Abstract
Bariatric surgery is the most effective treatment for obesity and improves several manifestations of the metabolic syndrome, including nonalcoholic fatty liver disease. Strict nutritional counseling after bariatric surgery is a key in realizing these outcomes. When postoperative nutrient intake or nutrient uptake is compromised, bariatric surgery can also lead to severe hepatic complications. Here, we describe 3 cases of acute liver injury and acute liver failure caused by bariatric surgery, all with different management strategies and outcomes.
Keywords: Liver injury, Gastric bypass, Liver transplantation, Steatohepatitis, Protein malnutrition
Introduction
Bariatric surgery is the most effective modality for reducing obesity-related comorbidities [1, 2, 3]. The goal of bariatric surgery is to maximize weight loss while retaining nutritional health, by preventing postoperative malnutrition and loss of lean body mass. Bariatric procedures are categorized as either restrictive (limiting stomach capacity), malabsorptive (bypassing a portion of the small intestines), or a combination of both. Weight loss after bariatric surgery generally has a beneficial effect on the hepatic complications of obesity, with improvement of steatosis, inflammation, and fibrosis [3]. However, a combination of malnutrition, malabsorption, and a compromised intestinal barrier function after surgery occasionally also results in the deterioration of liver function [4, 5]. Acute liver failure (ALF) is a rare but life-threatening complication of bariatric surgery. We present three cases of severe hepatic dysfunction after bariatric surgery, all with different management strategies and outcomes.
Case Series
Case 1
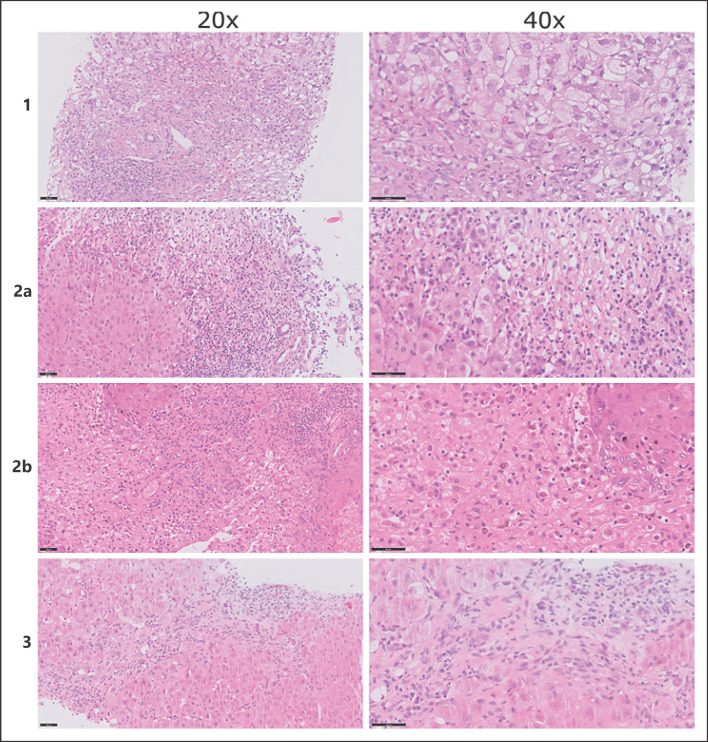
A 37-year-old female was referred to our liver transplantation ward because of progressive jaundice. Seven months prior, she underwent a laparoscopic one-anastomosis gastric bypass for morbid obesity with a body mass index (BMI) of 45.5 kg/m2 and multiple obesity-related comorbidities, including type-2 diabetes mellitus, diabetic nephropathy, and hypertension. The one-anastomosis gastric bypass was performed in the standard fashion with a biliopancreatic limb of 180 cm. Postoperative compliance to nutritional supplements was subpar. Within 6 months, she experienced a total weight loss of 45 kg, corresponding to a reduction in BMI to 29 kg/m2. During follow-up, liver biochemistry and liver function tests initially remained normal. Two weeks prior to admission, she noticed progressive jaundice accompanied by fatigue and postprandial pain, followed by diarrhea. There was no history of alcohol or drug use, and she did not use of over-the-counter medication or herbal supplements. Clinical examination showed jaundice without signs of chronic liver disease or hepatic encephalopathy. Laboratory results showed acute hepatitis with coagulopathy (Table 1). Serology tests for infectious (e.g., viral) and noninfectious causes of hepatitis and stool examination were negative. Serum acetaminophen concentration was <0.3 mg/L. Abdominal computed tomography (CT) showed periportal edema and mild ascites, without signs of chronic liver disease. Upon referral, a percutaneous liver biopsy was performed, and total parenteral nutrition (TPN) was started, based on the hypothesis that severe acute liver injury (ALI) might be caused by extensive protein malnutrition and a consequent amino acid shortage [4]. Autoimmune hepatitis was initially also considered (IgG 15.4 g/L, anti-smooth muscle antibody positive at 1:40), and treatment with prednisone was initiated with a dose of 40 mg daily. Liver biopsy showed periportal and lobular hepatitis with prominent hepatocyte ballooning with Mallory-Denk bodies, bilirubinostasis, and no steatosis (<5%), in a panacinar pattern (Fig. 1). Since the liver biopsy was not compatible with autoimmune hepatitis, prednisone treatment was discontinued. After 2 weeks of TPN, liver biochemistry normalized without developing hepatic encephalopathy. She was referred to her bariatric surgeon for a redo procedure by lengthening the common loop. Currently, several years after revision surgery, liver function tests remain normal.
Table 1.
Evolution of liver function tests
| Case 1 | Unit | Reference range | DO | D2 | D3 | D7 | D14 | D23 | D28 | D39 | D48 | D74 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Admission | Transfer | Start TPN | Transfer | Redo | ||||||||
| AST | U/L | <31 | 723 | 778 | 629 | 143 | 150 | 116 | 71 | NA | 40 | 30 |
| ALT | U/L | <34 | 428 | 526 | 393 | 187 | 109 | 90 | 57 | NA | 32 | 26 |
| Total bilirubin | µmol/L | <17 | 247 | 325 | 270 | 238 | 143 | 73 | 42 | NA | 16 | 9 |
| Albumin | g/L | 34–38 | 24 | 26 | 20 | 25 | 27 | 36 | 27 | NA | 30 | 40 |
| INR | Ratio | 0.8–7.0 | 1.9 | 1.6 | 1.3 | 1.0 | 0.9 | NA | NA | NA | NA | 1.0 |
| Case 2 | Unit | Reference range | DO | D2 | D3 | D4 | D6 | D7 | D10 | D40 | D102 | |
| Admission | Transfer | Start TPN | HU-list | Tx | Tx + 30D | Tx + 92 D | ||||||
| AST | U/L | <31 | 1,539 | 1,394 | 1,294 | 636 | 335 | 220 | 116 | 27 | 106 | |
| ALT | U/L | <34 | 1,168 | 1,014 | 997 | 733 | 528 | 429 | 220 | 241 | 375 | |
| Total bilirubin | µmol/L | <17 | 178 | 265 | 260 | 265 | 307 | 320 | 369 | 50 | 16 | |
| Albumin | g/L | 34–38 | 32 | 28 | 26 | 24 | 19 | 20 | 18 | 28 | 40 | |
| INR | Ratio | 0.8–7.0 | 2.6 | 3.1 | 2.9 | 3.2 | 4.0 | 4.1 | 3.7 | 1.1 | 1.0 | |
| Case 3 | Unit | Reference range | DO Dl | D2 | D5 | D7 | D13 | D19 | D27 | |||
| Admission | Transfer | Start TPN | ||||||||||
| AST | U/L | <31 | 161 | 190 | 185 | 238 | 232 | 120 | 121 | 100 | ||
| ALT | U/L | <34 | 30 | 36 | 34 | 38 | 37 | 30 | 36 | 32 | ||
| Total bilirubin | µmol/L | <17 | 93 | 127 | 129 | 160 | 237 | 183 | 184 | 143 | ||
| Albumin | g/L | 34–38 | NA | 26 | 26 | 30 | 35 | 29 | 31 | 26 | ||
| INR | Ratio | 0.8–7.0 | 1.7 | 1.7 | 1.8 | 1.7 | 1.4 | 1.4 | 1.4 | 1.4 |
AST, aspartate transaminase; ALT, alanine transaminase; INR, international normalized ratio; D, day; TPN, total parenteral nutrition; HU-list, high-urgency list; Tx, transplantation; NA, not available.
Fig. 1.
Histological images. Case 1 (top row): periportal and lobular inflammation with prominent hepatocyte ballooning and Mallory-Denk bodies (HE staining, left colum ×20,right column ×40). Case 2A (second row): severe acute hepatitis with extensive collapse and confluent hepatocellular necrosis (HE staining, left column ×20, right column ×40). Case 2B (third row): necrotic liver parenchyma with extensive inflammation without destruction of the bile ducts or blood vessels (HE staining, left column ×20, right column ×40). Case 3 (bottom row): periportal hepatitis with septating fibrosis (HE staining, left column ×20, right column ×40).
Case 2
A 49-year-old female was referred to our liver transplantation ward because of progressive jaundice. Seven months prior, she underwent a Roux-en-Y gastric bypass for morbid obesity with a BMI of 62.1 kg/m2. Her past medical history showed hypothyroidism for which she was on treatment. Thyroid function was well regulated. Surgery was performed using a standard laparoscopic Roux-en-Y gastric bypass technique, creating a biliopancreatic limb of 150 cm and an alimentary limb of 75 cm. After surgery, she achieved a total weight loss of 40 kg, resulting in a BMI of 47.2 kg/m2. During follow-up, liver biochemistry and function tests initially remained normal. However, adherence to nutritional supplements was subpar. Clinical examination revealed jaundice without signs of chronic liver disease or hepatic encephalopathy. Laboratory results revealed acute hepatitis with coagulopathy (Table 1). Serum markers for common infectious (e.g., viral) and noninfectious causes of hepatitis and stool examination were negative. Serum acetaminophen levels were <0.3 mg/L. Abdominal CT showed a normal liver with patent hepatic vasculature and no intrahepatic or extrahepatic biliary dilatation. Percutaneous liver biopsy revealed severe acute hepatitis with extensive collapse, causing a fibrotic morphology and confluent hepatocellular necrosis. The liver parenchyma showed a nodular aspect and prominent ductular reaction, without ballooning of hepatocytes or Mallory-Denk bodies and no steatosis (<2%, Fig. 1). As in the first case, TPN was promptly started. However, she developed hepatic encephalopathy and was listed for high-urgency liver transplantation within 5 days after referral. Three days after listing, she underwent a liver transplantation. The postoperative course was uncomplicated, and she was discharged after 5 weeks following satisfactory clinical recovery. Histopathological examination of the explanted liver revealed a similar morphology as the previous liver biopsy, with increased bilirubinostasis and steatosis (<10%, Fig. 1). She is now under strict nutritional surveillance with additional supplements and has not undergone revision surgery yet. Currently, several years after liver transplantation, the liver function tests remain normal.
Case 3
A 59-year-old female was referred from a regional hospital to our ICU because of ALF. Two years earlier, she underwent a sleeve gastrectomy for morbid obesity with a BMI of 70.1 kg/m2. Weight regain prompted revision surgery with conversion to a single anastomosis duodeno-ileal bypass with a sleeve gastrectomy, with a biliopancreatic limb of 170 cm and an alimentary limb of 80 cm. Her medical history further included heterozygosity for hemochromatosis without cirrhosis, for which phlebotomies were performed. There was no history of an alcohol use disorder, and she did not use any over-the-counter medication or herbal supplements. Three months after revision surgery, she experienced severe dumping syndrome, leading to insufficient nutriment intake for which enteral tube feeding was initiated. Because of frequent dislodgement of the nasal tube, enteral feeding was discontinued. Six months after revision surgery, she was admitted to a regional hospital with refractory hypotension and required inotropic support. Clinical examination revealed jaundice and mild hepatic encephalopathy. Laboratory results showed acute hepatitis, with coagulopathy, and acute kidney injury (Table 1). Signs of preexisting liver disease were absent. Serology tests for common infectious (e.g., viral) and noninfectious causes of hepatitis were negative. Abdominal CT showed a normal liver and biliary system, without radiological signs of cirrhosis or portal hypertension. No infectious substrate for the hypotension was identified. Percutaneous liver biopsy was performed and revealed periportal hepatitis with bilirubinostasis and mild steatosis (<10%), without signs of ballooning. The liver parenchyma showed a nodular aspect with septating fibrosis and a prominent ductular reaction (Fig. 1. As in the first 2 cases, treatment with TPN was started directly after liver biopsy. Over the next couple of weeks, there was a gradual clinical recovery. She has been referred to her bariatric surgeon for a redo procedure by lengthening the common loop.
Discussion/Conclusion
ALI and ALF are uncommon but life-threatening complications of bariatric surgery [4]. In contrast to other case reports, only 1 out of 3 patients required liver transplantation in this case series. Based on the histological images showing an emaciated steatohepatitis, or so called “depleted nonalcoholic steatohepatitis (depleted NASH),” we decided to start treatment with TPN rich in amino acids directly after referral in all 3 cases, in order to “recharge” the hepatocytes. We assume that malabsorption, combined with nonadherence to nutritional supplements, results in protein-energy malnutrition and exhaustion of intrahepatic amino acid stores [4]. The lack of available resources in our cases, on average, emerged 8 months after bariatric surgery, which is relatively early as compared to the available literature [5]. Several cellular alterations seem to take place in protein-starved livers [6, 7]. Our cases represent different stages of the hepatocyte response to a lack of resources, ranging from an acute phase with prominent necrosis (Fig. 1) to a more chronic phase with fibrotic changes of the liver parenchyma (Fig. 1). The first case showed a (N)ASH like morphology as has been described in similar cases [7].
A major risk factor for the development of malabsorption and liver dysfunction after bariatric surgery is a (relatively) short common loop [8], causing malnutrition in general and amino acid deficiencies in particular [9]. Furthermore, small-intestinal bacterial overgrowth (SIBO) may contribute to malabsorption and thereby the development of ALF. SIBO is a common complication after either a malabsorptive or combined procedure, leading to loss of nutrients due to intestinal barrier alterations and increased intestinal permeability [10]. Bacterial translocation into the portal venous system may induce hepatocellular damage, followed by liver dysfunction [4]. Preoperative deficiencies and reduced dietary intake may also contribute to enhanced depletion of liver energy reserves, in addition to inadequate supplement intake. Of note, mental illness is prevalent after bariatric surgery and may negatively impact adherence to nutritional support, increasing the risk for hepatic dysfunction and highlighting the importance of dedicated guidance and counseling after bariatric surgery [11, 12].
Our second case developed ALF within 2 days after referral, with rapid clinical deterioration. Unfortunately, there is no marker or clinical scoring system available to predict whether a patient will improve with TPN or will develop ALF, necessitating a liver transplant to prevent a fatal outcome. It is important to realize that there is a delicate balance in these patients, requiring intensive clinical and biochemical monitoring in a liver transplant center. To prevent recurrence of ALI following discharge, we referred our patients for a redo procedure with lengthening the common loop. Lengthening the common loop decreases the risk of malnutrition, malabsorption, and amino acid deficiency and also is a curative treatment for SIBO [10].
In conclusion, this case series illustrates the importance of recognizing the onset of ALI and ALF after bariatric surgery. It emphasizes the crucial role of adequate and timely (parenteral) nutrition in these patients, which may avoid liver transplantation in case of this life-threatening complication.
Statement of Ethics
Ethics approval by an Institutional Review Board was not required for this retrospective case series in accordance with local guidelines. Written informed consent was obtained from the patients for publication of this case report and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors have no funding sources to disclose.
Author Contributions
Rowan F. van Golen, Nadine E. de Waard, Laura R. Moolenaar, Akin Inderson, Stijn Crobach, Alexandra M.J. Langers, Bart van Hoek, and Maarten E. Tushuizen contributed to data collection, data analysis, data interpretation, and critical revision of the manuscript. Rowan F. van Golen, Nadine E. de Waard, and Maarten E. Tushuizen.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1.Carlsson LMS, Sjöholm K, Jacobson P, Andersson-Assarsson JC, Svensson P-A, Taube M, et al. Life expectancy after bariatric surgery in the Swedish obese subjects study. N Engl J Med. 2020 Oct 15;383((16)):1535–43. doi: 10.1056/NEJMoa2002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes: 5-year outcomes. N Engl J Med. 2017 Feb 16;376((7)):641–51. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aminian A, Al-Kurd A, Wilson R, Bena J, Fayazzadeh H, Singh T, et al. Association of bariatric surgery with major adverse liver and cardiovascular outcomes in patients with biopsy-proven nonalcoholic steatohepatitis. JAMA. 2021 Nov 23;326((20)):2031–42. doi: 10.1001/jama.2021.19569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moolenaar LR, de Waard NE, Heger M, de Haan LR, Slootmaekers CPJ, Nijboer WN, et al. Liver injury and acute liver failure after bariatric surgery: an overview of potential injury mechanisms. J Clin Gastroenterol. 2022 Apr 1;56((4)):311–23. doi: 10.1097/MCG.0000000000001662. [DOI] [PubMed] [Google Scholar]
- 5.Addeo P, Cesaretti M, Anty R, Iannelli A. Liver transplantation for bariatric surgery-related liver failure: a systematic review of a rare condition. Surg Obes Relat Dis. 2019 Aug;15((8)):1394–401. doi: 10.1016/j.soard.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Corcos O, Cazals-Hatem D, Durand F, Kapel N, Guinhut M, Stefanescu C, et al. Intestinal failure after bariatric surgery. Lancet. 2013;382((9893)):742. doi: 10.1016/S0140-6736(13)61214-3. [DOI] [PubMed] [Google Scholar]
- 7.Tsai JH, Ferrell LD, Tan V, Yeh MM, Sarkar M, Gill RM. Aggressive non-alcoholic steatohepatitis following rapid weight loss and/or malnutrition. Mod Pathol. 2017;30((6)):834–42. doi: 10.1038/modpathol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahawar KK. Liver dysfunction with both Roux-en-Y and one-anastomosis gastric bypass is almost exclusively seen with longer than standard limb lengths. Obes Surg. 2018;28((2)):548–9. doi: 10.1007/s11695-017-3028-4. [DOI] [PubMed] [Google Scholar]
- 9.Nicoletti CF, Morandi Junqueira-Franco MV, dos Santos JE, Marchini JS, Salgado W, Nonino CB. Protein and amino acid status before and after bariatric surgery: a 12-month follow-up study. Surg Obes Relat Dis. 2013 Dec;9((6)):1008–12. doi: 10.1016/j.soard.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Alcaraz F, Frey S, Iannelli A. Surgical management of small intestinal bacterial overgrowth after Roux-en-Y gastric bypass. Obes Surg. 2020 Nov;30((11)):4677–8. doi: 10.1007/s11695-020-04809-5. [DOI] [PubMed] [Google Scholar]
- 11.Mingrone G, Bornstein S, Le Roux CW. Optimisation of follow-up after metabolic surgery. Lancet Diabetes Endocrinol. 2018 Jun;6((6)):487–99. doi: 10.1016/S2213-8587(17)30434-5. [DOI] [PubMed] [Google Scholar]
- 12.Lammers WJ, van Tilburg AJ, Apers JA, Wiebolt J. Liver failure caused by prolonged state of malnutrition following bariatric surgery. World J Hepatol. 2018;10((3)):396–9. doi: 10.4254/wjh.v10.i3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.