Abstract
Staphylococcus epidermidis is a major cause of infections associated with indwelling medical devices. Biofilm production is an important virulence attribute in the pathogenesis of device-related infections. Therefore, elimination of these biofilms is an ideal treatment. Salicylate (5 mM) combined with 1 μg of vancomycin per ml inhibited biofilm formation by S. epidermidis (RP62A) by ≥99.9%. When biofilm-coated polystyrene beads were exposed to 5 mM sodium salicylate and 4 μg of vancomycin per ml (one-half the minimum biofilm eradication concentration), there was a >99.9% reduction in viable count.
Catheter-related infections are among the most common nosocomial infections, accounting for significant morbidity and mortality (27, 32). In 1992, the annual cost incurred by these infections in the United States was estimated to exceed $4.5 billion (24). The most common etiologic agent of catheter-related infection is Staphylococcus epidermidis (15, 20, 35). Vancomycin is often used to treat these infections because of the frequent occurrence of methicillin-resistant coagulase-negative staphylococci, including S. epidermidis. Vancomycin efficacy is reduced when S. epidermidis exists within a biofilm on the surfaces of indwelling medical devices (18, 31). Biofilm-producing S. epidermidis is usually involved in catheter-related infections (1, 33, 36). Resistance of biofilm bacteria to antibiotics may be due to a variety of factors, including changes in cell wall composition and surface structures (1, 2, 33). In view of the difficulty of the treatment of infections due to biofilm-producing bacteria, various measures for the prevention and treatment of catheter-related infections are being investigated. One intervention uses implants coated or impregnated with antimicrobial agents (8, 16, 22, 23, 26, 27).
Sodium salicylate has been demonstrated to have remarkable antibacterial activity, including the ability to enhance the activities of certain antibiotics. This drug inhibits adherence (55%), growth, and biofilm production of S. epidermidis (13, 28). It also enhances the in vitro and in vivo activities of amikacin against Klebsiella pneumoniae (10, 11) and increases the synergistic activity of imipenem and amikacin when they are used to treat K. pneumoniae infections in animals. The combined effect of vancomycin and sodium salicylate on S. epidermidis biofilms has not been reported. This study was designed to investigate the effect of sodium salicylate on the ability of vancomycin to inhibit biofilm production by S. epidermidis and to kill the bacteria.
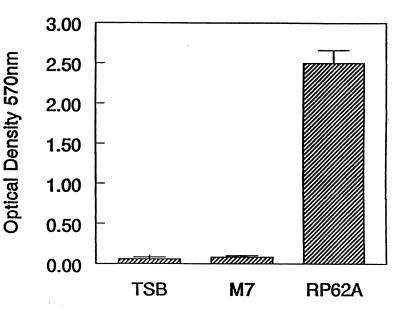
S. epidermidis RP62A (ATCC 35984) was obtained from the American Type Culture Collection (ATCC), and S. epidermidis (M7) and Staphylococcus aureus (ATCC 29213) were kind contributions of M. Hussain (Institute of Medical Microbiology, Muenster, Germany) and S. L. Josephson (Rhode Island Hospital, Providence, R.I.), respectively. Inhibition of biofilm formation was confirmed by an adherence-biofilm assay described previously (3, 4, 5 6, 21). Biofilm-negative mutant S. epidermidis M7 (34) served as a control. Briefly, aliquots (300 μl) of overnight cultures of S. epidermidis RP62A and M7 diluted (1:100) in Trypticase soy broth (TSB; Difco, Detroit, Mich.) were dispensed into each well of a sterile 96-well polystyrene microtiter plate (Corning, Corning, N.Y.). The plates were incubated in humidified conditions at 37°C for 24 h with shaking at 150 rpm. Wells with sterile TSB alone served as controls, and the mean optical density (OD) values for these wells was subtracted from the OD values for the test wells. Following incubation, the liquid was gently aspirated and replaced with sterile phosphate-buffered saline (PBS; pH 7.3). Each well was rinsed three times and air dried. Adherent bacteria were fixed with 95% ethanol and then stained with crystal violet. The OD at 570 nm (OD570) was measured with a Micro-ELISA AutoReader (DYNEX MRX). Biofilm-producing strains were defined as those with a mean OD570 value >0.1 (21). Biofilm production by S. epidermidis (RP62A) was confirmed by an OD of 2.5 ± 0.16. Strain M7 strain did not form a biofilm (OD, 0.08 ± 0.01).
The MIC of vancomycin (Sigma Diagnostics, St. Louis, Mo.) was determined by broth microdilution in cation-adjusted Mueller-Hinton broth (CAMHB; Difco) by the procedures recommended by the National Committee for Clinical Laboratory Standards (NCCLS) (29). The MIC of vancomycin for S. epidermidis (RP62A) was 2 μg/ml. The effect of 5 mM salicylate on the ability of vancomycin to inhibit biofilm formation was evaluated. Bacterial suspensions were added to serial dilutions of vancomycin such that the final inoculum was between 5 × 105 and 1 × 106 CFU/ml. For each trial, performed in triplicate, viable counts were performed with the inoculum. The following treatment regimens (final concentrations) in CAMHB were used: treatment A contained 1 μg of vancomycin per ml, treatment B contained 5 mM sodium salicylate and 1 μg of vancomycin per ml, treatment C contained 5 mM sodium salicylate, and treatment D contained CAMHB alone. The plates were incubated as described above. The relative inhibition of biofilm production (expressed as mean percentage) was determined as follows: 100 − [(OD570 of treated well/OD570 of reference well) × 100]. All treatment regimens inhibited biofilm production (Table 1). However, sodium salicylate was slightly more effective than 1 μg of vancomycin per ml (one-half the MIC). Vancomycin alone exerted a limited effect on the adherence and biofilm formation observed here and in previous studies (3, 28, 33). The combination treatment (treatment B) was more effective (P = 0.022) than treatment with vancomycin alone. Compared to the reference well, combination treatment reduced biofilm formation by >99.9%, giving an OD570 value <<0.1. Treatments A and C resulted in some degree of biofilm inhibition, but the bacteria were still producing a biofilm (Table 1). The OD values produced by the strain receiving treatment D were lower than those produced by the same bacterial strain in this assay (Fig. 1). This was most likely due to the presence of glucose in the TSB used in this assay but not in CAMHB used in the other assays. Glucose enhances biofilm production (21). Despite the absence of glucose in CAMHB, remarkable biofilm production was still observed, and treatment regimen D remained appropriate as a reference for comparison of inhibition of biofilm production.
TABLE 1.
Summary data (OD values) on inhibition of S. epidermidis biofilm formationa
| No. of triplicate evaluation | Mean OD570 value (mean % inhibition) for various treatment regimens
|
|||
|---|---|---|---|---|
| A | B | C | D (reference) | |
| Run 1 | 0.179 (65.4) | 0.000 (99.9) | 0.062 (88.0) | 0.517 |
| Run 2 | 0.383 (35.3) | 0.001 (99.9) | 0.329 (44.4) | |
| Run 3 | 0.469 (46.8) | 0.001 (99.9) | 0.225 (74.5) | |
| Total mean (SEM) % inhibition | 49.2 (8.8)b | 99.9 (0.04)b | 69.1 (12.9) | |
An OD570 ≥0.1 represents biofilm production. S. epidermidis suspensions that gave a final inoculum size that ranged between 5 × 105 and 1 × 106 CFU/ml were introduced to one of the four treatment regimens and were incubated with shaking (150 rpm) for 24 h at 37°C. Control wells containing CAMHB only, but no bacteria, were also included. The average ODs derived from these provided the zero values, which enabled circulation of the relative OD570 values.
The statistical significance of the relative percentage of biofilm inhibition was determined, and the difference between treatments A and B was significant (P = 0.022). However, only treatment B prevented biofilm formation. Comparisons of the means among the groups were done by one-way analysis of variance by Bonferroni multiple separation tests. Statistical analyses were performed with Stata software (version 7; Stata Corp., College Station, Tex.).
FIG. 1.
Biofilm production by S. epidermidis (RP62A). S. epidermidis M7 was included as a negative control. TSB represents the microtiter well that contained only TSB. Strains were tested in quadruplicate. An OD570 ≥0.1 represents biofilm production.
A polystyrene bead adherence assay was set up with bacterial suspensions between 5 × 105 and 1 × 106 CFU/ml. An aliquot (15 μl) of diluted cell suspensions (∼107 CFU/ml) was dispensed into each culture tube containing one sterile polystyrene bead (diameter, 5.5 mm; Precision Plastic Ball Co., Franklin Park, Ill.) immersed in 300 μl of CAMHB, and the treatment regimens described above were used. The tubes were incubated in humidified conditions at 37°C for 24 h with shaking at 150 rpm (model G-10; New Brunswick Scientific Co., Inc.). Following incubation, the medium was gently aspirated and replaced three times with sterile PBS, and then the beads were placed into a solution (500 μl) containing 0.5% Tween 80 and 10 mM EDTA for 10 min. The number of bacteria that adhered to and formed a biofilm on the beads after treatment was determined by vigorously vortexing (Fischer Vortex-Genie 2, model G-560; Scientific Industries, Inc., Bohemia, N.Y.) the beads for 3 min; the liquid was serially diluted and the bacteria were enumerated by the viable count method. Ultrasonic treatment was unnecessary for the release of bacteria, since our preliminary study showed that vortexing had a recovery efficiency >97%. This is consistent with the level of biofilm cell removal reported previously (37). When the effects of the treatments on biofilm formation were determined, the mean numbers CFU released from the bead in each control tube served as the reference inoculum for the corresponding experiment. Relative inhibition of biofilm production was determined as follows: 100 − [(CFU of treated bead/CFU of reference bead) × 100]. In the viable count assay, the level of inhibition by treatment A was 64.1% and the level of inhibition by treatment C was 82%. Treatment B was most effective (significantly more effective than treatment A [P = 0.03]), inhibiting biofilm formation ≥99.9% (Table 2).
TABLE 2.
Summary data (polystyrene beads) on inhibition of S. epidermidis biofilm formationa
| No. of duplicate evaluation | Mean no. of CFU/bead from various treatment regimens
|
|||
|---|---|---|---|---|
| A | B | C | D (reference) | |
| Run 1 | 1.9 × 105 (78.4) | 7.4 × 102 (99.9) | 1.7 × 105 (80.7) | 8.8 × 105 |
| Run 2 | 5.5 × 104 (72.9) | 4.8 × 102 (99.8) | 4.1 × 104 (79.8) | 2.1 × 105 |
| Run 3 | 1.3 × 105 (40.9) | 2.1 × 102 (99.9) | 3.2 × 104 (85.5) | 2.2 × 105 |
| Total mean (SEM) % inhibition | 64.1 (11.7)b | 99.9 (0.04)b | 82.0 (1.8) | |
The viable counts were compared with those recovered from beads incubated in the reference treatment (treatment D). S. epidermidis suspensions that gave a final inoculum size that ranged between 5 × 105 and 1 × 106 CFU/ml were introduced to one of the four treatment regimens and incubated with shaking (150 rpm) for 24 h at 37°C.
The statistical significance of the relative percentage of biofilm inhibtion was determined, and the difference between treatments A and B was significant (P = 0.03). However, only treatment B effectively inhibited biofilm formation. Comparisons of the mean among the groups were tested by one-way analysis of variance by Bonferroni multiple separation tests. Statistical analyses were performed with Stata software (version 7; Stata Corp.).
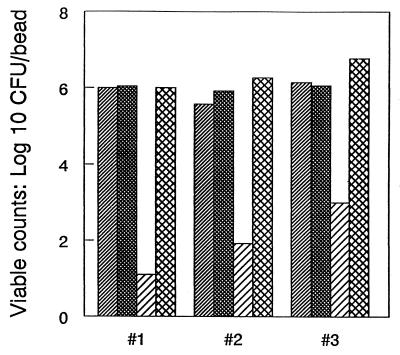
The minimum biofilm eradication concentration (MBEC) of vancomycin was determined by a broth macrodilution method in CAMHB, as described by NCCLS, with some modifications. The MBEC of vancomycin for S. epidermidis biofilms was 8 μg/ml and the MIC was 4 μg/ml (Table 3). This allowed us to assess the effect of sodium salicylate on the bactericidal activity of one-half the MBEC of vancomycin (4 μl/ml). Adherent inocula (between 5 × 105 and 1.5 × 106 CFU/bead) were generated by incubating each bead (with shaking at 37°C) for 18 to 20 h with bacteria (∼107 CFU/ml) suspended in CAMHB. Following incubation, biofilm-coated beads were rinsed to remove the nonadherent bacteria (37). The number of bacteria in the biofilm was determined as described above. Two beads were randomly selected and were used to establish a representative, mean adherent inoculum for that evaluation. A standard inoculum size, verified by determination of viable counts, served as a reference point for assessment of bacterial killing. Beads colonized with an S. epidermidis biofilm were placed in selected dilutions of vancomycin, and the mixtures were incubated at 37°C for 24 h with shaking at 150 rpm. After incubation, the beads were rinsed as described above. Adherent bacteria were released and enumerated, and the percent killing of adherent bacteria was calculated as follows: 100 − [(CFU of treated bead/CFU of untreated bead [reference inoculum]) × 100]. The MBEC was defined as the minimum concentration of vancomycin required to reduce biofilm cell numbers (initial inoculum size) ≥99.9%. Assays were performed in parallel against adherent standard inoculum; treatment A contained 4 μg of vancomycin per ml, treatment B contained 5 mM sodium salicylate and 4 μg of vancomycin per ml, treatment C contained 5 mM sodium salicylate, and treatment D contained CAMHB alone. After incubation, the beads were processed as described above. Treatment D served as a reference for evaluation of the efficacy of treatment on biofilm bacteria. Percent biofilm growth reduction was defined as: 100 − [(CFU of treated bead/CFU of reference bead of regimen D) × 100]. Treatment B exerted a pronounced bactericidal effect on biofilm bacteria, resulting in a mean reduction in viable count of >3 log10 CFU/bead (>99.9%) (Fig. 2). Neither treatment A nor treatment C had any significant effect on biofilm eradication. However, they both demonstrated some bacteriostatic activity against biofilm bacteria (Table 4). In this work, inhibition of biofilm formation and eradication of established biofilms were evaluated with S. epidermidis RP62A (ATCC 35984), which was isolated from a patient with intravascular catheter-associated sepsis (6). It has been characterized as a proficient biofilm producer, thereby making it an ideal strain for studies on the prevention and treatment of device-related infections involving bacterial biofilms.
TABLE 3.
MBECs of vancomycin for S. epidermidis biofilmsa
| No. of duplicate evaluation | Inoculum sizeb | No. of viable cells recovered (no. of CFU/bead) for vancomycin concn (μg/ml) of:
|
|||||
|---|---|---|---|---|---|---|---|
| 10 | 8 | 6 | 4 | 2 | 0 | ||
| Run 1 | 7 × 105 | <1 × 101 (−, 99.9)c | 9 × 101 (−, 99.9) | 4 × 102 (−, 99.9) | 3 × 106 (−, 0) | NA (+) | NA (+) |
| Run 2 | 5.3 × 105 | 1.9 × 101 (−, 99.9) | 2.5 × 101 (−, 99.9) | 7.5 × 103 (−, 98.59) | 1.9 × 106 (−, 0) | NA (+) | NA (+) |
| Run 3 | 1.2 × 106 | <1 × 101 (−, 99.99) | 3.1 × 102 (−, 99.97) | 9.1 × 102 (−, 99.92) | 6.5 × 105 (−, 45.8) | NA (+) | NA (+) |
| Mean percent biofilm eradication | >99.9 | 99.9 | 99.48 | 15.3 | |||
S. epidermidis biofilms growing on beads (5.3 × 105 to 1.2 × 106 CFU/bead) were subjected to three independent test evaluations.
For each evaluation, a representative inoculum size was established by determining the mean number of CFU per bead for at least two untreated, biofilm-colonized beads that were randomly selected. This value served as a reference for comparison with viable biofilm cell counts for beads exposed to the various vancomycin concentrations. NA, not applicable (therefore, no further assessment was done).
Information in parentheses represents the growth turbidity (+, turbid [visible growth in tube]; −, no visible growth, i.e., inhibition of growth), percent eradication.
FIG. 2.
Number of viable biofilm cells recovered following a 24-h exposure to vancomycin, sodium salicylate, and a combination thereof. Each bar represents viable counts obtained from tests in the three independent evaluations. The representative inoculum size ( ) was determined for each of the evaluations.
) was determined for each of the evaluations.  , vancomycin (4 μg/ml) only;
, vancomycin (4 μg/ml) only;  , salicylate (5 mM) plus vancomycin (4 μg/ml);
, salicylate (5 mM) plus vancomycin (4 μg/ml);  , salicylate (5 mM) only.
, salicylate (5 mM) only.
TABLE 4.
Effect of salicylate in combination with one-half the MBEC of vancomycin on S. epidermidis biofilms
| No. of duplicate evaluation | Inoculum size (reference biofilm cell eradication)a | No. of viable cells recovered (no. of CFU/bead) for treatment regimen:
|
|||
|---|---|---|---|---|---|
| A | B | C | D (reference biofilm growth inhibition) | ||
| Run 1 | 9.5 × 105 | 1.0 × 106 (−, 0)b | 1.3 × 101 (−, 99.9) | 1.0 × 106 (++, 0) | 9.0 × 106 (++++) |
| Run 2 | 5.0 × 105 | 9.0 × 105 (−, 0) | 8.3 × 101 (−, 99.98) | 2.0 × 106 (++, 0) | 1.1 × 107 (++++) |
| Run 3 | 1.5 × 106 | 1.3 × 106 (−, 13.33) | 9.5 × 102 (−, 99.94) | 6.5 × 106 (++, 0) | (++++) |
| Mean no. of log10 CFU/bead | 5.99 | 6.04 | 2.54 | 6.51 | 7.15 |
| Mean % eradication of biofilm cellsc | <10 | >99.9 | 0 | NAd | |
| Mean % inhibition of biofilm growthe | 92 | NA | 85 | ||
For each evaluation, a representative inoculum size was determined as described in the text. This value served as a reference. S. epidermidis biofilms growing on beads (5 × 105 to 1.5 × 106CFU/bead) were subjected to three independent test evaluations.
Information in parentheses represents the growth turbidity (++++ or ++, turbid [visible growth in tube]; (−) no visible growth), percent eradication.
The statistical significance of the percent reduction of the inoculum in size medium (CAMHB) only was determined, and the percent reduction from treatment B was significantly greater than that from treatment A or C (P = 0.000). Comparison of the means among the groups was tested by one-way analysis of variance by Bonferroni multiple separation tests. Statistical analyses were performed with Stata software (version 7; Stata. Corp.).
NA, not applicable (therefore, no further assessment was done).
Relative to reference treatment D.
The data presented in Table 4 and Fig. 2 provide compelling evidence that one-half the MBEC of vancomycin combined with 5 mM salicylate reduced the viable counts of biofilm cells >99.9%, therefore effectively eradicating established S. epidermidis biofilms. All treatments had some bacteriostatic effect on biofilm growth (Table 4).
The mechanisms behind reduced antibiotic susceptibility remain a topic of ongoing debate, but unlike the genetically mediated antibiotic resistance developed by vancomycin-resistant enterococci (VRE) (30), resistance in biofilm-producing bacteria may be a function of the biofilm itself (7, 14). The bacteria in biofilms acquire attachment-specific phenotypes, such as a reduced growth rate, which, in concert with the extracellular components, make them resistant to conventional treatment (7, 14). In the biofilm milieu, the extracellular substance may act as an ion-exchange matrix and may bind to charged antibiotics, limiting antibiotic availability, diffusion, and penetration (14).
The concerted effects of salicylate in combination, as presented here, are not fully understood. Salicylate is a chelator of divalent cations, and this may have influenced the assay system in one or more ways, including distortion of the surface charge on bacterial cell membranes, thereby impairing nutrient uptake, translocation, adherence, and biofilm formation (9, 12). As a chelator of divalent cations, salicylate may have depleted the pool of potential cofactors for enzymes essential for synthesis of the polysaccharide constituents of the biofilm. This study used 5 mM salicylate, equivalent to ∼800 μg/ml, a concentration above the therapeutic range for aspirin (200 to 350 μg/ml). However, 5 mM has been among the lower concentrations of this drug used in studies of bacteriology (17, 28), suggesting its appropriateness in our research efforts to better understand salicylate's potential role in combined therapy.
Prophylactic administration of vancomycin or teicoplanin during catheter insertion fails to prevent intravascular catheter-related bloodstream infections (19, 25; G. Pellizzer, R. Nicolin, A. D'Emilio, G. Figoli, L. Bragagnolo, and F. Merio, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. J89, p. 273, 1995). To reduce the risk of acquisition of VRE, the Centers for Disease Control and Prevention has recommended against the use of these antibiotics as prophylaxis (4). Our in vitro studies, however, indicate that the antistaphylococcal efficacy of vancomycin is significantly enhanced when vancomycin is used in conjunction with salicylate. Further work is needed to determine if this combination is clinically useful for the prevention or treatment of intravascular device-related infections.
In conclusion, this study has shown that (i) sodium salicylate significantly enhances the antistaphylococcal activity of vancomycin, (ii) a combination of one-half the MIC of vancomycin and 5 mM salicylate effectively prevents biofilm formation, and (iii) a combination of one-half the MBEC of vancomycin and 5 mM sodium salicylate effectively kills the bacteria in biofilms, reducing the viable biofilm cell numbers by >3 log10 CFU. If the in vitro data presented herein could be confirmed in vivo with an appropriate animal model, the salicylate-vancomycin combination may be useful for the prevention and treatment of intravascular catheter-related infections caused by S. epidermidis.
Acknowledgments
We thank David Laux (Department of Biochemistry, Microbiology and Molecular Genetics, University of Rhode Island) and Harrold Bibb (Department of Biological Sciences, University of Rhode Island) for constructive criticism of the manuscript, Clinton Chichester III (Biomedical Sciences, University of Rhode Island) for assistance with the Micro-ELISA AutoReader that he kindly provided, and Steven Reinert (Lifespan Medical Computing, Providence, R.I.) for the statistical analysis of the data.
This study was supported by the Department of Biochemistry, Microbiology and Molecular Genetics at the University of Rhode Island, Kingston.
REFERENCES
- 1.Anwar H, Costerton J W. Effective use of antibiotics in the treatment of biofilm-associated infections. ASM News. 1992;58:665–668. [Google Scholar]
- 2.Carlsson J. Bacterial metabolism in dental biofilms. Adv Dent Res. 1997;11:75–80. doi: 10.1177/08959374970110012001. [DOI] [PubMed] [Google Scholar]
- 3.Carsenti-Etesse H, Durant J, Entenza J, Mondain V, Padier C, Bernard E, Dellamonica P. Effects of subinhibitory concentrations of vancomycin and teicoplanin on adherence of staphylococci tissue culture plates. Antimicrob Agents Chemother. 1993;37:921–923. doi: 10.1128/aac.37.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC) Morbid Mortal Wkly Rep. 1994;44:1–13. [PubMed] [Google Scholar]
- 5.Christensen G D, Parisi J T, Bisno A L, Simpson W A, Beachey E H. Characterization of clinically significant strains of coagulase-negative staphylococci. J Clin Microbiol. 1985;18:258–269. doi: 10.1128/jcm.18.2.258-269.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen G D, Simpson W A, Younger J J, Baddour L M, Barrett F F, Melton D M, Beachey E H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costerton J W. Overview of microbial biofilms. J Ind Microbiol. 1995;15:137–140. doi: 10.1007/BF01569816. [DOI] [PubMed] [Google Scholar]
- 8.Darouiche R O, Raad I I, Heard S O, Thornby J I, Wenker O C, Gabrielli A, Berg J, Khardori N, Hanna H, Hachem R, Harris R L, Mayhall G. A comparison of two antimicrobial-impregnated central venous catheters. N Engl J Med. 1999;340:1–8. doi: 10.1056/NEJM199901073400101. [DOI] [PubMed] [Google Scholar]
- 9.Deighton M, Borland R. Regulation of slime production in Staphylococcus epidermidis by iron limitation. Infect Immun. 1993;61:4473–4479. doi: 10.1128/iai.61.10.4473-4479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domenico P, Straus D C, Woods D E, Cunha B A. Salicylate potentiates amikacin therapy in rodent models of Klebsiella pneumoniae infection. J Infect Dis. 1993;168:766–769. doi: 10.1093/infdis/168.3.766. [DOI] [PubMed] [Google Scholar]
- 11.Domenico P, Hopkins T, Cunha B A. The effect of sodium salicylate on antibiotic susceptibility and synergy in Klebsiella pneumoniae. J Antimicrob Chemother. 1990;26:343–351. doi: 10.1093/jac/26.3.343. [DOI] [PubMed] [Google Scholar]
- 12.Domenico P, Schwartz S, Cunha B A. Reduction of capsular polysaccharide production in Klebsiella pneumoniae by sodium salicylate. Infect Immun. 1989;57:3778–3782. doi: 10.1128/iai.57.12.3778-3782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farber B F, Wolff A G. The use of nonsteroidal antiinfammatory drugs to prevent adherence of Staphylococcus epidermidis to medical polymers. J Infect Dis. 1992;166:861–865. doi: 10.1093/infdis/166.4.861. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert P, Das J, Foley I. Biofilm susceptibility to antimicrobials. Adv Dent Res. 1997;11:160–167. doi: 10.1177/08959374970110010701. [DOI] [PubMed] [Google Scholar]
- 15.Huebner J, Goldmann D A. Coagulase-negative staphylococci: role as pathogens. Annu Rev Med. 1999;50:223–236. doi: 10.1146/annurev.med.50.1.223. [DOI] [PubMed] [Google Scholar]
- 16.Jansen B, Peters G. Modern strategies in the prevention of polymer associated infections. J Hosp Infect. 1991;19:83–88. doi: 10.1016/0195-6701(91)90100-m. [DOI] [PubMed] [Google Scholar]
- 17.Kang G, Balasubramanian K A, Koshi R, Mathan M M, Mathan V I. Salicylate inhibits fimbriae mediated HEp-2 cell adherence of and haemagglutination by enteroaggregative Escherichia coli. FEMS Microbiol Lett. 1998;16:257–265. doi: 10.1111/j.1574-6968.1998.tb13899.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee C K, Rubin L G, Molwin R M. Synergy between protamine and vancomycin in the treatment of Staphylococcus epidermidis biofilms. Urology. 1994;45:720–724. doi: 10.1016/S0090-4295(99)80074-0. [DOI] [PubMed] [Google Scholar]
- 19.Ljungman P, Hagglund H, Bjorkstrand B, Lonnqvist B, Ringden O. Perioperative teicoplanin for prevention of gram-positive infections in neutropenic patients with indwelling central venous catheters: a randomized controlled study. Supp Care Cancer. 1997;5:485–488. doi: 10.1007/s005200050117. [DOI] [PubMed] [Google Scholar]
- 20.Mack D. Molecular mechanisms of Staphylococcus epidermidis biofilm formation. J Hosp Infect. 1999;43(Suppl. SI):13–25. doi: 10.1016/s0195-6701(99)90074-9. [DOI] [PubMed] [Google Scholar]
- 21.Mack D, Siemseen N, Laufs R. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adherence. Infect Immun. 1992;60:2048–2057. doi: 10.1128/iai.60.5.2048-2057.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maki D G, Garman J K, Shapiro J M, Ringer M, Helgerson R B. An attachable silver-impregnated cuff for prevention of infection with central venous catheters: a prospective randomized multicenter trial. Am J Med. 1988;85:307–314. doi: 10.1016/0002-9343(88)90579-7. [DOI] [PubMed] [Google Scholar]
- 23.Maki D G, Stolz S M, Wheeler S, Mermel L A. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized controlled trial. Ann Intern Med. 1997;127:257–266. doi: 10.7326/0003-4819-127-4-199708150-00001. [DOI] [PubMed] [Google Scholar]
- 24.Martone W J, Jarvis W O, Edwards J R, Culver D H, Haley R H. Incidence and nature of endemic and epidemic nosocomial infection. In: Bennet J W, Bruchman P S, editors. Hospital infection. 4th ed. Philadelphia, Pa: Lippincott Raven; 1998. pp. 461–476. [Google Scholar]
- 25.McKee R, Dunsmuir R, Wnitby M, Garden O J. Does antibiotic prophylaxis at the time of catheter insertion reduce the incidence of catheter-related sepsis in intravenous nutrition? J Hosp Infect. 1985;6:419–425. doi: 10.1016/0195-6701(85)90059-3. [DOI] [PubMed] [Google Scholar]
- 26.Mermel L A, Stolz S M, Maki D G. Surface antimicrobial activity of heparin-bonded and antiseptic-impregnated vascular catheters. J Infect Dis. 1993;167:920–924. doi: 10.1093/infdis/167.4.920. [DOI] [PubMed] [Google Scholar]
- 27.Mermel L A. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132:391–402. doi: 10.7326/0003-4819-132-5-200003070-00009. [DOI] [PubMed] [Google Scholar]
- 28.Muller E, Al-Attar J, Wolff A G, Farber B F. Mechanism of salicylate-mediated inhibition of biofilm in Staphylococcus epidermidis. J Infect Dis. 1998;117:501–503. doi: 10.1086/517386. [DOI] [PubMed] [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. Approved standard M7–A4. 4th ed. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 30.Noble W C, Virani Z, Cree R. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol Lett. 1992;93:195–198. doi: 10.1016/0378-1097(92)90528-v. [DOI] [PubMed] [Google Scholar]
- 31.Raad I, Alarahwan A, Rolston K. Staphylococcus epidermidis: emerging resistance and need for alternative agents. Clin Infect Dis. 1998;26:1182–1187. doi: 10.1086/520285. [DOI] [PubMed] [Google Scholar]
- 32.Richards M J, Edwards J R, Culver D H, Gaynes R P. Nosocomial infections in medical intensive care units in the United States. National nosocomial infections surveillance system. Crit Care Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 33.Rupp M E, Hamer K E. Effect of subinhibitory concentrations of vancomycin, cefazolin, ofloxacin, l-ofloxacin and d-ofloxacin on adherence to intravascular catheters and biofilm formation by Staphylococcus epidermidis. J Antimicrob Chemother. 1998;41:155–161. doi: 10.1093/jac/41.2.155. [DOI] [PubMed] [Google Scholar]
- 34.Schumacher-Pedreau F, Heilmann C, Peters G, Gotz F, Pulverer G. Comparative analysis of a biofilm-forming Staphylococcus epidermidis strain and its adhesion-positive, accumulation-negative mutant M7. FEMS Microbiol Lett. 1994;117:71–78. doi: 10.1111/j.1574-6968.1994.tb06744.x. [DOI] [PubMed] [Google Scholar]
- 35.Sitges-Serra A, Girvent M. Catheter-related blood stream infections. World J Surg. 1999;23:589–595. doi: 10.1007/pl00012352. [DOI] [PubMed] [Google Scholar]
- 36.Soboh F, Khoury A E, Zamboni A C, Davidson D, Mittleman M W. Effects of ciprofloxacin and protamine sulfate combinations against catheter-associated Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 1995;39:1281–1286. doi: 10.1128/aac.39.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmerli W, Frei R, Widmer A F, Rajacic Z. Microbiological tests to predict treatment outcome in experimental device-related infections due to Staphylococcus aureus. J Antimicrob Chemother. 1994;33:959–967. doi: 10.1093/jac/33.5.959. [DOI] [PubMed] [Google Scholar]