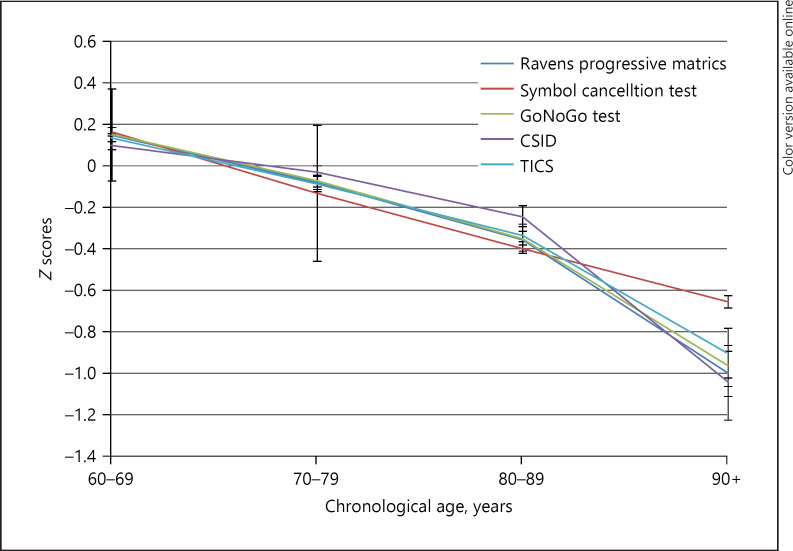
To observe the interrelationships among fluid and crystallized variables, Pearson's bivariate correlation analysis was performed (shown in Table 1). For fluid intelligence, Raven's Progressive Matrices were significantly correlated with Symbol Cancellation test (r = 0.49), Go-No-Go test (r = 0.48), and Hand Sequencing Test (r = 0.39). Similarly, for crystallized intelligence variables, CSI-D was significantly correlated with Health and Retirement Study-Telephone Interview for Cognitive Status (HRS-TICS) (r = 0.41), Token test (r = 0.36), and Retrieval fluency test (r = 0.26). Further, all these variables were found to be positively correlated with education and negatively correlated with increasing age (shown in Fig. 1). While with IQCODE, significant negative correlation was evident as hypothesized as per the LASI-DAD protocol (shown in Table 2).
Table 1.
Correlation among fluid and crystallized intelligence variables (n = 3,223)
| Fluid intelligence | Raven's Progressive Matrices | Symbol Cancellation | Go-No-Go test |
|---|---|---|---|
| Raven's Progressive Matrices | − | − | − |
| Symbol Cancellation | 0.49** | − | − |
| Go-No-Go test | 0.48** | 0.55** | − |
| Hand Sequencing Test | 0.39** | 0.41** | 0.37** |
|
| |||
| Crystallized intelligence | CSI-D | HRS-TICS | Token test |
|
| |||
| CSI-D HRS-TICS Token test Retrieval Fluency |
− 0.41 ** 0.36** 0.26** |
− − 0.47** 0.31** |
− − − 0.33** |
All values were significant with p < 0.001.
Fig. 1.
Graphical representation of fluid and crystallized ability scores across the age-groups.
Table 2.
Correlation across fluid intelligence and crystallized intelligence variables with age, education, and IQCODE
| Age | Years of education | IQCODE | |
|---|---|---|---|
| gf | |||
| Raven's Progressive Matrices | −0.16** | 0.43** | −0.34** |
| Symbol Cancellation test | −0.21** | 0.57** | −0.32** |
| Go-No-Go test | −0.16** | 0.48** | −0.37** |
| Hand Sequencing Test | −0.18** | 0.26** | −0.21** |
| gc | |||
| CSID | −0.12** | 0.24** | −0.47** |
| TICS | −0.13** | 0.42** | −0.31** |
| Token test | −0.14** | 0.49** | −0.37** |
| Retrieval Fluency | −0.16** | 0.32** | −0.31** |
All values were significant with p < 0.001. gf, fluid intelligence; gc, crystallized intelligence.
In factor analysis, sampling adequacy was confirmed using Kaiser-Meyer Olkin value, which was 0.84 and Bartlett's test of sphericity was also found to be significant (p = 0.001). For the first factor, i.e., fluid ability, the zero order factor loadings (structure coefficients) were largest for Symbol Cancellation (0.84) followed by Raven's Progressive Matrices (0.72) and Go-No-Go test (0.70). For the second factor, i.e., crystallized ability, the factor loading in order of the magnitude was CSI-D (0.94) and TICS (0.55). For discrepancy score, we subtracted the fluid score from the crystallized score for each participant. Increasingly, values more than 0.99 SD represented an increasingly greater discrepancy in ability.
As evident in Tables 3 and 4, females outnumbered males in both the groups. The sample was drawn from 14 States of India which were categorized into 6 geographic regions. North and south region of India comprised maximum draw of sample for the study in both the population. In the total sample, there was no significant difference in educational criteria. However, in the derived population of pure normal, there was an evidence of significant statistical difference, which depicts that education has a crucial role in ruling in cognitive impairment.
Table 3.
Categorization of total sample (n = 3,223) as normal and controls on discrepancy analysis
| Variable | Full sample, (N = 3,223) | Discrepancy score ≤0.99 SD (N = 2,56) 1 (normal) | Discrepancy score >0.99 SD (N = 662) (impaired) | p value | ||||
|---|---|---|---|---|---|---|---|---|
| Age, years | 69.82±7.73 | 69.56±7.61 | 70.82±8.10 | 0.002 | ||||
| Gender, % | ||||||||
| Males | 46.12 | 48.07 | 38.67 | <0.001 | ||||
| Females | 53.88 | 51.93 | 61.33 | |||||
| Education, years | 4.03±4.73 | 4.22±4.79 | 3.37±4.44 | 0.003 | ||||
| State, % | ||||||||
| North | 30.00 | 27.37 | 40.18 | |||||
| North-East | 6.21 | 5.62 | 8.46 | |||||
| East | 17.06 | 17.61 | 14.95 | <0.001 | ||||
| West | 9.31 | 10.78 | 3.63 | |||||
| Central | 3.10 | 3.55 | 1.36 | |||||
| South | 34.32 | 35.06 | 31.42 | |||||
| HMSE | 22.82±5.51 | 23.33±5.57 | 20.84±4.82 | <0.001 | ||||
| IQCODE | 3.45±0.56 | 3.42±0.55 | 3.56±0.59 | <0.001 | ||||
| Clock drawing | 0.06±1.07 | 0.20±1.10 | −0.46±0.68 | <0.001 | ||||
| Constructional praxis | 1.65±2.71 | 1.95±2.82 | 0.49±1.87 | <0.001 | ||||
| Retrieval fluency | 11.50±4.91 | 11.84±5.09 | 10.18±3.88 | <0.001 |
All values were significant with p < 0.001.
Table 4.
Derived pure normal on discrepancy analysis (n = 1,621)
| Variable | Full sample, N = 1,621 | Discrepancy score ≤0.99 SD, N = 1,362 | Discrepancy score >0.99 SD, N = 259 | p value | |
|---|---|---|---|---|---|
| Age, years | 68.21±6.45 | 68.13±6.40 | 68.61±6.72 | <0.001 | |
| Gender, % | |||||
| Males | 50.59 | 52.72 | 39.38 | <0.001 | |
| Females | 49.41 | 47.28 | 60.62 | ||
| Education, years | 4.57±4.97 | 4.70±5.00 | 3.90±4.72 | <0.001 | |
| State, % | |||||
| North | 32.63 | 31.79 | 37.07 | ||
| North-East | 4.69 | 4.11 | 7.72 | ||
| East | 11.66 | 11.67 | 11.58 | ||
| West | 8.82 | 10.06 | 2.32 | ||
| Central | 2.10 | 2.06 | 2.32 | ||
| South | 40.10 | 40.31 | 39.00 | ||
| HMSE | 25.39±3.55 | 25.81±3.39 | 23.17±3.54 | <0.001 | |
| IQCODE | 3.05±0.24 | 3.04±0.23 | 3.05±0.25 | <0.001 | |
| Clock drawing | 0.33±1.13 | 0.48±1.14 | −0.41±0.74 | <0.001 | |
| Constructional praxis | 2.38±2.90 | 2.69±2.95 | 0.76±1.94 | <0.001 | |
| Retrieval fluency | 12.83±4.79 | 13.17±4.87 | 11.04±3.87 | <0.001 |
All values were significant with p < 0.001.
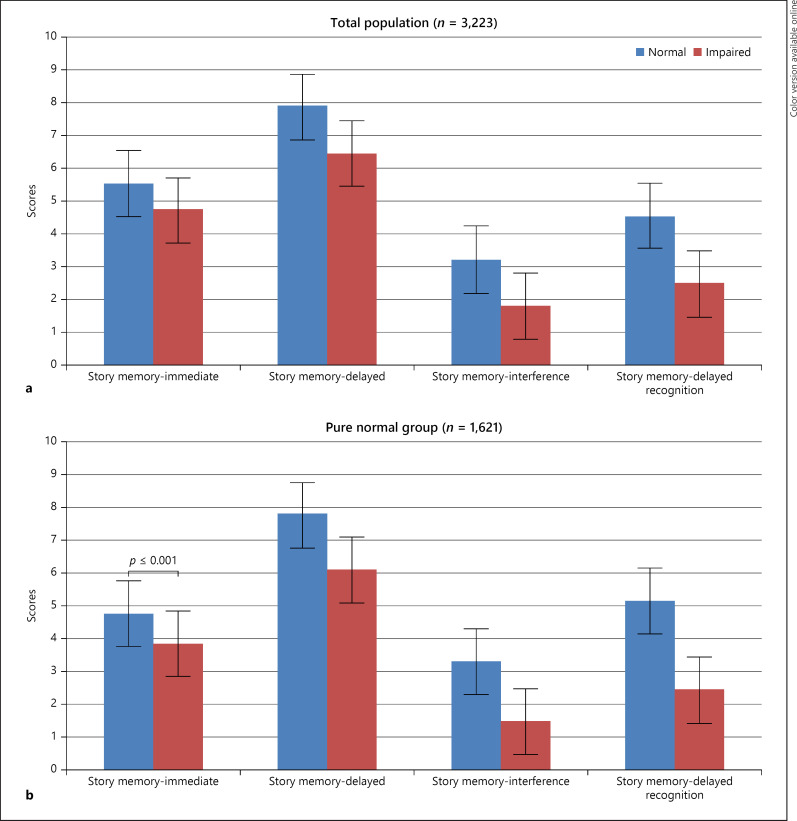
Further, with increased discrepancy score, each cognitive parameter score declined which were found to be clinically and statistically significant. In HMSE (Normal = 25.81 ± 3.39; Impaired = 23.17 ± 3.54), there is a drop of 2 point scores in identifying cognitive impairment in the population sample. Similar finding was seen in language (Retrieval fluency-Normal = 13.17 ± 4.87; Impaired = 11.04 ± 3.87) and in executive functions (clock drawing-Normal = 0.48 ± 1.14; Impaired = −0.41 ± 0.74), constructional praxis (Normal = 2.69 ± 2.95; Impaired = 0.76 ± 1.94). Further, in subdomains of memory − story memory-immediate recall (Normal = 4.79 ± 2.91; Impaired = 3.86 ± 3.01); story memory-delayed (Normal = 7.79 ± 4.67; Impaired = 6.11 ± 4.53); story memory-interference (Normal = 3.33 ± 3.84; Impaired = 1.50 ± 3.29); story memory-delayed recognition (Normal = 5.17 ± 5.79; Impaired = 2.46 ± 4.75), there was significant difference between the normal and impaired group (shown in Fig. 2a, b) with an average difference of 1.78 score point.
Fig. 2.
Trend of discrepancy scores in memory domain in total population group (a) and derived pure normal sample (b) on discrepancy analysis.
Discussion
Over the last few years, the aging research has expanded on the relationship between cognition and aging [23]. The emerging theme in this area has been the emphasis on the fluid (gf) and crystallized intelligence (gc) which have proven especially insightful regarding developmental changes in intelligence throughout the lifespan [24]. According to the long-standing age differentiation hypothesis [25, 26], the relations between cognitive functions change with age. The gf tends to decline, while the gc stays stable as a person grows older [27]. However, the correlation among them is considerably less known with preclinical dementia [28]. Contemporary approach in identifying preclinical dementia relies on radiological biomarkers that are costly, often invasive, and are not accessible by all members of a given community [29]. One way to mitigate this issue is to investigate a cognitive marker that can predict future cognitive impairment [30]. This study focuses on exclusive approach of fluid-crystallized intelligence discrepancy analysis which has a potential to precisely predict preclinical dementia and fundamentally challenges the criteria on the basis of which we consider individuals to be cognitively healthy.
Fluid ability relies on working memory and abstract reasoning. These tests often are intentionally developed in such a way that the prior knowledge or learned skills are at minimal use [31, 32]. Conversely, crystallized ability encompasses vocabulary, analogies, and general knowledge which are learned through experience and education [31]. Since these two abilities are highly correlated with one another across the lifespan, it is expected that with higher crystallized ability, the fluid ability should also be higher [33, 34]. Due to aging, the difference between the two widens up (in the direction of crystallized > fluid) which tend to reflect abnormal cognitive decline.
This is further supported by a Network Neuroscience Theory which states that fluid intelligence exhibits greater variability than crystallized ability with advancing age and across generations [35]. Age-related decline in fluid ability is due to alterations in the neuronal resilience whereas generational change has a beneficial effect on fluid intelligence owing to better dietary intake, educational achievements, and other lifestyle factors which tend to enhance network flexibility [36]. This trend was evident in our present study wherein (Table 1) gf and gc ability was found to be highly correlated. However, with increasing age with mean age of 69.82 ± 7.7 years, the gf declined more than gc (shown in Fig. 1). Conversely, with education years, gc was more maintained than gf (shown in Table 2). Our study followed “classic intellectual aging pattern” [37]-lower functioning on performance tests, than on verbal scales (shown in Table 2).
The major finding of the present study is the implication of the discrepancy score (gc-gf) in relation to cognitive decline. Such discrepancy analysis is a precise method of predicting mild cognitive impairment before it is clinically manifested in a more comprehensive and objective manner. In routine clinical practice, HMSE is widely used to detect cognitive impairment. The standard cut off of ≤24 is considered as presence of any cognitive impairment. A similar trend was evident in our study as well (shown in Table 3). However, with the use of the present discrepancy analysis, a 2 point of difference was evident in both groups (normal = 25.81 ± 3.39 vs. impaired = 23.17 ± 3.54) and (normal = 23.33 ± 5.57 vs. impaired = 20.84 ± 4.82), respectively (shown in Tables 3, 4). This shows that as a widely used gold standard test, it emphasizes on correct number of answers given and then compares the person's performance with the range of the healthy population. However, these tests do not consider age or intellectual ability which actually has implication in predicting cognitive decline early. Further, when other neuropsychological tests assessing memory (shown in Fig. 2a, b), language, visuospatial ability, and executive functions were administered individually, similar results were obtained. A 2-3 point of difference was evident in those cognitive tests as well (shown in Tables 3, 4). Such findings are supported by a recent biological marker study which demonstrated positive correlation between intelligence discrepancy score with global Aβ deposition and cortical thickness in AD-vulnerable regions in cognitively normal population [12]. It has also been stated that in healthy older adults greater intelligence discrepancy is associated with reduced functional abilities, due to physical and social engagement [38]. Altogether, these findings indicate the preclinical dementia may be associated with increased age-related decline in fluid intelligence, but due to scarcity of such studies, the undetected preclinical dementia could have biased previous normal aging studies [28, 39].
In conclusion, crystallized-fluid intelligence discrepancy analysis has a strong potential in predicting the onset of cognitive decline. It provides a roadmap to predict the latent cognitive decline in older populations ahead of time, who may not be clinically manifesting symptoms of cognitive decline at the start of follow-up, since such analysis considers peak level of intellectual aptitude of an individual; hence, it can possibly become a potential predictor of a cognitive marker in dementia research. The current study supplemented the existing substantial aging research. Nevertheless, the association between neurocognitive functions and neuropathological progression in dementia research is yet to be studied with more empirical approach.
Statement of Ethics
Ethics approval was obtained from the Institute Ethics Committee, All India Institute of Medical Sciences, New Delhi − IEC-284/06.05.2016, RP-33/2016. All mentioned research participants have given written informed consent.
Conflict of Interest Statement
The authors have no potential conflicts of interest to disclose.
Funding Sources
This project is funded by the National Institute on Aging and the National Institutes of Health (R01 AG051125, 1RF1AG055273).
Author Contributions
S.B. and A.B.D. were involved in the overall concept of the study. A.D.U. was involved in complete statistical analysis. J.B., J.L., A.C., and P.C. contributed on the manuscript revision and final approval.
Data Availability Statement
The dataset is available in the public domain and can be accessed through the Gateway to Global Aging Data website (https://g2aging.org/). The Harmonized Diagnostic Assessment of Dementia for the Longitudinal Aging Study in India (LASI-DAD) Wave 1 Version A Data, doi:10.25549/h5wx-ay45, are produced and distributed by the University of Southern California with funding from the National Institute on Aging (R01AG051125, RF1AG055273, U01AG065958).
Acknowledgments
The authors would like to acknowledge the USC team and AIIMS LASI-DAD investigative team for their contribution in conducting this study.
References
- 1.World Health Organization . World report on ageing and health. World Health Organization; 2015. Oct 22, [Google Scholar]
- 2.Rodriguez FS, Lachmann T. Systematic review on the impact of intelligence on cognitive decline and dementia risk. Front Psychiatry. 2020;11:658. doi: 10.3389/fpsyt.2020.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lezak MD, Howieson DB, Loring DW, Fischer JS. Neuropsychological assessment. USA: Oxford University Press; 2004. [Google Scholar]
- 4.Mazzonna F, Peracchi F. Oxford research encyclopedia of economics and finance. Oxford University Press; 2018. The economics of cognitive aging. [Google Scholar]
- 5.Schneider WJ, McGrew KS. The Cattell-Horn-Carroll theory of cognitive abilities. The Guilford Press; 2018. [Google Scholar]
- 6.Jensen AR. Human evolution, behavior, and intelligence. The g factor: the science of mental ability. CT, USA: Greenwood Publishing Group Westport; 1998. [Google Scholar]
- 7.Shakeel MK, Goghari VM. Measuring fluid intelligence in healthy older adults. J Aging Res. 2017;2017:8514582. doi: 10.1155/2017/8514582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Postlethwaite BE. Fluid ability, crystallized ability, and performance across multiple domains: a meta-analysis [Doctoral dissertation] The University of Iowa; 2011. [Google Scholar]
- 9.Murman D. The impact of age on cognition. Semin Hear. 2015;36:111–21. doi: 10.1055/s-0035-1555115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manard M, Carabin D, Jaspar M, Collette F. Age-related decline in cognitive control: the role of fluid intelligence and processing speed. BMC Neurosci. 2014;15:7–6. doi: 10.1186/1471-2202-15-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bugg JM, Zook NA, DeLosh EL, Davalos DB, Davis HP. Age differences in fluid intelligence: contributions of general slowing and frontal decline. Brain Cogn. 2006;62:9–16. doi: 10.1016/j.bandc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 12.McDonough IM, Popp TE. Linear and nonlinear relationships between cognitive subdomains of ability discrepancy and Alzheimer's disease biomarkers. Neuropsychology. 2020;34:211. doi: 10.1037/neu0000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korologou-Linden R, Howe LD, Millard LAC, Ben-Shlomo Y, Williams DM, Smith GD, et al. The causes and consequences of Alzheimer's disease: phenome-wide evidence from Mendelian randomization. medRxiv. 2020 doi: 10.1038/s41467-022-32183-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reuben A, Brickman AM, Muraskin J, Steffener J, Stern Y. Hippocampal atrophy relates to fluid intelligence decline in the elderly. J Int Neuropsychol Soc. 2011;17:56–61. doi: 10.1017/S135561771000127X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cervilla J, Prince M, Joels S, Lovestone S, Mann A. Premorbid cognitive testing predicts the onset of dementia and Alzheimer's disease better than and independently of APOE genotype. J Neurol Neurosurg Psychiatry. 2004;75:1100–6. doi: 10.1136/jnnp.2003.028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amieva H, Jacqmin-Gadda H, Orgogozo JM, Le Carret N, Helmer C, Letenneur L, et al. The 9 year cognitive decline before dementia of the Alzheimer type: a prospective population-based study. Brain. 2005;128:1093–101. doi: 10.1093/brain/awh451. [DOI] [PubMed] [Google Scholar]
- 17.Ganguli M, Ratcliff G, Chandra V, Sharma S, Gilby J, Pandav R, et al. A Hindi version of the MMSE: the development of a cognitive screening instrument for a largely illiterate rural elderly population in India. Int J Geriatr Psychiatr. 1995;10:367–77. [Google Scholar]
- 18.Jorm AF, Jacomb PA. The informant questionnaire on cognitive decline in the elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–22. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 19.Weir DR, Langa KM, Ryan LH. Harmonized cognitive assessment protocol (hcap): study protocol summary. Vol. 10. Institute for Social Research, University of Michigan; 2016. [Google Scholar]
- 20.Lee J, Banerjee J, Khobragade PY, Angrisani M, Dey AB. LASI-DAD study: a protocol for a prospective cohort study of late-life cognition and dementia in India. BMJ Open. 2019;9:e030300. doi: 10.1136/bmjopen-2019-030300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carroll JB. The three-stratum theory of cognitive abilities. In: Flanagan DP, Genshaft JL, Harrison PL, editors. Contemporary intellectual assessment: theories, tests, and issues. New York, NY: Guilford Press; 1997. pp. p. 122–30. [Google Scholar]
- 22.Schneider WJ, McGrew KS. The Cattell-Horn-Carroll model of intelligence. In: Flanagan DP, Harrison PL, editors. Contemporary intellectual assessment: theories, tests, and issues. 3rd ed. New York, NY: Guilford Press; 2012. pp. p. 99–144. [Google Scholar]
- 23.Blum D, Holling H. Spearman's law of diminishing returns. A meta-analysis. Intelligence. 2017;65:60–6. [Google Scholar]
- 24.Belbase A, Sanzenbacher GT. Cognitive aging: a primer. Vol. 16. Center for Retirement Research at Boston College; 2016. pp. p. 1–9. Research brief. [Google Scholar]
- 25.Garrett HE. Differentiable mental traits. Psychol Rec. 1938;2:257. [Google Scholar]
- 26.Garrett HE. A developmental theory of intelligence. Am Psychol. 1946;1:372–8. doi: 10.1037/h0056380. [DOI] [PubMed] [Google Scholar]
- 27.Hartshorne JK, Germine LT. When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychol Sci. 2015;26:433–43. doi: 10.1177/0956797614567339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrington KD, Dang C, Lim YY, Ames D, Laws SM, Pietrzak RH, et al. The effect of preclinical Alzheimer's disease on age-related changes in intelligence in cognitively normal older adults. Intelligence. 2018;70:22–9. [Google Scholar]
- 29.Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer's disease: implications for prevention trials. Neuron. 2014;84:608–22. doi: 10.1016/j.neuron.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mortamais M, Ash JA, Harrison J, Kaye J, Kramer J, Randolph C, et al. Detecting cognitive changes in preclinical Alzheimer's disease: a review of its feasibility. Alzheimers Dement. 2017;13:468–92. doi: 10.1016/j.jalz.2016.06.2365. [DOI] [PubMed] [Google Scholar]
- 31.Wechsler D. The measurement of adult intelligence. Baltimore: Williams & Wilkins; 1944. [Google Scholar]
- 32.Wechsler D. Adult intelligence scale. Vol. 21. Psychological Corporation; 1997. p. p. 504. [Google Scholar]
- 33.Cattell RB. The scientific study of personality. Hammondsworth; 1965. [Google Scholar]
- 34.Kaufman AS, McLean JE, Lincoln A. The relationship of the Myers-Briggs type indicator (MBTI) to IQ level and the fluid and crystallized IQ discrepancy on the Kaufman adolescent and adult intelligence test (KAIT) Assessment. 1996;3:225–39. [Google Scholar]
- 35.Chai LR, Khambhati AN, Ciric R, Moore TM, Gur RC, Gur RE, et al. Evolution of brain network dynamics in neurodevelopment. Netw Neurosci. 2017;1:14–30. doi: 10.1162/NETN_a_00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zamroziewicz MK, Talukdar MT, Zwilling CE, Barbey AK. Nutritional status, brain network organization, and general intelligence. Neuroimage. 2017;161:241–50. doi: 10.1016/j.neuroimage.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 37.Botwinick J. Handbook of the psychology of aging. Van Nostrand Reinhold; 1977. Intellectual abilities; pp. p. 508–605. [Google Scholar]
- 38.O'Shea DM, Fieo R, Woods A, Williamson J, Porges E, Cohen R. Discrepancies between crystallized and fluid ability are associated with frequency of social and physical engagement in community dwelling older adults. J Clin Exp Neuropsychol. 2018;40:963–70. doi: 10.1080/13803395.2018.1452195. [DOI] [PubMed] [Google Scholar]
- 39.Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80:1341–8. doi: 10.1212/WNL.0b013e31828ab35d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset is available in the public domain and can be accessed through the Gateway to Global Aging Data website (https://g2aging.org/). The Harmonized Diagnostic Assessment of Dementia for the Longitudinal Aging Study in India (LASI-DAD) Wave 1 Version A Data, doi:10.25549/h5wx-ay45, are produced and distributed by the University of Southern California with funding from the National Institute on Aging (R01AG051125, RF1AG055273, U01AG065958).