Abstract
Background
CD38+ NK (CD3− CD16+ CD38+ CD56+) cells were increased in rheumatoid arthritis (RA), which suppressed Treg cell differentiation. This study explored how CD38+ NK cells regulated CD4+ T-cell differentiation into Treg cells in RA.
Methods
Proportions of CD38+ NK cells and their counterpart CD38+ NK-like T (CD3+ CD16+ CD38+ CD56+) cells were measured in RA and rats with collagen-induced arthritis (CIA). CD38+ NK cells and CD38+ NK-like T cells were cocultured with CD4+ T cells, respectively.
Results
A significantly increased proportion of CD38+ NK cells and a decreased proportion of CD38+ NK-like T cells were detected in RA and CIA blood and synovial fluids. When CD4+ T cells were cocultured with CD38+ NK cells, mammalian target of rapamycin (mTOR) signaling was activated, and Th1/Th2 and Th17/Treg ratios were increased. When CD38+ NK cells were pretreated with anti-CD38 antibody, Treg cell proportion was increased, and Th1/Th2 and Th17/Treg ratios were decreased. CD38+ NK-like T cells showed the opposite results. CD38+ NK cells and CD38+ NK-like-T cells activated differential gene expressions and pathways in CD4+ T cells and initiated Th1 and Th2 cell differentiation by differential gene nodes.
Conclusions
This study suggest that the high CD38+ NK cell proportion and low CD38+ NK-like T cell proportion in RA suppress Treg cell differentiation by stimulating mTOR signaling in CD4+ T cells, which consequentially disturbs the immune tolerance.
Keywords: Cluster of differentiation 38, Collagen-induced arthritis, CD38+ NK cells, CD38+ NK-like T cells, Immune tolerance, Rheumatoid arthritis, Treg cells
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic joint inflammation. Helper cells (Th) differentiate into Th1, Th2, Th17, and Treg cells [1]. Tregs are important regulators of immune homeostasis, and they can regulate the immune response by inhibiting inflammation and promoting self-tolerance [1, 2, 3]. Many studies have demonstrated that abnormal Th1/Th2, Th17/Treg ratios, and Treg cells play important roles in the occurrence and development of RA and other autoimmune diseases [4, 5, 6, 7, 8]. When the number and function of Treg cells are damaged, the Th17/Treg balance is disrupted, resulting in immune imbalance and damage to immune tolerance [9]. The proportion of Treg cells in RA was significantly decreased, which was significantly related to the degree of RA activity [10, 11, 12, 13, 14]. Many studies have found that increasing the proportion of Treg cells or repairing Treg cell function is an effective strategy for the treatment of autoimmune diseases [8, 15, 16, 17]. We previously injected human Treg cells into collagen-induced arthritis (CIA) rats and found that exogenous Treg cells significantly alleviated CIA, indicating that cross-species Treg cells can exert therapeutic effect on CIA by restoring immune balance [18]. We also reported that injection of Xuebijing, a traditional Chinese medicine, could significantly treat RA and CIA by elevating Treg cells in RA patients and rat models [19]. The above studies support the notion that maintenance of the proportion and function of Treg cells plays an important role in preventing and treating RA. However, the mechanism by which Th1/Th2 and Th17/Treg balance is maintained is unclear, and the pathological mechanism responsible for the imbalance of Th1/Th2 and Th17/Treg cells is also unknown. This has been the subject of long-term efforts in basic immunology and clinical immunology.
Cluster of differentiation 38 (CD38) is a multifunctional ectoenzyme that catalyzes conversion of β-nicotinamide adenine dinucleotide (β-NAD) to nicotinamide adenine dinucleotide phosphate (NADP). The metabolites NAD and NADP have roles in calcium signaling in different cell types [20, 21]. CD38 is mainly expressed in natural killer (NK) cells, B cells, T cells, monocyte-macrophages and dendritic cells (DCs) and is highly expressed in multiple myeloma (MM) cells [22, 23, 24]. CD38 is an emerging therapeutic target through which metabolism is altered in relation to infection, aging and tumorigenesis [20]. Through transcriptomic analyses, we discovered that CD38 is specifically overexpressed in synovial tissues in RA compared to ankylosing spondylitis and osteoarthritis (OA) samples; likewise, the proportions of CD38+ cells and CD38+ CD56+ cells are significantly elevated in the peripheral blood of RA patients, and the level of CD38+ cells is positively correlated with the level of rheumatoid factor in the patients. Furthermore, suppressing CD38 expression in cultured RA synovial fibroblast cells decreased IL-1α and IL-1β secretion [25]. Other studies have shown elevated CD38 expression in B and T cells from patients with autoimmune diseases [26, 27]. CD38 knockout mice have significantly reduced occurrence and development of CIA [28, 29, 30]. These studies suggest that CD38 may play an important role in RA pathogenesis. Moreover, we recently found that CD38+ NK cells (CD38+ CD3− CD16+ CD56) were overabundant in RA. CD38 decreased TNF-α level and increased IFN-γ level by suppressing Sirtuin 6 (Sirt6) expression in CD38+ NK cells, which consequently inhibited mononuclear cell (MNC) differentiation into Treg cells. This study suggested that high numbers of CD38+ NK cells as well as their inhibition on Treg cell differentiation in MNCs were potential causes of the immune imbalance and disrupted immune tolerance in RA and CIA [31]. However, the following questions remain unclear: (i) How do CD38+ NK cells suppress MNC differentiation to Treg cells? (ii) Does CD38 antibody have a therapeutic effect in RA and CIA? (iii) Do CD38+ NK-like T cells (CD3− CD16+ CD38+ CD56+ cells), another CD38+ CD56 cell subtype, play a role in RA?
This study simultaneously studied CD38+ CD3− CD16+ CD56+ and CD38+ CD3+ CD16+ CD56+ cells, which are also known as CD38+ NK cells and CD38+ NK-like T cells, respectively. Proportions of CD38+ NK cells and their counterpart, CD38+ NK-like T (CD3+ CD16+ CD38+ CD56+) cells were measured in RA patients and rats with CIA. CD38+ NK cells and CD38+ NK-like T cells were cocultured with CD4+ T cells or MNCs depleted of CD38+ CD16+ CD56+ cells from RA synovial fluid and blood. Levels of Th1, Th2, Th17, Treg cells, inflammatory cytokines, and TGF-β were measured in the culture. Activation of the mammalian target of rapamycin (mTOR) signaling pathway has been demonstrated to promote the differentiation of primary T cells, and blocking mTOR activity can reverse this inhibitory effect on Treg cells [32, 33]. This study examined mTOR signaling activity in CD4+ T cells following the coculture. Expression profiles of CD4+ T cells following the coculture were examined using Transcriptomic analysis. The purpose of this study was to investigate the roles and regulatory mechanism of CD38+ NK cells and their counterpart, CD38+ NK-like T cells, in RA.
Materials and Methods
Sample Collection
Peripheral blood and synovial fluid were collected from RA patients (n = 30), and synovial fluid was collected from OA patients (n = 30) in the Rheumatology Department of the Affiliated Hospital of Qingdao University in Qingdao, China. All enrolled RA patients met the 1987 RA classification criteria of the American College of Rheumatology (ACR) and the 2010 classification criteria of the American Association of Rheumatology (AAR)/the European League Against Rheumatism (EULAR). Peripheral blood samples were collected from healthy volunteers (n = 30) at the Physical Examination Center of the hospital. The sample collection and experimental design were approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (Approval No. 20190105), and all patients gave written informed consent. Clinical information of the patients who donated blood samples and synovial fluid samples is shown in online suppl. Table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000516642 and online suppl. Table 2, respectively. The peripheral blood and synovial fluid samples from patients with RA were not paired.
Generating a Rat Model of Bovine Type 2 CIA
Six-week-old Sprague Dawley (SD) rats (200 ± 16 g) (n = 48, male) were purchased from the Shandong Laboratory Animal Center (China). The rats were randomly divided into 2 groups: normal control (NC, n = 24) and CIA model (n = 24) groups. Rats in the CIA model group were intradermally injected at the tail root with an emulsified and equal mixture of bovine type 2 collagen (Chondrex, USA) (0.2 mg/per rats) and complete Freund's adjuvant (Sigma, USA). One week later, the same amount of the emulsified and equally mixed bovine type 2 collagen and incomplete Freund's adjuvant (Sigma) was injected. To measure disease activity, an inflammation curve was drawn based on changes in the thickness of the toe. These rats were sacrificed at 16 days following the first time of collagen injection. Histochemical observation for the joint tissues was performed by routine way. The rats in the control group were injected with the same volume of physiological saline (PBS).
Detection of Lymphocyte Subtypes in Rat Blood
Rats were euthanized with lethal doses of ketamine and xylazine, and peripheral blood was collected from the inferior vena cava. The cells were collected by centrifugation and lysed using red blood cell lysis buffer (BioLegend, USA). Antibodies and isotype controls for flow cytometry were added (1.0 μg per 106 cells in 100 μL volume), and the mixture was incubated at 4°C for 30 min in the dark. CD38+ CD3+ T (CD38+ T) cells were detected with an APC anti-rat CD3 antibody and PE anti-rat CD38 antibody (BioLegend), CD3+ CD4+ T cells with APC anti-rat CD3 and FITC anti-rat CD4 antibodies (BioLegend), CD3+ CD8+ T cells with APC anti-rat CD3 and PerCP anti-rat CD8a antibodies (BioLegend), CD38+ CD3-CD45RA+ B (CD38+ B) cells with PE anti-rat CD38, APC anti-rat CD3 and FITC anti-rat CD45RA antibodies (BioLegend), and CD38+ CD3− CD161+ NK (CD38+ NK) cells and CD38+ CD3+ CD161+ NK-like T cells (CD38+ NK-like T) cells with PE anti-rat CD38, APC anti-rat CD3 and FITC anti-rat CD161 antibodies (BioLegend). FITC IgG1, PE IgG1, APC IgG1, and PerCP/Cy5.5 IgG1 antibodies were used as isotype controls. Data were acquired using NovoCyte flow cytometry system (NovoCyte D2040R; American ACEA BIO).
Detection of Rat Treg Cells
APC anti-rat CD4 and FITC anti-rat CD25 antibodies (BioLegend) or APC IgG2a and FITC IgG2a isotype controls (BioLegend) were added to the blood samples with anticoagulation treatment (1 μg per 106 cells in 100 μL volume). The mixture was incubated at room temperature for 15 min in the dark. Then, 1× red blood cell lysis/fixation solution (BioLegend) was added, and the mixture was incubated at room temperature for 15 min. After centrifugation, cell staining buffer (BioLegend) was added to resuspend the pellet, and the mixture was centrifuged at 300 g for 5 min. The cell pellet was then fixed with fixation buffer (BioLegend) and incubated for 20 min at room temperature. Following centrifugation, the fixed cells were resuspended in permeabilization wash buffer (BioLegend), and PE anti-rat FOXP3 antibody or PE IgG2a isotype control (BioLegend) was added (1 μg per 106 cells in 100 μL volume). The mixture was incubated at room temperature for at least 30 min and resuspended in 1× flow cytometry staining buffer. CD4+ CD25+ FOXP3+ Treg cells were detected by flow cytometry.
Detection of Lymphocyte Subtypes in RA Synovial Fluid or Blood
Peripheral blood of RA patients or healthy volunteers (n = 30, from patients No. 1 to No. 30 in online suppl. Table 1) and RA or OA synovial fluid samples (n = 30, from patients No. 1 to No. 30 in online suppl. Table 2) were collected and washed 3 times with PBS. The cell pellet was resuspended in PBS. The sample to be tested was incubated with the following antibodies and isotype controls at 4°C for 30 min (1 μg per 106 cells in 100 μL volume). CD45+ lymphocytes, CD3+ T cells, CD3+ CD4+ T cells, and CD3+ CD8+ T cells were detected with a FITC anti-CD3/PE anti-CD8/PerCP anti-CD45/APC anti-CD4 detection kit (ACEA Biosciences, China). CD3− CD16+ CD56+ NK cells, CD3+ CD16+ CD56+ NK-like T cells, and CD3− CD19+ B cells were detected using a FITC anti-CD3/PE anti-CD16+ CD56/PerCP anti-CD45/APC anti-CD19 detection kit (ACEA biosciences). FITC IgG1, PE IgG1, APC IgG1, and PerCP/Cy5.5 IgG1 antibodies were used as isotype controls (BioLegend). Lymphocyte subtypes within MNCs were measured using flow cytometry.
The proportions of CD38+ T cells (CD3+ CD38+), CD38+ B cells (CD3− CD19+ CD38+), CD38+ NK cells (CD38+ CD3− CD16+ CD56+), and CD38+ NK-like T cells (CD38+ CD3+ CD16+ CD56+) were detected using flow cytometry as described above. PerCP anti-human CD38 (BioLegend), FITC anti-human CD3/PE (CD16+ CD56) cocktail (BioLegend), FITC anti-human-CD3 (BioLegend), and APC anti-human-CD19 antibodies (BioLegend) were added to the samples.
Detection of RA Treg Cells
This study used a human Treg cell assay kit (Multiscience Biotech). FITC anti-human CD4 and APC anti-human CD25 antibodies or FITC IgG2a and APC IgG2a isotype controls were added to blood samples with anticoagulation treatment, synovial fluids, cultured MNCs, or CD4+T cells (1 μg per 106 cells in 100 μL volume). The mixture was incubated at room temperature for 15 min in the dark. Next, 1× flow cytometry lysing solution was added, and the mixtures were incubated at room temperature for 15 min. Then, 1× fixation/permeabilization working solution was added, and the mixtures were incubated at room temperature for 30 min. Following centrifugation, the pellets were resuspended in 1× permeabilization buffer, and PE anti-human FOXP3 antibody or rat PE IgG2a isotype control was added (1 μg per 106 cells in 100 μL volume). The mixture was incubated at room temperature for 30 min and centrifuged. The pellet was resuspended in 1× flow cytometry staining buffer. CD4+ CD25+ FOXP3+ Treg cells were detected using flow cytometry.
Separation of MNCs from Synovial Fluid or Peripheral Blood
Heparin sodium-anticoagulated peripheral blood (n = 9, patients No. 22 to No. 30 in online suppl. Table 1) was added to an equal volume of PBS. Synovial fluids (n = 9, patients No. 1 to No. 9 in online suppl. Table 2) were collected and pretreated with hyaluronidase (10 U/mL) at 37°C for 30 min, and 3 volumes of PBS were added. The blood or synovial samples were centrifuged and resuspended with lymphocyte separation solution (Tianjin Haoyang, China). The mixture was centrifuged at 800 g for 20 min, and the white circular layer was collected to obtain peripheral blood MNCs or synovial fluid SMNCs.
Flow Cytometric Preparation of CD38+ NK Cells, CD38+ NK-Like T Cells, and CD38+ CD16+ CD56+ Cell-Depleted MNCs
The peripheral blood or synovial fluid of RA patients was collected to isolate MNCs as described above. The pellet was resuspended in PBS. PerCP anti-human CD38, PE anti-human CD3 and FITC/(CD16+ CD56) antibodies (10 μg per 107 cells in 500 μL volume) (1:1) were added, and the mixture was incubated for 30 min at 4°C. The cells were washed, collected by centrifugation, and resuspended in PBS. The labeled cells were sorted using a BD FACS ARIA II cell sorter with BD FACS Diva V6 software (BD Biosciences, USA). CD38+ and CD16+ CD56+ and CD16+ CD56-gates were established, and the CD38+ NK and CD38+ NK-like T cells were differentiated by CD3+/− in the CD38+ and CD16+ CD56+ cell populations. The cell population in the negative gate was composed of MNCs depleted of CD38+ CD16+ CD56+ cells. The CD38+ NK cells contained a CD3− CD38+ CD16+ CD56+ population, and the CD38+ NK-like T cells contained a CD3+ CD38+ CD16+ CD56+ population. The sorted populations were collected into RPMI-1640 medium (Gibco) containing 10% human heat-inactivated pooled AB serum (Gibco). The viability of the sorted cells was determined by trypan blue exclusion (Thermo Fisher Scientific) using a routine protocol. After sorting, the samples were tested for purity using the BD FACS ARIA II cell sorter with BD FACS Diva V6 software (BD Biosciences, USA). The MNCs prior to co-culturing with NK/NKT cells contained other non-CD38+ CD16+ CD56+ including T-helper cell populations.
Sorting of Naïve CD4+ T Cells
A human naïve CD4+ T Cell Isolation Kit II (Miltenyi Biotec, Germany) was used to isolate naïve CD4+ T cells. The RA synovial MNCs were obtained as described above and were resuspended in the magnetic-activated cell sorting buffer (PBS with 5% fetal calf serum) provided in the kit. CD4 and CD45RA magnetic beads were added to the cells, and the mixture was incubated at 4°C for 15 min. The sample was loaded into the sorting column, and the negative cells were collected. Magnetic-activated cell sorting buffer was added to the sorting column, and the cells were quickly forced into the positive collection tube with the piston. The purity of the freshly sorted naïve CD4+ T cells was determined by flow cytometry.
Coculture of CD38+ NK Cells or CD38+ NK-Like-T Cells with CD38+ CD16+ CD56+ Cell-Depleted MNCs or CD4+ T Cells
CD38+ NK or CD38+ NK-like T cells were treated with anti-CD38 monoclonal antibody (CD38 mAb; Janssen Pharmaceutical Companies of Johnson & Johnson) at a final concentration of 1 μg/mL for 24 h. The anti-CD38 monoclonal antibody has the trade name of daratumumab and is a humanized monoclonal antibody. The anti-CD38 antibody was removed by centrifugation at 200 g for 5 min, and the cells were plated in the upper chamber of a 0.4 μm transwell apparatus (Corning Costar®, USA). Cells that were treated with an equivalent amount of PBS were used as controls. Homologous CD38+ CD16+ CD56+ cell-depleted MNCs or CD4+ T cells were seeded into the lower chamber. After 48 h of coculture, the cells were harvested, and the lymphocyte subtypes among the MNCs or CD4+ T cells were detected using flow cytometry. The supernatant was also collected, and cytokines (IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ) were detected using flow cytometry according to the following description.
Detection of RA Th17 Cells
A human Th17 cell assay kit (Multiscience Biotech) was used to identify RA Th17 cells. MNCs were placed into a flow cytometry tube, and serum-free medium, phorbol myristate acetate/ionomycin mixture and brefeldin A/monensin mixture from the kit were added. The mixture was incubated at 37°C for 4 h and centrifuged. The pellet was resuspended in medium containing 10% fetal bovine serum. FITC anti-human CD3 and PerCP-Cy5.5 anti-human CD4 antibody were added, and FITC IgG1 and PerCP/Cy5.5 IgG1 isotype control antibodies were added as controls (1 μg per 106 cells in 100 μL volume). The mixture was incubated at room temperature for 15 min. The FIX & PERM Medium A from the kit was added, the mixtures were incubated for 15 min at room temperature, and 1× flow cytometry staining buffer was then added. Following centrifugation, FIX & PERM Medium B and PE anti-human IL-17A antibody from the kit were added, and PE IgG1 isotype control antibody was added as a control (1 μg per 106 cells in 100 μL volume). The mixtures were incubated for 15 min at room temperature. Flow cytometry staining buffer was added, and the mixture was centrifuged. The contents were resuspended in 1× flow cytometry staining buffer, and CD3+ CD4+ IL-17A+ Th17 cells were detected using flow cytometry.
Detection of Cytokines in Culture Medium
A human Th1/Th2 cytokine detection kit (Hangzhou Cell Gene, China) was used for cytokine detection. The corresponding standard in the kit was added to each standard tube, and the samples were added to each sample tube. Fluorescence detection reagents that were conjugated to microcapsules in the kit were added to all tubes, and the mixture was incubated at room temperature for 2.5 h. Following centrifugation, the samples were resuspended in PBS, and the concentrations of IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ were measured by flow cytometry.
Treg-Cell Isolation and Sorting
MNCs were obtained from the peripheral blood of RA patients. The MNCs were incubated with FITC anti-human-CD25 (BioLegend), PerCP/Cy5.5 anti-human-CD4 (BioLegend), and FE anti-human-CD127 (BioLegend) antibodies for 30 min at room temperature. The cells were collected by centrifugation and collected in PBS. The labeled cells were sorted using a BD FACS ARIA II cell sorter with BD FACS Diva V6 software (BD Biosciences, USA). The Treg cells were analyzed for purity.
Cell Count Kit-8 Assay for Treg Cells
CD38+ NK or CD38+ NK-like T cells were treated with anti-CD38 monoclonal antibody (CD38 mAb; TheraMabs Bio-Technology, China) at a final concentration of 1 μg/mL or an equal volume of PBS at 37°C for 24 h. CD38+ NK or CD38+ NK-like T cells were then washed and cocultured with Treg cells in a transwell apparatus at 37°C in the presence of 5% CO2 for 24 h. Treg cells were suspended and washed, and Cell Count Kit-8 solution (Dojindo, Japan) was added. After incubation for 3 h, OD values were measured at 450 nm using a spectrophotometer (BioTek, USA).
Detection of TGF-β Expression in Culture Medium
TGF-β expression in the culture medium was detected by flow cytometry using Human TGF−β1 capture bead A4 (BioLegend). The TGF-β1 magnetic beads, anti-TGF-β1 antibody, assay buffer, and standard or tested sample were incubated together for 3 h at room temperature with shaking. Fluorescence detection reagents in the kit were added and incubated with samples for 0.5 h at room temperature with shaking. Following centrifugation, the samples were resuspended in PBS, and TGF-β concentrations were measured using flow cytometry. The data were analyzed using LEGENDplex v 8.0 software (BioLegend).
Detection of RA Th1 and Th2 Cells
A human Th1/Th2 cell assay kit (Multiscience Biotech, China) was used to detect RA Th1 and Th2 cells. Serum-free medium and the phorbol myristate acetate/ionomycin/brefeldin A/monensin mixture in the kit was added to blood samples, MNCs, or CD4+ T cells. The mixture was incubated at 37°C for 4 h and collected by centrifugation. The pellet was resuspended in medium containing 10% fetal bovine serum. FITC anti-human CD3 and PerCP-Cy5.5 anti-human CD4 antibodies were added, and FITC IgG1 isotype control and PerCP-Cy5.5 antibody were added as controls (1.0 μg per 106 cells in 100 μL volume). The mixture was incubated at room temperature for 15 min. The FIX & PERM Medium A in the kit was added, and the mixture was incubated for 15 min at room temperature. Next, 1× flow cytometry staining buffer was added, and the mixture was centrifuged. The FIX & PERM Medium B in the kit, PE anti-human IFN-γ, and APC anti-human IL-4 were added, and PE IgG1 isotype control and APC antibody were added as controls (1 μg per 106 cells in 100 μL volume). The mixtures were incubated for 15 min at room temperature, centrifuged, and resuspended with flow cytometry staining buffer. CD3+ CD4+ IFN-γ+ Th1 cells and CD3+ CD4+ IL-4+ Th2 cells were detected using flow cytometry. The Th1/Th2 ratio was calculated according to the proportions of Th1 and Th2 cells among the CD4+ T cells.
Real-Time PCR Detection
CD38+ NK or CD38+ NK-like T cells were collected from the upper chamber of a transwell apparatus. Total RNA was extracted and reverse transcribed into cDNA (Vazyme, China). Real-time quantitative PCR (Thermo Fisher Scientific, USA) was used to detect the CD3γ, CD28, and TGF-β mRNA expression levels. The PCR primer sequences were as follows: CD3 forward 5′-GCATTTTCGTCCTTGCTGTTGGG-3′ and reverse 5′-GGTCATCTTCTCGATCCTTGAGG-3′; CD28 forward 5′-GAGAAGAGCAATGGAACCATTATC-3′ and reverse 5′-TAGCAAGCCAGGACTCCACCAA-3′; and TGF-β forward 5′-TACCTGAACCCGTGTTGCTCTC-3′ and reverse 5′-GTTGCTGAGGTATCGCCAGGAA-3′.
Western Blot Analysis
Cocultured CD4+ T cells were collected, and RIPA lysis buffer (Beyotime, China) and PMSF (phenylmethanesulfonyl fluoride) were added. The cells were lysed on ice for 30 min. Following centrifugation, the supernatant was collected, and the protein samples were subjected to SDS-PAGE (Beyotime) and transferred to a PVDF membrane (Boster, China). Protein expression changes in the mTOR pathway were detected using an mTOR substrate antibody sampler kit (Cell Signaling Technology, USA). The membranes were incubated with the phospho-mTOR (Ser2448), total mTOR, phospho-p70S6 kinase (Thr389 and Ser371), and phospho-4E-BP1 (Thr37/46) antibodies in the kit. Followed washing 3 times in Tris-buffered saline, the membrane was incubated with anti-rabbit IgG and horseradish peroxidase-linked antibodies from the kit. A gel imaging system (GelDoc XR+; Bio-Rad, USA) was used to expose and develop the blots. Glyceraldehyde-3-phosphate dehydrogenase was used as an internal reference to normalize the expression levels of the target proteins. The expression normalization was performed with ImageJ software (NIH, Bethesda, MD, USA).
The expression of CD38 was examined using a similar protocol, and the antibody was obtained from Santa Cruz Biotechnology. The anti-CD38 antibody is a mouse monoclonal IgG1 (kappa light chain) that was raised against amino acids 1–170 of CD38 of human origin.
Transcriptomic Analysis
CD4+ T Cells were cocultured with CD38+ NK cells or CD38+ NK-like T cells as mentioned above. Non-cocultured CD4+T cells were used as controls. Total RNA was extracted from CD4+ T cells and digested DNA with DNase. Eukaryotic mRNA was enriched with magnetic beads with Oligo(dT). The mRNA was broken into short segments, reverse-transcripted into one-strand cDNA using a 6-base random primer, and prepared into two-strand cDNA. The purified double-stranded cDNA is added with a tail with sequencing connector. PCR amplification is carried out to amplify the sample. The library was qualified by Agilent 2100 Bioanalyzer. After the quality inspection, the transcriptome sequencing and sequencing analysis were carried out with Illumina sequencer. Clean readings were mapped to the reference genome (Sscrofa 10.2). Gene expression level was quantified by the Fragments Per kb Per Million Reads method. The DESeq software (DESeq version 1.39.0) was used to standardize the counts number of each sample gene. Basemean value was used to estimate the gene expression level, and the fold change (FC) was calculated. Negative binomial distribution test (NB) was used to test the difference significance of reads number. The differential protein coding genes were screened according to the FC and difference significance test results. Differentially expressed genes (DEGs) were selected from those protein coding genes with features of the variable importance in the projection values FC >2 or <0.5 and p < 0.05. Kyoto Encyclopedia of Genes and Genomes (KEGG) were used to analyze the enrichment of ontology (GO) biological processes and pathways. DEG with FC ≥1.5 was used for the pathway analysis. The significance of DEG enrichment in each pathway entry was calculated by hypergeometric distribution test (p value <0.05). Bubble chart shows the top 20 of KEGG enrichment analysis, in which the number of DEG >2.
Statistical Analysis
Normal and variance homogeneity tests were performed using SPSS 17.0 software (IBM, USA). One-way ANOVA was used to perform multigroup significance tests. The least significant difference method was used for pairwise comparisons. Pearson correlation analysis was performed, and |r| ≥ 0.8 indicated that 2 variables were highly correlated; 0.8 > |r| ≥ 0.5 indicated that 2 variables were moderately correlated; 0.5 > |r| ≥ 0.3 indicated low correlation of 2 variables; and 0.3 > |r| indicated that there was no correlation between 2 variables. The test standard was α = 0.05, and p < 0.05 indicated statistical significance.
Results
Phenotypic Changes in CD38+ NK and CD38+ NK-Like T Cells in CIA Peripheral Blood
We collected peripheral blood from 24 CIA rats and 24 normal rats. The inflammation curve is shown online suppl. Figure 1. The proportions (percentage) of CD4+ T cells (32.29 ± 2.13), CD3-CD19+ B cells (19.97 ± 0.98) and CD3-CD161+ NK cells (11.15 ± 1.23) in total lymphocytes (CD45+ lymphocytes) in the CIA group were significantly higher than those in the normal control group (26.72 ± 1.699, 15.99 ± 1.63, and 7.04 ± 1.10. p = 0.0467, 0.0415, and 0.0164, respectively), whereas the proportions of CD8+ T cells (14.42 ± 1.14), CD3+ CD161+ NK-like T cells (1.69 ± 0.14), and CD4+ CD25+ FOXP3+ Treg cells (2.213 ± 0.59) were significantly lower than those in the normal controls (19.07 ± 1.84, 2.28 ± 0.25, and 5.17 ± 0.49. p = 0.037, 0.0436, and 0.0004, respectively). There was no significant difference in the proportion of CD3+ T cells (34.60 ± 2.54 and 36.39 ± 2.50) (p = 0.618). The results are shown in Figure 1a.
Fig. 1.
Changes in CD38+ NK and CD38+ NK-like T cells in peripheral blood lymphocytes from CIA rats. a The proportions of lymphocyte subtypes in peripheral blood from the normal control (n = 24) and CIA rats (n = 24). b The proportions of CD38+ cell subtypes in peripheral blood from the normal control and CIA rats. c Changes in the ratios of lymphocyte subtypes in peripheral blood from the normal control and CIA rats. d Correlation analysis of the CD38+ NK/CD38+ NK-like T cell ratio in peripheral blood from the CIA rats and the disease activity. *Represents a p value <0.05, **<0.01, and ***<0.001. CIA, collagen-induced arthritis.
Compared with the normal control group (3.54 ± 0.52, 0.91 ± 0.09, 0.82 ± 0.08, and 6.46 ± 0.45), the proportions of CD38+ CD3− CD19+ B (CD38+ B) cells (5.48 ± 0.44) and CD38+ CD3− CD161+ NK (CD38+ NK) cells (2.24 ± 0.22) in the peripheral blood of CIA rats were significantly increased (p = 0.0062 and <0.0001, respectively), the proportion of CD38+ CD3+ CD161+ NK-like T (CD38+ NK-like T) cells (0.47 ± 0.03) was significantly decreased (p < 0.0001), and there was no significant change in the proportion of CD38+ CD3+ (CD38+ T) cells (7.19 ± 0.66, p = 0.3639) (Fig. 1b). The CD38+ NK/CD38+ NK-like T-cell ratio values in the CIA rats ranged from 0.83 to 16.28, with an average of 5.16, which was significantly higher than that in the normal control group (range: 0.08–5.62, mean: 1.66, p = 0.0012). Meanwhile, the NK/NK-like T-cell ratio in the CIA rats ranged from 1.13 to 26.69, with an average of 8.34, which was significantly higher than that in the normal control group (range: 0.18–14.39, mean: 3.86, p = 0.007) (Fig. 1c). In addition, the ratio of CD38+ NK/CD38+ NK-like T cells in the CIA rats was positively correlated with the CIA inflammation index (r = 0.76, p < 0.0001), and the NK/NK-like T cell ratio was positively correlated with the disease activity score (r = 0.69, p = 0.0002) (Fig. 1d).
Phenotypic Changes in CD38+ NK and CD38+ NK-Like T Cells in RA Peripheral Blood and Synovial Fluid
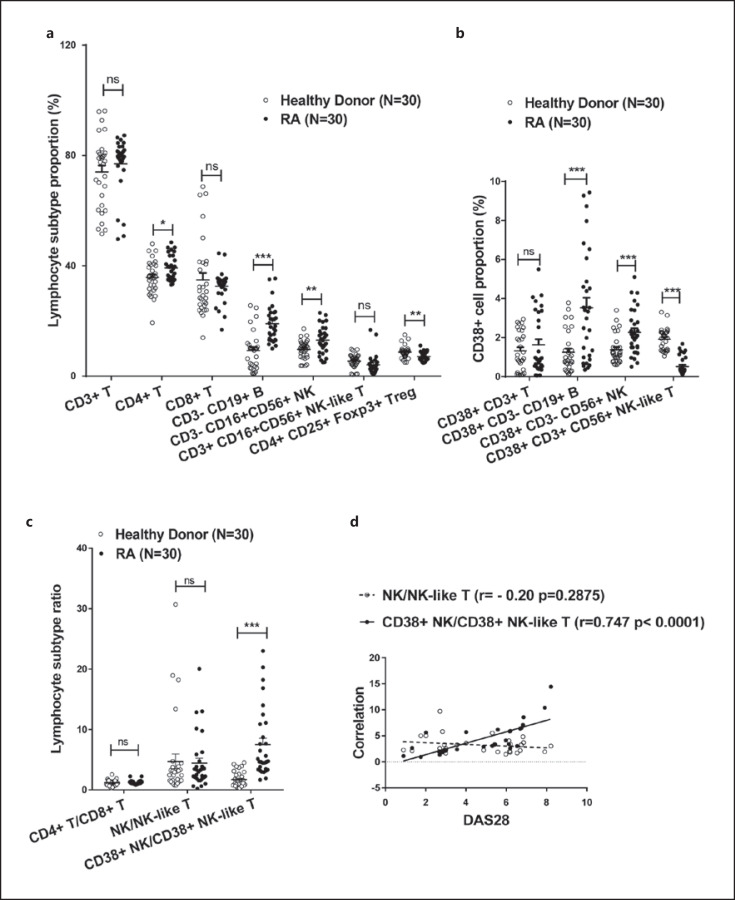
We collected peripheral blood from 30 RA patients and 30 healthy volunteers. The proportions of CD4+ T (39.29 ± 4.55), CD3− CD19+ B (9.30 ± 6.85), and CD3− CD16+ CD56+ NK cells (13.08 ± 5.04) in total lymphocytes (CD45+ lymphocytes) in the RA group were significantly higher than those in the control group (35.83 ± 6.19, 9.03 ± 6.84, and 9.71 ± 3.40. p = 0.0167, <0.0001, and 0.0037, respectively), whereas the proportions of CD4+ CD25+ FOXP3+ Treg cells (7.15 ± 1.59) in the RA group were significantly lower than those in the healthy controls (8.722 ± 2.27, p = 0.0029). There were no significant differences in the proportions of CD3+ T (76.99 ± 10.21 and 74.03 ± 12.86), CD8+ T (32.65 ± 5.66 and 34.88 ± 14.06) and CD3+ CD16+ CD56+ (NK-like T) cells (4.14 ± 3.61 and 5.50 ± 2.53) between the 2 groups (p > 0.05). The results are shown in Figure 2a.
Fig. 2.
Changes in CD38+ NK and CD38+ NK-like T cells in peripheral blood lymphocytes of RA patients. a The proportions of lymphocyte subtypes in peripheral blood from healthy individuals (n = 30) and RA patients (n = 30). b The proportions of CD38+ cell subtypes in peripheral blood from healthy individuals and RA patients. c Changes in the CD38+ NK/CD38+ NK-like T-cell ratio in peripheral blood from healthy individuals and RA patients. d Correlation analysis of the CD38+ NK/CD38+ NK-like T-cell ratio in peripheral blood from RA patients with DAS28. *Represents a p value <0.05, **<0.01, and ***<0.001. RA, rheumatoid arthritis.
Compared with healthy volunteers (1.25 ± 1.06, 1.378 ± 0.74, 1.92 ± 0.56, and 1.32 ± 0.988), CD38+ CD3− CD19+ B (CD38+ B) cells (35.29 ± 2.83) and CD38+ CD3− CD16+ CD56+ NK (CD38+ NK) cells (2.27 ± 1.17) were significantly elevated in the peripheral blood of RA patients (p = 0.0001 and 0.0008, respectively), the proportion of CD38+ CD3+ CD16+ CD56+ NK-like T (CD38+ NK-like T) cells (0.60 ± 0.69) was significantly reduced (p < 0.0001), and there was no significant change in the proportion of CD38+ CD3+ cells (1.63 ± 1.57) (p = 0.3689) (Fig. 2b). The ratio of CD38+ NK cells/CD38+ NK-like T cells in RA patients ranged from 0.62 to 23, was significantly higher than that in the healthy control group (range: 0.31–11.26, mean: 1.153, p = 0.0004). There was no significant difference in the NK/NK-like T cell ratio between the RA patients and healthy subjects (p = 0.9227) (Fig. 2c). The CD38+ NK/CD38+ NK-like T cell ratio was positively correlated with the RA DAS28 (r = 0.716, p < 0.0001), whereas the NK/NK-like T cell ratio was slightly correlated with DAS28 (r = 0.465, p = 0.0096) (Fig. 2d).
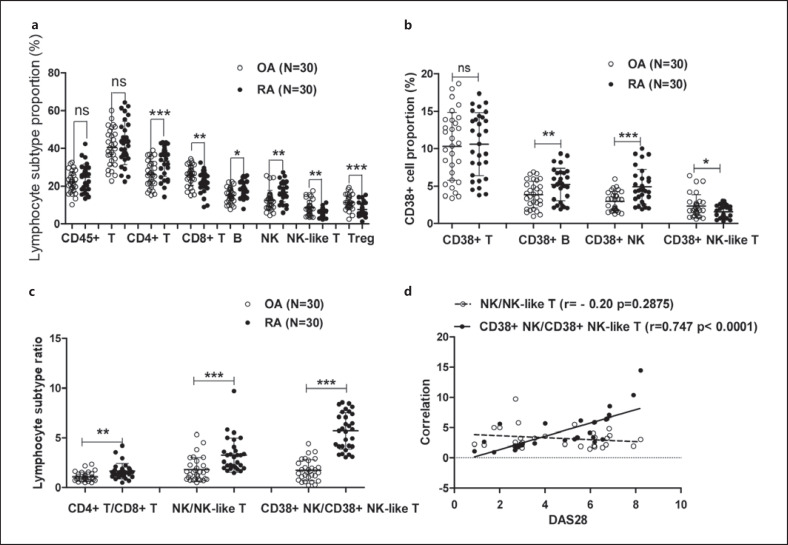
We collected synovial fluid samples from 30 RA patients and 30 OA patients. The proportions of CD4+ T (33.15 ± 7.99), CD3− CD19+ B (17.77 ± 4.37), and CD3− CD16+ CD56+ NK cells (16.76 ± 5.10) among total lymphocytes (CD45+ lymphocytes) in the RA group were significantly higher than those in the OA group (26.29 ± 6.83, 14.99 ± 3.96, and 12.46 ± 5.22. p = 0.0007, 0.0124, and 0.0021, respectively), whereas the proportions of CD3+ CD16+ CD56+ (NK-like T) (5.83 ± 2.14), Treg (7.58 ± 3.37), and CD8+ T cells (21.99 ± 5.37) were significantly lower than those in the control group (8.51 ± 3.95, 11.31 ± 3.95, and 25.71 ± 5.34. p = 0.0019, 0.0002, and 0.0094, respectively). There was no significant difference in the proportion of CD3+ T cells (42.79 ± 11.5 and 40.42 ± 9.71) between the 2 groups (p > 0.05). The results are shown in Figure 3a.
Fig. 3.
Changes in CD38+ NK and CD38+ NK-like T cells in synovial fluid lymphocytes of RA patients. a The proportions of various lymphocyte subtypes in synovial fluid from OA (n = 30) and RA patients (n = 30). b The proportions of CD38+ cell subtypes in synovial fluid of OA and RA patients. c Changes in the CD38+ NK/CD38+ NK-like T-cell ratio in synovial fluid of patients with OA or RA. d Correlation analysis of the CD38+ NK/CD38+ NK-like T-cell ratio in synovial fluid of RA patients with DAS28. *Represents a p value <0.05, **<0.01, and ***<0.001. RA, rheumatoid arthritis; OA, osteoarthritis.
Compared with the OA control group (3.84 ± 1.77, 2.98 ± 1.29, 2.33 ± 1.52), the proportions of CD38+ CD3− CD19+ (CD38+ B) (5.22 ± 2.16) and CD38+ CD3− CD16+ CD56+ NK (CD38+ NK) cells (4.92 ± 2.33) in synovial fluid from RA patients were significantly increased (p = 0.0088 and 0.0002, respectively), the proportion of CD38+ CD3+ CD16+ CD56+ NK-like T cells (1.55 ± 0.85) was significantly reduced (p = 0.0189), and there was no significant change in the proportion of CD38+ CD3+ cells (10.3 ± 4.55 and 10.6 ± 4.22) (p = 0.7905) (Fig. 3b). The ratio of CD38+ NK/CD38+ NK-like T cells in RA patients ranged from 0.942 to 14.45, with an average of 3.07, which was significantly higher than that in the OA group (range: 0.234–4.407, mean: 1.58, p < 0.0001). The NK/NK-like T-cell ratios in RA patients ranged from 1.463 to 9.713, with an average of 2.82, which was significantly higher than that in OA group (range: 0.503–5.326, mean: 1.54, p = 0.0004) (Fig. 3c). The CD38+ NK/CD38+ NK-like T-cell ratio in RA patients was positively correlated with DAS28 (r = 0.747, p < 0.0001), whereas the NK/NK-like T-cell ratio was not associated with DAS28 (r = −0.20, p = 0.2875) (Fig. 3d).
Effects of CD38+ NK and CD38+ NK-Like T Cells on MNCs
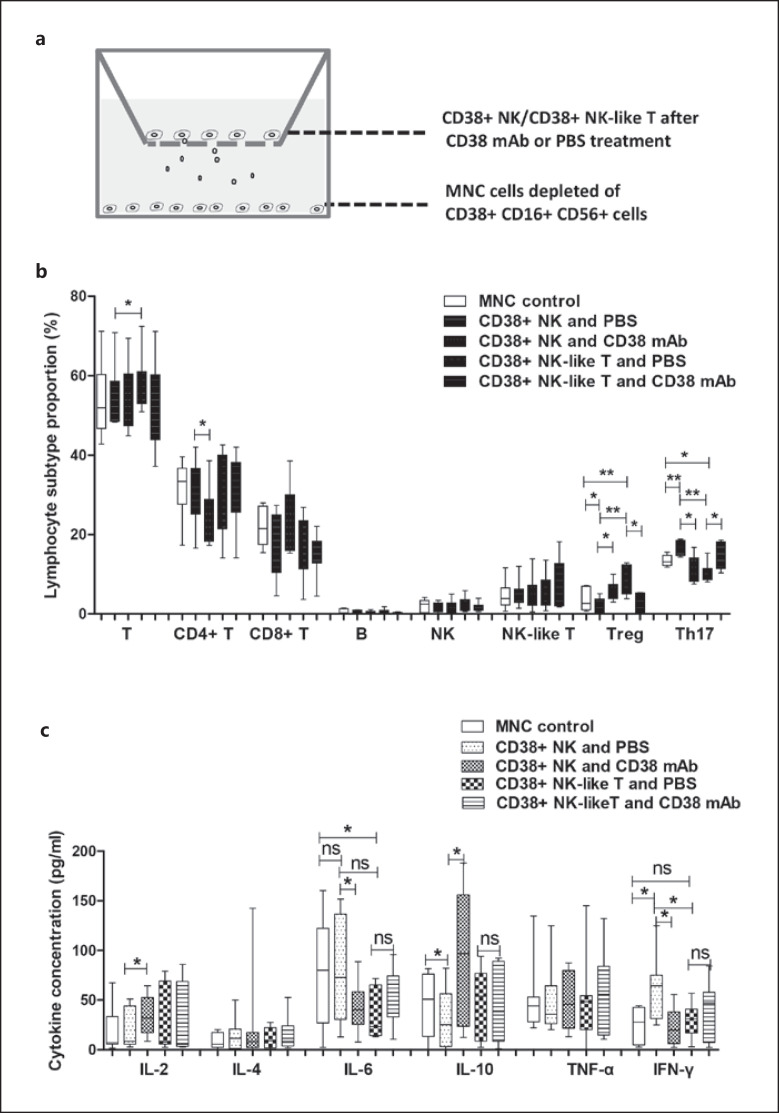
We prepared CD38+ NK cells, CD38+ NK-like T cells, and MNCs depleted of CD38+ cells from synovial fluids of 9 patients with RA. The purity of the CD38+ NK cells was 99.28%, whereas that of the CD38+ NK-like T cells was 98.9%. The results are shown in online suppl. Figure 2a, b. The CD38+ NK and CD38+ NK-like T cells were pretreated with anti-CD38 monoclonal antibody or PBS as a control and then cocultured with the MNCs. The experimental design is shown in Figure 4a. Compared with non-cocultured synovial MNCs, the proportion of CD4+ CD25+ FOXP3+ Treg cells among total lymphocytes (CD45+ lymphocytes) in the MNCs was significantly decreased (p = 0.033), and the proportion of CD4+ IL-17+ Th17 (Th17) cells among the lymphocytes was significantly increased (p = 0.0055) in MNCs after coculture with the CD38+ NK cells. Meanwhile, the proportion of Treg cells was significantly increased (p = 0.0021), and the proportion of Th17 cells was significantly decreased (p = 0.0152) in the MNCs after coculture with CD38+ NK-like T cells. Compared with the MNCs cocultured with CD38+ NK cells, the proportions of CD3+ T cells and Treg cells were significantly higher (p = 0.0252 and 0.0021, respectively), and the proportion of Th17 cells was significantly reduced (p = 0.0025) in MNCs cocultured with CD38+ NK-like T cells. Compared with MNCs cocultured with PBS-pretreated CD38+ NK cells, the proportions of CD4+ T cells and Th17 cells were significantly reduced (p = 0.0402 and 0.0137, respectively), and the proportion of Treg cells was significantly elevated (p = 0.0479) in MNCs after coculture with the antibody-pretreated CD38+ NK cells. Conversely, compared with MNCs cocultured with PBS-pretreated CD38+ NK-like T cells, the proportion of Treg cells was significantly reduced (p = 0.0139), and the proportion of Th17 cells was significantly increased (p = 0.0224) in MNCs after coculture with the antibody-pretreated CD38+ NK-like T cells. The above results are shown in Figure 4b.
Fig. 4.
Effect of CD38+ NK and CD38+ NK-like T cells on lymphocyte subtypes and cytokine secretion in MNCs from synovial fluids. CD38+ NK cells, CD38+ NK-like T cells, and MNCs were collected from RA synovial fluids. a Illustration of coculture of CD38+ NK cells or CD38+ NK-like T cells with MNCs depleted of CD16+ CD56+ CD38+ cells. b Effects of CD38+ NK cells and CD38+ NK-like T cells on lymphocyte subtypes in synovial MNCs. c Effects of CD38+ NK cells and CD38+ NK-like T cells on cytokine secretion in the coculture medium. *Represents a p value <0.05, **<0.01, and ***<0.001. RA, rheumatoid arthritis; MNCs, mononuclear cells.
Compared with non-cocultured synovial MNCs, the IFN-γ level in the medium was significantly increased (p = 0.0114) and the IL-10 level was significantly decreased (p = 0.0377) when MNCs were cocultured with CD38+ NK cells. In contrast, the IL-6 level was significantly decreased (p = 0.0434) when MNCs were cocultured with CD38+ NK-like T cells. Compared with MNCs cocultured with CD38+ NK cells, the IFN-γ level was significantly decreased (p = 0.0193) when MNCs were cocultured with CD38+ NK-like T cells. Compared with MNCs cocultured with PBS-pretreated with CD38+ NK cells, the levels of IL-2 and IL-10 in the culture medium were significantly increased (p = 0.0492 and 0.0275, respectively) when MNCs were cocultured with CD38+ NK cells pretreated with the anti-CD38 antibody, and the levels of IL-6 and IFN-γ were significantly reduced (p = 0.0200 and 0.0119, respectively). Compared with MNCs cocultured with PBS-pretreated CD38+ NK-like T cells, the cytokine levels in the culture medium were not changed when MNCs were cocultured with anti-CD38 antibody-pretreated CD38+ NK-like T cells. The above results are shown in Figure 4c.
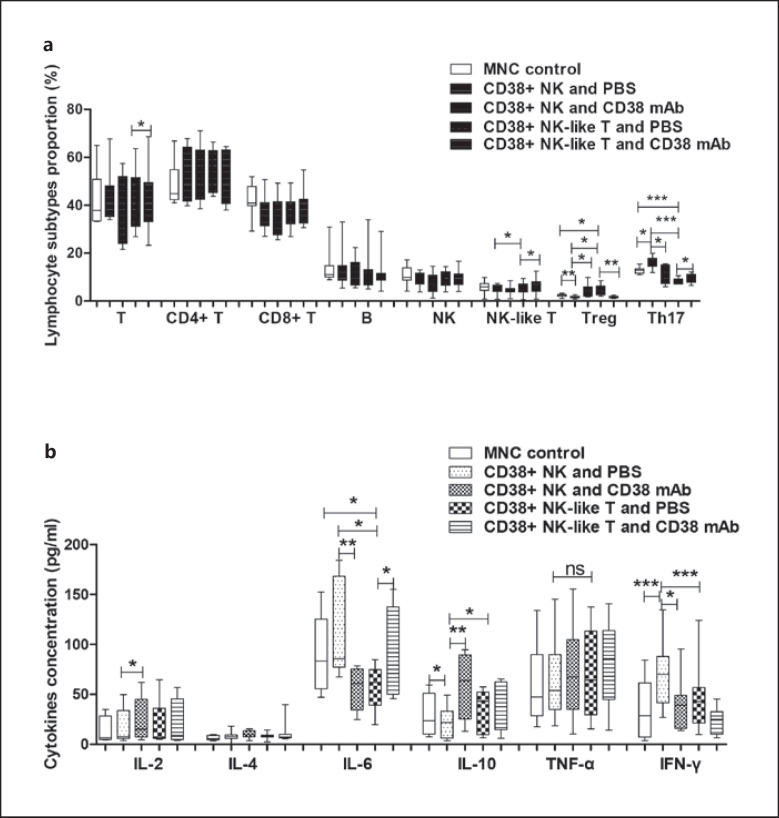
We prepared CD38+ NK cells, CD38+ NK-like T cells, and MNCs depleted of CD38+ cells from peripheral blood collected from 9 patients with RA. These 2 CD38+ cell subsets were separately cocultured with the MNCs. Compared with non-cocultured peripheral MNCs, the proportion of Treg cells among lymphocytes was significantly decreased (p = 0.0007), and the proportion of Th17 cells was significantly increased (p = 0.0162) in the blood MNCs after coculture with CD38+ NK cells. The proportion of Treg cells was significantly increased (p = 0.0269) and the proportion of Th17 cells was significantly decreased (p = 0.0006) in MNCs after coculture with CD38+ NK-like T cells. Compared with MNCs cocultured with CD38+ NK cells, the proportion of Treg cells was significantly increased (p = 0.0327) in MNCs after coculture with CD38+ NK-like T cells, and the proportion of Th17 cells was significantly decreased (p = 0.0004). Compared with MNCs cocultured with PBS-pretreated CD38+ NK cells, the proportion of Treg cells was significantly increased (p = 0.0454) after coculture with anti-CD38 antibody-pretreated CD38+ NK cells, and the proportion of Th17 cells was significantly decreased (p = 0.0381). Compared with MNCs cocultured with PBS-pretreated CD38+ NK-like T cells, the proportion of Treg cells was significantly decreased (p = 0.008) and the proportion of Th17 cells was significantly increased (p = 0.0342) in MNCs after coculture with anti-CD38 antibody-treated CD38+ NK-like T cells. The results are shown in Figure 5a.
Fig. 5.
Effect of CD38+ NK and CD38+ NK-like T cells on lymphocyte subtypes and cytokine secretion in MNCs from peripheral blood. CD38+ NK cells, CD38+ NK-like T cells, and MNCs were collected from RA peripheral blood. a Effects of CD38+ NK cells and CD38+ NK-like T cells on lymphocyte subtypes in peripheral blood MNCs. b Effects of CD38+ NK cells and CD38+ NK-like T cells on cytokine secretion in the coculture medium. *Represents a p value <0.05, **<0.01, and ***<0.001. RA, rheumatoid arthritis; MNCs, mononuclear cells.
Compared with non-cocultured blood MNCs, the level of IFN-γ in the culture medium was significantly increased (p < 0.0001) and the IL-10 level was significantly decreased (p = 0.0164) when MNCs were cocultured with CD38+ NK cells. The IL-6 level was significantly decreased (p = 0.0434) when MNCs were cocultured with CD38+ NK-like T cells. Compared with MNCs cocultured with CD38+ NK cells, IL-6, and IFN-γ levels were significantly decreased (p = 0.0155 and p = 0.0006, respectively) when MNCs were cocultured with CD38+ NK-like T cells, whereas the IL-10 level was significantly increased (p = 0.0416). Compared with MNCs cocultured with PBS-pretreated CD38+ NK cells, the levels of IL-2 and IL-10 were significantly increased (p = 0.0499 and p = 0.0013, respectively) and the IL-6 and IFN-γ levels were significantly decreased (p = 0.0051 and p = 0.0454, respectively) when MNCs were cocultured with anti-CD38 antibody-pretreated CD38+ NK cells. Compared to MNCs cocultured with PBS-pretreated CD38+ NK-like T cells, the IL-6 level was significantly increased (p = 0.0341) when MNCs were cocultured with anti-CD38 antibody-pretreated CD38+ NK-like T cells, but the levels of other cytokines were not significantly changed. The above results are shown in Figure 5b.
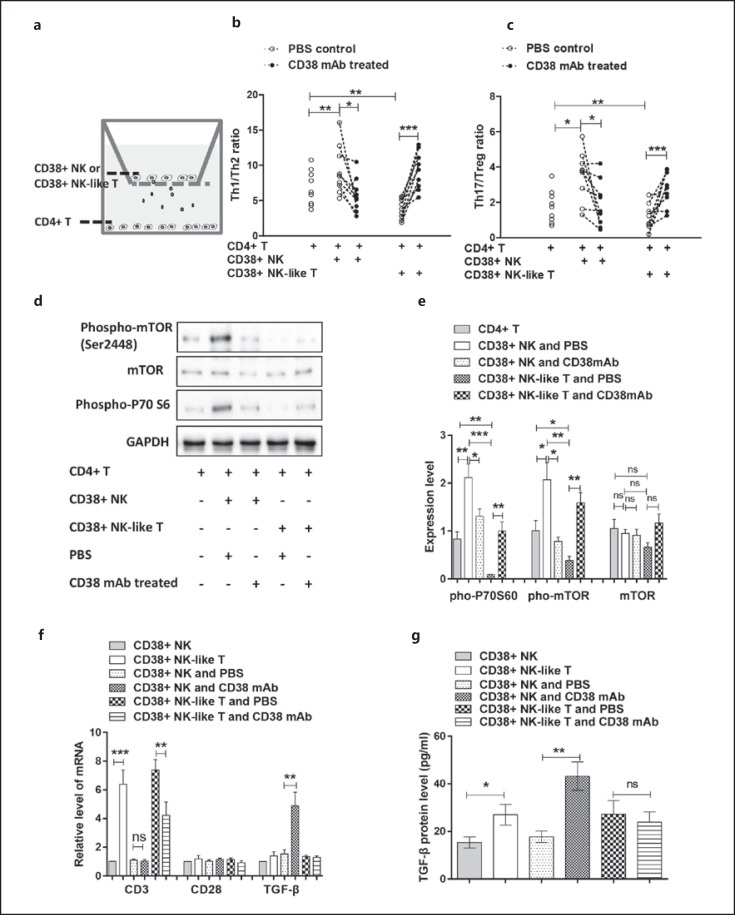
Effect of CD38+ NK and CD38+ NK-Like T Cells on CD4+ T Cells
We collected synovial fluid from 9 RA patients and sorted homogenous CD38+ NK cells, CD38+ NK-like T cells and CD4+ T cells. The CD38+ NK and CD38+ NK-like T cells were treated with PBS or anti-CD38 antibody and were then cocultured with CD4+ T cells. The experimental design is shown in Figure 6a. Compared with the non-cocultured CD4+ T cells, the ratios of Th1/Th2 and Th17/Treg cells were increased significantly (p = 0.0086 and 0.0186, respectively) in the CD4+ T cells cocultured with CD38+ NK cells, whereas the Th1/Th2 and Th17/Treg cell ratios were significantly decreased (p = 0.0061 and 0.0074, respectively) after coculture with CD38+ NK-like T cells. Compared with CD4+T cells cocultured with PBS-pretreated CD38+ NK cells, the Th1/Th2, and Th17/Treg cell ratios were significantly decreased (p = 0.0325 and 0.0259, respectively) after coculture with anti-CD38 antibody-treated CD38+ NK cells. Compared with CD4+ cells cocultured with PBS-pretreated CD38+ NK-like T cells, the Th1/Th2 and Th17/Treg cell ratios were significantly increased (p = 0.0006 and 0.0002, respectively) after coculture with anti-CD38 antibody-pretreated CD38+ NK-like T cells. The results are shown in Figure 6b, c.
Fig. 6.
Effect of CD38+ NK and CD38+ NK-like T cells on CD4+ T cells. CD38+ NK cells, CD38+ NK-like T cells and MNCs were collected from RA synovial fluids. a Illustration of the coculture of CD38+ NK cells or CD38+ NK-like T cells with CD4+ T cells. b Effects of CD38+ NK and CD38+ NK-like T cells on the Th1/Th2 cell ratio in CD4+ T cells. c Effect of CD38+ NK and CD38+ NK-like T cells on the Th17/Treg cell ratio in CD4+ T cells. d Effect of CD38+ NK and CD38+ NK-like T cells on mTOR signaling in CD4+ T cells assessed via Western blot analysis. e Expression levels of phospho-mTOR, mTOR, and phospho-P70 S6 determined by densitometry (after normalization) in comparison to the respective GAPDH expression. f Expression levels of CD3, CD28, and TGF-β mRNA in CD38+ NK and CD38+ NK-like T cells determined using real-time PCR. g Statistical analysis of TGF-β expression levels in culture medium using flow cytometry. mAb indicates anti-CD38 antibody. *Represents a p value <0.05, **<0.01, and ***<0.001. RA, rheumatoid arthritis; MNCs, mononuclear cells; mTOR, mammalian target of rapamycin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
To determine whether CD38+ NK or CD38+ NK-like T cells have a direct effect on Treg cell proliferation, we cocultured CD38+ NK cells or CD38+ NK-like T cells and Treg cells in place of CD4+ T cells in a transwell apparatus. The experimental design is shown in online suppl. Figure 3a. We examined the proliferation of Treg cells using a Cell Count Kit-8 kit. Following coculture with CD38+ NK cells, Treg cells showed no significant change in cell proliferation compared with the Treg cells without coculture or Treg cells following coculture with anti-CD38 antibody-pretreated CD38+ NK cells. The effect of CD38+ NK-like T cells on Treg cell proliferation was similar to that of CD38+ NK cells. The results are shown in online suppl. Figure 3b.
To investigate the molecular mechanisms by which CD38+ NK and CD38+ NK-like T cells act on CD4+ T cell differentiation, we collected the cocultured CD4+ T cells and then examined changes in the mTOR signaling pathway using Western blot analysis. Compared with CD4+ T cells cocultured with PBS-pretreated CD38+ NK cells, CD4+ T cells showed a significant decrease in phospho-P70S6, phospho-mTOR and total mTOR protein expression levels (p = 0.0473, 0.0127 and 0.0094, respectively) after coculture with anti-CD38 antibody-pretreated CD38+ NK cells. Compared with CD4+ T cells cocultured with CD38+ NK cells, CD4+ T cells also showed a significant decrease in phospho-P70S6, phospho-mTOR, and total mTOR protein expression levels (p = 0.0004, 0.0037 and 0.0012, respectively) after coculture with CD38+ NK-like T cells. Compared with CD4+ T cells cocultured with PBS-pretreated CD38+ NK-like T cells, CD4+ T cells showed significant increases in phospho-P70S6, phospho-mTOR, and total mTOR protein expression levels (p = 0.003, 0.0019 and 0.0059, respectively) after coculture with anti-CD38 antibody-pretreated CD38+ NK-like T cells. Compared with non-cocultured CD4+T cells, CD4+ T cells showed significant increases in the phospho-P70S6, phospho-mTOR, and total mTOR protein expression levels (p = 0.0075, 0.0425 and 0.0216, respectively) after coculture with CD38+ NK cells, while CD4+ T cells also showed significant increases in phospho-P70S6, phospho-mTOR, and total mTOR protein levels (p = 0.0029, 0.0358, and 0.0116, respectively) after coculture with anti-CD38 antibody-pretreated CD38+ NK-like T cells. The above results are shown in Figure 6d, e.
We also collected CD38+ NK and CD38+ NK-like T cells from the cocultures described above and used real-time PCR to detect the CD3, CD28, and TGF-β mRNA levels. Compared with the CD38+ NK cells, the CD38+ NK-like T cells showed a significant increase in CD3 mRNA expression (p = 0.0002), while the CD38+ NK-like T cells with CD38 antibody pretreatment showed decreased CD3 mRNA expression (p = 0.0079). Regardless of the presence or absence of the anti-CD38 antibody, the CD28 level in CD38+ NK and CD38+ NK-like T cells remained unchanged. Compared with PBS-pretreated CD38+ NK cells, CD38+ NK cells with anti-CD38 antibody pretreatment showed significantly higher levels of TGF-β (p = 0.0056), but the antibody pretreatment did not affect TGF-β expression in CD38+ NK-like T cells. The above results are shown in Figure 6e. Additionally, flow cytometry analysis of cytokines detected an increased TGF-β level in the cultured medium of CD38+ NK-like T cells compared with CD38+ NK cells (p = 0.0321). CD38+ NK cells following the anti-CD38 antibody pretreatment produced more TGF-β compared with the PBS-treated control (p = 0.0011) in the cultured medium. However, the antibody treatment did not affect TGF-β level in the cultured medium of CD38+ NK-like T cells. The above results are shown in online suppl. Figures 4 and Figure 6f.
Transcriptomic Analysis for CD4+ T Cells
To investigate the molecular mechanisms by which CD38+ NK and CD38+ NK-like T cells act on CD4+ T cells, we collected the cocultured CD4+ T cells and then examined expression profiles of the T cells using transcriptomic analysis. Compared with non-cocultured CD4+T cells, 226 DEGs showed significantly elevated levels in CD4+ T cells following coculture with CD38+ NK cells, meanwhile the 289 DEGs showed significantly decreased levels in the T cells. Compared with non-cocultured CD4+T cells, 324 DEGs showed elevated levels in CD4+ T cells following coculture with CD38+ NK-like T cells, meanwhile 316 DEGs showed significantly decreased levels in the T cells. The analytic result was shown in online suppl. Figure 5 and the detailed information was shown in online suppl. Table 3.
Pathway analysis was conducted using KEGG pathway database. TOP 20 pathways significantly related to DEGs were summarized in online suppl. Figure 6. The functional analysis (TOP 20) indicated that DEGs between CD4+ T cells cocultured with CD38+ NK and non-cocultured CD4+ T cells were strictly related to Human immunodeficiency virus 1 infection, Epstein-Barr virus infection, hepatitis B, toxoplasmosis, leishmaniasis, pertussis, shigellosis, amyotrophic lateral sclerosis, Fc epsilon RI signaling pathway, Th1 and Th2 cell differentiation, VEGF signaling pathway, lysosome, non-homologous end-joining, ribosome biogenesis in eukaryotes, biosynthesis of unsaturated fatty acids, glycosylphosphatidylinositol-anchor biosynthesis, glycosaminoglycan degradation, amino sugar and nucleotide sugar metabolism, other types of O-glycan biosynthesis, and steroid biosynthesis. The functional analysis (TOP 20) indicated that the DEGs between CD4+ T cells cocultured with CD38+ NK-like T cells and non-cocultured CD4+ T cells were strictly related to measles, Th1 and Th2 cell differentiation, apoptosis − multiple species, base excision repair, RNA polymerase, antifolate resistance, aminoacyl-tRNA biosynthesis, glyoxylate and dicarboxylate metabolism, sphingolipid metabolism, inositol phosphate metabolism, amino sugar and nucleotide sugar metabolism, N-glycan biosynthesis, cysteine and methionine metabolism, glycine, serine and threonine metabolism, alanine, aspartate and glutamate metabolism, purine metabolism, steroid biosynthesis, fructose and mannose metabolism, pentose phosphate pathway, and citrate cycle (TCA cycle). The analytic result was shown in online suppl. Figure 6 and the detailed information was shown in online suppl. Table 4.
Discussion
This study examined changes in CD38+ cell subtypes in peripheral blood and synovial fluid. The proportions of CD38+ B cells and CD38+ NK lymphocytes in the peripheral blood and synovial fluid of RA patients were significantly increased, and the proportion of CD38+ NK-like T cells was significantly decreased. Furthermore, the CD38+ NK/CD38+ NK-like T-cell ratio was also increased in RA and positively correlated with DAS28, an index of RA clinical performance. Similar results were obtained in CIA rats, and the CD38+ NK/CD38+ NK-like T-cell ratio was positively correlated with the disease activity. Thus, a high proportion of CD38+ NK cells and low proportion of CD38+ NK-like T cells may be a common phenomenon in RA.
To explore the function of CD38+ NK and CD38+ NK-like T cells in RA, we cocultured CD38+ NK cells or CD38+ NK-like T cells with CD38+ CD16+ CD56+ cell-depleted MNCs from RA synovial fluid or peripheral blood. After coculture with CD38+ NK cells, the proportion of Treg cells in MNCs was significantly decreased, and the proportion of Th17 cells was significantly increased. After coculture with CD38+ NK-like T cells, the proportion of Treg cells in MNCs was significantly increased, and the proportion of Th17 cells was significantly decreased. The opposite results were observed in the MNCs when CD38+ NK or CD38+ NK-like T cells were pretreated with anti-CD38 antibody. Meanwhile, the IL-10 level in the culture medium was significantly increased, and IFN-γ and IL-6 levels were significantly decreased after coculture of MNCs with anti-CD38 antibody-pretreated CD38+ NK cells. IL-10 levels were also significantly increased after coculture with CD38+ NK-like T cells, and IFN-γ levels were significantly reduced. Additionally, our study indicates that CD38+ NK cells have no direct effect on Treg-cell proliferation. We previously found that CD38+ NK cells did not alter Treg-cell apoptosis [31]. Our results suggest that a high proportion of CD38+ NK cells and a low proportion of CD38+ NK-like T cells might contribute to decreased Treg cells and IL-10 secretion and elevated proportions of Th17 cells, IL-6, and IFN-γ levels in RA, all of which aggravate the disease activity. NK cells have been reported to prevent CD28-mediated Foxp3 transcription in CD4+CD25− T lymphocytes and aggravate the inflammatory response in RA by secreting IFN-γ [34, 35]. Studies have shown that IL-10 is downregulated in RA synovial fluid and peripheral blood [36, 37, 38], and CD38 promotes IFN-γ secretion in NK cells [39, 40]. Previous findings support our results.
It has been reported that the function of Treg cells in RA is partially impaired or that Treg cell numbers are decreased [16]. Animal studies in CIA rats have shown that Treg cell treatment effectively alleviates the inflammatory response [41]. IL-10 is an immunoregulatory cytokine that plays a central role in pathogenesis of autoimmune diseases and IL-10 reduces the inflammatory response in RA [42]. The current study detected a decreased number of Treg cells in CIA rats. Furthermore, coculture of CD38+ NK cells reduced Treg cells and IL-10 production, elevated Th17 cells and IFN-γ production in MNC, and increased Th17/Treg in CD4+ T cells. On the other hand, coculture of CD38+ NK-like T cells increased Treg cells and IL-10 production; decreased Th17 cells, IL-6 and IFN-γ production in MNC; and reduced Th17/Treg in CD4+ T cells. We thus propose that CD38+ NK cells can exert proinflammatory effects by inhibiting either MNCs or CD4+ T-cell differentiation into Treg cells, whereas CD38+ NK-like T cells promote the differentiation of MNCs or CD4+ T cells into Treg cells.
Disruption in the Th1/Th2 cell and Th17/Treg cell balance plays an important role in RA pathogenesis. The present study found that CD38+ NK cells increased the Th1/Th2 and Th17/Treg cell ratios in CD4+ T cells, and the ratios significantly decreased after the CD38+ NK cells were pretreated with anti-CD38 antibody. CD38+ NK-like T cells had an effect opposite that of CD4+ T cells. These results suggest that the increased CD38+ NK cell proportion and decreased CD38+ NK-like T cell proportion may be important reasons for the high Th1/Th2 and Th17/Treg cell ratios in RA. NK cells have been reported to participate in pneumonia caused by chlamydia by regulating the Th1/Treg and Th17/Treg cell balance [43]. One study reported that CD38 participates in the pathogenesis of CIA by promoting Th1 inflammatory responses [28].
mTOR signaling inhibits the expansion of CD4+ CD25+ FOXP3+ T cells [44, 45]. mTOR pathway blocking agents are being developed for RA treatment and prevention [45]. The present study found that CD38+ NK cells activated mTOR signaling in CD4+ T cells following coculture and elevated Th1/Th2 and Th17/Treg ratios. Pretreatment of CD38+ NK cells with an anti-CD38 antibody inactivated the mTOR signaling and reduced the Th1/Th2 and Th17/Treg ratios. CD38+ NK-like T cells and treatment of CD38+ NK-like T cells with anti-CD38 antibody had the opposite effects on mTOR activities as well as the Th1/Th2 and Th17/Treg ratios in CD4+ T cells. These observations suggest that the high CD38+ NK proportion and low CD38+ NK-like T proportion in RA may inhibit the differentiation of CD4+ T cells into Treg cells and disturb the immune balance by downregulating mTOR activity.
Naïve CD4+ T cells can be induced to differentiate into Treg cells with TGF-β and into Th17 cells with a combination of TGF-β and IL-6 or with IL-21 [46, 47, 48]. Anti-CD3 antibodies, CD28, IL-2, and TGF-β are usually used to induce CD4+ T cell differentiation into Treg cells in vitro [49, 50]. We found that anti-CD38 antibody treatment significantly increased TGF-β expression in CD38+ NK cells but not CD3 or CD28 expression. Furthermore, our study found that anti-CD38 antibody pretreatment significantly increased IL-2 secretion and decreased IL-6 and IFN-γ secretion by MNCs cocultured with CD38+ NK cells. IFN-γ has been reported to activate the mTOR signaling pathway [46]. Therefore, this study suggests that CD38+ NK cells downregulate TGF-β and IL-2 expression and upregulate IFN-γ secretion to retard CD4+ T cell differentiation into Treg cells and stimulate Th17 cell development. Additionally, the present study found that CD38+ NK-like T cells produced more TGF-β and produced lower IL-6 and IFN-γ levels compared with CD38+ NK cells, which can stimulate more CD4+ T cells to develop into Treg cells rather than Th17 cells. Decreased serum TGF-β levels have been detected in RA [51, 52].
NK cells play important roles in autoimmune diseases [53, 54]. High percentages of synovial fluid NK cells with strong activity are present in patients with advanced stage active RA [55]. CD38+ NK cells are increased in HIV-1-infected partners. Moreover, CD38+ NK cells of HIV-1-infected partners are associated with increased expression of inhibitory molecules, such as NKG2A, PD-1, and Tim-3 [56]. CD38+ NK cells were recently detected in MM patients. CD38 protein degradation was associated with NK cell activation, leaving an activated CD38-negative NK cell population. Thus, CD38+ NK cells may be an unexplored therapeutic target for priming the immune system against disease [57].
NK-like T cells are usually defined as CD3+ CD56+ cells and have also been referred to as CD56(+) T, NKT-like cells, and even NKT in published references. Studies on NK-like T cells in RA are scarce, and related knowledge is limited, controversial, and heterogeneous [58]. Accumulation of CD56 (+) T cells was detected in RA and may be involved in maladaptive immune responses, and these T cells have been suggested as potential targets for therapy [59]. CD3+CD56+ NKT-like cells were also detected in systemic lupus and bronchiolitis obliterans syndrome [60, 61]. NKT cells can influence the differentiation of CD4+ T cells via cytokines in vitro. These findings suggest that NK-like T cells play an important role in RA by polarizing Th1, Th2, Th17, and Treg cells [8], consistent with our results about the induction of CD4+ T-cell differentiation by CD38+ NK-like T cells.
Transcriptomic analysis revealed that the pathways in CD4+ T cells following coculture with CD38+ NK cells were differential from that in the T cells following coculture with CD38+ NK-like T cells, indicating that both CD38-expressing cell types promoted differential function profiles in the CD4+ T cells. This analytic result demonstrated that CD38+ NK cells and CD38+ NK-like T cells were 2 differential cell subtypes with distinct functions. Most of all, both CD38+ NK cells and CD38+ NK-like T cells activated Th1 and Th2 cell differentiation pathway in CD4+ T cells. However, CD38+ NK cells changed the gene expressions of RBPJ, HLA-DRA, IFNGR1, PRKCQ, CHUK, IFNG, NFATC3 Th1, and Th2 cell differentiation, and CD38+ NK-like T cells s changed the gene expressions of MAPK9, HLA-DQB1, HLA-DMA, MAPK14, DLL1, GATA3, PPP3CB, and NFATC3 in the process (see online suppl. Tables 3 and 4). The differential expression profiles in Th1 and Th2 cell differentiation can explain why CD38+ NK cells and CD38+ NK-like T cells had contrary results on CD4+ T-cell differentiation.
Another study of ours detected increased INF-γ and decreased TNF-α production in cultured CD38+ NK cells. In that study, we cocultured MNCs with CD38+ NK cells in the presence of TNF-α and an anti-IFN-γ antibody, whereupon the IL-10+ Treg cell proportion significantly increased. When MNCs were cocultured with CD38+ NK cells in the presence of IFN-γ and an anti-TNF-α antibody, the IL-10+ Treg cell proportion sharply decreased [31]. Combined with the current results, these findings suggest that CD38+ NK cells produce low levels of TGF-β and TNF-α and high levels of IFN-γ through downregulation of Sirt6 expression, which suppresses Treg cell differentiation via mTOR signaling in CD4+ T cells (online suppl. Fig. 7).
The CD38-blocking antibody daratumumab has been used to treat MM [62, 63]. A recent study demonstrated that administration of anti-CD38 antibody prevented arthritis development in Cynomolgus monkeys [64]. Anti-CD38 antibody consumes plasmablasts in MNCs of RA patients in a dose-dependent manner in vitro [26]. Our study also detected an increased proportion CD38+ B cells in RA and CIA. We further found that CD38+ NK cells pretreated with anti-CD38 antibody led to elevated Treg cell proportions and IL-10 secretion and restored the Th1/Th2 and Th17/Treg balance in MNC and CD4+ T cells. Although CD38+ NK-like T cells had the opposite effect on immune balance following CD38 antibody treatment, the antibody treatment in vivo could restore Th1/Th2 and Th17/Treg balance in RA because the proportion of CD38+ NK-like T cells was relatively low in the patients. We suggest that anti-CD38 antibody has potential for treating RA by inactivating both CD38+ NK cells and CD38+ B cells.
Morandi et al. [65] found that peripheral blood CD56(dim)CD16(+) and CD56(bright)CD16(−) NK cells expressed similar levels of CD38. CD56(bright)CD16(−) NK cells produce adenosine (ADO) and inhibit autologous CD4(+) T cell proliferation. Such inhibition was reverted through pretreatment of CD56(bright)CD16(−) NK cells with a CD38 inhibitor. Postigo et al. [28] demonstrated that CD38 KO mice developed an attenuated CIA that is accompanied by a lack of IL-1β, IL-6, and IFN-γ production in the joints and by a reduction in the percentages of invariant NKT (iNKT) cells in the spleen. Immunized CD38 KO mice produced low percentages of Th1 cells in the draining lymph nodes. In our study, we not only detected a low percentage of CD38+ NK-like T cells in CIA rats and RA patients but also found that a high CD38+ NK cell proportion and low CD38+ NK-like T cell proportion in RA inhibited development of CD4+ T cells into Treg cells and elevated Th1/Th2 cells and Th17/Treg ratios by activating mTOR activity.
Conclusion
Our study found that CD38+ NK cells were significantly elevated and that CD38+ NK-like T cells were significantly decreased in RA and CIA. Coculture with CD38+ NK cells activated mTOR signaling in CD4+ T cells and increased the Th1/Th2 and Th17/Treg cell ratios in the CD4+ T cells. CD38+ NK-like T cells and anti-CD38 antibody-pretreated CD38+ NK cells showed the opposite effect on MNCs and CD4+ T cells. The anti-CD38 antibody also stimulated TGF-β expression in CD38+ NK cells. The above results suggest that a high level of CD38+ NK cells and a low level of CD38+ N-like T cells in RA activate the mTOR pathway in CD4+ T cells among MNCs. This subsequently inhibits the differentiation of CD4+ T cells into Treg cells, enhances Th1/Th2 and Th17/Treg ratios, and ultimately exacerbates RA pathogenesis, including immune imbalance and tolerance disorder. Thus, inhibiting the number of CD38+ NK cells or their function using anti-CD38 antibody may be a therapeutic approach for RA.
Statement of Ethics
The experimental procedures involving human tissue and animals were approved by the Ethic Committee at The Affiliated Hospital of Qingdao University. Informed written consent was obtained from each human subject.
Conflict of Interest Statement
The authors declare that they have no competing interests.
Funding Sources
This study was supported by Shandong Provincial Key R & D programs (2017CXGC1202 and 2017GSF18174).
Author Contributions
X.C. involved in conception and design; X.C., H.W., K.F., and W.Y. involved in analysis and interpretation; X.C. and H.W involved in drafting the manuscript for important intellectual content; all authors have reviewed the manuscript and agree with its submission.
Data Availability Statement
All data used during the current study are available from the corresponding author on reasonable request.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
References
- 1.Rosenblum MD, Way SS, Abbas AK. Regulatory T cell memory. Nat Rev Immunol. 2016;16((2)):90–101. doi: 10.1038/nri.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lužnik Z, Anchouche S, Dana R, Yin J. Regulatory T cells in angiogenesis. J Immunol. 2020;205:2557–65. doi: 10.4049/jimmunol.2000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alijotas-Reig J, Llurba E, Gris JM. Potentiating maternal immune tolerance in pregnancy: a new challenging role for regulatory T cells. Placenta. 2014;35((4)):241–8. doi: 10.1016/j.placenta.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8((4)):345–50. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Bozec A, Ramming A, Schett G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol. 2019;15((1)):9–17. doi: 10.1038/s41584-018-0109-2. [DOI] [PubMed] [Google Scholar]
- 6.Boissier MC. Cell and cytokine imbalances in rheumatoid synovitis. Joint Bone Spine. 2011;78((3)):230–4. doi: 10.1016/j.jbspin.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Yang J, Qiao Y, Li X. Understanding the regulatory roles of natural killer T cells in rheumatoid arthritis: T helper cell differentiation dependent or independent? Scand J Immunol. 2016;84((4)):197–203. doi: 10.1111/sji.12460. [DOI] [PubMed] [Google Scholar]
- 8.Vyas SP, Hansda AK, Goswami R. Rheumatoid arthritis: “melting pot” of T helper subsets. Int Rev Immunol. 2019;38((5)):212–31. doi: 10.1080/08830185.2019.1621865. [DOI] [PubMed] [Google Scholar]
- 9.Scheinecker C, Göschl L, Bonelli M. Treg cells in health and autoimmune diseases: new insights from single cell analysis. J Autoimmun. 2020;110:102376. doi: 10.1016/j.jaut.2019.102376. [DOI] [PubMed] [Google Scholar]
- 10.Serrano Hernández A. Helper (TH1, TH2, TH17) and regulatory cells (Treg, TH3, NKT) in rheumatoid arthritis. Reumatol Clin. 2009;5((Suppl 1)):1–5. doi: 10.1016/j.reuma.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Sun H, Gao W, Pan W, Zhang Q, Wang G, Feng D, et al. Tim3 (+) Foxp3 (+) Treg cells are potent inhibitors of effector T cells and are suppressed in rheumatoid arthritis. Inflammation. 2017;40:1342–50. doi: 10.1007/s10753-017-0577-6. [DOI] [PubMed] [Google Scholar]
- 12.Liu C, Wang D, Lu S, Xu Q, Zhao L, Zhao J, et al. Increased circulating follicular treg cells are associated with lower levels of autoantibodies in patients with rheumatoid arthritis in stable remission. Arthritis Rheumatol. 2018;70((5)):711–21. doi: 10.1002/art.40430. [DOI] [PubMed] [Google Scholar]
- 13.Kanjana K, Chevaisrakul P, Matangkasombut P, Paisooksantivatana K, Lumjiaktase P. Inhibitory activity of FOXP3+ regulatory T cells reveals high specificity for displaying immune tolerance in remission state rheumatoid arthritis. Sci Rep. 2020;10((1)):19789. doi: 10.1038/s41598-020-76168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zare HR, Habibagahi M, Vahdati A, Habibagahi Z. Patients with active rheumatoid arthritis have lower frequency of nTregs in peripheral blood. Iran J Immunol. 2015;12((3)):166–75. [PubMed] [Google Scholar]
- 15.Malemud C. Defective T-cell apoptosis and T-regulatory cell dysfunction in rheumatoid arthritis. Cells. 2018;7((12)):223. doi: 10.3390/cells7120223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miao J, Zhu P. Functional defects of treg cells: new targets in rheumatic diseases, including ankylosing spondylitis. Curr Rheumatol Rep. 2018;20((5)):30. doi: 10.1007/s11926-018-0729-1. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133((5)):775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Wang H, Wu H, Chang X. Therapeutic effect of exogenous regulatory T cells on collagen-induced arthritis and rheumatoid arthritis. Cell Transplant. 2020 Jan-Dec;29:963689720954134. doi: 10.1177/0963689720954134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Wang H, Sun Q, Liu B, Chang X. Therapeutic effect of Xuebijing, a Traditional Chinese Medicine Injection, on rheumatoid arthritis. Evid Based Complement Alternat Med. 2020;2020:2710782. doi: 10.1155/2020/2710782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogan KA, Chini CCS, Chini EN. The Multi-faceted Ecto-enzyme CD38: Roles in Immunomodulation, Cancer, Aging, and Metabolic Diseases. Front Immunol. 2019;10:1187. doi: 10.3389/fimmu.2019.01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei W, Graeff R, Yue J. Roles and mechanisms of the CD38/cyclic adenosine diphosphate ribose/Ca(2+) signaling pathway. World J Biol Chem. 2014;5((1)):58–67. doi: 10.4331/wjbc.v5.i1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deterre P, Berthelier V, Bauvois B, Dalloul A, Schuber F, Lund F. CD38 in T- and B-cell functions. Chem Immunol. 2000;75:146–68. doi: 10.1159/000058767. [DOI] [PubMed] [Google Scholar]
- 23.Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev. 2008;88((3)):841–86. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- 24.Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128((3)):384–94. doi: 10.1182/blood-2015-12-687749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang X, Yue L, Liu W, Wang Y, Wang L, Xu B, et al. CD38 and E2F transcription factor 2 have uniquely increased expression in rheumatoid arthritis synovial tissues. Clin Exp Immunol. 2014;176((2)):222–31. doi: 10.1111/cei.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domínguez-Pantoja M, López-Herrera G, Romero-Ramírez H, Santos-Argumedo L, Chávez-Rueda AK, Hernández-Cueto Á, et al. CD38 protein deficiency induces autoimmune characteristics and its activation enhances IL-10 production by regulatory B cells. Scand J Immunol. 2018;87((6)):e12664. doi: 10.1111/sji.12664. [DOI] [PubMed] [Google Scholar]
- 27.Cole S, Walsh A, Yin X, Wechalekar MD, Smith MD, Proudman SM, et al. Integrative analysis reveals CD38 as a therapeutic target for plasma cell-rich pre-disease and established rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther. 2018;20((1)):85. doi: 10.1186/s13075-018-1578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Postigo J, Iglesias M, Cerezo-Wallis D, Rosal-Vela A, García-Rodríguez S, Zubiaur M, et al. Mice deficient in CD38 develop an attenuated form of collagen type II-induced arthritis. PLoS One. 2012;7((3)):e33534. doi: 10.1371/journal.pone.0033534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du Y, Dai Q, Zhang H, Li Q, Song K, Fu Y, et al. CD38 Deficiency Downregulates the Onset and Pathogenesis of Collagen-Induced Arthritis through the NF-κB Pathway. J Immunol Res. 2019 Mar 5;2019:7026067. doi: 10.1155/2019/7026067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosal-Vela A, García-Rodríguez S, Postigo J, Iglesias M, Longobardo V, Lario A, et al. Distinct serum proteome profiles associated with collagen-induced arthritis and complete Freund's adjuvant-induced inflammation in CD38-/- mice: the discriminative power of protein species or proteoforms. Proteomics. 2015;15((19)):3382–93. doi: 10.1002/pmic.201400536. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Li S, Zhang G, Wu H, Chang X. Potential therapeutic effects of cyanidin-3-O-glucoside on rheumatoid arthritis by relieving inhibition of CD38+ NK cells on Treg cell differentiation. Arthritis Res Ther. 2019;21((1)):220. doi: 10.1186/s13075-019-2001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA, et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10((7)):769–77. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang P, Zhang Q, Tan L, Xu Y, Xie X, Zhao Y. The regulatory effects of mTOR complexes in the differentiation and function of CD4(+) T cell subsets. J Immunol Res. 2020;2020:3406032. doi: 10.1155/2020/3406032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brillard E, Pallandre JR, Chalmers D, Ryffel B, Radlovic A, Seilles E, et al. Natural killer cells prevent CD28-mediated Foxp3 transcription in CD4+CD25− T lymphocytes. Exp Hematol. 2007;35((3)):416–25. doi: 10.1016/j.exphem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Thanapati S, Ganu M, Giri P, Kulkarni S, Sharma M, Babar P, et al. Impaired NK cell functionality and increased TNF-α production as biomarkers of chronic chikungunya arthritis and rheumatoid arthritis. Hum Immunol. 2017;78((4)):370–4. doi: 10.1016/j.humimm.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Pandiyan P, Zhu J. Origin and functions of pro-inflammatory cytokine producing Foxp3+ regulatory T cells. Cytokine. 2015;76((1)):13–24. doi: 10.1016/j.cyto.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng G, Song X, Fujimoto S, Piccirillo CA, Nagai Y, Greene MI. Foxp3 post-translational modifications and treg suppressive activity. Front Immunol. 2019;10:2486. doi: 10.3389/fimmu.2019.02486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stadhouders R, Lubberts E, Hendriks RW. A cellular and molecular view of T helper 17 cell plasticity in autoimmunity. J Autoimmun. 2018;87:1–15. doi: 10.1016/j.jaut.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Deaglio S, Zubiaur M, Gregorini A, Bottarel F, Ausiello CM, Dianzani U, et al. Human CD38 and CD16 are functionally dependent and physically associated in natural killer cells. Blood. 2002;99((7)):2490–8. doi: 10.1182/blood.v99.7.2490. [DOI] [PubMed] [Google Scholar]
- 40.Sconocchia G, Titus JA, Mazzoni A, Visintin A, Pericle F, Hicks SW, et al. CD38 triggers cytotoxic responses in activated human natural killer cells. Blood. 1999;94((11)):3864–71. [PubMed] [Google Scholar]
- 41.Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, et al. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52((7)):2212–21. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 42.Tian G, Li JL, Wang DG, Zhou D. Targeting IL-10 in auto-immune diseases. Cell Biochem Biophys. 2014;70((1)):37–49. doi: 10.1007/s12013-014-9903-x. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Dong X, Zhao L, Wang X, Wang Y, Yang X, et al. Natural killer cells regulate Th1/Treg and Th17/Treg balance in chlamydial lung infection. J Cell Mol Med. 2016;20((7)):1339–51. doi: 10.1111/jcmm.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delgoffe GM, Powell JD. mTOR: taking cues from the immune microenvironment. Immunology. 2009;127((4)):459–65. doi: 10.1111/j.1365-2567.2009.03125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perl A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat Rev Rheumatol. 2016;12((3)):169–82. doi: 10.1038/nrrheum.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kroczynska B, Kaur S, Platanias LC. Growth suppressive cytokines and the AKT/mTOR pathway. Cytokine. 2009 Oct–Nov;48((1–2)):138–43. doi: 10.1016/j.cyto.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Wing JB, Tanaka A, Sakaguchi S. Human FOXP3+ regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity. 2019;50((2)):302–16. doi: 10.1016/j.immuni.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 48.Schiavinato JLDS, Haddad R, Saldanha-Araujo F, Baiochi J, Araujo AG, Santos Scheucher P, et al. TGF-beta/atRA-induced Tregs express a selected set of microRNAs involved in the repression of transcripts related to Th17 differentiation. Sci Rep. 2017;7((1)):3627. doi: 10.1038/s41598-017-03456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanjabi S, Oh SA, Li MO. Regulation of the immune response by TGF-β: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol. 2017;9:a022236. doi: 10.1101/cshperspect.a022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheng J, Chen W, Zhu HJ. The immune suppressive function of transforming growth factor-β (TGF-β) in human diseases. Growth Factors. 2015;33:92–101. doi: 10.3109/08977194.2015.1010645. [DOI] [PubMed] [Google Scholar]
- 51.Sun WK, Bai Y, Yi MM, Wu LJ, Chen JL, Wu DM, et al. Expression of T follicular helper lymphocytes with different subsets and analysis of serum IL-6, IL-17, TGF-β and MMP-3 contents in patients with rheumatoid arthritis. Eur Rev Med Pharmacol Sci. 2019;23((1)):61–9. doi: 10.26355/eurrev_201901_16748. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalo-Gil E, Galindo-Izquierdo M. Role of transforming growth factor-beta (TGF) beta in the physiopathology of rheumatoid arthritis. Reumatol Clin. 2014 May–Jun;10((3)):174–9. doi: 10.1016/j.reuma.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 53.Fogel LA, Yokoyama WM, French AR. Natural killer cells in human autoimmune disorders. Arthritis Res Ther. 2013;15((4)):216. doi: 10.1186/ar4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gianchecchi E, Delfino DV, Fierabracci A. NK cells in autoimmune diseases: linking innate and adaptive immune responses. Autoimmun Rev. 2018;17((2)):142–54. doi: 10.1016/j.autrev.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 55.Lima JF, Oliveira LMS, Pereira NZ, Duarte AJS, Sato MN. Polyfunctional natural killer cells with a low activation profile in response to Toll-like receptor 3 activation in HIV-1-exposed seronegative subjects. Sci Rep. 2017;7((1)):524. doi: 10.1038/s41598-017-00637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viola D, Dona A, Caserta E, Troadec E, Besi F, McDonald T, et al. Daratumumab induces mechanisms of immune activation through CD38+ NK cell targeting. Leukemia. 2021;35((1)):189–200. doi: 10.1038/s41375-020-0810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamin R, Berhani O, Peleg H, Aamar S, Stein N, Gamliel M, et al. High percentages and activity of synovial fluid NK cells present in patients with advanced stage active Rheumatoid Arthritis. Sci Rep. 2019;9((1)):1351. doi: 10.1038/s41598-018-37448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Almeida JS, Couceiro P, López-Sejas N, Alves V, Růžičková L, Tarazona R, et al. NKT-like (CD3+CD56+) cells in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Front Immunol. 2019;10:2493. doi: 10.3389/fimmu.2019.02493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Michel JJ, Turesson C, Lemster B, Atkins SR, Iclozan C, Bongartz T, et al. CD56-expressing T cells that have features of senescence are expanded in rheumatoid arthritis. Arthritis Rheum. 2007;56((1)):43–57. doi: 10.1002/art.22310. [DOI] [PubMed] [Google Scholar]
- 60.Lin SJ, Chen JY, Kuo ML, Hsiao HS, Lee PT, Huang JL. Effect of interleukin-15 on CD11b, CD54, and CD62L expression on natural killer cell and natural killer T-like cells in systemic lupus erythematosus. Mediators Inflamm. 2016;2016:9675861. doi: 10.1155/2016/9675861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hodge G, Hodge S, Holmes-Liew CL, Reynolds PN, Holmes M. Bronchiolitis obliterans syndrome is associated with increased peripheral blood natural killer and natural killer T-like granzymes, perforin, and T-helper-type 1 pro-inflammatory cytokines. J Heart Lung Transplant. 2012;31((8)):888–95. doi: 10.1016/j.healun.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 62.Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–31. doi: 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- 63.Plesner T, Arkenau HT, Gimsing P, Krejcik J, Lemech C, Minnema MC, et al. Phase 1/2 study of daratumumab, lenalidomide, and dexamethasone for relapsed multiple myeloma. Blood. 2016;128((14)):1821–8. doi: 10.1182/blood-2016-07-726729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korver W, Carsillo M, Yuan J, Idamakanti N, Wagoner M, Shi P, et al. A reduction in B, T, and natural killer cells expressing CD38 by TAK-079 inhibits the induction and progression of collagen-induced arthritis in cynomolgus monkeys. J Pharmacol Exp Ther. 2019;370((2)):182–96. doi: 10.1124/jpet.119.256602. [DOI] [PubMed] [Google Scholar]
- 65.Morandi F, Horenstein AL, Chillemi A, Quarona V, Chiesa S, Imperatori A, et al. CD56brightCD16- NK cells produce adenosine through a CD38-mediated pathway and act as regulatory cells inhibiting autologous CD4+ T cell proliferation. J Immunol. 2015;195((3)):965–72. doi: 10.4049/jimmunol.1500591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
All data used during the current study are available from the corresponding author on reasonable request.