Abstract
Blood‐based phosphorylated tau (Ptau) 181 and 217 biomarkers are sensitive and specific for Alzheimer's disease. In this racial/ethnically diverse cohort study, participants were classified as biomarker positive (Ptau+) or negative (Ptau‐) based on Ptau 181 and 217 concentrations and as cognitively impaired (Sym) or unimpaired (Asym). The four groups, Ptau‐/Asym, Ptau+/Asym, Ptau‐/Sym, and Ptau+/Sym, differed by age, APOE‐4 allele frequency, total tau, neurofilament light chain, and cortical thickness measured by MRI. Our results add to increasing evidence that plasma Ptau 181 and 217 concentrations are valid Alzheimer's disease biomarkers in diverse populations.
Introduction
The development of blood‐based biomarkers to estimate the amyloid (‘A'), tau (‘T'), and neurodegenerative (‘N') features of Alzheimer's disease (AD) has increased the ability to study disease in diverse populations. Plasma concentrations of phosphorylated tau at threonine 181 and 217 (Ptau 181 and Ptau 217) along with neurofilament light (NfL) chain were elevated in multi‐racial/ethnic, community based, older adults diagnosed clinically, and pathologically with AD, 1 consistent with other studies. 2 , 3 , 4 , 5 , 6 , 7 We found increased Ptau 181 and Ptau 217 concentrations and decreased Aβ42/Aβ40 ratios were associated with higher risk of AD after an average of 4 years.
Fluid and imaging biomarkers allow for incorporation of pathophysiological data into diagnosis according to the ATN framework without explicit consideration of cognitive or clinical symptoms. 8 The goal of this study was to examine demographic (i.e., age, sex/gender, race/ethnicity), genetic (i.e., APOE Ɛ4 allele), amyloid (Aβ42/Aβ40), tau (total tau concentration), neurodegeneration (NfL, cortical atrophy) variables across older adults classified by Ptau cutpoint and clinical status in a multi‐ethnic cohort.
Methods
Participants and diagnostic procedures
This follow‐up study included 300 participants from the Washington Heights‐Inwood Columbia Aging Project (WHICAP) selected for equal representation from race/ethnicity groups: non‐Latinx White, Latinx/Hispanic, and non‐Latinx African American/Black) with similar numbers with an AD clinical diagnosis at their last longitudinal assessment. Race and ethnicity were self‐reported and coded via standardized criteria. 9 A goal for the current analyses was to define four groups based on symptom and biomarker status. A consensus committee, including clinicians with expertise in dementia, reviewed neuropsychological, medical, and neurological data (but not biomarker data) for clinical diagnoses. A clinical diagnosis of AD was assigned using research criteria 10 and a Clinical Dementia Rating 11 of 0.5 or higher. To derive a diagnostic threshold score for Ptau biomarker concentration, we examined data from autopsied WHICAP participants with stored plasma (n = 113) as described previously. 1 A pathological assignment of AD was made according to the National Institute on Aging‐Alzheimer's Association criteria, as previously described, 1 with case status assigned to those deemed with high AD neuropathological changes (ADNC) and controls to those without pathological AD or with low or intermediate ADNC.
Plasma biomarkers
Plasma biomarker concentrations were measured as described previously. 1 Briefly stored aliquoted plasma was used to determine Aβ42, Aβ40, t‐tau, NfL, Ptau 181, and Ptau 217 concentrations. For Aβ42, Aβ40, and t‐tau we used Simoa (Quanterix) technology on the SR‐X platform with the multiplex Neuro 2‐plex A (#101995) and NfL (#103400) kits. Each plate assayed in duplicate 38 samples, 8 calibrators, and 2 controls. The ratio of Aβ42 to Aβ40 was considered as the primary biomarker for amyloid. Ptau 181 and Ptau 217 concentrations were determined on a Meso Scale Discovery (MSD) platform as described previously. 1 Briefly, for Ptau 181, Biotinylated‐AT270 (MN1050, ThemoFisher) was used as a capture antibody and SULFOTAG‐Ru‐4G10‐E2 (Eli Lilly) for the detector. For Ptau217, Biotinylated‐IBA493 (Eli Lilly) was used as a capture antibody and SULFO‐TAG‐Ru‐4G10‐E2 (Eli Lilly) for the detector.
Neuropsychological assessment
At each visit, participants are evaluated with a comprehensive set of neuropsychological tests 12 in their preferred language (English or Spanish). Based on the factor structure of the neuropsychological battery, 13 summary scores were derived for memory, language, visuospatial function, and speed/executive function by averaging z‐scores for individual tests in each domain.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) was available for a subset of participants (n = 223) included in the analyses. T1‐weighted anatomical images were acquired on a 3 T Philips (Best, the Netherlands) Intera scanner (TR = 6.6 ms; TE = 3.0 ms; flip angle = 8°; FOV = 256 × 200 × 165 mm; slice thickness = 0.6 mm) or 1.5 T Philips Intera scanner (TR = 20 ms, TE = 2.1 ms, FOV = 240 × 256 × 160 mm, slice thickness = 1.3 mm). FreeSurfer 14 was used to derive regional cortical thickness and an “AD signature” was calculated by averaging cortical thickness in the entorhinal cortex, parahippocampus, inferior parietal lobe, pars opercularis, pars orbitalis, pars triangularis, inferior temporal lobe, temporal pole, precuneus, supramarginal gyrus, superior parietal lobe, and superior frontal lobe across hemispheres.
Statistical analysis
We used receiver operating characteristic (ROC) curves to evaluate the area under the curve (AUC) to assess Ptau 217 and Ptau 181 concentrations with high ADNC relative to controls. We calculated Youden's index 15 to derive the respective Ptau concentration cutoff scores with the highest combination of sensitivity and specificity. This allowed us to define four distinct groups: low Ptau concentration (Ptau181 and Ptau217) without clinical diagnosis of AD (Ptau‐, Asym), high Ptau concentration without clinical diagnosis (Ptau+, Asym), low Ptau concentration with clinical diagnosis (Ptau‐, Sym), and high Ptau concentration with clinical diagnosis (Ptau+, Sym). We compared age, sex/gender, and race/ethnicity across the four groups using chi‐squared and general linear models. Next, we examined differences in AD‐relevant variables, including APOE ε4 allele frequency; plasma biomarker concentrations for Aβ42/Aβ40, t‐tau, and NfL; cognitive domain scores; and cortical thickness on MRI using general linear models and Chi‐squared tests. Post hoc analyses contrasted participant groups to Ptau‐/Asym group as reference.
Results
The Youden Index for Ptau 217 was 0.21 pg./mL, yielding a diagnostic sensitivity and specificity of 0.84 and 0.77, respectively, as previously reported. 1 Relative to individuals classified as Ptau‐/Asym, those who were Ptau+/Sym were older, but similar in sex/gender and race/ethnicity (Table 1). Ptau+ individuals regardless of cognitive status were more likely to have at least one APOE ε4 allele. The four groups did not differ in Aβ42/Aβ40 ratio. Compared with participants classified as Ptau‐/Asym, Ptau+/Asym, and Ptau+/Sym had higher total tau concentrations. Participants classified as Ptau+/Asym, Ptau‐/Sym, or Ptau+/Sym had higher NfL concentrations compared with the Ptau‐/Asym group. Cortical thickness was lower in the Ptau‐/Sym and Ptau+/Sym compared with the Ptau‐/Asym group, respectively (Fig. 1 and Table 1). Cortical thickness values decreased linearly (p = 0.02) across groups as seen in Figure 1. For Ptau 181, the Youden Index was calculated as 0.95 pg./mL, yielding a sensitivity and specificity of 0.84 and 0.59, respectively. Results were similar for groups defined by Ptau 181 compared with Ptau 217 with the exception of a slight difference in the age and the significant difference in cortical thickness (Table 1).
Table 1.
Differences across biomarker/clinical groups in demographic and Alzheimer's disease‐related measures.
| Ptau‐/Asym | Ptau+/Asym | Ptau‐/Sym | Ptau+/Sym | Total sample | Statistic | |||
|---|---|---|---|---|---|---|---|---|
| Ptau 217 | N | 119 (40%) | 54 (18%) | 60 (20%) | 67 (22%) | 300 | ‐ | |
| Age mean (SD) yrs | 80.75 (6.36) | 81.41 (6.15) | 82.00 (6.36) | 84.13 (6.51) | 81.87 (6.45) | F = 4.17, p = 0.006 | ||
| N (%) women | 81 (68%) | 31 (57%) | 42 (70%) | 46 (69%) | 200 (66.7%) | Χ2 = 2.60, p = 0.45 | ||
| Race/ethnicity N (%) | White N (%) | 46 (39%) | 18 (33%) | 14 (23%) | 22 (33%) | 100 (33.3%) | Χ2 = 5.90, p = 0.43 | |
| Black N (%) | 38 (32%) | 17 (32%) | 20 (33%) | 25 (37%) | 100 (33.3%) | |||
| Hispanic N (%) | 35 (29%) | 19 (35%) | 26 (43%) | 20 (30%) | 100 (33.3%) | |||
| APOE, N (%) with at least one ε4 allele | 21 (18%) | 28 (52%) | 8 (13%) | 27 (40%) | 84 (28%) | Χ2 = 32.99, p < 0.001 | ||
| Aβ42/Aβ40 mean (SD) concentration | 0.060 (0.01) | 0.057 (0.01) | 0.63 (0.02) | 0.057 (0.01) | 0.059 (0.01) | F = 2.39, p = 0.06 | ||
| T‐tau mean (SD) concentration, pg./mL | 4.63 (1.91) | 5.54 (2.39) | 4.58 (2.05) | 5.24 (2.08) | 4.92 (2.09) | F = 3.40, p = 0.01 | ||
| NfL mean (SD) concentration, pg./mL | 27.53 (19.12) | 38.45 (41.99) | 35.57 (30.81) | 38.43 (18.07) | 33.48 (27.25) | F = 3.52, p = 0.01 | ||
| Cognition mean (SD) standard score | Memory | 0.46 (0.54) | 0.32 (0.57) | −0.69 (0.67) | −0.89 (0.70) | −0.07 (0.85) | F = 92.73, p < 0.001 | |
| Language | 0.52(0.51) | 0.55 (0.56) | −0.41 (0.60) | −0.24 (0.50) | 0.17 (0.68) | F = 60.21, p < 0.001 | ||
| Speed/executive function | 0.44 (0.80) | 0.51 (0.69) | −0.59 (1.08) | −0.49 (1.06) | 0.12 (0.99) | F = 23.71, p < 0.001 | ||
| Visuospatial function | 0.58 (0.43) | 0.54 (0.56) | −0.18 (0.61) | −0.03 (0.62) | 0.30 (0.63) | F = 37.15, p < 0.01 | ||
| Cortical thickness mean (SD) mma | 2.60 (0.14) | 2.57 (0.14) | 2.53 (0.14) | 2.52 (0.16) | 2.57 (0.14) | F = 3.50, p = 0.016 | ||
| Ptau 181 | N | 123 (41%) | 49 (16%) | 68 (23%) | 59 (20%) | 300 | ‐ | |
| Age mean (SD) yrs | 80.40 (6.26) | 82.33 (6.25) | 82.34 (6.56) | 84.03 (6.37) | 81.87 (6.47) | F = 4.68, p = 0.08 | ||
| Race/ethnicity N (%) | White N (%) | 45 (36.7) | 19 (39) | 18 (26.5) | 18 (30.5) | 100 (33.3) | Χ2 = 5.53, p = 0.47 | |
| Black N (%) | 39 (32) | 15 (31) | 21 (31) | 24 (41) | 100 (33.3) | |||
| Hispanic N (%) | 39 (32) | 15 (31) | 29 (42.6) | 17 (29) | 100 (33.3) | |||
| APOE, N (%) with at least one ε4 allele | 26 (21) | 22 (45) | 12 (17.6) | 23 (39) | 83 (28) | Χ2 = 17.04, p = 0.001 | ||
| Aβ42/Aβ40 mean (SD) concentration | 0.060 (0.01) | 0.058 (0.01) | 0.062 (0.02) | 0.057 (0.01) | 0.059 (0.01) | F = 1.57, p = 0.196 | ||
| T‐tau mean (SD) concentration, pg./mL | 4.58 (1.88) | 5.75 (2.41) | 4.40 (1.68) | 5.54 (2.23) | 4.92 (2.09) | F = 7.21, p < 0.001 | ||
| NfL mean (SD) concentration, pg./mL | 26.84 (18.57) | 40.56 (43.87) | 32.51 (26.52) | 42.30 (21.75) | 33.46 (27.29) | F = 5.85, p < 0.001 | ||
| Cognition mean (SD) standard score | Memory | 0.44 (0.54) | 0.35 (0.58) | −0.72 (0.63) | −0.88 (0.75) | −0.07 (0.85) | F = 90.81, p < 0.001 | |
| Language | 0.49 (0.49) | 0.60 (0.62) | −0.37 (0.57) | −0.27 (0.53) | 0.17 (0.68) | F = 59.45, p < 0.001 | ||
| Speed/executive function | 0.41 (0.79) | 0.56 (0.71) | −0.59 (1.03) | −0.47 (1.11) | 0.12 (0.99) | F = 23.79, p < 0.001 | ||
| Visuospatial function | 0.55 (0.46) | 0.62 (0.51) | −0.16 (0.62) | −0.04 (0.61) | 0.30 (0.63) | F = 36.58, p < 0.001 | ||
| Cortical thickness mean (SD) mma | 2.60 (0.12) | 2.56 (0.18) | 2.54 (0.13) | 2.51 (0.16) | 2.57 (0.14) | F = 4.20, p = 0.006 | ||
The four groups: Ptau‐/Asym, Pta+/Asym, Ptau‐/Sym, and Ptau+/Sym, differed significantly by age, APOE‐4 allele frequency, total tau, NfL, and cortical thickness on MRI. Ptau+ regardless of their symptomatic status were similar with only slight differences in cortical thickness. There were no difference by sex/gender or race/ethnicity across the four groups.
Available for n = 223 participants.
Figure 1.
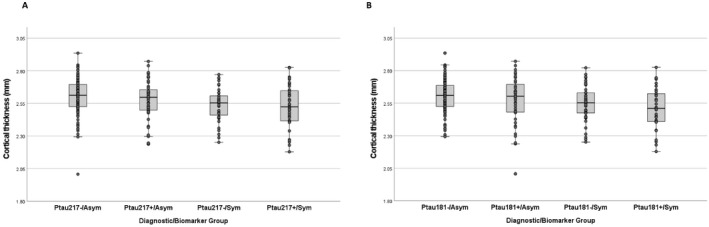
Differences across biomarker/clinical groups in cortical thickness in Alzheimer's signature regions for groups defined by Ptau 217 cutpoint (A) and by Ptau 181 cutpoint (B).
Discussion
Groups defined by Ptau 217 and Ptau 181 yielded similar results. Compared to those with low Ptau concentrations, with elevated Ptau concentrations, were slightly older but did not differ in race/ethnicity or sex/gender especially when symptomatic. The results here indicate that in a community‐representative sample, biomarkers and clinical diagnoses do not vary by demographic factors. Groups with increased Ptau concentration irrespective of dementia status had a higher APOE Ɛ4 allele frequency. Interestingly, those diagnosed clinically without biomarker evidence of AD had a similar proportion of APOE Ɛ4 carriers as those who were asymptomatic and biomarker negative.
In assessing biological differences among groups, several important patterns emerged. Our previous analyses showed that Aβ42/Aβ40 concentration ratios may be prognostically useful; those with lower values were more likely to progress to dementia but the values did not discriminate prevalent cases from unimpaired control participants. 1 The analyses here confirm that these plasma amyloid biomarkers in this community‐based sample do not track as well with other AD biomarkers or with clinical status, providing additional evidence that implementation of a single Ptau value may be sufficient for characterizing AD pathophysiology.
Mass spectrometry approaches to estimate amyloid pathology from plasma samples may be more accurate than immunoassays. 16 Consideration of the three biological markers that reflect neurodegeneration: t‐tau, NfL, and cortical thickness, provides additional insight into the biological basis of the diagnostic groups. Although t‐tau measurements are considered markers of neurodegeneration 17 they appear to track most with Ptau elevation and not with clinical status. Neurofilament light chain concentrations, conversely, appeared elevated among individuals with either elevated Ptau concentrations or a dementia diagnosis. Similarly, cortical thickness, a reflection of neurodegeneration in the form of brain atrophy, decreased across diagnostic groups (Fig. 1), but tracked with dementia. We conclude that plasma NfL concentrations and cortical thinning reflect neurodegeneration, irrespective of a specific AD pathophysiological process. Our observed sensitivities and specificities are slightly lower than what has been reported previously, including recent papers from Thijssen et al. 18 and Palmqvist et al. 19 We speculate that differences reflect the design and composition of our study cohort. Ours is a community‐based study, the average age at death is older than the other two studies, and the interval between the antemortem collection and death is somewhat longer. These factors are likely contributors to the differences in diagnostic accuracy among studies.
The existence of two discordant groups: those with elevated Ptau concentrations but asymptomatic and those who were symptomatic but without elevated Ptau concentrations are of great interest. In the first group, despite evidence of neurodegenerative changes, there may be factors that mitigate the impact of neurodegenerative pathology on its clinical manifestations, while in the second group, other pathophysiological factors are likely driving the diagnosis of dementia. AD pathology per se is only one of the myriad factors that contributes to emergence of dementia. Indeed, pathological data suggest that the defining feature of symptomatic AD in older adults is the presence of multiple pathologies. 20 Future studies should continue to examine biological and social correlates of biomarker and clinical features of AD without discounting either.
Author Contributions
AMB: Conception and design, acquisition and analysis of data, drafting manuscript. JJM: Conception and design, acquisition and analysis of data, drafting manuscript. LSH: Analysis of data. DS: Acquisition of data. DR‐D: Acquisition of data. RAL: Acquisition and analysis of data. JPV: Acquisition and analysis of data. AFT: Acquisition and analysis of data. MSK: Analysis of data. JLD: Acquisition and analysis of data. RM: Conception and design, acquisition and analysis of data, drafting manuscript.
Conflict of Interest
Jeffrey L. Dage is a minor stockholder and receives some research support from Eli Lilly and Company. Additionally, Jeffrey L. Dage is an inventor on patents and/or patent applications of Eli Lilly and Company relating to the ptau217 assay, methods, reagents, and/or compositions of matter used in this research.
Acknowledgments
Data collection and sharing for this project were supported by the Washington Heights‐Inwood Columbia Aging Project (WHICAP, R01 AG072474 P01AG07232, R01AG037212, RF1AG054023, R56AG034189, R01AG034189, R01AG054520) funded by the National Institute on Aging (NIA). This manuscript has been reviewed by WHICAP investigators for scientific content and consistency of data interpretation with previous WHICAP Study publications. We acknowledge the WHICAP study participants and the WHICAP research and support staff for their contributions to this study. The authors are grateful to Marielba Zerlin‐Esteves for her expert handling of the Human Genetic Resources Core at Columbia University Irving Medical Center.
Funding InformationNational Institutes of Health, National Institute on Aging R01 AG072474 P01AG07232, R01AG037212, RF1AG054023
Funding Statement
This work was funded by National Institutes of Health, National Institute on Aging grant R01 AG072474 P01AG07232, R01AG037212, RF1AG054023,.
References
- 1. Brickman AM, Manly JJ, Honig LS, et al. Plasma p‐tau181, p‐tau217, and other blood‐based Alzheimer's disease biomarkers in a multi‐ethnic, community study. Alzheimers Dement. 2021;17(8):1353‐1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cullen NC, Leuzy A, Janelidze S, et al. Plasma biomarkers of Alzheimer's disease improve prediction of cognitive decline in cognitively unimpaired elderly populations. Nat Commun 2021;12(1):3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P‐tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med. 2020;26(3):379‐386. [DOI] [PubMed] [Google Scholar]
- 4. Mielke MM, Hagen CE, Xu J, et al. Plasma phospho‐tau181 increases with Alzheimer's disease clinical severity and is associated with tau‐ and amyloid‐positron emission tomography. Alzheimers Dement. 2018;14(8):989‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mielke MM, Frank RD, Dage JL, et al. Comparison of plasma phosphorylated tau species with amyloid and tau positron emission tomography, neurodegeneration, vascular pathology, and cognitive outcomes. JAMA Neurol. 2021;78(9):1108‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Palmqvist S, Tideman P, Cullen N, et al. Prediction of future Alzheimer's disease dementia using plasma phospho‐tau combined with other accessible measures. Nat Med. 2021;27(6):1034‐1042. [DOI] [PubMed] [Google Scholar]
- 7. Pereira JB, Janelidze S, Stomrud E, et al. Plasma markers predict changes in amyloid, tau, atrophy and cognition in non‐demented subjects. Brain. 2021;144:2826‐2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jack CR Jr, Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bureau USC, Census of Population and Housing . Summary Population and Housing Characteristics. Bureau of the Census; 2001. [Google Scholar]
- 10. Manly JJ, Tang MX, Schupf N, et al. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63(4):494‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43:2412‐2414. [DOI] [PubMed] [Google Scholar]
- 12. Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm‐based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49(5):453‐460. [DOI] [PubMed] [Google Scholar]
- 13. Avila JF, Rentería MA, Witkiewitz K, Verney SP, Vonk JMJ, Manly JJ. Measurement invariance of neuropsychological measures of cognitive aging across race/ethnicity by sex/gender groups. Neuropsychology. 2020;34(1):3‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dale AM, Fischl B, Sereno MI. Cortical surface‐based analysis. I Segmentation and surface reconstruction neuroimage. Neuroimage. 1999;9(2):179‐194. [DOI] [PubMed] [Google Scholar]
- 15. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32‐35. [DOI] [PubMed] [Google Scholar]
- 16. Janelidze S, Teunissen CE, Zetterberg H, et al. Head‐to‐head comparison of 8 plasma amyloid‐β 42/40 assays in Alzheimer disease. JAMA Neurol. 2021;78:1375‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hampel H, Cummings J, Blennow K, Gao P, Jack CR Jr, Vergallo A. Developing the ATX(N) classification for use across the Alzheimer disease continuum classification for use across the Alzheimer disease continuum. Nat Rev Neurol. 2021;17:580‐589. [DOI] [PubMed] [Google Scholar]
- 18. Thijssen EH, La Joie R, Strom A, et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer's disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol. 2021;20(9):739‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma Phospho‐tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324(8):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017;134(2):171‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]


