Abstract
Objective
Royal demolition explosive (RDX) can induce seizures in wildlife and humans following release into the environment or after voluntary consumption. During the Vietnam War, RDX intoxication was the most common cause of generalized seizures in US service personnel, and in some sections of the armed forces, eating of RDX has continued as “a dare” to this day. After its mechanism of action was long unknown, RDX was recently shown to be a GABAA receptor antagonist. We here determined the GABAA receptor subtype‐selectivity of RDX and mapped its functional binding site.
Methods
We used whole‐cell patch‐clamp to determine the potency of RDX on 10 recombinantly expressed GABAA receptors and mapped the RDX binding site using a combination of Rosetta molecular modeling and site‐directed mutagenesis.
Results
RDX was found to reversibly inhibit the α1β2γ2 GABAA receptor with an IC50 of 23 μmol/L (95% CI 15.1–33.3 μmol/L), whereas α4 and α6 containing GABAA receptor combinations were 4–10‐fold less sensitive. RDX is binding to the noncompetitive antagonist (NCA) site in the pore. In a molecular model based on the cryo‐EM structure of the resting state of the α1β2γ2 receptor, RDX forms two hydrogen bonds with the threonines at the T6’ ring and makes hydrophobic interactions with the valine and alanine in 2′ position of the α1 or β2 subunits.
Interpretation
Our findings characterize the mechanism of action of RDX at the atomistic level and suggest that RDX‐induced seizures should be susceptible to treatment with GABAA modulating drugs such as benzodiazepines, barbiturates, propofol, or neurosteroids.
Introduction
The high‐energy explosive hexahydro‐1,3,5‐trinitro‐1,3,5‐triazine, also known as cyclonite or RDX (Royal Demolition Explosive), is the major component of plastic explosives such as composition C‐4 which contains ~90% RDX and ~10% plasticizers. 1 Without a detonator, RDX is relatively insensitive to impact and can be transported and even burned without exploding. Due to these properties, RDX has been extensively employed in every major war since World War II and is also widely used for civilian purposes. In 2018 RDX had an estimated market of $10.4 billion. 2 RDX release as waste during manufacture, via explosion of ordinances, and during disposal of munitions constitutes an environmental concern for exposure of both humans and wildlife. 2 , 3 At 32 Environmental Protection Agency (EPA) listed National Priority Sites, RDX has been found to leach into soil and groundwater and, in some cases, to induce seizures in fish, rodents, lizards, or birds. 3 , 4 , 5 There also is suggestive evidence of carcinogenic potential based on an EPA assessment performed in 2018. 3
In addition to these chronic environmental exposures, acute RDX intoxications have occurred in workers in ammunition factories, in soldiers who used RDX as cooking fuel or voluntarily consumed it. 1 , 6 Following oral or inhalation exposure, RDX causes dizziness, headaches, vomiting, and confusion and, at high doses, generalized tonic–clonic seizures. During the Vietnam War, it apparently was common knowledge among field troops that small quantities of RDX would produce a “high” similar to ethanol. 1 When gram quantities were consumed with standard issued beer, soldiers supposedly could achieve increased inebriation, but often went on to develop seizures that required hospitalization. In a report from 1969, army physicians recognized RDX intoxication as the most common cause of generalized seizures in US service personnel in Vietnam. 1 “Eating” of RDX has continued in some sections of the armed services to this day as “a dare” or rite of passage. 7 For example, in 2019, a 22‐year‐old active‐duty male who was training with explosives was documented to exhibit status epileptics with RDX plasma concentrations of 3.06 μg/mL following consumption of a piece of C‐4. 8 Another case of a 20‐year old combat engineer on a demolition range eating C‐4 under peer pressure was reported as we were writing this paper. 9
The molecular mechanism of RDX action was long unknown, and its elucidation was initially confused by anecdotal reports that RDX increased salivation and lacrimation 10 similar to organophosphate pesticides or nerve agents targeting acetylcholine esterase (AChE). In a seminal study in 2011, Williams et al. clarified this question by demonstrating that RDX administration to rats does not result in salivation or inhibition of brain AChE. 11 Instead, RDX was shown to displace [35S]‐t‐butylbicyclophosphoorothothionate (TBPS) in rat brain membrane preparations with a K i of 21 μmol/L and to inhibit GABA‐induced currents in basolateral amygdala neurons. This suggested that RDX is binding to the noncompetitive antagonist (NCA) site in the pore of GABAA receptors similar to picrotoxinin. 11 Using molecular modeling and site‐directed mutagenesis, we here confirm that RDX is binding to the NCA site in the pore of GABAA receptors, specifically to the so‐called threonine ring where all five subunits of the GABAA receptor channel contain a pore lining threonine in 6′ position in the M2 segment. In whole‐cell patch‐clamp experiments with 10 recombinantly expressed GABAA receptors, we also determined the subtype‐selectivity of RDX and found that it blocks α1β2γ2, the most common GABAA receptor combination in the mammalian CNS, with an IC50 of 23 μmol/L in a fully reversible manner. Our findings thus characterize the mechanism of action of RDX at the molecular level and suggest that RDX‐induced seizures should be susceptible to treatment with GABAA modulating drugs such as benzodiazepines, neurosteroids, propofol, or barbiturates.
Materials and Methods
Chemicals
RDX (Hexahydro‐1,3,5‐trinitro‐1,3,5‐triazine) was purchased as certified reference material (Cerilliant® from MilliporeSigma) in a 1‐mg/mL solution in acetonitrile (1 mL ampules, chromatographic purity 99.9%). For electrophysiological experiments, the acetonitrile was evaporated and RDX immediately reconstituted in 1 mL DMSO rendering 0.1 molar stocks. RDX waste was treated with nitric acid and disposed of using the waste accumulation program at UC Davis. Bicuculline, picrotoxinin, and GABA were purchased from MilliporeSigma. Diazepam was purchased from Tocris Bioscience.
Clones and cell lines
The sources and recombinant expression of the human GABAA receptors α1, α2, α4, α6, β1, β3, γ1, γ2L, and δ and the rat GABAA receptor β2 were previously described. 12 Briefly, GABAA receptors were expressed in L929 cells, a mouse fibroblast cell line (CCL‐1, American Type Culture Collection, Manassas, VA). Cells were transfected using FuGENE 6 (ThermoFisher) transfection reagent with an equal amount of each of the subunits (1:1:1) in combination with green fluorescent protein (GFP) expressed from the pEGFP‐C1 vector (Invitrogen). Cells were detached by trypsinization 48 h post‐transfection, washed, and plated onto poly‐L‐lysine coated glass coverslips. Transfected cells were identified as GFP‐expressing cells, using an epifluorescence microscope for the whole‐cell voltage‐clamp studies. Correct subunit composition of the various GABAA receptors was tested by their sensitivity to diazepam, propofol, allopregnanolone, DS2, fipronil, bicuculline, and Zn2+ as previously described. 12
Electrophysiology
Whole‐cell voltage‐clamp experiments were performed with an EPC‐10 HEKA amplifier (HEKA Elektronik) as previously described. 12 Cells were bathed in an external Ringer solution consisting of 160 mmol/L NaCl, 4.5 mmol/L KCl, 1 mmol/L MgCl2, 2 mmol/L CaCl2, 10 mmol/L HEPES, pH 7.4, 310 mOsm. Recording electrodes were pulled from soda lime glass micro‐hematocrit tubes (Kimble Chase, Rochester, NY) and fire‐polished to resistances of 1.6–2.9 MΩ. Electrodes were filled with an internal solution consisting of 154 mmol/L KCl, 2 mmol/L CaCl2, 1 mmol/L MgCl2, 10 mmol/L HEPES and 10 mmol/L EGTA, pH 7.2, and 301 mOsm. Cells were voltage‐clamped at −80 mV, and currents recorded under the local 5‐sec applications of varying GABA concentrations to the patch‐clamped cell using an 8‐channel pinch valve controlled gravity perfusion system (VC3‐8xG system, ALA Scientific). The GABA concentration–response relationships for the GABAA receptor subunit combinations used here were previously published by our group, 12 and the blocking potency of RDX was tested at the respective GABA EC90. RDX additions and washes were performed through a separate, syringe‐driven perfusion system and with a volume (2 mL) that exchanged the chamber volume five times. Varying concentrations of RDX were allowed to sit for 3 min on the cell before re‐application of EC90 GABA directly onto the cell through the gravity perfusion system. For testing or washing out of RDX concentrations of 100 μmol/L or more we added 1% of DMSO to the Ringer solution to keep RDX in solution. One cell was used per concentration. Cells that became leaky during the experiment or that did not produce the same magnitude of response to EC90 GABA twice before the experiment and after washout of RDX were excluded from the analysis. For analysis of current blockade, the area under the current curve (AUCMax) was determined for the control (EC90 GABA) and the AUCEx after exposure. [AUCEx]/[AUCMax] × 100 = % Current Blocked. Data analysis and data fitting to the Hill equation to obtain IC50 values was performed using GraphPad Prism8 (GraphPad Software, La Jolla, CA). Individual data points are presented as mean ± SD from 5 to 8 independent recordings. IC50 values are presented with 95% confidence intervals. For screening of mutant channels 100 μmol/L RDX and EC90 GABA were used to evaluate if the mutation affected RDX potency. Percentage of current blocked (mean ± SD from n = 5–8 cells per mutant) was analyzed with one‐way ANOVA followed by Dunnett's test to compare the means to the WT control and to correct for multiple comparisons. *p < 0.05, **p < 0.01, ***p < 0.001.
Molecular modeling
Using the Rosetta molecular modeling suite 13 with membrane environment‐specific energy functions 14 , 15 we generated a model of the α1β2γ2 GABAA receptor in the resting state based on the cryo‐EM structure of the α1β3γ2 receptor 16 with picrotoxinin bound (pdb id: 6X40). Structural refinement and docking of RDX with the RosettaLigand application 17 using the Talaris2014 energy function was performed as previously described in detail. 18 Molecular graphics were rendered with the UCSF Chimera software (Resource for Biocomputing, Visualization, and Informatics, San Francisco, CA) (Pettersen et al., 2004). Protein Data Bank (pdb) format files of the model are available upon request.
Mutagenesis
Mutagenesis primers (20–30 base pairs in length, with a 5–8 base pair overhang on the 3′ end) were designed with PrimerX software (http://www.bioinformatics.org/primerx), synthesized by ThermoFisher, and mutagenesis performed as previously described. 18 Mutant sequences were confirmed via sequencing using ABI 3730 Capillary Electrophoresis Genetic Analyzers (UC Davis DNA Sequencing Facility). Mutant α1β2γ2 GABAA receptors were deemed functional if they produced at least 200 pA of current in response to 100 μmol/L GABA and were sensitive to positive modulation by diazepam. The following mutants did not produce functional currents in our hands: α1L9’F, α1L9’C, β2A2’E, β2L9’Q, β2L9’F, β2L9’G, and β2L9’V.
Plasma protein binding
RDX plasma protein binding was determined with rat plasma in triplicate using rapid equilibrium devices with a molecular weight cutoff of 8 kD (RED, Fisher Scientific). RDX concentrations were determined by liquid chromatography using Hewlett Packard 1100 series HPLC equipped with a C‐18 Zorbax Eclipse XDB column (5 μm, 4.6 × 150 mm, Agilent) and an isocratic mobile phase consisting of 50/50 water/acetonitrile for 6.0 min at 25°C. RDX had a retention time of 3.1 min, and UV absorption was monitored 234 nm.
Results
RDX displays selectivity for α1β2γ2 among recombinantly expressed GABAA receptors
We previously recombinantly expressed and characterized a panel of synaptic and extrasynaptic GABAA receptor subtype combinations. 12 Correct incorporation of γ and δ subunits was confirmed by their respective sensitivity to diazepam, propofol, allopregnanolone, DS2, fipronil, bicuculline, and Zn2+. 12 The GABA concentration–response curves were in good agreement with literature. 12 We here used these established assays to study RDX. For each subtype combination, increasing concentrations of RDX were tested for their ability to inhibit chloride currents elicited by the GABA EC90 for this respective receptor subtype in whole‐cell patch‐clamp experiments. RDX was found to be most potent on the α1β2γ2 subtype combination (Fig. 1) which was blocked with an IC50 of 22.9 μmol/L (95% CI 15.1–33.3 μmol/L, Emax 95%). RDX blocked GABAA receptors consisting of only α1β2 with slightly higher potency (IC50 13.2 μmol/L) but reduced efficacy (Emax ~70%) and was 3–10‐fold less potent on α1 receptor combinations containing β1 or β3 subunits (Fig. 1). GABAA receptors containing α4 and α6 subunits (either with γ or δ subunits) were 4–8‐fold less sensitive to RDX than α1β2γ2 receptors (Fig. 1), while there was no significant selectivity over α2β3γ2 receptors.
Figure 1.
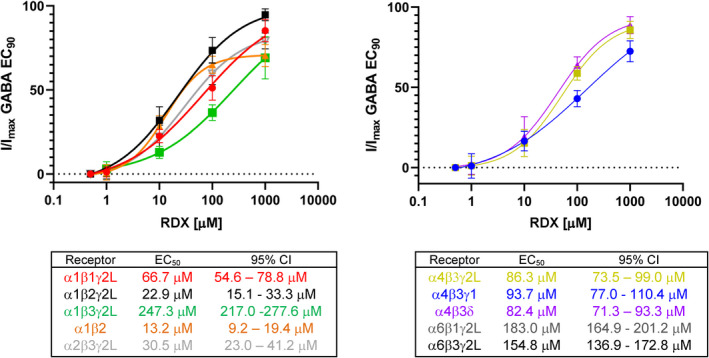
Concentration–response curves and IC50 values for RDX‐mediated inhibition of currents evoked by EC90 GABA for α1 and α2 (left) or α4 and α6 (right) containing GABAA receptors. Data points are mean ± SD from 3 to 8 independent recordings. IC50 values are presented with 95% confidence intervals. The EC90 GABA concentration was 110 μmol/L for α1β1γ2L, 100 μmol/L for α1β2γ2L, 60 μmol/L for α1β3γ2L, 40 μmol/L for α1β2, 50 μmol/L for α2β3γ2L, 10 μmol/L for α4β3γ2L, 10 μmol/L for α4β3γ1, 5 μmol/L for α4β3δ, 15 μmol/L for α6β1γ2L, and 10 μmol/L for α6β3γ2L. [Colour figure can be viewed at wileyonlinelibrary.com]
RDX is a fully reversible GABAA receptor blocker
We next probed the mechanism of RDX inhibition in more detail. When determining the subtype‐selectivity of RDX, we had observed that RDX was more potent when GABAA receptors were preincubated with RDX before GABA application than if GABA and RDX were applied simultaneously. As shown in Figure 2A, if α1β2γ2L receptors were first activated by application of 100 μmol/L GABA directly to the patch‐clamped cell to elicit a control current and RDX was then perfused into the recording chamber following washout of GABA, reapplication of GABA after 3 min of incubation with RDX induced a smaller current with virtually no further enhancement of current decay during the 5‐sec duration of the GABA application. RDX inhibition was fully reversible by a washout with 2 mL of external Ringer solution. With these fast kinetics, especially the quick washout, RDX resembles the pore blockers picrotoxinin 16 , 19 and TETS 18 suggesting that it could be binding to the NCA site in keeping with its ability to displace [35S]‐TBPS in binding assays with rat brain membrane preparations. 11 In order to confirm that RDX is indeed a noncompetitive antagonist we next compared the effect of RDX and the competitive antagonist bicuculline on the GABA concentration–response curve for the α1β2γ2L receptor (Fig. 2B). While bicuculline shifted the curve to the right without decreasing the maximum GABA response in keeping with its binding to the same site as GABA, 100 μmol/L of RDX drastically reduced the maximum response elicited by 50 μmol/L or even 1 mM GABA demonstrating that RDX indeed behaves like a NCA that cannot be competed off by GABA.
Figure 2.
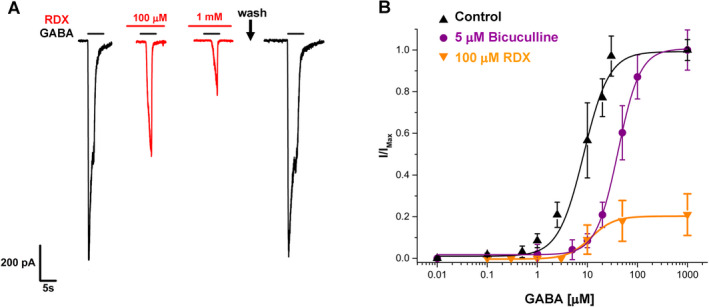
RDX is a reversible, noncompetitive inhibitor of the α1β2γ2L GABAA receptor. (A) Example recording showing that RDX is reversible on washout. Currents were elicited by 100 μmol/L GABA. (B) Comparison of the effects of bicuculline (5 μmol/L) and RDX (100 μmol/L) on the GABA concentration–response curve. Data points are mean ± SD from 3 to 8 independent recordings. [Colour figure can be viewed at wileyonlinelibrary.com]
RDX positively enhances GABA activity at low concentrations
Interestingly, in addition to blocking GABA‐induced currents through the α1β2γ2 receptor at high μmol/L concentrations, RDX potentiates currents induced by GABA EC90 at low concentrations (Fig. 3A). This GABA potentiating effect occurred in a concentration range of between 500 nmol/L and 2 μmol/L as shown in Figure 3B and in Figure S1, which contrasts the biphasic effects of RDX with the straightforward inhibitory effects of picrotoxinin. A full GABA concentration–response curve, obtained in the presence of 1 μmol/L RDX, revealed that the potentiating effects of RDX were most pronounced at saturating GABA concentrations (Fig. 3C). Low‐concentration RDX thus differs in its behavior from benzodiazepines and somewhat resembles the neurosteroid allopregnanolone. However, unlike allopregnanolone, which changes from being a positive allosteric modulator (PAM) to being a direct activator at higher concentrations, higher concentrations of RDX have no effect in the absence of GABA and block GABA‐induced currents in the presence of GABA (Figs. 1 and 2). A similar GABA potentiating effect at low RDX concentrations was observed on all other tested GABAA receptor combinations including the α4β3δ receptor (Fig. S1).
Figure 3.
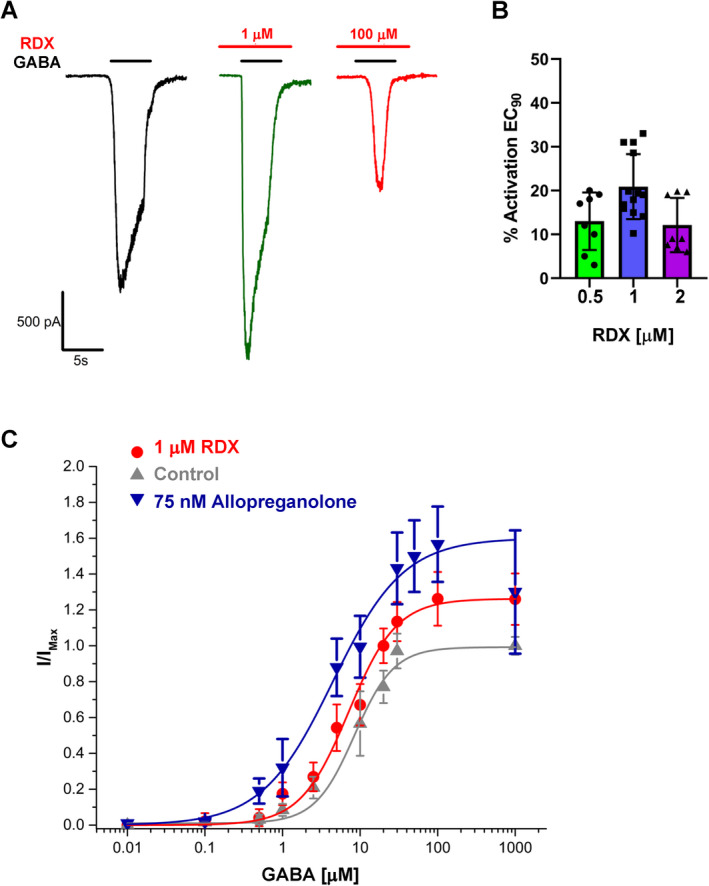
RDX enhances GABA effects at low concentrations. (A) Example recording showing that 1 μmol/L of RDX enhances chloride currents elicited by 100 μmol/L GABA while 100 μmol/L RDX inhibits currents. (B) Scatterplots showing the percentage of current activation by 0.5, 1, and 2 μmol/L of RDX (n = 8 to 13 cells, error bars are ±SD). (C) Comparison of the effects of allopregnanolone (75 nmol/L) and RDX (1 μmol/L) on the GABA concentration–response curve of the α1β2γ2L GABAA receptor. Data points are mean ± SD from 3 to 8 independent recordings. [Colour figure can be viewed at wileyonlinelibrary.com]
Docking of RDX into a model of the α1β2γ2 GABAA receptor with the RosettaLigand method
We next decided to probe the mechanism of RDX action at the atomistic level by docking RDX into a model of the resting state of the α1β2γ2 GABAA receptor with the RosettaLigand application. Our model was based on the recently published cryo‐electron microscopy structure of the human α1β2γ2 receptor in lipid nanodiscs. 16 The receptor had been captured in multiple states including a probably resting state structure with picrotoxinin in the pore at a resolution of 2.86 Å (pdb id: 6X40). We removed picrotoxinin, refined the structure, allowed the side chains to relax, and then docked RDX in 50,000 random starting positions in the pore. Rosetta identified a binding site in the permeation pathway (Fig. 4A) with two alternative, frequently sampled, and low‐energy binding poses (Fig. 4B and C). In both poses, RDX is accepting two hydrogen bonds from the T6’ ring (residues are counted starting from the cytoplasmic entry to the pore) where all five subunits have a threonine at the same position in their highly homologous M2 segments. In the first binding pose, RDX is positioned somewhat “higher up” and is making hydrophobic van der Waals interactions with the leucines at the L9’ ring and accepting two hydrogen bonds at the T6’ ring from the α1 and γ2 subunits, which are colored blue and yellow, respectively, in Figure 4B. In the second binding pose, RDX is hydrogen bonding with two other threonine residues at the T6’ ring, β2T6’, and α1T6’, while one nitro‐group is turned “down” toward the 2′ ring where it is interacting with α1V2′ and β2A2’.
Figure 4.
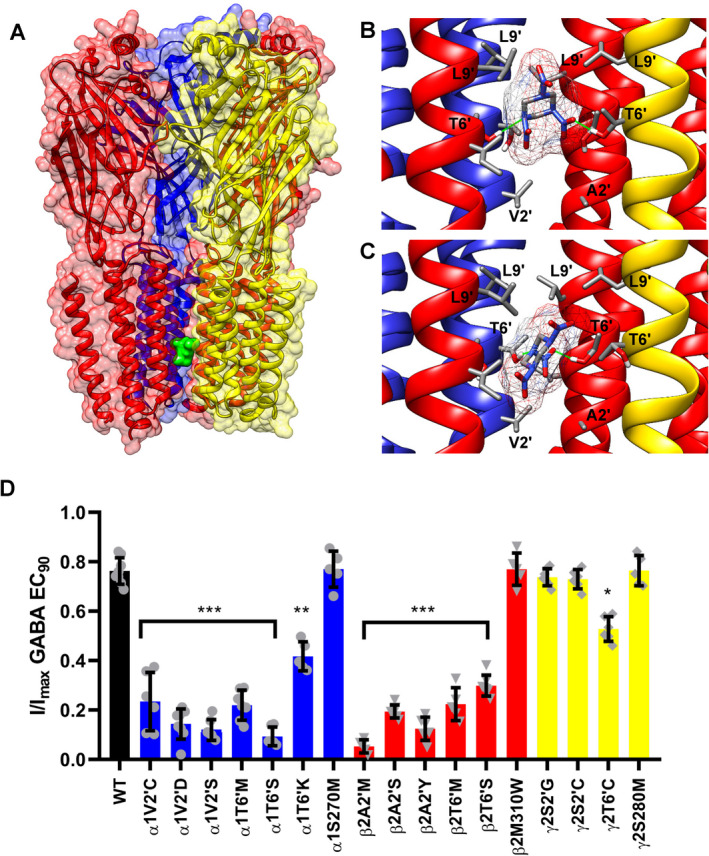
The RDX binding site. (A) Molecular model of the RDX binding site in the pore of the α1β2γ2 receptor identified of RosettaLigand. The receptor is color‐coded as follows: α1 (blue), β2 (red), and γ2 (yellow). RDX is shown as a green space‐filled model. One α1 subunit is removed to allow a view of the permeation pathway. (B and C) Closeup views of the two alternative, low‐energy binding poses of RDX at T6’ ring. RDX is shown in stick representation, hydrogen bonds are shown in green. (D) Site‐directed mutagenesis of the α1β2γ2L receptor. Percentage of current blocked by 100 μmol/L RDX (mean ± SD from n = 5–8 cells per mutant) was analyzed with one‐way ANOVA followed by Dunnett's test to compare the means to the WT control and to correct for multiple comparisons. *p < 0.05, **p < 0.01, ***p < 0.001. [Colour figure can be viewed at wileyonlinelibrary.com]
RDX exerts its inhibitory activity by binding to the T6′ ring of the α1β3γ2 GABAA receptor
In order to determine whether RDX is interacting with any of the residues identified by RosettaLigand we mutated the respective residues as well as control residues in the α1β2γ2L GABAA receptor and compared the ability of RDX at 100 μmol/L, which corresponds to IC80, to block chloride currents carried by wild‐type and mutant channels (Fig. 4D). When interpreting the results, it should be kept in mind that mutations in the α or β subunit always introduce changes in two of the five subunits of the heteropentameric channel consisting of two α, two β, and one γ subunit. We studied a total of 17 mutations. For the α1 subunit we made seven mutation: six mutations in the NCA site (V2’C, V2’D, V2’S, T6’M, T6’K, and T6’S) and one mutation (S270M) outside of the pore. For the β2 subunit, we studied a total of six mutants: T6’S, A2’M, A2’Y, A2’S, T6’M, and M310W. All α1 or β2 mutations at the 2′ and 6′ ring significantly reduced the response to 100 μmol/L RDX (Fig. 4D). For the γ2 subunit, which only contributes one subunit to the pentameric receptor, we made four mutations: S2’C, S2’G, and T6’C and S280M. While mutating S2′ had no effect, the γ2T6’C mutant reduced RDX potency, but not as drastically as the α1 or β2 T6′ mutations in keeping with the fact that γ2 only contributes one residue at the 2′ and 6’ ring. Figure 5 shows representative currents and concentration–response curves from the α1T6’M, β2A2’M, and γ2S2’G mutants. Our modeling had also shown possible interactions for RDX at the L9′ ring, but unfortunately all the L9′ mutations we were able to generate failed to express functional channels. Control mutations at the top of the TMD domain in the M3‐M1 interfaces in the phenobarbital binding site 16 did not affect RDX potency (Fig. 4D). Taken together, our mutagenesis studies demonstrate that RDX is binding to the NCA site in the pore of the α1β2γ2 GABAA receptor and confirm the RosettaLigand predictions of molecular interactions at the 2′ and 6′ ring.
Figure 5.
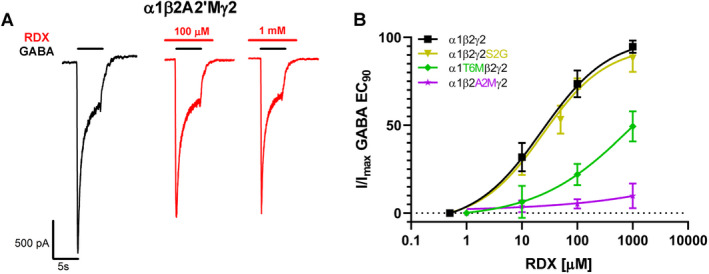
Representative examples from the mutagenesis. (A) The β2A2’M mutant is insensitive to RDX. Currents were elicited by 100 μmol/L GABA. (B) RDX concentration–response curves for the wild‐type α1β2γ2L receptor and the α1T6’M, β2A2’M, and γ2S2’G mutants. Data points are mean ± SD from 3 to 8 independent recordings. [Colour figure can be viewed at wileyonlinelibrary.com]
While we were thus able to identify the site of action for the inhibitory activity of RDX, our attempts of identifying the site mediating its GABA potentiating activity at low concentrations failed. None of the mutations reducing the inhibitory effect of RDX affected its potentiator activity at low concentrations (data not shown) indicating that the potentiator effect is mediated through a different site. However, none of the mutations that are known to render propofol or barbiturates (αS270M, β2M310W, and γ2LS280W) less active or inactive, 20 reduced the activating activity of RDX in our hands suggesting that RDX is exerting its GABA potentiating activity through a novel site, which we were not able to identify through molecular modeling or mutagenesis.
Discussion
RDX, the major component of plastic explosives, induces seizures following accidental or intentional consumption. RDX intoxication therefore should be on the differential diagnosis for new onset seizures in military personnel or anybody handling explosives. 8 Using a combination of electrophysiology, molecular modeling, and site‐directed mutagenesis, we here show that RDX inhibits the α1β2γ2 GABAA receptor with an IC50 of 23 μmol/L by binding to the threonine ring in the channel pore, at the same site as picrotoxinin. 16 , 21 In comparison to picrotoxinin, which we previously tested by electrophysiology on the same recombinantly expressed GABAA receptor subtype combinations, 12 RDX is roughly eightfold less potent but somewhat more selective in its preference for the α1β2γ2 subtype. Since α1‐subunit containing GABAA receptors are most highly expressed in the cortex, thalamus, pallidum, and hippocampus, and the α1β2γ2 subunit combination is estimated to constitute about 60% of all GABAA receptors, 22 , 23 this subtype combination likely constitutes the most relevant pharmacological target for the seizure‐inducing activity of RDX. The second most abundantly expressed GABAA subtype combination, α2β3γ2, is only slightly less sensitive to RDX (IC50 30 μmol/L) and therefore is also likely to contribute to its pharmacodynamic activity following intoxication.
In a pharmacokinetic/pharmacodynamic correlation study in rats, Burdette et al. demonstrated in 1988 that oral RDX administration at doses of 25 and 50 mg/kg resulting in plasma concentrations of 5–8 μg/mL (22–36 μmol/L) induced intense and sustained seizure activity. In humans, an explosive ordinance who had consumed 1.6 g of RDX (~20 mg/kg) and who exhibited persistent seizure activity for 2 days, has been documented to have RDX plasma concentrations of 3.06 μg/mL (13.77 μmol/L) while showing frontal intermittent rhythmic delta activity with intermittent sharp waves on the EEG. 8 Another study in rats measured RDX blood and cortical tissue concentrations and found that RDX penetrates well into the brain and achieves brain concentrations that are roughly twofold higher than plasma concentrations. 11 A brain concentration of 8 μg/g (36 μmol/L) was more than sufficient to initiate seizures. 11 In order to correlate these brain and plasma concentrations to the functional activity observed in our patch‐clamp assays, we determined the plasma protein binding of RDX using equilibrium dialysis and found that when using rat plasma RDX is 40.0 ± 2.7% protein bound leaving 60% free. Considering this low protein binding, the good brain penetration, and the observation, that for other GABAA antagonists like picrotoxinin, brain concentrations that are able to block roughly 10–20% of GABAA currents in electrophysiological experiments are sufficient to trigger seizures, 24 the brain concentrations (16–27 μmol/L) that are likely to have been achieved in the above described patient 8 with matched EEG and RDX plasma levels would have been sufficient to block 10–50% of α1β2γ2‐mediated GABAA currents. The brain concentrations found in rats exhibiting seizures, which ranged from 36 to 45 μmol/L, 11 are well above the IC50 for α1β2γ2 receptors and are also sufficient for significantly inhibiting α2β3γ2 receptors.
RDX had been previously suggested to bind to the NCA site in the pore of GABAA receptors based on the observation that it can displace [35S]‐TBPS in binding assays with rat brain membranes. 11 We here confirmed this hypothesis by taking advantage of the tremendous advances that have been made in the last few years in elucidating GABAA receptor channel structures. After initially using a homology model based on the closed/resting state structure of the α1β3γ2 GABAA receptor, 21 in which we had replaced the β3 subunits with β2 subunits to guide our mutational work, we generated the final models shown in Figure 4 by directly docking RDX into the recently published structure of the α1β2γ2 GABAA receptor in the resting state. 16 Both the α1β3γ2 receptor (pdb id:6HUG) and the α1β2γ2 receptor (pdb id: 6X40) have been captured with picrotoxinin bound but differ somewhat in the width of the conduction pathway. The 6HUG structure is constricted to ~1.5 Å at both the desensitization gate at −2’ and the activation gate at 9’ and is therefore assumed to represent the closed/resting state. In contrast, the 6X40 structure is less tightly closed and the activation gate at the 9’ ring is partially open, suggesting that the channel was captured in a transition from the resting to the desensitized state. 16 RosettaLigand identified low‐energy binding poses that show RDX interacting with residues at the 2’ and 6’ ring in agreement with the mutagenesis in both models, but we favored the real structure over the homology model for the final model.
Given its electrophysiological behavior as a noncompetitive antagonist and its binding to the NCA site in the pore of α1β3γ2 receptor, RDX is thus a “classic” GABAA receptor blocker that even structurally resembles other “caged” convulsants like picrotoxinin or the rodenticide TETS (tetramethylenedisulfotetramine). So why would military personnel voluntarily consume RDX? There is, of course, the thrill of doing something as daring as “eating explosive” in front of a peer group, which based on case reports between 1969 and 2019, is particularly appealing to young males since no intentional ingestions have ever been reported in females. 9 The practice of field troops in the Vietnam War of consuming RDX to simulate or enhance the effects of ethanol 1 could have been based on interactions between RDX and ethanol. We here found that RDX in a concentration range of between 500 nmol/L and 2 μmol/L potentiates GABAA receptor activity, and it is therefore perceivable that low RDX concentrations enhance the relaxing and sedating effects of ethanol. Soldiers who had consumed RDX are described as having a reduced attention span, a reduced ability to perform simple arithmetic and being disorientated, 1 at 24 h after exposure when they had stopped seizing and when RDX brain and plasma levels were presumably again in the range where RDX potentiates GABAA receptors. At higher RDX concentrations, where RDX is a GABAA receptor antagonist, there might be a small window where GABA inhibition enhances neural excitability and balances with the intoxicating, disinhibitory, and depressant effects of ethanol, an interaction which has been suggested for α‐thujone and ethanol in absinthe. 25 The terpenoid α‐thujone, which is found in wormwood oil and in sage, inhibits GABAA receptors with an IC50 of 21 μmol/L and induces seizures, 26 very similar to RDX. Whatever the reasons for voluntary RDX consumption, our findings suggest that RDX‐ induced seizures should be treated with GABAA modulating drugs such as benzodiazepines, barbiturates, propofol, or with neurosteroids, such as alloprenanolone or ganaxolone. In several of the recent case reports where RDX had been consumed as “a dare”, seizures were treated with diazepam 8 or lorazepam 7 , 9 or, on recurrence, terminated with propofol. 8
Conflict of Interest
None of the authors have any conflict of interest to disclose related to this work.
Author Contributions
BP, INP, and HW developed the concept and designed the study. BP performed the electrophysiology and the molecular modeling. RDL performed the mutagenesis. VS determined the plasma protein binding. BP, RDL, and VS acquired and analyzed data. BP and HW prepared the Figures. BP and HW wrote the manuscript with input from all co‐authors.
Supporting information
Figure S1. RDX acts as a positive allosteric modulator at low concentrations. (A) Comparison of the concentration response curve for picrotoxinin (PTX) and RDX on currents evoked by EC90 GABA for the α1β2γ2L GABAA receptor. Data points are mean ± SD from 3 to 8 independent recordings. (B) Percentage current activation by 1 μmol/L RDX (mean ± SD from 4 to 8 independent recordings) for the different GABAA receptor combinations.
Funding Information
This work was supported by the CounterACT Program, National Institutes of Health Office of the Director [U54NS079202 and R21 NS110647]. BP was supported by a National Institute of General Medical Sciences funded Pharmacology Training Program [T32GM099608]].
Funding Statement
This work was funded by National Institute of General Medical Sciences grant T32GM099608; NIH Office of the Director grants R21NS110647 CounterACT Program and U54NS079202 CounterACT Program.
References
- 1. Stone WJ, Paletta TL, Heiman EM, Bruce JI, Knepshield JH. Toxic effects following ingestion of C‐4 plastic explosive. Arch Intern Med. 1969;124:726‐730. [PubMed] [Google Scholar]
- 2. Cary TJ, Rylott EL, Zhang L, et al. Field trial demonstrating phytoremediation of the military explosive RDX by XplA/XplB‐expressing switchgrass. Nat Biotechnol. 2021;39:1216‐1219. [DOI] [PubMed] [Google Scholar]
- 3. EPA US . Toxicological review of hexahydro‐1,3,5‐trinitro‐1,3,5‐triazine (RDX). EPA/635/R‐18/211Fa. 2018; www.epa.gov/iris:CASRN 121‐182‐124.
- 4. McFarland CA, Quinn MJ Jr, Bazar MA, Talent LG, Johnson MS. Toxic effects of oral hexahydro‐1,3,5‐trinitro‐1,3,5‐triazine in the Western fence lizard (sceloporus occidentalis). Environ Toxicol Chem. 2009;28:1043‐1050. [DOI] [PubMed] [Google Scholar]
- 5. Quinn MJ, Hanna TL, Shiflett AA, et al. Interspecific effects of 4a‐DNT (4‐amino‐2,6‐dinitrotoluene) and RDX (1,3,5‐trinitro‐1,3,5‐triazine) in Japanese quail, northern bobwhite, and zebra finch. Ecotoxicology. 2013;22:231‐239. [DOI] [PubMed] [Google Scholar]
- 6. Von Oettingen WF, Donahue DD, Yagoda H, Monaco AR, Harris MR. Toxocity and potential dangers of cyclotrimethylenetrinitramine. J Ind Hyg Toxicol. 1949;31:21‐31. [PubMed] [Google Scholar]
- 7. Kasuske L, Schofer JM, Hasegawa K. Two marines with generalized seizure activity. J Emerg Nurs. 2009;35:542‐543. [DOI] [PubMed] [Google Scholar]
- 8. Garcia R, Karimian A, Donaldson C, Preston K, Scully S. Status epilepticus after C‐4 ingestion: using liquid chromatography to quantify toxicity. Clin Toxicol. 2019;57:819‐821. [DOI] [PubMed] [Google Scholar]
- 9. Whitesides JD, Turner MN, Watts S, Watkins SA. Peer pressure = explosive consequences: a case report of toxic ingestion of cyclonite (C‐4) explosive on a dare. Clin Pract Cases Emerg Med. 2021;5:43‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burdette LJ, Cook LL, Dyer RS. Convulsant properties of cyclotrimethylenetrinitramine (RDX): spontaneous audiogenic, and amygdaloid kindled seizure activity. Toxicol Appl Pharmacol. 1988;92:436‐444. [DOI] [PubMed] [Google Scholar]
- 11. Williams LR, Aroniadou‐Anderjaska V, Qashu F, et al. RDX binds to the GABAA receptor‐convulsant site and blocks GABAA receptor‐mediated currents in the amygdala: a mechanism for RDX‐induced seizures. Environ Health Perspect. 2011;119:357‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pressly B, Nguyen HM, Wulff H. GABAA receptor subtype selectivity of the proconvulsant rodenticide tets. Arch Toxicol. 2018;92:833‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rohl CA, Strauss CE, Misura KM, Baker D. Protein structure prediction using Rosetta. Methods Enzymol. 2004;383:66‐93. [DOI] [PubMed] [Google Scholar]
- 14. Yarov‐Yarovoy V, Schonbrun J, Baker D. Multipass membrane protein structure prediction using Rosetta. Proteins. 2006;62:1010‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yarov‐Yarovoy V, DeCaen PG, Westenbroek RE, et al. Structural basis for gating charge movement in the voltage sensor of a sodium channel. Proc Natl Acad Sci USA. 2012;109:E93‐E102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim JJ, Gharpure A, Teng J, et al. Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature. 2020;585:303‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davis IW, Baker D. Rosettaligand docking with full ligand and receptor flexibility. J Mol Biol. 2009;385:381‐392. [DOI] [PubMed] [Google Scholar]
- 18. Pressly B, Lee RD, Barnych B, Hammock BD, Wulff H. Identification of the functional binding site for the convulsant tetramethylenedisulfotetramine in the pore of the alpha2 beta3 gamma2 GABAA receptor. Mol Pharmacol. 2021;99:78‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olsen RW, Lindemeyer AK, Wallner M, Li X, Huynh KW, Zhou ZH. Cryo‐electron microscopy reveals informative details of GABAA receptor structural pharmacology: implications for drug discovery. Ann Transl Med. 2019;7:S144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szabo A, Nourmahnad A, Halpin E, Forman SA. Monod‐Wyman‐Changeux allosteric shift analysis in mutant alpha1beta3gamma2l GABAA receptors indicates selectivity and crosstalk among intersubunit transmembrane anesthetic sites. Mol Pharmacol. 2019;95:408‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Masiulis S, Desai R, Uchanski T, et al. GABAA receptor signalling mechanisms revealed by structural pharmacology. Nature. 2019;565:454‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olsen RW, Sieghart W. GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov. 2011;10:685‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pressly B, Vasylieva N, Barnych B, et al. Comparison of the toxicokinetics of the convulsants picrotoxinin and tetramethylenedisulfotetramine (TETS) in mice. Arch Toxicol. 2020;94:1995‐2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olsen RW. Absinthe and gamma‐aminobutyric acid receptors. Proc Natl Acad Sci USA. 2000;97:4417‐4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hold KM, Sirisoma NS, Ikeda T, Narahashi T, Casida JE. Alpha‐thujone (the active component of absinthe): gamma‐aminobutyric acid type a receptor modulation and metabolic detoxification. Proc Natl Acad Sci USA. 2000;97:3826‐3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. RDX acts as a positive allosteric modulator at low concentrations. (A) Comparison of the concentration response curve for picrotoxinin (PTX) and RDX on currents evoked by EC90 GABA for the α1β2γ2L GABAA receptor. Data points are mean ± SD from 3 to 8 independent recordings. (B) Percentage current activation by 1 μmol/L RDX (mean ± SD from 4 to 8 independent recordings) for the different GABAA receptor combinations.


