Abstract
Seventy-two Enterococcus faecium isolates of different origins highly resistant to nourseothricin and streptomycin were studied. Sequencing of a genomic fragment from two isolates identified a gene cluster, aadE–sat4–aphA-3, which has been isolated recently in staphylococci and Campylobacter coli. Patterns of digested PCR products of aadE–sat4–aphA-3 were identical for all isolates.
Resistance to streptothricin antibiotics has been reported from gram-negative bacteria following use of nourseothricin as an antimicrobial feed additive on industrial animal farms in the former East Germany (12, 16). Streptothricins are antibiotics consisting of a streptolidine ring, a gulosamine, and a polylysine side chain (6). Resistance is due to N acetylation of lysine (11) and is mediated in gram-negative bacteria via different streptothricin acetyltransferases, Sat1 to Sat4 (5, 8–10). In staphylococci, a (truncated) sat4 allele has been shown to be flanked by the aminoglycoside resistance genes aadE, encoding a 6′ adenyltransferase [AAD(6′)] conferring resistance to streptomycin, and aphA, encoding a 3′ phosphotransferase [APH(3′)-III] conferring resistance to kanamycin. This gene cluster was part of a transposon structure, Tn5405, found on the chromosomes of staphylococci (2–4). Previously, Tn5405 has been found in close proximity to an erm(B) gene cluster in different isolates of Staphylococcus intermedius (1). In this paper, we describe the aadE–sat4–aphA-3 gene cluster, which is identical to the one described by Boerlin et al. (1), disseminated among 72 multiresistant, independent isolates of Enterococcus faecium originating from different ecological origins.
MICs of nourseothricin were determined for 95 E. faecium isolates of different origins (14, 15). Susceptible isolates of Staphylococcus aureus NCTC 8325, Escherichia coli NCTC 10418, and E. faecium ATCC 19434 served as reference isolates (not shown in Table 1). All MIC tests were done by broth microdilution as described elsewhere (14), following instructions of the German Institute for Standards (Deutsches für Normen).
TABLE 1.
Seventy-two nourseothricin-resistant E. faecium isolates investigated in this study
| Isolate type | No. of isolates | Origin | Genotype (PCR)a |
|---|---|---|---|
| Animal and sewage | |||
| 7 | Poultry manure | vat(E), sat4, aadE-aphA, erm(B), cat | |
| 5 | Pig manure | vat(E), sat4, aadE-aphA, erm(B) | |
| 2 | Poultry meat | vat(E), sat4, aadE-aphA, erm(B) | |
| 4 | Sewage | vat(E), sat4, aadE-aphA, erm(B) | |
| Human | |||
| 10 | Stool samples from outpatients | vat(E) [8]/vat(D) [1]d, sat4, aadE-aphA, erm(B) | |
| 7 | Hospitalized patientsb | vat(E) [3]/vat(D) [1]d, sat4, aadE-aphA, erm(B) | |
| 37 | Hospitalized patientsc | vanA, sat4, aadE-aphA, erm(B) |
Gene names indicate a positive PCR product according to a resistance phenotype: aadE codes for streptomycin resistance, aphA codes for kanamycin resistance, cat codes for chloramphenicol resistance, erm(B) codes for macrolide-lincosamide-streptogramin B resistance, sat4 codes for streptothricin (nourseothricin) resistance, vanA codes for vancomycin resistance, and vat(D) and vat(E) codes for streptogramin A resistance. The number of positive isolates is given in brackets; if no number is indicated, all isolates were positive. Primers are given in the text or were according to reference 16.
From stool samples and colonizations.
14 from stool samples, 23 from infections.
Four isolates with an unknown genotype for streptogramin A resistance.
Preparation of samples and subsequent macrorestriction analysis was done in a CHEF II apparatus (Bio-Rad, Munich, Germany) as previously described (7) with the following modifications: agarose gel concentration was 1%, and ramped pulse times were 1 to 11 s for 13 h and then 11 to 30 s for 13 more h.
Genomic DNA of two nourseothricin-resistant E. faecium isolates, UW1965 and UW786, was digested with HindIII, and fragments were randomly cloned into pUC18. E. coli transformants were detected on agar plates supplemented with kanamycin (20 mg/liter) and ampicillin (50 mg/liter).
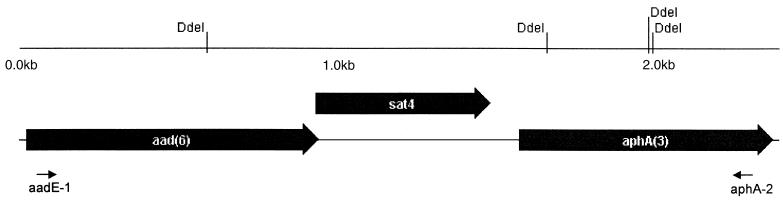
The aadE-sat4-aphA cluster and the sat4 gene were amplified by PCR with the use of the following primers: primers aadE-1 (identical to Ps1 in reference 4; 5′-GCAGAACAGGATGAACGTATTCG) and aphA-2 (identical to Pn2; 5′-CCCAATCAGGCTTGATCCCC) and primers sat4R (5′-GTTGGCGTATAACATAGTATCG) and sat4F (5′-CTGCGAAAAAATTGGAACC), respectively. PCR was done using ready-to-go beads from Amersham Pharmacia Biotech Inc. (Piscataway, N.J.), adding a 100 pM concentration of each primer and 10 ng of DNA. Cycle conditions included an annealing temperature of 50°C for a fragment of the aadE-sat4-aphA cluster and 55°C for a fragment of the sat4 gene. As a reference, the product for sat4 was compared with the corresponding PCR product amplified with DNA of plasmid pAT132 (5). Polymorphisms in the composition of the aadE-sat4-aphA resistance gene cluster were investigated by digesting purified PCR products (PCR Product Purification Kit; Qiagen, Hilden, Germany) with DdeI, an endonuclease having four sites in this fragment (see Fig. 2). Five microliters of the PCR product, 1× Dde buffer, 1× bovine serum albumin solution, and 10 U of DdeI (New England Biolabs, Frankfurt, Germany) in a final solution of 20 μl were digested for 2 h at 37°C. Ten microliters per sample was resolved on an 1% agarose gel.
FIG. 2.
Genetic structure of the three open reading frames (ORFs) from the sequenced fragments of E. faecium UW786 and UW1965. Putative amino acid sequence of ORF1 to -3 is shown in accordance with results from databank screens (100% identical to parts of GenBank accession number AF299292). Restriction endonuclease sites for DdeI (CTNAG) are shown. Small black arrows indicate the binding sites for the two internal primers, aadE-1 and aphA-2. The start codon of ORF2 (sat4) overlaps with the stop codon of ORF1 (aadE). The complete nucleotide composition and putative protein sequences are given in GenBank under accession number AF330699.
Filter mating experiments were done as described elsewhere (13). All donor strains were susceptible to rifampin (MICs ≤ 0.25 mg/liter) and fusidic acid (MICs of 1 to 2 mg/liter). Isolate 64/3 which is resistant to fusidic acid and rifampin (both MICs ≥ 128 mg/liter) was the recipient. Transconjugants were selected on brain heart infusion agar (Difco Laboratories, Detroit, Mich.) supplemented with nourseothricin (500 mg/liter) and rifampin (30 mg/liter). Transconjugants were checked for growth separately on agar plates with fusidic acid (20 mg/liter) and nourseothricin (500 mg/liter).
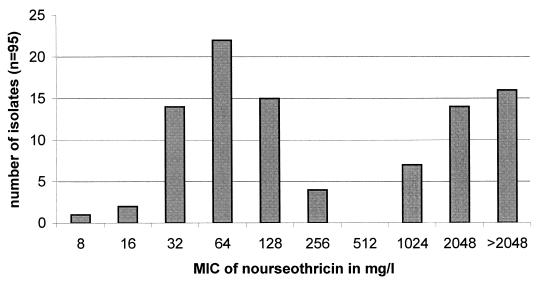
The E. coli and S. aureus isolates exhibited a nourseothricin MIC of 2 mg/liter, whereas the MIC for E. faecium ATCC 19434 was 64 mg/liter. All 95 E. faecium isolates showed a bimodal distribution of MICs (Fig. 1). According to Fig. 1 and to the results for the reference isolates, enterococcal isolates with nourseothricin MICs of ≥1,024 mg/liter were regarded as streptothricin resistant. E. faecium displays a higher level of intrinsic resistance to nourseothricin than S. aureus and gram-negative bacteria such as E. coli and Campylobacter coli (ca. 2 mg/liter).
FIG. 1.
Distribution of MICs of nourseothricin among 95 E. faecium isolates.
Among the above-mentioned 95 E. faecium isolates, 35 were nourseothricin resistant and high-level streptomycin resistant (MIC of streptomycin of ≥2,048 mg/liter). We also included 37 nonrelated, isolates resistant to high levels of streptomycin (and vancomycin) in our study which originated from our strain culture collection of human E. faecium isolates (Table 1). These isolates were also known to be nourseothricin resistant. Distribution of MICs of nourseothricin for all 72 nourseothricin-resistant E. faecium isolates were as follows: 1 isolate with a MIC of 128 mg/liter (PCR positive for sat4), 11 isolates with a MIC of 1,024 mg/liter, 30 isolates with a MIC of 2,048 mg/liter, and 30 isolates with a MIC of >2,048 mg/liter. Except for five isolates with a streptomycin MIC of 2,048 mg/liter, all the others exhibited a MIC of >2,048 mg/liter. All isolates were unrelated based on different antibiotic resistance phenotypes and macrorestriction patterns resolved by pulsed-field gel electrophoresis (14; unpublished data).
Cloned genomic DNA fragments of E. faecium UW1965 and UW786, which encoded kanamycin resistance in E. coli transformants, revealed identical nucleotide sequences (GenBank accession number AF330699). Three open reading frames were identified which showed almost complete identity with the resistance genes aadE, sat4, and aphA-3 (Fig. 2). All putative proteins from sat4 of C. coli BE/G4 (5), S. aureus BM3505 (3), and the two enterococcal isolates described here consist of 180 amino acids. Due to a point mutation at nucleotide 949 (A→G) in the two sequences described here, as well as at the corresponding position in the staphylococcal sat4 gene on plasmid pIP1718 in S. aureus BM3505, the amino acid Glu (GAG) at position eight in the putative C. coli protein is changed to Gly (GGG). This indicates a close relationship between the sat4 alleles in this Staphylococcus and in the two investigated enterococcal isolates. The complete nucleotide sequence of the aadE–sat4–aphA-3 cluster of UW786 and UW1965 showed 100% identity with a cluster which has been isolated recently from different canine S. intermedius isolates (1). In contrast to this, a number of corresponding clusters in other staphylococci possessed a truncated and nonfunctional sat4 allele (3, 4). In most staphylococci, the gene cluster of aadE–sat4–aphA-3 is integrated into a transposon structure, Tn5405, flanked by two copies of the IS element IS1182. Whether the aadE–sat4–aphA-3 cluster in the described enterococcal isolates is also integrated into a Tn5405-like element has not yet been investigated and is the subject of ongoing studies.
Arrangement of resistance genes in the other 70 E. faecium isolates was investigated by PCR for aadE–sat4–aphA-3 followed by a restriction digestion with endonuclease DdeI. All the isolates investigated showed an identical pattern, suggesting a highly conserved gene cluster among nonrelated E. faecium of different origins (not shown).
Four of five nourseothricin-resistant E. faecium transferred their nourseothricin resistance determinant into a recipient isolate, 64/3 (mating frequencies from 6.36 × 10−8 to 1.44 × 10−3 per recipient). One transconjugant per mating experiment was characterized in more detail. All transconjugants were resistant to fusidic acid (MIC ≥ 16 mg/liter) and rifampin (MIC ≥ 4 mg/liter) and to quinupristin-dalfopristin, erythromycin, clindamycin, oxytetracycline (all MICs ≥ 8 mg/liter), as well as to high levels of nourseothricin and streptomycin (MIC ≥ 2,048 mg/liter). A PCR for the sat4 gene and the aad–sat4–aphA-3 cluster using genomic DNA from the transconjugants gave positive results for both fragments (not shown). The transconjugants exhibited SmaI macrorestriction patterns that were different from those of the donors and related to the pattern of recipient 64/3 (not shown).
It remains unclear why enterococci still harbor resistance determinants against antibiotics that were not or only rarely used for treating enterococcal infections. Streptothricins have never been used for veterinary or human therapy in Middle European countries. A selective pressure resulting from use of aminoglycosides is more reasonable. However, the standard aminoglycoside treatment for enterococcal infections in humans in Europe involves gentamicin, for which the genes aadE and aphA-3 do not confer resistance. It is still not known if the described gene cluster is integrated into a larger composite element, where other as-yet-unknown determinants may promote dissemination among enterococci. This hypothesis is being investigated.
Nucleotide sequence accession number. The identical genomic fragments of E. faecium UW786 and UW1965 have been assigned GenBank accession no. AF330699.
Acknowledgments
This work was partly supported by grants from the Federal Ministry for Health and the Federal Office for the Environment.
REFERENCES
- 1.Boerlin P, Burnens A P, Frey J, Kuhnert P, Nicolet J. Molecular epidemiology and genetic linkage of macrolide and aminoglycoside resistance in Staphylococcus intermedius of canine origin. Vet Microbiol. 2001;79:155–169. doi: 10.1016/s0378-1135(00)00347-3. [DOI] [PubMed] [Google Scholar]
- 2.Derbise A, Dyke K G H, El Solh N. Characterization of a Staphylococcus aureus transposon, Tn5405, located within Tn5404 and carrying the aminoglycoside resistance genes, aphA-3 and aadE. Plasmid. 1996;35:174–188. doi: 10.1006/plas.1996.0020. [DOI] [PubMed] [Google Scholar]
- 3.Derbise A, de Cespedes G, El Solh N. Nucleotide sequence of a Staphylococcus aureus transposon, Tn5405, carrying aminoglycoside resistance genes. J Basic Microbiol. 1997;37:379–384. doi: 10.1002/jobm.3620370511. [DOI] [PubMed] [Google Scholar]
- 4.Derbise A, Aubert S, El Solh N. Mapping the regions carrying three contiguous antibiotic resistance genes aadE, sat4, and aphA-3 in the genomes of staphylococci. Antimicrob Agents Chemother. 1997;41:1024–1032. doi: 10.1128/aac.41.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacob J, Evers S, Bischoff K, Carlier C, Courvalin P. Characterization of the sat4 gene encoding a streptothricin acetyltransferase in Campylobacter coli BE/G4. FEMS Microbiol Lett. 1994;120:13–17. doi: 10.1111/j.1574-6968.1994.tb07000.x. [DOI] [PubMed] [Google Scholar]
- 6.Khokhlov A S, Shutova K J. Chemical structure of streptothricins. J Antibiot (Tokyo) 1972;25:501–508. doi: 10.7164/antibiotics.25.501. [DOI] [PubMed] [Google Scholar]
- 7.Klare I, Heier H, Claus H, Reissbrodt R, Witte W. vanA-mediated high-level glycopeptide resistance in Enterococcus faecium from animal husbandry. FEMS Microbiol Lett. 1995;125:165–172. doi: 10.1111/j.1574-6968.1995.tb07353.x. [DOI] [PubMed] [Google Scholar]
- 8.Tietze E, Brevet J, Tschaepe H. Relationship among the streptothricin resistance transposon Tn1825 and Tn1826 and the trimethoprim resistance transposon Tn7. Plasmid. 1987;18:246–249. doi: 10.1016/0147-619x(87)90067-9. [DOI] [PubMed] [Google Scholar]
- 9.Tietze E, Brevet J, Tschaepe H, Voigt W. Cloning and preliminary characterization of the streptothricin resistance determinants of the transposons Tn1825 and Tn1826. J Basic Microbiol. 1988;28:129–136. doi: 10.1002/jobm.3620280116. [DOI] [PubMed] [Google Scholar]
- 10.Tietze E, Brevet J. Nucleotide sequence of the bacterial streptothricin resistance gene sat3. Biochim Biophys Acta. 1995;1263:176–178. doi: 10.1016/0167-4781(95)00103-n. [DOI] [PubMed] [Google Scholar]
- 11.Tschäpe H, Tietze E, Prager R, Voigt W, Wolter E, Seltmann G. Plasmid borne streptothricin resistance in gram-negative bacteria. Plasmid. 1984;12:189–196. doi: 10.1016/0147-619x(84)90043-x. [DOI] [PubMed] [Google Scholar]
- 12.Tschäpe H. The spread of plasmids as a function of bacterial adaptability. FEMS Microbiol Ecol. 1994;15:23–32. [Google Scholar]
- 13.Werner G, Klare I, Witte W. Arrangement of the vanA gene cluster in enterococci of different ecological origin. FEMS Microbiol Lett. 1997;155:55–61. doi: 10.1111/j.1574-6968.1997.tb12685.x. [DOI] [PubMed] [Google Scholar]
- 14.Werner G, Klare I, Heier H, Hinz K-H, Böhme G, Wendt M, Witte W. Quinupristin/dalfopristin-resistant enterococci of the satA (vatD) and satG (vatE) genotypes from different ecological origins in Germany. Microb Drug Resist. 2000;6:37–47. doi: 10.1089/mdr.2000.6.37. [DOI] [PubMed] [Google Scholar]
- 15.Werner G, Hildebrandt B, Klare I, Witte W. Linkage of determinants for streptogramin A, macrolide-lincosamide-streptogramin B, and chloramphenicol resistance on a conjugative plasmid in Enterococcus faecium and dissemination of this cluster among streptogramin-resistant enterococci. Int J Med Microbiol. 2000;290:543–548. doi: 10.1016/S1438-4221(00)80020-X. [DOI] [PubMed] [Google Scholar]
- 16.Witte W, Tschaepe H, Klare I, Werner G. Antibiotics in animal feed. Acta Vet Scand. 2000;93(Suppl.):37–45. [PubMed] [Google Scholar]