Abstract
Nanoparticles are tested in mice and non-human primates (NHPs) before being selected for clinical trials. Yet the extent to which mRNA delivery as well as the cellular response to mRNA drug delivery vehicles is conserved across species in vivo is unknown. Using a species-independent DNA barcoding system, we compare how 89 lipid nanoparticles (LNPs) deliver mRNA in mice with humanized livers, primatized livers, and four controls: mice with “murinized” livers as well as wildtype BL/6, Balb/C, and NZB/BlNJ mice. We measure how functional delivery results in murine, NHP, and human hepatocytes predict one another in vivo. By analyzing in vivo hepatocytes with RNA-Seq, we identify species-dependent responses to LNPs, including mRNA translation and endocytosis. These data support an evidence-based approach to making small animal pre-clinical nanoparticle studies more predictive, thereby accelerating the development of RNA therapies.
Introduction
mRNA, siRNA, and antisense oligonucleotides have treated disease in humans1-8. The subset of these drugs administered intravenously requires nucleic acid delivery to diseased cells. To date, FDA-approved siRNA drugs utilize carbohydrate conjugates9 or lipid nanoparticles (LNPs)10, both of which exploit endogenous trafficking pathways to deliver siRNA into hepatocytes11, 12. Specifically, ionizable LNPs can bind serum ApoE in the bloodstream; LNP–ApoE complexes are endocytosed by cells expressing low-density lipoprotein receptor, including hepatocytes10. siRNA conjugated to modified N-Acetylgalactosamine (GalNAc) enters hepatocytes after binding asialoglycoprotein receptor, which is rapidly endocytosed. These insights into evolutionary conserved pathways that promote drug delivery have proven valuable as drugs progressed from mice to non-human primates (NHPs) and humans. Furthermore, by comparing delivery in murine hepatocytes and human hepatocytes from “humanized” mice13-16, which are mice engineered to include human cells, often hepatocytes, the AAV field has demonstrated the importance of understanding potential disparities between species. Specifically, comparing AAV variants in both mice models revealed that humanized mice are more predictive of AAV delivery in human cells than wildtype mice. Those studies resulted in the successful selection of an AAV variant that effectively delivered a therapeutic gene in hemophilia B patients17, demonstrating the value of humanized mice in drug development.
These examples underscore the value of understanding how genes that alter nanoparticle delivery vary between pre-clinical species and humans. Yet no study has systematically (i.e., using dozens of chemically distinct nanoparticles) compared LNP delivery across species in vivo. We therefore tested three hypotheses. First, we would observe species-dependent differences in hepatocyte LNP delivery. Second, the differences between NHP delivery and human delivery would be smaller than the differences between murine and human delivery. Third, transcriptomic analyses of hepatocytes from different species would identify cell signaling pathways associated with differences in delivery. However, testing these hypotheses using traditional one-by-one in vivo nanoparticle studies is challenging since humanized mice cost several thousand dollars per animal.
To overcome these issues, we developed Species-Agnostic Nanoparticle Delivery Screening (SANDS). SANDS has several traits specifically required for these studies. First, it is high throughput and in vivo. Second, it reports functional LNP delivery (in this case, mRNA translated into functional protein) at the cell-type level, in this case hepatocytes. Third, it is species independent, as it does not require Cre reporters or other genomic DNA constructs. These studies also required mice with humanized, primatized, and murinized livers that were generated in the same way. Murinized mice were used as a control because they have the same immune system as primatized and humanized mice and can be used to validate that the cell engraftment process does not alter delivery. Using SANDS, we quantified how 89 chemically distinct LNPs functionally deliver mRNA in six murine models: BL/6, Balb/C, NZB/BlNJ (NZB), Fah/Rag2/interleukin gamma chain (FRG) knockout mice on a BL/6 background with humanized livers (humanized), FRG knockout mice on a BL/6 background with NHP livers (primatized), and FRG knockout mice on a BL/6 background with murine livers (murinized). FRG mice, which have been previously described18, 19, were acquired from Yecuris. In addition to quantifying how well mouse, NHP, and human hepatocytes predict delivery in one another, we identified species-dependent responses to LNPs.
Species-agnostic nanoparticle delivery screening (SANDS)
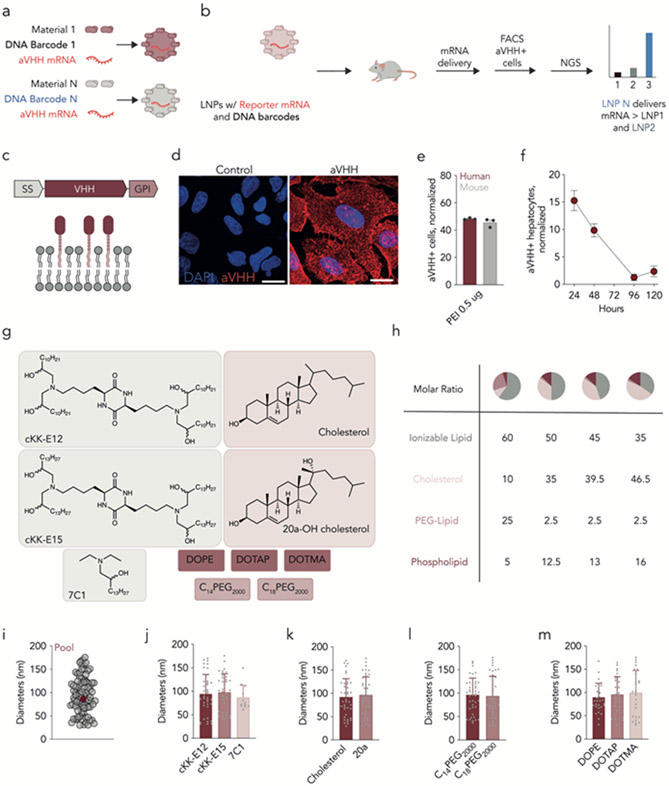
We designed SANDS to quantify functional hepatocyte mRNA delivery mediated by dozens of LNPs across species. Each LNP was formulated by microfluidic mixing20 and carried a functional mRNA encoding a reporter and a DNA barcode; LNP-1 carried mRNA and DNA barcode 1, whereas LNP-N carried mRNA and DNA barcode N. We performed quality control on individual LNPs by quantifying hydrodynamic diameter and polydispersity with dynamic light scattering; stable, monodisperse LNPs with a hydrodynamic diameter less than 200 nm were pooled together. Twenty-four hours after LNPs were administered, reporter+ cells were isolated using fluorescence-activated cell sorting (FACS). By sequencing the DNA barcodes in these cells, we identified barcodes, associated with specific LNPs, that colocalized with cells in which functional mRNA delivery occurred (Fig. 1a,b). Using DNA barcodes chemically and computationally optimized to facilitate sensitive in vivo readouts21, 22, we formulated LNPs carrying much more mRNA than barcode (mRNA: DNA mass ratio of 10:1)23, 24.
Figure 1.
Characterizing species-agnostic nanoparticle delivery screening (SANDS). (a) LNP-N is formulated to carry mRNA encoding aVHH as well as DNA barcode N. (b) Stable LNPs are pooled and injected into mice; aVHH+ cells are later isolated by FACS, and the barcodes within the cells are identified using next-generation sequencing. (c) Anchored VHH (aVHH) was selected as a reporter since it is embedded into the cell bilayer and can be quantified using antibodies against VHH. (d) aVHH protein expression, measured by immunofluorescent staining, 24 hours after A549 cells were transfected with aVHH mRNA. (e) aVHH protein expression, measured by flow cytometry, in Fa2N-4 human and AML-12 mouse hepatocytes 24 hours after transfection with aVHH mRNA. (f) The percentage of aVHH+ hepatocytes (i.e., CD31−CD45−aVHH+) at different timepoints following treatment with LNPs carrying 1 mg/kg aVHH mRNA. N=3/group, average +/− SEM. (g) The combinatorial design of 144 chemically distinct LNPs formulated carrying aVHH and DNA barcodes. (h) Hydrodynamic diameters of 89 LNPs that were deemed stable enough to pool together, as well as the diameter of the pooled LNP control. (i-l) Hydrodynamic diameter of the 89 LNPs plotted as a function of LNP chemical property. N=3/group, average +/− STD.
We considered several reporter mRNAs for SANDS. We excluded mRNA encoding Cre25, which requires transgenes unavailable in humanized mice. We also excluded mRNA encoding luciferase, which requires permeabilization and intracellular staining for cell-level readouts using flow cytometry. Instead, we formulated LNPs to carry mRNA encoding a glycosylphosphatidylinositol (GPI)-anchored camelid VHH antibody (anchored-VHH, aVHH). Linking the VHH domain with a GPI anchor induces cell-surface aVHH expression26, allowing aVHH+ cells to be detected with an anti-camelid VHH antibody (Fig. 1c). We first quantified aVHH signal in vitro. We formulated aVHH mRNA with Lipofectamine MessengerMax and transfected A549 cells with 1 μg/well of mRNA. Twenty-four hours later, we observed aVHH expression by immunofluorescent staining (Fig. 1d). We then confirmed that the expression of the mRNA reporter was similar in human and mouse cells in vitro. We formulated aVHH mRNA with PEI and transfected Fa2N-4 and AML-12 cells, which are derived from human and mouse hepatocytes respectively, with 0.5 μg/well of mRNA. Twenty-four hours later, we quantified aVHH expression by flow cytometry and found 48% of aVHH+ cells in human and 46% in mouse (Fig. 1e). Finally, we quantified aVHH expression in vivo after formulating aVHH mRNA into a previously reported27 LNP and intravenously injecting BL/6 mice with 1 mg/kg mRNA. The percentage of functionally transfected hepatocytes (CD31−CD45−aVHH+ cells) was highest at 24 hours, decreased slightly between 24 and 48 hours, and was near zero at 96 and 120 hours (Fig. 1f). We therefore selected a 24-hour timepoint for subsequent studies.
In vivo LNP delivery across cells from different species
We used SANDS to quantify how 89 LNPs delivered mRNA in vivo in cells derived from species. We chose a combinatorial library design, utilizing four components that traffic to the liver without targeting ligands 28 (Fig. 1g,h): lipomers (7C1, cKK-E12, cKK-E15) 29-31, cholesterol (cholesterol, 20α-hydroxycholesterol) 27, 32, 33, PEG-lipids (C14PEG2000, C18PEG2000) 34 and helper lipids (DOPE, DOTAP, DOTMA) 35-37. Of the 144 barcoded LNPs we formulated, 89 met quality control criteria and were pooled together and sterile filtered. As a control, we plotted the hydrodynamic diameter of the LNP pool; it was within the range of the 89 individual LNPs, indicating that pooling LNPs did not cause them to come out of solution (Fig. 1i). We then analyzed the hydrodynamic diameter as a function of ionizable lipid (Fig. 1j), cholesterol (Fig. 1k), PEG-lipid (Fig. 1l), and helper lipid (Fig. 1m) and found that LNP stability was not significantly impacted by any individual component.
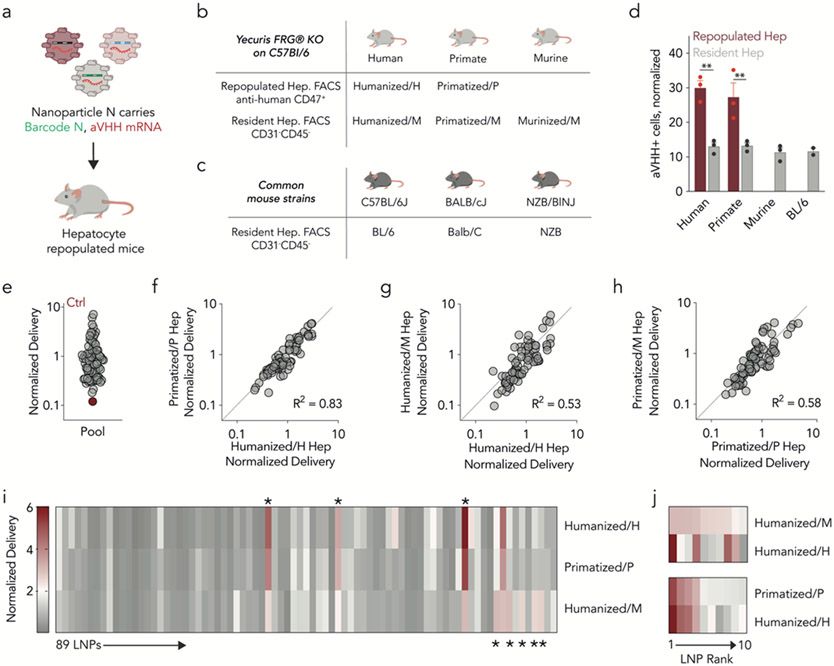
We intravenously injected the LNP pool, containing aVHH mRNA and DNA barcodes, into six mouse strains at a total nucleic acid dose of 1.5 mg/kg (an average of 0.017 mg total nucleic acid/kg/particle) (Fig. 2a). We injected control mice for each strain with PBS. After 24 hours, we isolated human- and NHP-repopulated (anti-human CD47+) as well as murine resident (anti-mouse CD31−CD45−) hepatocytes from FRG mice using FACS (Supplementary Figs. 1,2). We named human- and NHP-repopulated hepatocytes humanized/H and primatized/P, respectively; murine hepatocytes found in human and NHP FRG mice were named humanized/M and primatized/M. Finally, mouse hepatocytes from murinized mice were named murinized/M (Fig. 2b,c). We observed a significant increase in the percentage of aVHH+ humanized/H and primatized/P hepatocytes compared to humanized/M and primatized/M hepatocytes (Fig. 2d). These data compared hepatocytes isolated from the same animal, excluding the possibility that differences were due to tissue handling or missed injections. One possibility is that physical alterations in the repopulated livers promoted delivery to human and NHP cells, relative to mouse cells. We therefore performed an in-situ hybridization analysis of delivered aVHH mRNA and species-specific cell markers (Supplementary Fig. 3,4). Consistent with our aVHH flow cytometry data, we observed increased aVHH colocalization with human or NHP hepatocytes. We did not observe overt changes to liver tissue structure. As an additional control, we compared the percentage of aVHH+ murinized/M and BL/6 hepatocytes and observed similar delivery profile humanized/M and primatized/M hepatocytes (Fig. 2d). These lines of evidence do not support the hypothesis that species-dependent differences were driven by liver structural alterations.
Figure 2.
Nanoparticle delivery across murine, NHP, and human hepatocytes in vivo. (a) Eighty-nine LNPs labeled with SANDS were administered to six different murine models, including mice with (b) humanized, primatized livers, as well as murinized control mice that underwent the same procedures as humanized mice and (c) three wildtype strains. (d) Percentage of aVHH+ hepatocytes across the three species. Two-way ANOVA **P < 0.007, ns = 0.9755. N=3/group, average +/− SEM. (e) Normalized delivery for all 89 LNPs, averaged across all samples. Unencapsulated DNA barcode, acting as a negative control (Ctrl), was delivered into cells less efficiently than barcodes encapsulated by LNPs. (f-h) R2 analyses comparing delivery in human, NHP, and murine hepatocytes. (i) Heatmap of normalized delivery for all 89 LNPs in human, NHP, and control mouse cells. *Denotes LNPs that delivered mRNA differently between the three species. (j) Normalized delivery for the top 10 performing LNPs, ranked by delivery in murine or NHP cells. Delivery into human cells does not consistently align with murine delivery but does align more consistently with NHP cells.
We then quantified how delivery varied across species by calculating the correlation between normalized delivery in humanized/H, primatized/P, and murinized/M hepatocytes. Normalized delivery is a validated way to quantify LNP delivery using DNA barcodes24 (Supplementary Fig. 5). As expected, unencapsulated barcodes entered cells less efficiently than barcodes formulated into LNPs (Fig. 2e). We then calculated the correlation of normalized delivery for primatized/P hepatocytes and humanized/H hepatocytes and found it to be high (R2=0.83) (Fig. 2f). The correlation between humanized/H and humanized/M hepatocytes was lower (R2=0.53, Fig. 2g) even though these cells were taken from the same mice, excluding the possibility that species differences were caused by injection or tissue handling. A similar correlation (R2=0.58, Fig. 2h) was observed between primatized/P and primatized/M hepatocytes, again isolated from the same animals. We calculated correlations for humanized/H hepatocytes and four additional mouse strains, including murinized/M control hepatocytes; these values ranged between 0.31 and 0.64 (Supplementary Fig. 6). These data suggest that delivery to humanized/H cells was best predicted by primatized/P cells relative to all tested murine cells.
These correlations include all 89 LNPs. However, the best LNPs in mice are selected for NHP or human studies. We therefore compared delivery across species for top-performing LNPs. As a qualitative first step, we generated a heatmap of normalized delivery for all 89 LNPs in humanized/M, primatized/P, and humanized/H hepatocytes (Fig. 2i). We noted that five LNPs exhibited high delivery in humanized/M hepatocytes but low delivery in primatized/P or humanized/H hepatocytes. Additionally, we found three LNPs that had high delivery in humanized/H and low delivery in humanized/M hepatocytes. This suggests that screening in mice can generate false positive LNPs (i.e., LNPs predicted to deliver mRNA in NHP or human hepatocytes that fail to do so) as well as false negative LNPs and provides one preliminary line of evidence that the false positive rate may be somewhat higher. To further evaluate potential false positive LNPs, we rank ordered the top 10 LNPs in humanized/M hepatocytes and plotted their normalized delivery in humanized/H hepatocytes (Fig. 2j). Some top LNPs performed well in murine and human hepatocytes. However, several LNPs performed well in murine hepatocytes but not human. We similarly compared primatized/P and humanized/H hepatocytes and found that NHP delivery was similar to human delivery (Fig. 2j), even though these cells were isolated from different mice. These analyses led us to conclude that murine hepatocytes do not predict delivery in human cells as well as NHP hepatocytes, and that even top-performing LNPs in mice may be false positives.
To confirm that SANDS can predict NHP or human results more accurately, we performed two control experiments. First, we analyzed mRNA delivery mediated by the top LNP from our screen in primary human and mouse hepatocytes in vitro. (Supplementary Fig. 7a). We found that this LNP preferentially delivered mRNA to primary human hepatocytes, relative to mouse hepatocytes. By contrast, a control transfection reagent did not show a species-dependent delivery. Second, we found a Moderna patent that compared hepatocyte mRNA delivery in NHP livers using a lipid called lipid H and the FDA-approved Dlin-MC3-DMA formulation38. We therefore formulated two LNP libraries, using 32 LNPs per lipid, and compared delivery in humanized hepatocytes. The Moderna library resulted in twice as many aVHH+ cells in humanized hepatocytes, relative to MC3 (Supplementary Fig. 7b). These data suggest that SANDS can recapitulate previously reported datasets.
In addition to selecting individual LNPs for studies in larger animals, a second common practice is to use LNP delivery in mice to extract relationships between LNP chemical structures and in vivo delivery, in order to inform the design of future LNPs. We therefore inquired whether LNP physical and chemical traits were overrepresented in murinized/M hepatocytes, relative to primatized/P and humanized/H hepatocytes. We plotted normalized delivery for all 89 LNPs as a function of lipomer, cholesterol, PEG, helper lipid and molar ratio, and looked for relationships between LNP structure and normalized delivery differences across species. We did not observe any species-dependent differences in cholesterol (Supplementary Fig. 8a), which is consistent with the fact that the ApoE and cholesterol trafficking that influences LNP delivery10 is conserved across species. The relationship between helper lipid and delivery was also consistent across all three species; we were unable to come up with a credible biological hypothesis explaining this (Supplementary Fig. 8b). However, we observed species differences in PEG-lipids; PEG-lipids with a C14 lipid tail tended to deliver barcodes to humanized/H hepatocytes significantly more than PEG-lipids with a C18 lipid tail. This difference was consistent but not significant in primatized/P hepatocytes and was not observed in murinized/M hepatocytes (Supplementary Fig. 8c). Similarly, the type of lipomer that promoted delivery in murinized/M hepatocytes was distinct from the lipomer that promoted delivery in humanized/H and primatized/P hepatocytes (Supplementary Fig. 8d). Finally, we found a statistical difference in molar ratios but were unable to clearly interpret these differences (Supplementary Fig. 8e-g). Additional studies are required to understand whether the PEG- and lipomer-based differences we observed are consistent across LNPs. However, these data suggest it is possible to identify preliminary evidence of species-dependent LNP traits using this study design.
Species-dependent cellular responses to LNPs
We then returned to our observation that LNP delivery led to a higher percentage of aVHH+ humanized/H and primatized/P hepatocytes, relative to humanized/M, primatized/M, and murinized/M hepatocytes (Fig. 2d). We hypothesized that the difference in aVHH+ hepatocytes was driven by overt differences in how much nucleic acid reached the cells. We therefore quantified LNP biodistribution in aVHH+ hepatocytes using droplet digital PCR (ddPCR); the barcodes included a ddPCR probe site for sensitive biodistribution readouts in FACS-sorted cells in vivo21. The increase in aVHH+ cells (Fig. 2d) was not observed when we measured biodistribution (Supplementary Fig. 9), which led us to discard this hypothesis. We then posited that the species-dependent difference in the percentage of aVHH+ cells was caused by species-dependent cell signaling responses to LNPs. This hypothesis is consistent with evidence that in vivo biodistribution is necessary but not sufficient for functional delivery39, 40 and that internal cell signaling influences LNP mRNA delivery31, 41.
We compared the transcriptomic profiles of humanized/H and humanized/M cells. Using a modified Smart-seq2 protocol, we sequenced 1,000 cells from three pooled biological replicates (Fig. 3a). Counts were analyzed using iDEP, which allows for differential expression and pathway analysis of RNA-Seq data42 (Fig. 3b, Supplementary Fig. 10). Due to the high number of samples, biological replicates were pooled before sequencing, therefore increasing the total number of reads per conditions. To ensure the accuracy of our conclusions, we performed a separate analysis using CORNAS, a tool specifically developed for analyzing gene expression data without biological replicate43. We obtained similar results for both CORNAS and DESeq2 analyses.
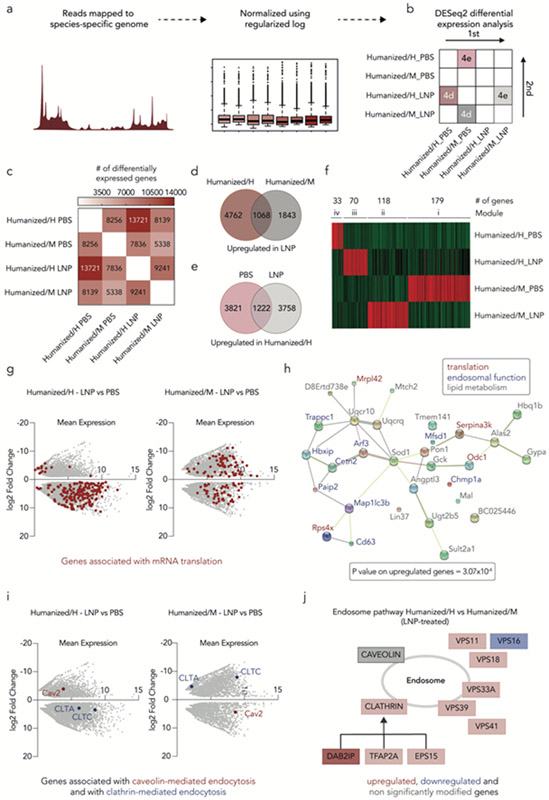
Figure 3.
Transcriptomics studies reveal species-dependent response to LNPs. (a) Schematic of Smart-seq2 protocol design. (b) DESeq2 differential expression analysis uses a pairwise comparison to determine the number of differentially expressed genes between different conditions. (c) DESeq2 differential expression analysis reveals that LNP-treated humanized/H contains the higher number of differentially expressed genes. (d) The number of upregulated genes was determined between both species (humanized/H and humanized/M) after LNP treatment. (e) The number of upregulated genes was determined between the humanized/H LNP-and PBS-treated samples. (f) Weighted correlation network analysis (WGCNA) shows the 400 most upregulated genes in humanized/H and humanized/M hepatocytes; four unique modules were identified. (g) Differential gene expression in the LNP-treated humanized/H and humanized/M hepatocytes was identified. Genes associated with mRNA translation are highlighted in red. (h) STRING analysis, based on the top 30 upregulated genes, across the LNP-treated humanized/H and humanized/M samples identifies interactions between genes associated with translation, lipid metabolism, and endosomal function. (i) Differential expression of genes associated with caveolin- and clathrin-mediated endocytosis in both humanized/H and humanized/M hepatocytes indicates receptor-mediated endocytosis-related difference in delivery across species. (j) As an additional line of evidence, Reactome database was utilized to illustrate the differences in endocytosis-related gene expression between LNP-treated humanized/H and humanized/M hepatocytes.
Using iDEP, we first performed a differential expression analysis (Fig. 3c), to determine the number of differentially expressed genes in humanized/H and humanized/M samples, treated with either PBS or LNPs. (Fig. 3c). We found 2.6x more differentially expressed genes (13,271) when comparing humanized/H hepatocytes treated with LNP, relative to their PBS control, than when we compared LNP-treated humanized/M hepatocytes to their PBS control (5,338) (Fig. 3c). These results provide a line of evidence that human and murine cells underwent different transcriptional responses to LNPs.
We noted that most of the differentially regulated genes, between the LNP- and PBS-treated samples, were upregulated (Supplementary Fig 10); we therefore focused our analysis on those genes. We quantified the number of upregulated genes in LNP-treated humanized/M cells, relative to PBS-treated humanized/M cells (Fig. 3d), and found 2,911 upregulated genes. Notably, the number of upregulated genes in LNP-treated humanized/H cells, relative to PBS-treated humanized/H cells, was 2.0x higher (5,830); of these upregulated genes, only 1,068 were shared, suggesting that upregulated genes differed between the two species. To control for “basal” species differences (i.e., differences not driven by response to LNPs), we compared humanized/H PBS-treated hepatocytes to humanized/M PBS-treated hepatocytes. We found that 5,043 genes were upregulated in humanized/H PBS-treated hepatocytes. We then compared humanized/H LNP-treated hepatocytes to humanized/M LNP-treated hepatocytes and found that 4,920 genes were upregulated. We reasoned that if the differences in gene expression were driven by “basal” species differences, then most of the upregulated genes would be shared. However, of these upregulated genes, only 1,222 were shared (Fig. 3e). Finally, we confirmed that the expression of 80 housekeeping genes was comparable across samples (Supplementary Fig. 11), demonstrating that the observed differences were not due to sample variability. To validate that cells exhibited different transcriptional profiles, we performed a weighted correlation network analysis (WGCNA) using the 400 most upregulated genes for both humanized/H and humanized/M LNP-treated samples. This algorithm separated the 400 genes into four distinct modules, providing a third line of evidence that murine and human hepatocytes responded differently to LNPs (Fig. 3f).
To understand the cellular functions of these genes, we analyzed the top 10 upregulated genes within the four WGCNA clusters. Five of the top 10 upregulated genes in the LNP-treated humanized/H cluster were related to translation or post-translational processes, whereas none of the top 10 humanized/M cluster genes were (Supplementary Fig. 10). To confirm this result, we entered read counts of all the genes into iDEP, for both humanized/H and humanized/M samples, with and without LNP treatment, and extracted a list of all the most upregulated genes. We considered genes significant if they changed by at least 2-fold with a false discovery rate (FDR) <0.1. The most differentially regulated pathway between humanized/H and humanized/M cells, both treated with LNPs relative to their own PBS controls, was again translation (Fig. 3g, Supplementary Fig. 12). Specifically, we found that 202 genes related to translation were upregulated in the LNP-treated humanized/H samples; 99 of these genes were upregulated in the LNP-treated humanized/M samples (Supplementary Fig. 13).
Given that mRNA translation is a key cellular function, we reasoned that some aspects of the cellular response to LNPs would be conserved. Therefore, we entered the list of the 99 genes upregulated in both humanized/H and humanized/M into the DAVID Bioinformatics database. The genes upregulated in both conditions were related to eukaryotic translation initiation factor eIF2/eIF5 and the eukaryotic elongation factor 1 (eEF1)44, suggesting that exogenously delivered mRNA is impacted by similar initiation and elongation mechanisms in both species (Supplementary Fig. 14). We then entered the 103 genes that were only upregulated in human cells, but were unable to identify specific aspects of translation that were clearly differentiated. We therefore explored interactions of these genes with other cellular functions. We used an unbiased Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) analysis, a database of known protein-protein interactions, to better understand how upregulated genes interact with each other. We took the top 30 upregulated genes in LNP-treated humanized/H and humanized/M hepatocytes45 and input them in the database (Fig. 3h). As a control, we first investigated whether STRING analysis identified genes associated with translation, which it did. Further, the STRING analysis highlighted that those genes were interacting with other genes related to lipid metabolism and endosomal function, which are both likely to affect LNP-mediated mRNA delivery. Based on this, we evaluated the expression of Cav2, CLTA, and CLTC, canonical genes associated with caveolin- and clathrin-mediated endocytosis, which LNPs use to enter cells. We found that CLTA and CLTC were upregulated in humanized/H hepatocytes treated with LNPs, relative to PBS, while Cav2 was downregulated (Fig. 3i). However, we observed the opposite expression profile in humanized/M hepatocytes (Fig. 3i). These data highlight that canonical clathrin- and caveolin-mediated endocytosis genes were not regulated similarly in murine and human hepatocytes after LNP exposure. To confirm these data, we used the Reactome database to analyze receptor-mediated endocytosis profiles in LNP-treated humanized/H and humanized/M samples. The Reactome data corroborated that humanized/H hepatocytes utilize clathrin-mediated endocytosis more predominantly than caveolin, relative to humanized/M hepatocytes after LNP exposure (Fig. 3j). To confirm our conclusions, we repeated the experiment (formulations of the same 89 LNPs, injection at 1.5 mg/kg, hepatocyte FACS sorting followed by library preparation and bulk RNA-Seq) in humanized mice, keeping biological replicates separated during sequencing (Supplementary Fig. 15,16). We again compared the transcriptomic profiles of humanized/H and humanized/M cells using the same modified Smart-seq2. We first performed a genome wide analysis and compared overall gene expression levels between each set of conditions (pooled and separated) and found them to be similar (R2 = 0.89 +/− 0.047, Supplementary Fig. 15). We therefore focused our analysis on the genes we previously observed. Particularly, we compared the expression level of genes related to caveolin- and clathrin-mediated endocytosis in both LNP-treated humanized/H and humanized/M to their respective PBS (Supplementary Fig. 16b,c). We again observed that CLTC and CLTA genes were upregulated in LNP-treated humanized/H samples, relative to PBS, while Cav2 was downregulated. The opposite trend was observed in LNP-treated humanized/M samples, compared to PBS. Lastly, we performed a STRING analysis to study the top 10 upregulated genes in LNP-treated humanized/H and humanized/M hepatocytes and found that four of those 10 genes were also observed in the previous experiment (Supplementary Fig. 16e). These genes were found to be associated with translation, lipid metabolism, and endosomal pathways that were also highlighted in our previous experiment.
Based on these data, we reached several conclusions. First, the number of genes that are differentially regulated in human hepatocytes in response to LNPs is higher than in murine hepatocytes. Second, the species-dependent increase in aVHH+ humanized/H hepatocytes is possibly driven by a combination of two non–mutually exclusive mechanisms. The first mechanism is a species-dependent reliance on either caveolin- or clathrin-mediated endocytosis. Although both pathways are present in both species, the relative in vivo activity of one pathway compared to the other may not be conserved. The second potential mechanism is to-be-determined differences in translation. We cannot identify the specific translational mechanism; however, we can exclude the possibility that species-dependent differences in translation are driven by initiation or elongation.
Strain-dependent cellular responses to LNPs
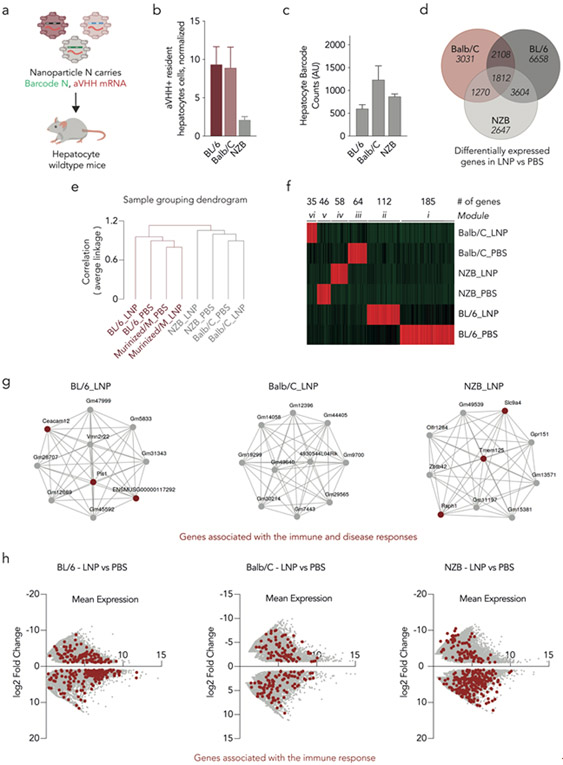
One key limitation to several of the unbiased RNA-Seq analyses above is that some cellular functions (e.g., translation) may have more genes associated with them and thus may come up more often as gene lists are surveyed. To control for this, we performed the same transcriptomic analyses but removed the species variable by comparing delivery to three wildtype immunocompetent mouse strains. Using the same pool of 89 LNPs, we injected wildtype BL/6, Balb/C, and NZB mice (Fig. 4a) and found a lower percentage of aVHH+ hepatocytes in NZB mice, compared to BL/6 and Balb/C (Fig. 4b). We quantified the biodistribution in hepatocytes using ddPCR and found that the biodistribution in aVHH+ cells between mice strains was similar and, once again, did not overlay with the percentage of aVHH+ hepatocytes (Fig. 4c).
Figure 4.
Inflammatory genes impact mRNA delivery across multiple mouse strains. (a) The 89 LNPs previously described, carrying a DNA barcode and aVHH mRNA, were intravenously administered to wildtype BL/6, Balb/C, and NZB mice. (b) Twenty-four hours later, the percentage of aVHH+ hepatocytes were analyzed. N=3/group, average +/− SEM. (c) Biodistribution of DNA barcodes was quantified using ddPCR. Average +/− SEM. (d) The number of upregulated genes was determined between the different mouse strains, after LNP treatment. (e) Hierarchal clustering analysis was performed indicating different gene expression profiles between Balb/C, NZB, and BL/6. (f) Weighted correlation network analysis (WGCNA) shows the 500 most upregulated genes in wildtype murine hepatocytes; six unique modules were identified. (g) Network analysis from the top 10 upregulated genes found in WGCNA for each cluster of the LNP-treated BL/6 (ii), Balb/C (vi), and NZB (iv). (h) Differential gene expression between the LNP- and PBS-treated samples was analyzed for all three strains. Genes associated with immune response are highlighted in red.
To analyze whether this difference in delivery was driven by the same cellular mechanisms observed between human and murine, we isolated hepatocytes and examined their transcriptomic profiles. We first quantified the number of differentially expressed genes and found differences in gene expression between the three mouse strains treated with LNPs, compared to their PBS controls (Fig. 4d). To better understand these differences and how they relate to each sample, we used hierarchal clustering to determine how similar each strain was to the others. We found that NZB and Balb/C clustered together, whereas BL/6 formed a separate cluster (Fig. 4e). As a control, we confirmed that murinized/M, which is on a BL/6 background, and wildtype BL/6 clustered together (Fig. 4e). To confirm different expression profiles between the three wildtype mice strains, we performed a WGCNA analysis and found unique gene clusters for each strain (Fig. 4f). We then looked at the top 10 genes found within each cluster; most of the genes were unannotated, and several of the annotated genes were related to immune and disease responses (Fig. 4g). Notably, we did not identify pathways for translation or endocytosis. Finally, to further explore the differences between mouse strains, we performed pathway analysis of all the genes using iDEP and found far more upregulated genes associated with immune response in BL/6 (273 genes) and NZB (324 genes) mice than in Balb/C (178 genes) mice (Supplementary Fig. 18). We confirmed these results by comparing MA plots of the differentially expressed genes in LNP-treated samples, relative to PBS, and highlighted different gene expression profiles between the three strains (Fig. 4h). These results, which implicate differential immune response as a potential mechanism for strain-dependent delivery, are consistent with the fact that immunity plays a key role in nanoparticle delivery46. These data led us to conclude that the translation and endocytosis differences observed in our species-to-species studies were not identified solely because these processes are well annotated in RNA-Seq analytical software.
Conclusions
SANDS may help solve a problem that slows the clinical development of RNA drugs: the hard-to-predict progression of drug delivery candidates from mice to NHPs to humans. NHP hepatocytes predicted delivery to human hepatocytes better than murine hepatocytes; however, murine hepatocytes were somewhat predictive. As a result, our results do not justify straight into NHP one-by-one LNP screening, which would come with a significant ethical cost. Instead, our data support a two-step model for selecting LNPs in mice. In the first step, dozens of LNPs are screened at once in humanized mice. In the second step, top LNPs are tested individually in humanized mice and immunocompetent mice. In the individual LNP studies, efficacy data in humanized mice supersede efficacy data from wildtype mice, and the safety data in wildtype mice supersede safety data in humanized mice.
Given that SANDS is species agnostic, future studies may evaluate hundreds of LNPs in combinations of animal models, including those with disease phenotypes. This confers two advantages. The first is the ability to screen in animals known to better recapitulate human physiology; this will provide more informative pre-clinical efficacy and toxicology data. The second advantage is the ability to test whether disease-specific genes influence delivery; we envision comparing delivery in diseased tissues and their healthy controls to identify (i) whether delivery changes as a function of disease, and (ii) if so, the genes responsible for that change. In this study, we used direct comparative experiments to identify translation and endocytosis as two cellular processes that may respond to LNPs in a species-dependent manner. More specifically, the data suggest that nanoparticles that preferentially interact with CLTA and CLTC may “underperform” in mice but may perform well in humans. Notably, while the data were consistent across several experiments and analyses, some data were generated with a pooled sample of cells, rather than biological replicates. These data were analyzed with software specifically designed for pooled samples (CORNAS and DESeq2) but may still be affected by biological variability and dispersion across samples. Thus, the specific genes listed need to be evaluated in future studies. If validated by other labs, then mice engineered to overexpress endocytosis genes used by human hepatocytes and to underexpress genes predominantly used by murine hepatocytes may be useful models for drug delivery. Specifically, we envision murine models with (i) a fully intact murine immune system and (ii) hepatocytes with “human-like” endocytosis profiles. Finally, we learned that we would have generated the opposite biological hypothesis if we only studied LNPs using murine hepatocytes.
It is important to acknowledge the limitations of the study. One limitation is the use of humanized and primatized mice as proxies for humans and NHPs. However, we believe our approach altered fewer variables, given that the rest of the system (i.e., background strain, treatment, etc.) was constant. One alternative is to screen directly in wildtype mice and NHPs, then compare results; however, this alternative would not include human cells. A second limitation is the use of LNPs we found likely to target hepatocytes using endogenous trafficking pathways. Other LNP chemical spaces may exhibit different species-to-species variability. A third limitation is our focus on hepatocytes. One alternative would be to perform similar studies using mice with other types of humanized cells, including mice with humanized immune systems. More generally, we hope to address whether mouse-NHP-human predictivity changes as a function of cell type. We focused on the difference in gene expression between species rather than the difference in gene expression driven by specific compounds. However, it is likely that different LNP chemistries will elicit different cellular responses. Future research should therefore expand the number of LNPs tested as well as the types of chemistries used to formulate those LNPs. Despite these limitations, we believe this work may help streamline the pre-clinical development of RNA delivery vehicles.
Materials and Methods
Synthesis.
7C1, cKK-E12, and cKK-E15 were prepared according to literature procedures (Supplementary Fig. 19) 29-31.
Preparation of 7C1.
C13 lipids and PEI600 were both combined and heated to 90°C in EtOH for 48–72 hours.
Preparation of cKK-e12.
Compound 1 (20 g, 41.9 mmol) was added into a 100 ml flask and trifluoroacetic acid (42 mL) was added slowly at 0 °C, then stirred at room temperature for 30 min. The solvent was evaporated under reduced pressure and the crude product, dissolved in DMF (5 mL), was added dropwise to pyridine (300 mL) at 0 °C. The reaction mixture was then stirred at room temperature overnight. The solvents were evaporated under reduced pressure and washed with ethyl acetate to give pure compound 2 (8.4 g, 31% yield). To a solution of compound 2 in acetic acid/CH2Cl2 (1/1, 300 mL) was added Pd/C (10 wt. %, 3.0 g). The black suspension was degassed for 5 min with hydrogen and stirred at room temperature overnight under hydrogen atmosphere. The reaction mixture was filtered on Celite and washed with MeOH. The combined filtrates were then concentrated to obtain a crude yellow viscous oil. The oil was washed with ethyl acetate to yield compound 3, which was used without further purification. To a solution of compound 3 (84 mg, 0.22 mmol) and 1,2-epoxydodecane (247 mg, 1.34 mmol) in EtOH (2 mL) was added triethylamine (0.12 mL, 0.88 mmol). After stirring for 30 min at room temperature, the reaction mixture was then irradiated in the microwave reactor at 150 °C for 5 h. Purification of the crude residue via flash column chromatography (gradient eluent: MeOH /DCM 20:1 to 7:1) afforded cKK-E12 (219 mg, 40%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 4.02 (br. s, 2H), 3.71 (br. s, 4H), 3.06–2.19 (m, 12H), 1.86 (br. s, 4H), 1.43–1.21 (m, 80H), 0.87 (t, J = 8 Hz, 12H). HRMS (ESI, m/z) calcd [M+2H]2+ for C60H121O6N4 497.4677, found 497.4662, m/z calcd [M+H]+ for C60H121O6N4 993.9272, found 993.9281 (Supplementary Figs. 20,21).
Preparation of cKK-e15.
Compound 1 (20 g, 41.9 mmol) was charged in a 100 ml flask and trifluoroacetic acid (42 mL) was added slowly at 0 °C and then stirred at room temperature for 30 min. The solvent was evaporated under reduced pressure and then the crude product, dissolved in DMF (5 mL), was added dropwise to pyridine (300 mL) at 0 °C. The reaction mixture was stirred at room temperature overnight. The solvents were evaporated under reduced pressure and the crude product washed with ethyl acetate to give pure compound 2 (8.4 g, 31% yield). To a solution of compound 2 in acetic acid/CH2Cl2 (1/1, 300 mL) was added Pd/C (10 wt. %, 3.0 g). The black suspension was degassed for 5 min with hydrogen and stirred at room temperature overnight under hydrogen atmosphere. The reaction mixture was filtered on Celite and washed with MeOH. The combined filtrates were concentrated, and the crude compound was washed with ethyl acetate to yield compound 3 (4.8 g, 98% yield). To a solution of compound 3 (84 mg, 0.22 mmol) and tridecyloxirane (302 mg, 1.34 mmol) in EtOH (2 mL) was added triethylamine (0.12 mL, 0.88 mmol). The reaction mixture was then irradiated in the microwave reactor at 150 °C for 5 h. Purification of the crude residue via flash column chromatography (gradient eluent: 1-2.0 % of MeOH/DCM then 2.0-4.0 % MeOH/DCM containing 0.5 % NH4OH) afforded cKK-E15 (200 mg, 78%) as a light-yellow oil. 1H NMR (500 MHz, CDCl3) δ 0.87 (t, J = 6.5 Hz, 12H), 1.22-1.32 (m, 88H), 1.36-1.56 (m, 16H), 1.73-1.99 (m, 4H), 2.17-2.68 (br. s, 12H), 3.62 (m, 4H), 3.98 (m, 2H). HRMS (ESI, m/z) calcd [M + H]+ for C72H145N4O6 1162.1159, found 1162.1153 (Supplementary Figs. 22, 23).
aVHH mRNA synthesis.
mRNA was synthesized as described previously 26. Briefly, the GPI-anchored VHH sequence was ordered as a DNA gBlock from IDT (Integrated DNA Technologies) containing a 5’ UTR with Kozak sequence, a 3’ UTR derived from the mouse alpha-globin sequence, and extensions to allow for Gibson assembly. The sequence was human codon optimized using the IDT website. The gBlock was then cloned into a PCR amplified pMA7 vector through Gibson assembly using NEB Builder with 3 molar excess of insert. Gibson assembly reaction transcripts were 0.8% agarose gel purified prior to assembly reaction. Subsequent plasmids from each colony were Sanger sequenced to ensure sequence identity. Plasmids were digested into a linear template using NotI-HF (New England Biolabs (NEB)) overnight at 37 °C. Linearized templates were purified by ammonium acetate (Thermo Fisher Scientific) precipitation before being resuspended with nuclease-free water. In vitro transcription was performed overnight at 37 °C using the HiScribe T7 kit (NEB) following the manufacturer’s instructions (full replacement or uracil with N1-methyl-pseudouridine). RNA product was treated with Dnase I (Aldevron) for 30 min to remove template and purified using lithium chloride precipitation (Thermo Fisher Scientific). RNA transcripts were heat denatured at 65 °C for 10 min before being capped with a Cap1 structure using guanylyl transferase (Aldevron) and 2’-O-methyltransferase (Aldevron). Transcripts were then polyadenylated enzymatically (Aldevron). mRNA was then purified by lithium chloride precipitation, treated with alkaline phosphatase (NEB), and purified a final time. Concentrations were measured using a Nanodrop, and mRNA stock concentrations were between 2–4 mg/mL. mRNA stocks were stored at −80 °C. Purified RNA products were analyzed by gel electrophoresis to ensure purity.
In vitro assay.
A549 cells were cultured in DMEM supplemented with 10% FBS and 5% penicillin-streptomycin. A549 cells were plated in 24-well plates and transfected with 1 μg/well of aVHH mRNA or left untransfected. mRNA was formulated with Lipofectamine MessengerMax at a ratio of 1.5μL/μg. After 24 hours incubation, cells were fixed with 4% paraformaldehyde and immunostained. Briefly, cells were permeabilized with 0.2% Triton-X 100 in 1X PBS, followed by nonspecific blocking with 5% BSA. Cells were then incubated with primary rabbit anti-VHH 647 antibody (Genscript Cat. No. A01994, 1:1000) for 30 minutes. Cells were washed with PBS, and nuclei were counterstained with DAPI before mounted with Prolong Gold antifade reagent. Finally, cells were imaged using a Perkin Elmer UltraVIEW spinning disk confocal microscope with a Zeiss 63x NA1.4 Plan-Apochromat objective lens with Volocity software. Images were linearly contrast enhanced equally across all images in Volocity.
Nanoparticle Formulation.
Nanoparticles were formulated with a microfluidic device as previously described20. Nucleic acids (DNA barcodes and mRNA) were diluted in 10mM citrate buffer (Teknova). Lipid-amine compounds (7C1, cKK-E12, and cKK-E15), PEG-lipids (1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol)-2000] and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol)-2000]), cholesterols (cholesterol and 20α-hydroxycholesterol), and helper lipids (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, 1,2-dioleoyl-3-trimethylammonium-propane, and 1,2-di-O-octadecenyl-3-trimethylammonium propane) were diluted in 100% ethanol. For mRNA screens, aVHH mRNA and DNA barcodes were mixed at a 10:1 mass ratio. All PEGs, cholesterols, and helper lipids were purchased from Avanti Lipids. Citrate and ethanol phases were combined in a microfluidic device by syringes (Hamilton Company) at a flow rate of 3:1.
DNA Barcoding.
Each LNP was formulated to carry its own unique DNA barcode. DNA barcodes were designed rationally with several characteristics as described21. All DNA barcodes were 91 nt long, single-stranded DNA sequences purchased from Integrated DNA Technologies. Briefly, the following modifications were on all barcodes: i) nucleotides on the 5’ and 3’ ends were modified with phosphorothioates to reduce exonuclease degradation, ii) universal forward and reverse primer regions were included to ensure equal amplification of each sequence, iii) 7 random nucleotides were included to monitor PCR bias, iv) a droplet digital PCR (ddPR) probe site was included for ddPCR compatibility, and v) a unique 8-nt barcode. An 8-nucleotide sequence can generate over 48 (65,536) distinct barcodes. We used only the 8-nucleotide sequences designed to prevent sequence bleaching and reading errors on the Illumina MiniSeq™ sequencing machine.
Nanoparticle Characterization.
LNP hydrodynamic diameter was measured using high-throughput dynamic light scattering (DLS) (DynaPro Plate Reader II, Wyatt). LNPs were diluted in sterile 1X PBS and analyzed. To avoid using unstable LNPs, and to enable sterile purification using a 0.22 μm filter, LNPs were included only if they met three criteria: diameter >20 nm, diameter <200 nm, and correlation function with 1 inflection point. Particles that met these criteria were pooled and dialyzed in a 20 kD dialysis cassette (Thermo Scientific) and a 100kD cassette (Thermo Scientific) in 1X PBS. The nanoparticle concentration was determined using NanoDrop (Thermo Scientific).
Animal Experiments.
Liver-xenograft FRG mice were generated and provided by Yecuris (Portland, Oregon) as described previously47. FRG KO on C57Bl/6 mice were repopulated with cryopreserved hepatocytes from either C57Bl/6 mice (murinized), rhesus macaques (primatized), or a human donor (HHM01008, a male 1-year-old donor). Balb/C (BALB/cJ), BL/6 (C57Bl/6J), and NZB (NZB/BlNJ) mice were acquired from Jackson Laboratories. All animal experiments were performed in accordance with the Georgia Institute of Technology’s IACUC. All animals were housed in the Georgia Institute of Technology Animal Facility. All mice were injected intravenously via the lateral tail vein with LNPs or 1X PBS.
Cell Isolation & Staining.
Cells were isolated 24 hours after injection with LNPs, unless otherwise noted. Mice were perfused with 20 mL of 1X PBS through the right atrium. Tissues were finely minced, and then placed in a digestive enzyme solution with Collagenase Type I (Sigma Aldrich), Collagenase XI (Sigma Aldrich) and Hyaluronidase (Sigma Aldrich) at 37 °C at 550 rpm for 45 minutes. Cell suspension was filtered through 70 μm mesh and red blood cells were lysed. Cells were stained to identify specific cell populations and sorted using the BD FacsFusion cell sorter at the Georgia Institute of Technology Cellular Analysis Core. The antibody clones used were anti-CD31 (390, BioLegend), anti-CD45.2 (104, BioLegend), anti-CD47 (CC2C6, BioLegend), and MonoRab™ Anti-Camelid VHH (96A3F5, GenScript). Representative flow gates are in Supplementary Fig. 1,2. PBS-injected mice were used to gate on aVHH positive populations.
Biodistribution Assay.
Biodistribution assays were executed using ddPCR as previously described 21. To summarize, DNA samples were prepared with 10 μL of ddPCR with ddPCR Supermix for Probes (Bio-Rad), 1 μL of primer and probe mix (solution of 10 μM target probe and 20 μM reverse/forward primers), 1 μL of template/TE buffer, and 8 μL of water. Twenty microliters of each reaction described and 70 μL of Droplet Generation Oil for Probes (Bio-Rad) were loaded into DG8 Cartridges (Bio-Rad) and covered with DG8 Gaskets (Bio-Rad). Using the QX200 Droplet Generator (BioRad), water–oil emulsion droplets were created. Cycle conditions for PCR were as follows: 1 cycle of 95 °C for 10 min followed by 40 cycles of 94 °C for 30 s, 60 °C for 1 min, and 1 cycle of 95 °C for 10 min. For each biological rep, 2-3 technical repetitions were completed. Unless stated otherwise, technical reps were averaged. Technical reps were only excluded if saturation was detected or there were inconsistent positive event amplitudes. The QX200 Droplet Digital PCR System (BioRad) was used to analyze all ddPCR results.
PCR Amplification.
All samples were amplified and prepared for sequencing using nested PCR. More specifically, 1 μL of primers (5 uM for Final Reverse/Forward) were added to 5 μL of Kapa HiFi 2X master mix (Roche), and 4 μL template DNA/water. During the second PCR Nextera XT chemistry, indices and i5/i7 adapter regions were added. Dual-indexed samples were run on a 2% agarose gel to ensure that PCR reaction occurred before being pooled and gel purified.
Deep Sequencing.
PCR samples were purified by AMPure XP beads. Final library QC was conducted using the Agilent Bioanalyzer 2100. Illumina deep sequencing was conducted on an Illumina MiniSeq™. Primers were designed based on Nextera XT adapter sequences.
Nanoparticle Data Analysis & Statistics.
Sequencing results were processed using a custom Python-based tool to extract raw barcode counts for each tissue. These raw counts were then normalized with an R script prior for further analysis. Counts for each particle, per tissue, were normalized to the barcoded LNP mixture we injected into the mouse. This “input” DNA provided the DNA counts and was used to normalize DNA counts from the cells and tissues. Statistical analysis was done using GraphPad Prism 8. Data is plotted as mean ± standard error mean unless otherwise stated.
Bulk RNA Sequencing Preparation.
A total of 10,000 cells were FACS sorted based on tissue and species-specific markers. Immediately after sorting, samples were flash frozen in an acetone/dry ice bath. Once all samples were collected, they were thawed on ice, lysed with 10x lysis buffer and vortexed for 30 seconds. One thousand cell equivalents of each biological replicate were pooled together and 2 uL (1000 cells of pool) of this mixture was used following the Smart-seq2 protocol as previously described 48. Each sample was normalized and pooled using Qubit, and library size was determined using a Bioanalyzer. Samples were sequenced using a NextSeq 550 instrument using the 150-cycle paired end high-throughput cartridge.
Bulk RNA Sequencing Analysis & Statistics.
Fastq files were trimmed and filtered based on quality and then mapped using the STAR aligner, and exon count tables were created using htseq-count. Transcripts were quantified with DESeq2. Genomes used for mapping were human (GRch38.9), mouse (GRCm38), or rhesus macaque (MMul10) based on sample species type. A control humanized/M and primatized/M were also mapped to the GRCm38 genome to compare native cell populations. Count tables were then used in iDEP.90 for normalization and analysis. Data were normalized using regularized log (rlog) calculation (Supplementary Fig. 10). Due to the high number of samples, biological replicates were pooled before sequencing, therefore increasing the total number of reads per conditions. To ensure the accuracy of our conclusions, we performed a separate analysis using CORNAS, a tool specifically developed for analyzing gene expression data without biological replicate. CORNAS uses the sequencing coverage information determined from the RNA concentration of each sample to estimate the posterior distribution of a true gene count43.
mRNA FISH Analysis.
Animals were injected through the lateral tail vein with 0.15 μg of aVHH mRNA LNPs. At 16 hours post-delivery, animals were sacrificed, perfused with 1x PBS, and livers were removed and incubated in 4% paraformaldehyde overnight at 4°C. Livers were incubated in 30% sucrose at 4°C for 24 hours, embedded in OCT medium, and processed into 10 μm frozen sections. Delivered aVHH mRNA and endogenous mRNA was visualized using RNAscope Multiplex Fluorescent Reagent Kit v2 (Advanced Cell Diagnostics 323136) according to manufacturer’s instructions. A custom probe set was designed against the synthetic aVHH mRNA sequence (ACD 879561). To distinguish engrafted human and rhesus cells within the mouse livers, species-specific probes were used. Human and mouse beta-2 microglobulin mRNA was used to positively identify human cells (ACD 484661-C3) and mouse cells (ACD 415191-C2), respectively. Rhesus ubiquitin C mRNA was used to identify rhesus cells (ACD 521081-C3). Images for cell-type staining were acquired using a Zeiss Plan-Apo 20x 0.8 NA air objective on an UltraVIEW Spinning Disk Confocal Microscope equipped with a Hamamatsu Flash 4.0v2 CMOS camera. Images were captured and processed using Volocity software (PerkinElmer).
Supplementary Material
Acknowledgments
The authors thank Karen E. Tiegreen at Georgia Tech, Sommer Durham at Georgia Tech, Robert Hughley at Georgia Tech. J.E.D. thanks Taylor E. Shaw and Jordan E. Cattie.
Funding
The work was funded by the National Institutes of Health (R01-GM132985, awarded to J.E.D.; UG3-TR002855, awarded to J.E.D. and P.J.S.) and DARPA (PREPARE grant number HR00111920008, awarded to P.J.S. and J.E.D.).
Footnotes
Competing Interests J.E.D. is a consultant for Beam Therapeutics and GV. All other authors declare no competing interests.
Data Availability
All RNA sequencing data have been deposited online at GEO (GSE178313). Scripts used to analyze barcodes are available at Github (https://github.com/Jack-Feldman/barcode_count). All other data are shown in the figures.
References
- 1.Adams D et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. The New England journal of medicine 379, 11–21 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Garrelfs SF et al. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. The New England journal of medicine 384, 1216–1226 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Balwani M et al. Phase 3 Trial of RNAi Therapeutic Givosiran for Acute Intermittent Porphyria. The New England journal of medicine 382, 2289–2301 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Ray KK et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N Engl J Med 382, 1507–1519 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Finkel RS et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. The New England journal of medicine 377, 1723–1732 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Baden LR et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New England Journal of Medicine (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polack FP et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine 383, 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahin U et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Nair JK et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc 136, 16958–16961 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Akinc A et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nature nanotechnology 14, 1084–1087 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Akinc A et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Molecular therapy: the journal of the American Society of Gene Therapy 18, 1357–1364 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willoughby JLS et al. Evaluation of GalNAc-siRNA Conjugate Activity in Pre-clinical Animal Models with Reduced Asialoglycoprotein Receptor Expression. Molecular therapy: the journal of the American Society of Gene Therapy 26, 105–114 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lisowski L et al. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature 506, 382–386 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulk NK et al. Bioengineered AAV Capsids with Combined High Human Liver Transduction In Vivo and Unique Humoral Seroreactivity. Mol Ther 26, 289–303 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vercauteren K et al. Superior In vivo Transduction of Human Hepatocytes Using Engineered AAV3 Capsid. Mol Ther 24, 1042–1049 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pei X et al. Development of AAV Variants with Human Hepatocyte Tropism and Neutralizing Antibody Escape Capacity. Molecular Therapy - Methods & Clinical Development 18, 259–268 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George LA et al. Hemophilia B Gene Therapy with a High-Specific-Activity Factor IX Variant. N Engl J Med 377, 2215–2227 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson EM et al. Extensive double humanization of both liver and hematopoiesis in FRGN mice. Stem Cell Res 13, 404–412 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foquet L et al. Successful Engraftment of Human Hepatocytes in uPA-SCID and FRG(®) KO Mice. Methods Mol Biol 1506, 117–130 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Chen D et al. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. J Am Chem Soc 134, 6948–6951 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Sago CD et al. Modifying a Commonly Expressed Endocytic Receptor Retargets Nanoparticles in Vivo. Nano letters (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sago CD et al. Barcoding chemical modifications into nucleic acids improves drug stability in vivo. J Mater Chem B 6, 7197–7203 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sago CD et al. Nanoparticles that deliver RNA to bone marrow identified by in vivo directed evolution. Journal of the American Chemical Society (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lokugamage MP, Sago CD & Dahlman JE Testing thousands of nanoparticles in vivo using DNA barcodes. Current Opinion in Biomedical Engineering 7, 1–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sago CD et al. High-throughput in vivo screen of functional mRNA delivery identifies nanoparticles for endothelial cell gene editing. Proceedings of the National Academy of Sciences (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiwari PM et al. Engineered mRNA-expressed antibodies prevent respiratory syncytial virus infection. Nature communications 9, 3999 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paunovska K et al. Nanoparticles Containing Oxidized Cholesterol Deliver mRNA to the Liver Microenvironment at Clinically Relevant Doses. Adv Mater 31, e1807748 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semple SC et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol 28, 172–176 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Dong Y et al. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America 111, 3955–3960 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dahlman JE et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat Nano 9, 648–655 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lokugamage MP et al. Mild Innate Immune Activation Overrides Efficient Nanoparticle-Mediated RNA Delivery. Advanced materials, e1904905 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paunovska K et al. Analyzing 2000 in Vivo Drug Delivery Data Points Reveals Cholesterol Structure Impacts Nanoparticle Delivery. ACS nano 12, 8341–8349 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel S et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nature communications 11, 983 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mui BL et al. Influence of Polyethylene Glycol Lipid Desorption Rates on Pharmacokinetics and Pharmacodynamics of siRNA Lipid Nanoparticles. Molecular therapy. Nucleic acids 2, e139 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaczmarek JC et al. Optimization of a Degradable Polymer-Lipid Nanoparticle for Potent Systemic Delivery of mRNA to the Lung Endothelium and Immune Cells. Nano Lett 18, 6449–6454 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kranz LM et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Cheng Q et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Compounds and compositions for intracellular delivery of therapeutic agents.
- 39.Gilleron J et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol 31, 638–646 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Wittrup A et al. Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat Biotechnol 33, 870–876 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel S et al. Boosting Intracellular Delivery of Lipid Nanoparticle-Encapsulated mRNA. Nano letters 17, 5711–5718 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ge SX, Son EW & Yao R iDEP: an integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC bioinformatics 19, 534 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Low JZB, Khang TF & Tammi MT CORNAS: coverage-dependent RNA-Seq analysis of gene expression data without biological replicates. BMC bioinformatics 18, 575 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang da W, Sherman BT & Lempicki RA Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols 4, 44–57 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Szklarczyk D et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res 45, D362–d368 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dobrovolskaia MA, Shurin M & Shvedova AA Current understanding of interactions between nanoparticles and the immune system. Toxicol Appl Pharmacol 299, 78–89 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azuma H et al. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nat Biotechnol 25, 903–910 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Picelli S et al. Full-length RNA-seq from single cells using Smart-seq2. Nature protocols 9, 171–181 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All RNA sequencing data have been deposited online at GEO (GSE178313). Scripts used to analyze barcodes are available at Github (https://github.com/Jack-Feldman/barcode_count). All other data are shown in the figures.