Abstract
Objective
To identify the rates of neurological events following administration of mRNA (Pfizer, Moderna) or adenovirus vector (Janssen) vaccines in the U.S.
Methods
We used publicly available data from the U.S. Vaccine Adverse Event Reporting System (VAERS) collected between January 1, 2021 and June 14, 2021. All free text symptoms that were reported within 42 days of vaccine administration were manually reviewed and grouped into 36 individual neurological diagnostic categories. Post‐vaccination neurological event rates were compared between vaccine types and to age‐matched baseline incidence rates in the U.S. and rates of neurological events following COVID.
Results
Of 306,907,697 COVID vaccine doses administered during the study timeframe, 314,610 (0.1%) people reported any adverse event and 105,214 (0.03%) reported neurological adverse events in a median of 1 day (IQR0‐3) from inoculation. Guillain‐Barre Syndrome (GBS), and cerebral venous thrombosis (CVT) occurred in fewer than 1 per 1,000,000 doses. Significantly more neurological adverse events were reported following Janssen (Ad26.COV2.S) vaccination compared to either Pfizer‐BioNtech (BNT162b2) or Moderna (mRNA‐1,273; 0.15% vs 0.03% vs 0.03% of doses, respectively, p < 0.0001). The observed‐to‐expected ratios for GBS, CVT and seizure following Janssen vaccination were ≥1.5‐fold higher than background rates. However, the rate of neurological events after acute SARS‐CoV‐2 infection was up to 617‐fold higher than after COVID vaccination.
Interpretation
Reports of serious neurological events following COVID vaccination are rare. GBS, CVT and seizure may occur at higher than background rates following Janssen vaccination. Despite this, rates of neurological complications following acute SARS‐CoV‐2 infection are up to 617‐fold higher than after COVID vaccination. ANN NEUROL 2022;91:756–771
Introduction
While vaccination against SARS‐CoV‐2 represents the best preventative strategy for COVID, there are still millions of people worldwide that are reluctant to receive inoculation. One concern may be the fear of vaccine‐related neurological side effects. 1 Although the U.S. Centers for Disease Control and Prevention (CDC) in the U.S. has issued warnings for Guillain‐Barre Syndrome (GBS) and vaccine‐induced immune thrombotic thrombocytopenia following Janssen/Johnson & Johnson (Ad26.COV2.S) vaccination, and myocarditis following mRNA vaccines (Pfizer‐BioNtech [BNT162b2] and Moderna [mRNA‐1,273]), 2 , 3 data addressing a wider spectrum of neurological symptoms/diagnoses, and comparing neurological events between mRNA and adenovirus vector vaccines in the U.S. is limited.
We used the U.S. Vaccine Adverse Event Reporting System (VAERS) 4 to identify neurological symptoms/syndromes reported for three FDA‐approved COVID vaccines: Pfizer, Moderna and Janssen. Our primary aim was to determine the incidence of a wide spectrum of neurological events reported within 42 days of COVID vaccination, and to compare these rates across the three vaccine types. Our secondary aims were to compare the reported post‐vaccination neurological event rates to pre‐pandemic background incidence rates in the U.S. and to neurological event rates during the acute phase of SARS‐CoV‐2 infection.
Methods
Study Design and Patient Cohort
We conducted a retrospective review of data collected in the VAERS 4 database between January 1, 2021‐June 14, 2021. VAERS is an online, publicly available database co‐managed by the U.S. CDC and the Food and Drug Administration FDA. 4 It is a passive reporting system in which clinicians, patients, and vaccine manufacturers can enter adverse event data related to any U.S. FDA‐approved vaccine. Healthcare providers are required by law to report serious adverse events following vaccination, regardless of causality including: death, a life‐threatening adverse event, inpatient hospitalization, or prolonged hospitalization following vaccination, persistent or significant incapacity, and/or disruption to normal life functions, congenital anomalies/birth defects, important medical events post‐vaccination that may jeopardize an individual or require medical or surgical intervention, vasovagal syncope or shoulder injury (within 7 days of inoculation). 4 Specific to COVID vaccines, healthcare providers are required to report post‐vaccination multisystem inflammatory syndrome, and breakthrough COVID that results in hospitalization or death. Apart from the legal reporting requirements, healthcare providers are encouraged to report any adverse event that occurs after vaccine administration, regardless of whether or not a causal relationship appears to be present. Data on clinician compliance with these regulations is limited. Vaccine manufacturers are required to report all adverse events that come to their attention. Additionally, the lay public may report adverse vaccine events to VAERS. However, knowingly filing a false VAERS report is a violation of federal law, punishable by fine and imprisonment. 4
Inclusion criteria for this study were: adverse events reported following administration of one or two doses of any COVID vaccine between January 1, 2021 and June 14, 2021, adverse events occurring within 42 days of vaccination, and aged ≥12 years (the minimum FDA‐approved age for COVID vaccination during the study time frame). We selected 42 days since this is considered a plausible timeframe within which symptoms might be reasonably associated with vaccination. 1 , 3 , 5 Exclusion criteria were: aged <12 years, vaccination type other than Pfizer, Moderna, or Janssen, timing of adverse event relative to inoculation unknown or >6 weeks from vaccination. Booster shots were neither approved nor available during the timeframe of this study.
The Pfizer vaccine (mRNA, two doses) received and FDA emergency use agreement (EUA) on December 11, 2020 for ages ≥16 years, Moderna (mRNA, two doses) received a FDA EUA for ages ≥18 years on December 18, 2020 and Janssen (adenovirus vector, one dose) received a FDA EUA for ages ≥18 years on February 27, 2021. 6 The Pfizer vaccine was approved for emergency use for children aged 12 to 15 on May 10, 2021. Vaccine rollout was staged with availability initially prioritized for healthcare workers, individuals in essential services, adults aged ≥65 years and immunocompromised people, prior to becoming available to the general public. 7
Neurological Diagnostic Categories
The VAERS database contains symptom/syndrome free text, as well as automated symptom coding. Both free text and symptom coding were manually reviewed by neurology‐trained physician abstractors. Symptoms/syndromes were categorized into nine broad neurological categories (demyelinating/inflammatory, neuromuscular, vascular, cranial nerve, seizure, cognitive disorder, movement disorder, general and other) and 36 sub‐categories (Table 1). Neurological events were coded only when the event was explicitly described. For example, Guillain‐Barre Syndrome was coded only when “Guillain‐Barré”, “GBS”, “AIDP”, “acute inflammatory demyelinating polyneuropathy”, “AMAN”, “AMSAN”, or “Miller‐Fisher” were documented. We did not code GBS when ambiguous terms such as “demyelinating polyneuropathy”, “neuropathy”, “acute polyneuropathy”, or “autoimmune neuropathy” were used. Similarly, cerebral venous thrombosis (CVT) was coded only when “cerebral venous thrombosis”, “CVT”, “cerebral vein thrombosis”, “cerebral sinus thrombosis”, or “cerebral venous sinus thrombosis” terms were used in the descriptive text. Seizure was coded when the term “seizure”, “status epilepticus”, “epileptic fit”, or “convulsion” was used. When symptom descriptions were unclear, another reviewer assessed the data, and coding was based on consensus opinion. Individual medical records were not available for review as data were anonymized and de‐identified. A subset of data was used to pilot and standardize data collection among the assessors.
TABLE 1.
Categories of Neurological Adverse Events Adjudicated Based on Manual Review of symptom Text
| Category | Complication |
|---|---|
| Demyelinating/inflammatory | Acute Disseminated Encephalomyelitis (ADEM) |
| Transverse myelitis | |
| Meningitis | |
| Encephalitis | |
| Multiple sclerosis | |
| Neuromuscular | Guillain‐Barre Syndrome |
| Peripheral neuropathy | |
| Radiculopathy | |
| Myopathy | |
| ALS/motor neuron disease | |
| Myasthenia Gravis | |
| Paresthesia/hypoesthesia | |
| Vascular | Ischemic stroke/TIA |
| Subarachnoid Hemorrhage | |
| Intracerebral hemorrhage/intraventricular hemorrhage | |
| Cerebral sinus thrombosis | |
| Subdural hematoma | |
| Cranial Neuropathy | Bell's Palsy |
| Anosmia | |
| Dysgeusia | |
| Hearing loss | |
| Vision abnormality | |
| Tinnitus | |
| Trigeminal Neuralgia | |
| Seizure | Seizure |
| Epilepsy | |
| Status epilepticus | |
| Cognitive | Encephalopathy/Delirium/Confusion/Brain fog |
| Acute psychosis | |
| Transient Global Amnesia | |
| Movement Disorder | Tremor/dystonia/blepharospasm/myoclonus |
| General | Headache |
| Dizziness/vertigo | |
| Syncope | |
| Fatigue | |
| Sleep disturbance | |
| Vivid dreams | |
| Other | Dysphagia |
| Worsening of underlying neurological disorder or deficit |
Study Outcomes
The primary outcome was the rate of reported neurological events within 42 days of COVID vaccination (reported as events per 1,000,000 vaccine doses).
Secondary outcomes included the observed to expected ratios (O:E) of adverse neurological events reported after COVID vaccination compared to published pre‐COVID background incidence rates in the U.S., 3 , 8 , 9 , 10 , 11 , 12 , 13 and incidence rates following acute SARS‐CoV‐2 infection. 14 , 15 , 16 , 17 , 18 , 19 , 20 The Global Burden of Disease (GBD) and Global Health Data Exchange (GHDx) data catalogs 8 were used for age‐matched U.S. background incidence rates in 2019, when possible. For neurological event rates acutely after SARS‐CoV‐2 infection, we used data from studies that allowed for calculation of incidence rates, (as opposed to pooled prevalence rates reported in most meta‐analyses), to remain consistent with our comparison to incidence rates of background disease.
Standard Protocol Approvals and Patient Consents
This study of de‐identified, anonymous data was not deemed to be human research by the NYU Grossman School of Medicine Institutional Review Board. Hence, it was not subject to institutional review board review, nor was patient consent required.
Statistical Analyses
Person‐years at risk for an adverse event within 42 days of vaccination were calculated as: number of persons who received at least one dose of vaccine × 42/365.25. The total number of individuals vaccinated, and the total number of doses administered in the U.S. during the timeframe of interest was obtained from publicly available CDC data. 21 The expected number of events was calculated as (person‐years x background rate)/100,000, where background rates were measured per 100,000 person‐years. Observed to expected ratios (O:E) were then calculated as the number of observed neurological cases divided by the number of expected cases. According to published methods, 3 , 22 95% confidence intervals (CI) were calculated for O:E ratios assuming a two‐sided type I error of 0.05. An O:E >1 indicates a higher than expected rate of a particular neurological complication following vaccination compared to either background or acute SARS‐CoV2 rates.
Reported neurological events were compared between different COVID vaccine types using chi‐squared and Fisher's exact tests, as appropriate. Age, sex, and days from vaccination to neurological adverse event were compared between vaccine types using chi‐squared or Fisher's exact tests, as appropriate, for dichotomous variables and Mann–Whitney U or Kruskal‐Wallis tests for non‐normally distributed continuous variables. For mRNA vaccines, the percentage of reported events occurring before the first or second dose of vaccine was documented, when data were available. Bonferroni corrections were made for multiple comparisons and significance was set at p < 0.0004. All analyses were conducted using JMP Pro 16 (SAS, Cary, NC, USA).
Results
From January 1, 2021 to June 14, 2021, 306,907,697 COVID vaccine doses were administered; 54% of doses were Pfizer, 42% Moderna and 4% Janssen (Table 2). A total of 314,610 adverse events following COVID vaccination were recorded in VAERS, representing 0.10% of all doses administered. Of these, 105,214/314,610 (33%) were neurological adverse events, representing 0.03% of all vaccine doses administered. The median age of patients with reported neurological adverse events was 50 years (interquartile range [IQR] 35–64), and 71% were female. The median time to onset of neurological symptoms from vaccination was 1 day (IQR 0–3). Individuals who received Janssen vaccination were significantly younger (aged 44 years vs 49 years for Pfizer and 52 years for Moderna, p < 0.001) and more often male (38% vs 29% for Pfizer and 26% for Moderna, p < 0.001) than those who received Pfizer or Moderna shots.
TABLE 2.
COVID Vaccine Administration and Adverse Events Reported in VAERS in the U.S. Between January 1, 2021 and June 14, 2021
| Pfizer | Moderna | Janssen | Any COVID vaccine a | |
|---|---|---|---|---|
| COVID Vaccine Administration 1/1/2021 to 6/14/2021 | ||||
| N doses of vaccine administered | 166,981,930 (54%) | 128,084,622 (42%) | 11,606,202 (4%) | 306,907,697 |
| N patients who received at least 1 dose | 90,063,644 | 72,705,161 | 11,465,768 | 174,234,573 |
| N patients who received full vaccine course | 75,374,466 | 58,015,983 | 11,465,768 | 144,919,339 |
| VAERS Vaccine Side Effect Data 1/1/2021 to 6/14/2021 | ||||
| N side effect entries (inclusive), % of vaccine doses administered | 126,901 (0.08%) | 148,674 (0.12%) | 38,194 (0.33%) | 314,610 (0.10%) |
| N side effect entries (neuro), % of vaccine doses administered | 44,896 (0.03%) ^# | 42,368 (0.03%) ^! | 17,622 (0.15%) #! | 105,214 (0.03%) |
| Age in VAERS‐yr, (median, IQR) | 49 (35–64)^# | 52 (37–66)^! | 44 (31–57)#! | 50 (35–64) |
| Sex in VAERS (% F) | 71%^# | 74%^! | 62%#! | 71% |
| # days from vaccine to symptom onset (median, IQR) | 1 (0–2)^# | 1 (0–6)^! | 0 (0–2)#! | 1 (0–3) |
| N (%) symptoms reported after: | ||||
| 1st dose | 50%^ | 57%^ | – | 54% |
| 2nd dose | 35% | 28% | 28% | |
| Missing | 15% | 15% | 18% | |
Includes Pfizer, Moderna, Janssen and unspecified manufacturer (n = 841); ^p < 0.001 Pfizer v Moderna, #p < 0.001 Pfizer v Janssen, !p < 0.001 Moderna v Janssen.
Overall, significantly fewer adverse neurological events were reported following Pfizer or Moderna vaccination compared to Janssen: 0.03% of doses or 269 neurological events per 1,000,000 doses for Pfizer versus 0.03% of doses or 331 events per 1,000,000 doses for Moderna versus 0.15% of doses or 1,518 events per 1,000,000 doses for Janssen, p < 0.001 (Table 2). There was also a small, but significantly lower proportion of individuals with adverse events reported following Pfizer vaccination compared to Moderna (p < 0.001). The most commonly reported adverse neurological events were headache, fatigue, dizziness, and syncope, all of which were more often reported following Janssen vaccination (Fig 1, Table 3). More severe adverse events including GBS, CVT, transverse myelitis and ADEM were reported in fewer than 1 per million vaccine doses for Pfizer and Moderna. Worsening of an underlying neurological condition was reported in 0.03 per 1,000,000 doses of vaccine. However, the Janssen vaccine had significantly higher reported rates of GBS (n = 61 events or 5.26 events per 1,000,000 doses), and CVT (n = 53 events or 4.57 events per 1,000,000 doses) than either Moderna (GBS n = 87 events or 0.68 per 1,000,000 doses; CVT n = 34 events or 0.27 per 1,000,000 doses), or Pfizer (GBS n = 115 events or 0.69 per 1,000,000 doses; CVT n = 40 events or 0.24 per 1,000,000 doses, all p < 0.0004; Tables 4 and 5).
FIGURE 1.
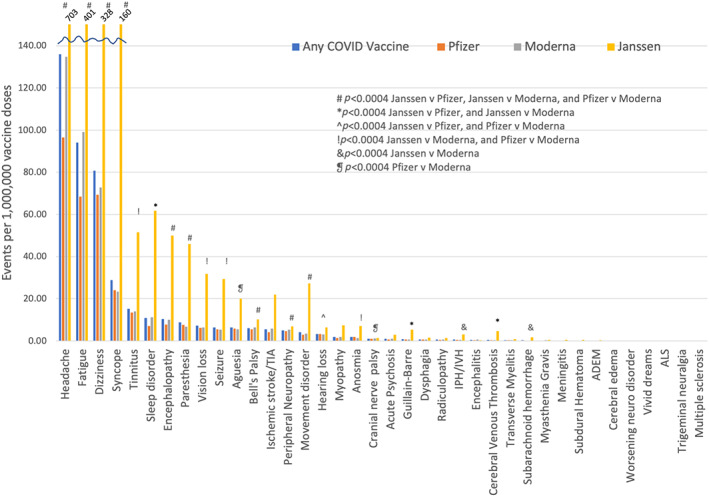
Neurological adverse events per 1,000,000 vaccine doses reported in VAERS stratified by COVID vaccine type. Note that the rates of headache, fatigue, dizziness and syncope exceed the y‐axis scale for the Janssen vaccine and event rates are listed above these bars. Significance was set at p < 0.0004 after Bonferroni correction for multiple comparisons. ADEM = acute disseminated encephalomyelitis; ALS = amyotrophic lateral sclerosis; IPH/IVH = intraparenchymal hemorrhage/intraventricular hemorrhage; TIA = transient ischemic attack. # p < 0.0004 Janssen v Pfizer, Janssen v Moderna, and Pfizer v Moderna; *p < 0.0004 Janssen v Pfizer, and Janssen v Moderna; ^p < 0.0004 Janssen v Pfizer, and Pfizer v Moderna; !p < 0.0004 Janssen v Moderna, and Pfizer v Moderna; & p < 0.0004 Janssen v Moderna; ❡ p < 0.0004 Pfizer v Moderna. [Color figure can be viewed at www.annalsofneurology.org]
TABLE 3.
Number of Adverse Events Per 1,000,000 Vaccine Doses
| Adverse event | Any COVID vaccine | Pfizer | Moderna | Janssen |
|---|---|---|---|---|
| Any neurological event | 342.82 | 268.87 | 330.78 | 1518.33 |
| Headache | 135.90 | 96.59 | 134.75 | 703.33 |
| Fatigue | 94.09 | 68.52 | 99.09 | 401.42 |
| Dizziness | 80.69 | 69.38 | 72.81 | 327.84 |
| Syncope | 28.87 | 23.94 | 23.30 | 160.35 |
| Tinnitus | 15.14 | 13.53 | 13.90 | 51.52 |
| Sleep disorder | 10.93 | 7.05 | 11.29 | 61.78 |
| Encephalopathy | 10.36 | 7.80 | 10.04 | 49.89 |
| Paresthesia | 8.72 | 7.58 | 6.78 | 45.92 |
| Vision loss | 7.24 | 6.19 | 6.35 | 31.79 |
| Seizure | 6.30 | 5.42 | 5.31 | 29.29 |
| Aguesia | 6.30 | 5.89 | 5.56 | 19.99 |
| Bell's Palsy | 6.01 | 5.49 | 6.32 | 10.08 |
| Ischemic stroke/TIA | 5.55 | 4.12 | 5.86 | 21.97 |
| Peripheral neuropathy | 4.98 | 4.57 | 5.36 | 6.81 |
| Movement disorder | 4.03 | 2.90 | 3.33 | 27.31 |
| Hearing loss | 3.26 | 3.20 | 3.08 | 6.29 |
| Myopathy | 1.84 | 1.40 | 1.90 | 7.41 |
| Anosmia | 1.81 | 1.88 | 1.35 | 6.98 |
| Cranial nerve palsy | 1.05 | 0.94 | 1.16 | 1.38 |
| Acute psychosis | 0.91 | 0.66 | 1.05 | 2.84 |
| Guillain‐Barre | 0.86 | 0.69 | 0.68 | 5.26 |
| Dysphagia | 0.67 | 0.60 | 0.69 | 1.46 |
| Radiculopathy | 0.57 | 0.43 | 0.66 | 1.38 |
| Intraparenchymal hemorrhage | 0.56 | 0.46 | 0.47 | 3.10 |
| Encephalitis | 0.44 | 0.34 | 0.59 | 0.26 |
| Cerebral venous thrombosis | 0.42 | 0.24 | 0.27 | 4.57 |
| Transverse myelitis | 0.28 | 0.22 | 0.31 | 0.78 |
| Subarachnoid hemorrhage | 0.23 | 0.18 | 0.17 | 1.64 |
| Myasthenia Gravis | 0.19 | 0.13 | 0.23 | 0.52 |
| Meningitis | 0.15 | 0.13 | 0.14 | 0.43 |
| Subdural Hematoma | 0.16 | 0.14 | 0.13 | 0.52 |
| ADEM | 0.06 | 0.06 | 0.03 | 0.26 |
| Cerebral edema | 0.05 | 0.04 | 0.07 | 0.00 |
| Worsening of underlying neurological disorder | 0.03 | 0.02 | 0.05 | 0.00 |
| Vivid dreams | 0.02 | 0.01 | 0.02 | 0.00 |
| ALS | 0.02 | 0.02 | 0.01 | 0.00 |
| Trigeminal neuralgia | 0.01 | 0.01 | 0.02 | 0.00 |
| Multiple sclerosis exacerbation/demyelinating event | 0.01 | 0.01 | 0 | 0 |
TABLE 4.
Observed to Expected (O:E) Adverse Event Rates Within 42 days of Inoculation for COVID Vaccines Compared to Background Rates in the U.S. Person‐years of Observation for all COVID Vaccines (N Patients Who Received at Least One Vaccine Dose × [42 days/365.25 days) was 20,036,976 Person‐years; for Pfizer: 10,357,319 Person‐years; for Moderna: 8,361,094 Person‐years; and for Janssen: 1,318,563 Person‐years
| Adverse event | Characteristic | All COVID vaccines^ (n = 306,907,697doses) | Pfizer (n = 166,981,930 doses) | Moderna (n = 128,084,622 doses) | Janssen (n = 11,606,202 doses) |
|---|---|---|---|---|---|
| Guillain‐Barre syndrome | Age, median (IQR) | 57 (42–66) | 57 (40–68) | 59 (47–66) | 57 (43–63) |
| Sex (% F) | 48% | 55% | 51% | 33% | |
| Days from vaccine, median (IQR) | 7 (2–14) | 4 (2–14) | 7 (1–12) | 13 (7–18) | |
| Observed N (% of doses) | 265 (0.00009%) | 115 (0.00007%) | 87 (0.00007%) | 61 (0.0005%) | |
| Expected N (background rate 1.513,12/100,000 person‐yr) | 302.6 | 156.4 | 126.3 | 19.9 | |
| O:E, (95% CI) | 0.88 (0.77–0.99) | 0.74 (0.61–0.88) | 0.69 (0.55–0.85) | 3.06 (2.34–3.93) | |
| ADEM | Age, median (IQR) | 47 (34–65) | 49 (36–67) | 42 (27–61) | 36 (32–54) |
| Sex (% F) | 71% | 70% | 75% | 67% | |
| Days from vaccine, median (IQR) | 3 (0–14) | 2 (0–24) | 3 (0–6) | 11 (0–15) | |
| Observed N (% of doses) | 17 (0.000006%) | 10 (0.000006%) | 4 (0.000003%) | 3 (0.000003%) | |
| Expected N (background rate 0.110/100,000 person‐yr) | 20.0 | 10.4 | 8.4 | 1.3 | |
| O:E, (95% CI) | 0.85 (0.49–1.36) | 0.97 (0.46–1.78) | 0.48 (0.13–1.22) | 2.28 (0.47–6.65) | |
| Bell's Palsy | Age, median (IQR) | 54 (41–66) | 52 (40–65) | 57 (45–68) | 47 (38–59) |
| Sex (% F) | 55% | 54% | 57% | 51% | |
| Days from vaccine, median (IQR) | 5 (1–14) | 4 (1–13) | 6 (1–17) | 9 (2–20) | |
| Observed N (% of doses) | 1846 (0.0006%) | 917 (0.0005%) | 809 (0.0006%) | 117 (0.001%) | |
| Expected N (background rate 25.029/100,000 person‐yr) | 5009.2 | 2589.3 | 2090.3 | 329.6 | |
| O:E, (95% CI) | 0.37 (0.35–0.39) | 0.35 (0.33–0.38) | 0.39 (0.36–0.41) | 0.35 (0.29–0.43) | |
| Transverse Myelitis | Age, median (IQR) | 54 (39–67) | 48 (39–66) | 58 (43–71) | 50 (33–62) |
| Sex (% F) | 62% | 59% | 60% | 78% | |
| Days from vaccine, median (IQR) | 6 (1–14) | 4 (1–15) | 6 (1–14) | 9 (6–12) | |
| Observed N (% of doses) | 86 (0.00003%) | 37 (0.00002%) | 40 (0.00003%) | 9 (0.00009%) | |
| Expected N (background rate 0.4611,13/100,000 person‐yr) | 92.2 | 47.6 | 38.5 | 6.1 | |
| O:E, (95% CI) | 0.93 (0.75–1.15) | 0.78 (0.55–1.07) | 1.04 (0.74–1.42) | 1.48 (0.68–2.82) | |
| Meningitis | Age, median (IQR) | 56 (36–69) | 45 (27–62) | 63 (37–69) | 65 (42–82) |
| Sex (% F) | 64% | 68% | 67% | 40% | |
| Days from vaccine, median (IQR) | 6 (1–10) | 6 (1–10) | 3 (1–15) | 6 (4–10) | |
| Observed N (% of doses) | 45 (0.00002%) | 22 (0.00001%) | 18 (0.00001%) | 5 (0.00004%) | |
| Expected N (background rate 1.18/100,000 person‐yr) | 220.4 | 113.9 | 91.9 | 14.5 | |
| O:E, (95% CI) | 0.20 (0.15–0.27) | 0.19 (0.12–0.29) | 0.20 (0.12–0.31) | 0.34 (0.11–0.80) | |
| Encephalitis | Age, median (IQR) | 62 (46–72) | 58 (46–68) | 64 (43–74) | 64 (64) |
| Sex (% F) | 60% | 63% | 59% | 33% | |
| Days from vaccine, median (IQR) | 1 (0–3) | 1 (0–3) | 1 (0–3) | 11 (0–39) | |
| Observed N (% of doses) | 135 (0.00004%) | 56 (0.00003%) | 76 (0.00006%) | 3 (0.00003%) | |
| Expected N (background rate 0.788 /100,000 person‐yr) | 156.3 | 80.8 | 65.2 | 10.3 | |
| O:E, (95% CI) | 0.86 (0.72–1.02) | 0.69 (0.52–0.90) | 1.16 (0.92–1.46) | 0.29 (0.06–0.85) |
O:E = observed to expected ratio; Bold represents O:E >1; ^note that the sum of events for Pfizer, Moderna and Janssen does not equal the total number of events because some entries did not specify COVID vaccine manufacturer.
TABLE 5.
Observed to Expected (O:E) Adverse Event Rates within 42 days of Inoculation for COVID Vaccines Compared to Background Rates in the U.S. Person‐years of Observation for all COVID Vaccines (N patients Who Received at Least One Vaccine Dose × [42 days/365.25 days) was 20,036,976 Person‐years; for Pfizer: 10,357,319 Person‐years; for Moderna: 8,361,094 Person‐years; and for Janssen: 1,318,563 Person‐years
| Adverse event | Characteristic | All COVID vaccines^ (n = 306,907,697 doses) | Pfizer (n = 166,981,930 doses) | Moderna (n = 128,084,622 doses) | Janssen (n = 11,606,202 doses) |
|---|---|---|---|---|---|
| Cerebral venous thrombosis | Age, median (IQR) | 52 (38–63) | 55 (40–68) | 54 (37–67) | 47 (38–59) |
| Sex (% F) | 62% | 60% | 53% | 68% | |
| Days from vaccine, median (IQR) | 7 (3–16) | 4 (2–12) | 8 (4–16) | 10 (5–16) | |
| Observed N (% of doses) | 128 (0.00004%) | 40 (0.00002%) | 34 (0.00003%) | 53 (0.0005%) | |
| Expected N (background rate 2.08/100,000 person‐yr) | 400.7 | 207.1 | 167.2 | 26.4 | |
| O:E, (95% CI) | 0.32 (0.27–0.38) | 0.19 (0.14–0.26) | 0.20 (0.14–0.28) | 2.01 (1.51–2.63) | |
| Ischemic Stroke | Age, median (IQR) | 68 (56–78) | 66 (55–77) | 71 (59–79) | 62 (52–74) |
| Sex (% F) | 55% | 54% | 57% | 54% | |
| Days from vaccine, median (IQR) | 4 (1–13) | 4 (1–12) | 4 (1–14) | 7 (2–16) | |
| Observed N (% of doses) | 1704 (0.0006%) | 688 (0.0004%) | 750 (0.0006%) | 255 (0.002%) | |
| Expected N (background rate 32.48 /100,000 person‐yr) | 6491.9 | 3355.8 | 2709.0 | 427.2 | |
| O:E, (95% CI) | 0.26 (0.25–0.28) | 0.21 (0.19–0.22) | 0.28 (0.26–0.30) | 0.60 (0.53–0.67) | |
| Intracerebral Hemorrhage | Age, median (IQR) | 65 (54–78) | 70 (59–78) | 66 (52–81) | 57 (40–64) |
| Sex (% F) | 57% | 47% | 62% | 72% | |
| Days from vaccine, median (IQR) | 4 (1–12) | 3 (1–12) | 3 (1–9) | 11 (4–15) | |
| Observed N (% of doses) | 173 (0.00006%) | 77 (0.00005%) | 60 (0.00005%) | 36 (0.0003%) | |
| Expected N (background rate 7.18 /100,000 person‐yr) | 1422.6 | 735.4 | 593.6 | 93.6 | |
| O:E, (95% CI) | 0.12 (0.10–0.14) | 0.10 (0.08–0.13) | 0.10 (0.08–0.13) | 0.38 (0.27–0.53) | |
| Subarachnoid Hemorrhage | Age, median (IQR) | 61 (49–74) | 65 (52–81) | 56 (47–66) | 59 (45–66) |
| Sex (% F) | 65% | 70% | 55% | 74% | |
| Days from vaccine, median (IQR) | 5 (2–14) | 4 (1–12) | 7 (2–15) | 7 (2–18) | |
| Observed N (% of doses) | 72 (0.00002%) | 30 (0.00002%) | 22 (0.00002%) | 10 (0.00009%) | |
| Expected N (background rate 10.28 /100,000 person‐yr 3 , 12 ) | 2043.8 | 1056.4 | 852.8 | 134.5 | |
| O:E, (95% CI) | 0.04 (0.03–0.04) | 0.03 (0.02–0.04) | 0.03 (0.02–0.04) | 0.07 (0.04–0.14) | |
| Headache | Age, median (IQR) | 49 (36–61) | 49 (36–61) | 52 (38–64) | 44 (32–55) |
| Sex (% F) | 75% | 77% | 76% | 68% | |
| Days from vaccine, median (IQR) | 1 (0–1) | 1 (0–1) | 1 (0–1) | 0 (0–1) | |
| Observed N (% of doses) | 41,710 (0.01%) | 16,128 (0.01%) | 17,260 (0.01%) | 8,163 (0.01%) | |
| Expected N (background rate 7368.48/100,000 person‐yr) | 1,476,404.5 | 763,168.7 | 616,078.9 | 97,156.9 | |
| O:E, (95% CI) | 0.03 (0.03–0.03) | 0.02 (0.02–0.02) | 0.03 (0.03–0.03) | 0.08 (0.08–0.09) | |
| Seizure | Age, median (IQR) | 39 (26–58) | 37 (23–57) | 45 (30–64) | 34 (26–47) |
| Sex (% F) | 55% | 57% | 56% | 47% | |
| Days from vaccine, median (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–2) | 0 (0–1) | |
| Observed N (% of doses) | 1933 (0.0006%) | 905 (0.0005%) | 680 (0.0005%) | 340 (0.003%) | |
| Expected N (background rate 13.88 /100,000 person‐yr) | 2765.1 | 1429.3 | 1153.8 | 181.9 | |
| O:E, (95% CI) | 0.699 (0.67–0.73) | 0.633 (0.59–0.68) | 0.59 (0.55–0.64) | 1.87 (1.68–2.08) |
O:E = observed to expected ratio; Bold represents O:E >1; ^note that the sum of events for Pfizer, Moderna and Janssen does not equal the total number of events because some entries did not specify COVID vaccine manufacturer.
The median age and sex proportions per individual reported adverse event are shown in Figure 2. Patients with subdural hemorrhage, myasthenia gravis, ischemic stroke, and intracerebral hemorrhage tended to be older, while those with syncope, multiple sclerosis, or ADEM were younger. There was a higher proportion of females reporting adverse events across the majority of event types. The median days from vaccination to adverse event onset and the proportion of events reported after the first or second dose of mRNA vaccines are shown in Figure 3. Data for event occurrence after the first or second vaccine dose was available in 235,293/275,575 (85%) subjects who reported events after either Pfizer or Moderna vaccination. Autoimmune or molecular mimicry‐related events including NMDA encephalitis, GBS, ADEM, and transverse myelitis were reported in in approximately 7 to 11 days following vaccination, while events such as syncope, dizziness, and confusion, which may represent vasovagal events, tended to occur immediately after inoculation. Among those who reported adverse events after a mRNA vaccine, 58% reported events after the first dose and 28% reported events after the second dose (data on dose series was missing in 18%, Table 2). Patients who received the Moderna vaccine were more likely to report events after the first dose compared to those who received the Pfizer vaccine (57% of events were reported after the first Moderna shot vs 50% after the first Pfizer shot, p < 0.001). Worsening of underlying neurological conditions, dysphagia, trigeminal neuralgia, and syncope were most often reported after the first dose of a vaccine, while myopathy, subarachnoid hemorrhage, and vivid dreams were more often reported after a second dose (Fig 3).
FIGURE 2.
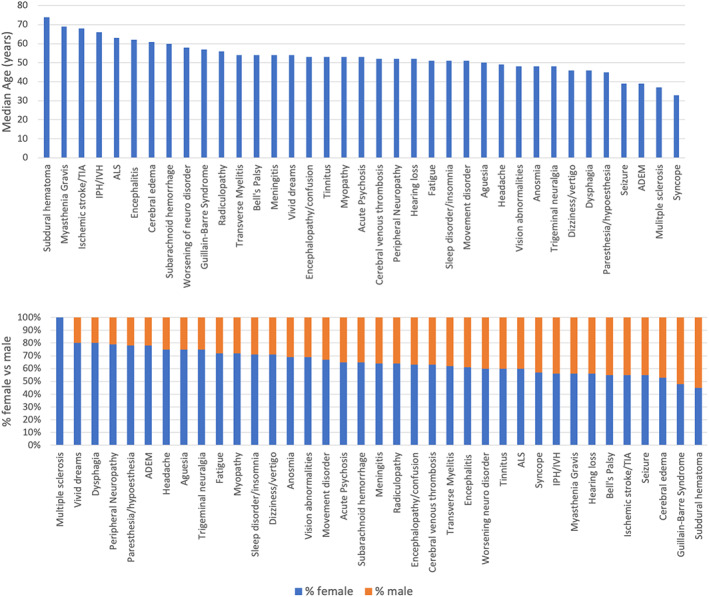
Median age and sex proportions for each adverse event reported in VAERS between January 1, 2021‐June 14, 2021, inclusive of all COVID vaccine brands. [Color figure can be viewed at www.annalsofneurology.org]
FIGURE 3.
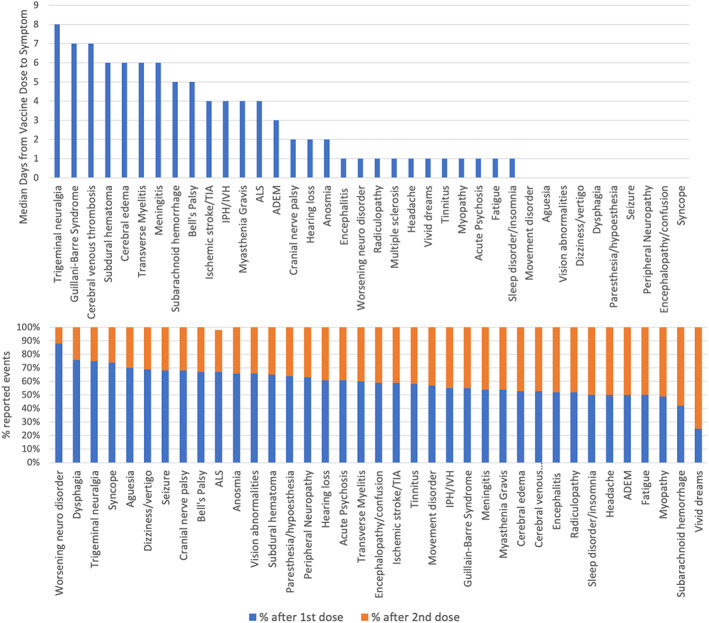
Median time (days) from vaccine injection to adverse event onset, inclusive of all COVID vaccine types, and proportion of events which were reported after the first or second dose of a mRNA vaccine (using data available in 235,293/275,575 [85%] of patients who received either Pfizer or Moderna vaccines). [Color figure can be viewed at www.annalsofneurology.org]
Person‐years of observation for all COVID vaccines was 20,036,976 person‐years; for Pfizer: 10,357,319 person‐years; for Moderna: 8,361,094 person‐years; and for Janssen: 1,318,563 person‐years. Compared to pre‐COVID background rates in the U.S., the O:E for GBS (3.06, 95% CI 2.34–3.93), CVT (2.01, 95% CI 1.51–2.63) and seizure (1.87, 95% CI 1.68–2.08) were all >1 following Janssen vaccination, indicating higher than expected rates (Tables 4 and 5). O:E rates were slightly above 1 for ADEM and transverse myelitis following Janssen vaccination, and for transverse myelitis and encephalitis following Moderna vaccination, though the confidence intervals for these events all crossed 1.0 and observed event rates were low.
Overall, compared to studies that reported a composite incidence of all neurological events acutely after SARS‐CoV‐2 infection, the rates of adverse neurological events reported after COVID vaccination were 132 to 617‐fold lower (O:E 0.002–0.008 with 105,214 observed cases compared to 13,908,927‐64,941,552 expected cases; Table 6). Examining specific neurological events, the O:E was lowest for encephalopathy (0.0004–0.00008), indicating that acute encephalopathy following SARS‐CoV‐2 infection was 2,230‐12,907‐fold more frequent that following COVID vaccination. The O:E for other neurological events post vaccination, compared to acutely after SARS‐CoV‐2 infection, ranged from 0.0004 for intracerebral hemorrhage to 0.11 for Bell's palsy.
TABLE 6.
Observed to Expected Rates of Neurological events Reported after COVID Vaccination Compared to Rates of Neurological Events During the Acute Phase of SARS‐CoV‐2 Infection. Person‐years of Observation for all COVID Vaccines
| Adverse event | Characteristic | All COVID vaccines^ (n = 306,907,697 doses) | Pfizer (n = 166,981,930 doses) | Moderna (n = 128,084,622 doses) | Janssen (n = 11,606,202 doses) |
|---|---|---|---|---|---|
| Any neurological event | Observed N | 105,214 | 44,896 | 42,368 | 17,622 |
| Expected N (post‐COVID rate 69,41614‐324,10919/100,000 person‐yr) | 13,908,927‐64,941,552 | 7,189,668‐33,568,956 | 5,803,962‐27,099020 | 915,298‐4,273,575 | |
| O:E, (95% CI) | 0.002 (0.002–0.002) to 0.008 (0.008–0.008) | 0.001 (0.001–0.001) to 0.006 (0.006–0.006) | 0.002 (0.002–0.002) to 0.007 (0.007–0.007) | 0.004 (0.004–0.004) to 0.02 (0.02–0.02) | |
| Encephalo‐pathy | Observed N | 3,180 | 1,303 | 1,286 | 579 |
| Expected N (post‐COVID rate 35,39514‐204,84419/100,000 person‐yr) | 7,092,176‐41,044,572 | 3,666,019‐21,216,361 | 2,959,446‐17,127,211 | 466,711‐2,700,999 | |
| O:E, (95% CI) | 0.0004 (0.0004–0.0005) to 0.00008 (0.00008–0.00008) | 0.0004 (0.0003–0.0004) to 0.00006 (0.00006–0.00006) | 0.0004 (0.0004–0.0005) to 0.00008 (0.00007–0.00008) | 0.001 (0.001–0.001) to 0.0002 (0.0002–0.0002) | |
| Guillain‐Barre Syndrome | Observed N | 265 | 115 | 87 | 61 |
| Expected N (post‐COVID rate 34414/100,000 person‐yr) | 68,847 | 35,588 | 28,729 | 4,531 | |
| O:E, (95% CI) | 0.004 (0.003–0.004) | 0.003 (0.003–0.004) | 0.003 (0.002–0.004) | 0.013 (0.01–0.02) | |
| Bell's Palsy | Observed N | 1846 | 917 | 809 | 117 |
| Expected N (post‐COVID rate 8217/100,000 person‐yr) | 16,430 | 8,493 | 6,856 | 1,081 | |
| O:E, (95% CI) | 0.11 (0.11–0.12) | 0.11 (0.10–0.11) | 0.12 (0.11–0.13) | 0.11 (0.09–0.13) | |
| Headache | Observed N | 41,710 | 16,128 | 17,260 | 8,163 |
| Expected N (post‐COVID rate 97,35820/100,000 person‐yr) | 19,507,599 | 10,083,679 | 8,140,194 | 1,284,727 | |
| O:E, (95% CI) | 0.002 (0.002–0.002) | 0.002 (0.002–0.002) | 0.002 (0.002–0.002) | 0.006 (0.006–0.006) | |
| Anosmia/dysgeusia | Observed N | 1932 | 983 | 712 | 232 |
| Expected N (post‐COVID rate 23,62120/100,000 person‐yr) | 4,732,934 | 2,446,502 | 1,974,974 | 311,458 | |
| O:E, (95% CI) | 0.0004 (0.0004–0.0004) | 0.0004 (0.0004–0.0004) | 0.0004 (0.0003–0.0004) | 0.0007 (0.0007–0.0008) | |
| Cerebral venous thrombosis | Observed N | 128 | 40 | 34 | 53 |
| Expected N (post‐COVID rate 11218/100,000 person‐yr) | 22,441 | 11,600 | 9,364 | 1,477 | |
| O:E, (95% CI) | 0.006 (0.005–0.007) | 0.003 (0.002–0.005) | 0.004 (0.003–0.005) | 0.04 (0.03–0.05) | |
| Ischemic Stroke | Observed N | 1704 | 688 | 750 | 255 |
| Expected N (post‐COVID rate 6987.414/100,000 person‐yr) | 1,400,064 | 723,707 | 584,223 | 92,133 | |
| O:E, (95% CI) | 0.001 (0.001–0.001) | 0.001 (0.001–0.001) | 0.001 (0.001–0.001) | 0.003 (0.002–0.003) | |
| Intracerebral Hemorrhage | Observed N | 173 | 77 | 60 | 36 |
| Expected N (post‐COVID rate 2290.914/100,000 person‐yr) | 459,027 | 237,276 | 191,544 | 30,207 | |
| O:E, (95% CI) | 0.0004 (0.0003–0.0004) | 0.0003 (0.0003–0.0004) | 0.0003 (0.0002–0.0004) | 0.001 (0.0008–0.001) | |
| Subarachnoid Hemorrhage | Observed N | 72 | 30 | 22 | 10 |
| Expected N (post‐COVID rate 343.614/100,000 person‐yr) | 68,847 | 35,588 | 28,729 | 4,531 | |
| O:E, (95% CI) | 0.001 (0.0008–0.001) | 0.0008 (0.0006–0.001) | 0.0008 (0.0005–0.001) | 0.002 (0.001–0.004) | |
| Seizure | Observed N | 1933 | 905 | 680 | 340 |
| Expected N (post‐COVID rate 4,38719–847714/100,000 person‐yr) | 878,956‐1,698,454 | 454,341‐877,949 | 366,774‐708,737 | 57,841‐111,769 | |
| O:E, (95% CI) | 0.001 (0.001–0.001) to 0.002 (0.002–0.002) | 0.001 (0.001–0.001) to 0.002 (0.002–0.002) | 0.001 (0.001–0.001) to 0.002 (0.002–0.002) | 0.003 (0.003–0.003) to 0.006 (0.005–0.007) |
O:E = observed to expected ratio; Bold represents O:E >1; ^note that the sum of events for Pfizer, Moderna and Janssen does not equal the total number of events because some entries did not specify COVID vaccine manufacturer.
Discussion
In this study, we found that reports of neurological adverse events were rare following COVID vaccination and serious events such as GBS, ADEM, transverse myelitis, and CVT were reported in less than 1 per 1,000,000 doses of vaccine administered. The chances of having a neurological event following acute SARS‐CoV‐2 infection were up to 617‐fold higher than after COVID vaccination, suggesting that the benefits of vaccination substantially outweigh the risks.
We found higher reported rates of adverse neurological events following the Janssen vaccine compared to the mRNA vaccines. Reporting bias may play some role in these findings, since the Janssen vaccine may have been viewed less favorably than the mRNA vaccines, particularly after distribution was temporarily halted April 13, 2021 to investigate reports of vaccine‐induced immune thrombotic thrombocytopenia (VITT). 23 Nonetheless, higher than expected rates of GBS, CVT, and seizure were reported following Janssen vaccination compared to background rates. Our findings align with those of the CDC, which reported higher than expected rates of both GBS and CVT (in the context of VITT) following Janssen vaccination. 2 , 3 , 24 While the CDC reported an O:E of 4.18 for GBS within a 42‐day window for adults aged ≥18 years, 3 we report a 42‐day O:E of 3.06. This discrepancy is likely due, in part, to the fact that the CDC had access to additional daily reports received by the FDA, which may not have been available in VAERS at the time of our search. Other adenovirus vector COVID vaccines, such as ChAdOx1 (Oxford/AstraZeneca), have also been reported to generate higher than expected rates of GBS. 25 , 26
We identified higher than expected rates of seizures following Janssen vaccination, which has not been previously reported. These findings are considered exploratory, however, since syncope or convulsive syncope may be confused with seizure. Without access to the source medical records, we cannot infer causality. The original trial that led to the approval of the Janssen vaccine reported only one GBS case, one CVT, and four seizure cases among 21,895 patients who received the vaccine, 27 which underscores the importance of post‐approval surveillance studies. Nonetheless, the incidence of seizure in the Janssen vaccine group was significantly higher than in the placebo group in this study (4 vs 1 participants), 27 suggesting that further exploration of a causal link between Janssen vaccination and seizure is warranted.
Though we also identified higher than expected rates of ADEM and transverse myelitis following Janssen vaccination, these findings should be interpreted with caution given rarity of the events, the wide confidence intervals (which cross 1.0) and the difficulty in establishing accurate background rates of these conditions.
Strengths of this study include the large sample size, the breadth of neurological symptoms/syndromes addressed and the manual abstraction and coding of symptoms by trained clinicians, which mitigates against coding error that can occur with natural language processing. Additionally, we provide context for adverse event rates by comparing them to the best available age‐matched data for background event rates, and incidence rates following severe COVID. These data may provide reassurance to individuals who experience vaccine hesitancy due to concerns regarding neurological side effects.
There are several limitations that should be mentioned. First, VAERS is a passive reporting system, which is subject to reporting biases and diagnostic inaccuracies. 28 For example, there was a higher proportion of females reporting adverse events, even for diseases that do not have a biological predilection for occurring at higher rates in women. This observation underscores the probability of some sex‐related reporting bias. Additionally, because VAERS is a passive system, it cannot completely capture all potential adverse events since data is restricted to patients and clinicians who voluntarily enter information, and data on clinician compliance with regulations guiding VAERS reporting is limited. Indeed, the CDC has posted the following disclaimer to consider when assessing VAERS data: “Reports may include incomplete, inaccurate, coincidental and unverified information”, and “VAERS data do not represent all known safety information for a vaccine.” 29 Second, due to the anonymized nature of the data, we did not have access to primary medical records to validate that specific diagnoses, such as GBS or ADEM followed established criteria. Indeed, reviewing over 105,000 patient records would have been prohibitively time consuming and expensive. Thus, verifying a causal link between a vaccination and an adverse event in this type of study is not possible, and associations should be considered exploratory. Additionally, because the data is de‐identified it is not possible to determine if one patient has multiple different VAERS entries. Third, though we used U.S. pre‐COVID age‐matched background event rates whenever possible, such data was not available for all neurological disease processes and less robust historical estimates were utilized. Additionally, the GBD/GHDx data we used were not stratified by sex, which may have impacted our estimates for certain adverse events. Incidence rates in a contemporaneous, unvaccinated, COVID‐negative control population would have been ideal, though impractical. We chose not to utilize background event rates that were derived from hospital‐based datasets, 30 since the denominator is typically “hospitalized patients”, rather than the general U.S. population, and hence, represent higher background rates. Use of these background rates, would falsely minimize O:E ratios. Fourth, we chose to compare post‐vaccination event rates to rates following acute COVID out of expediency, since incidence rates of neurological events in the post‐acute period are challenging to estimate given evolving definitions of Post‐Acute Sequelae of COVID‐19 (PASC), and ascertainment biases in reporting PASC prevalence rates. Because new neurological PASC symptoms may occur after the acute period of SARS‐CoV‐2 infection, rates of neurological events post COVID are likely higher that what we estimated, suggesting an even more favorable risk–benefit ratio for vaccination.
Conclusions
Reports of serious neurological events following COVID vaccination are rare. GBS, CVT and seizures may occur at higher than background rates following Janssen vaccination. Despite this, rates of neurological complications following acute SARS‐CoV‐2 infection are up to 617‐fold higher than rates following COVID vaccination, suggesting that the overall benefits of COVID vaccination outweigh the risks.
Author Contributions
JAF, AAT, MFD, DGA, HG, AG, AHK, JS, KT, EW, ASW, and EB contributed to the conception and design of the study.
JAF, AAT, MFD, DGA, HG, AG, AHK, and MS and Global COVID‐19 Neuro Research Coalition members (listed below) contributed to acquisition and analysis of data.
JAF and EW contributed to drafting of text and preparing of figures.
The contributors from the Global COVID‐19 Neuro Research Coalition and their institutional affiliations are included in the Supporting information.
Potential Conflict of Interest
JAF, AAT, MFD, DGA, HG, AG, AHK, MS, JS, KT, EW, ASW, EB and coalition members have no relevant conflicts to report.
Data Availability
De‐identified data will be made available to qualified investigators upon written request to the corresponding author.
Supporting information
Global COVID‐19 Neuro Research Coalition Co‐Authors for Indexing on Pubmed
Acknowledgements
We thank the patients and clinicians who contributed information to the VAERS dataset.
There was no funding for this project.
[Correction added on April 22, 2022, after first online publication: Tables 4, 5, and 6 have been included and Tables S1, S2 and S3 are removed from the supporting information.]
References
- 1. Butler M, Tamborska A, Wood GK, et al. Considerations for causality assessment of neurological and neuropsychiatric complications of SARS‐CoV‐2 vaccines: from cerebral venous sinus thrombosis to functional neurological disorder. J Neurol Neurosurg Psychiatry 2021;92:1144–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenblum HG, Hadler SC, Moulia D, et al. Use of COVID‐19 vaccines after reports of adverse events among adult recipients of Janssen (Johnson & Johnson) and mRNA COVID‐19 vaccines (Pfizer‐BioNTech and Moderna): update from the advisory committee on immunization practices – United States, July 2021. MMWR Morb Mortal Wkly Rep 2021;70:1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Woo EJ, Mba‐Jonas A, Dimova RB, et al. Association of Receipt of the Ad26.COV2.S COVID‐19 vaccine with presumptive Guillain‐Barre syndrome, February‐July 2021. JAMA 2021;326:1606–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vaccine Adverse Event Reporting System (VAERS) [online]. Available at: https://vaers.hhs.gov.
- 5. Sejvar JJ, Kohl KS, Gidudu J, et al. Guillain‐Barre syndrome and fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2011;29:599–612. [DOI] [PubMed] [Google Scholar]
- 6. FDA . Available at: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines.
- 7. Dooling K, Marin M, Wallace M, et al. The advisory committee on immunization Practices' updated interim recommendation for allocation of COVID‐19 vaccine – United States, December 2020. MMWR Morb Mortal Wkly Rep 2021;69:1657–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. GBD Compare/Viz Hub 2019 Incidence in U.S. ages 50–69 [online]. Available at: https://vizhub.healthdata.org/gbd-compare/. Accessed December 13, 2021.
- 9. Shahrizaila N, Lehmann HC, Kuwabara S. Guillain‐Barre syndrome. Lancet 2021;397:1214–1228. [DOI] [PubMed] [Google Scholar]
- 10. Dubey D, Pittock SJ, Kelly CR, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol 2018;83:166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeffery DR, Mandler RN, Davis LE. Transverse myelitis. Retrospective analysis of 33 cases, with differentiation of cases associated with multiple sclerosis and parainfectious events. Arch Neurol 1993;50:532–535. [DOI] [PubMed] [Google Scholar]
- 12. Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain‐Barre syndrome: a systematic review and meta‐analysis. Neuroepidemiology 2011;36:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gubernot D, Jazwa A, Niu M, et al. U.S. Population‐based background incidence rates of medical conditions for use in safety assessment of COVID‐19 vaccines. Vaccine 2021;39:3666–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frontera JA, Sabadia S, Lalchan R, et al. A prospective study of neurologic disorders in hospitalized patients with COVID‐19 in New York City. Neurology 2021;96:e575–e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manzano GS, McEntire CRS, Martinez‐Lage M, et al. Acute disseminated encephalomyelitis and acute hemorrhagic Leukoencephalitis following COVID‐19: Systematic review and meta‐synthesis. Neurol Neuroimmunol Neuroinflamm 2021;8(6):e1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roman GC, Gracia F, Torres A, et al. Acute Transverse myelitis (ATM):clinical review of 43 patients with COVID‐19‐associated ATM and 3 post‐vaccination ATM serious adverse events with the ChAdOx1 nCoV‐19 vaccine (AZD1222). Front Immunol 2021;12:653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tamaki A, Cabrera CI, Li S, et al. Incidence of bell palsy in patients with COVID‐19. JAMA Otolaryngol Head Neck Surg 2021;147:767–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taquet M, Husain M, Geddes JR, et al. Cerebral venous thrombosis and portal vein thrombosis: a retrospective cohort study of 537,913 COVID‐19 cases. EClinicalMedicine 2021;39:101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chou SH, Beghi E, Helbok R, et al. Global incidence of neurological manifestations among patients hospitalized with COVID‐19‐a report for the GCS‐NeuroCOVID consortium and the ENERGY consortium. JAMA Netw Open 2021;4:e2112131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance – United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep 2020;69:759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention : COVID‐19 Vaccinations in the United States [online]. Available at: https://data.cdc.gov/Vaccinations/COVID-19-Vaccinations-in-the-United-States-Jurisdi/unsk-b7fc/data.
- 22. Fleiss JLLB, Paik MC. Statistical methods for rates and proportions. 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc, 2003. [Google Scholar]
- 23. FDA and CDC Lift Recommended Pause on Johnson & Johnson (Janssen) COVID‐19 Vaccine Use Following Thorough Safety Review [online]. Available at: https://www.fda.gov/news-events/press-announcements/fda-and-cdc-lift-recommended-pause-johnson-johnson-janssen-covid-19-vaccine-use-following-thorough.
- 24. MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the advisory committee on immunization practices for use of the Janssen (Johnson & Johnson) COVID‐19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients – United States, April 2021. MMWR Morb Mortal Wkly Rep 2021;70:651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maramattom BV, Krishnan P, Paul R, et al. Guillain‐Barre syndrome following ChAdOx1‐S/nCoV‐19 vaccine. Ann Neurol 2021;90:312–314. [DOI] [PubMed] [Google Scholar]
- 26. Patone M, Handunnetthi L, Saatci D, et al. Neurological complications after first dose of COVID‐19 vaccines and SARS‐CoV‐2 infection. Nat Med 2021;27:2144–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single‐dose Ad26.COV2.S vaccine against Covid‐19. N Engl J Med 2021;384:2187–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singleton JA, Lloyd JC, Mootrey GT, et al. An overview of the vaccine adverse event reporting system (VAERS) as a surveillance system. VAERS Working Group. Vaccine 1999;17:2908–2917. [DOI] [PubMed] [Google Scholar]
- 29. About the Vaccine Adverse Event Reporting System [online] . Available at: https://wonder.cdc.gov/vaers.html. Accessed 2/11/22.
- 30. Li X, Ostropolets A, Makadia R, Shaoibi A, Rao G, et al. Characterizing the incidence of adverse events of special interest for COVID‐19 vaccines across eight countries: a multinational network cohort study. medRxiv 2021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Global COVID‐19 Neuro Research Coalition Co‐Authors for Indexing on Pubmed
Data Availability Statement
De‐identified data will be made available to qualified investigators upon written request to the corresponding author.


