Abstract
Coronavirus disease 2019 (COVID‐19) severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2 infection) can lead to intensive care unit (ICU) admission and critical illness myopathy (CIM). We examined 3 ICU patients with COVID‐19 who required mechanical ventilation for pneumonia and developed CIM. Pathological examination of the skeletal muscle biopsies revealed myopathic changes consistent with CIM, variable inflammation with autophagic vacuoles, SARS‐CoV immunostaining + fibers/granules, and electron microscopy findings of mitochondrial abnormalities and coronavirus‐like particles. Although mitochondrial dysfunction with compromised energy production is a critical pathogenic mechanism of non‐COVID‐19‐associated CIM, in our series of COVID‐19‐associated CIM, myopathic changes including prominent mitochondrial damage suggest a similar mechanism and association with direct SARS‐CoV‐2 muscle infection. ANN NEUROL 2022;91:568–574
The coronavirus disease 2019 (COVID‐19) pandemic due to severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) infection has caused tremendous challenges for healthcare systems. A significant proportion of patients with COVID‐19 require intensive care unit (ICU) admissions. ICU‐acquired weakness (ICUAW) is a common complication in critically ill patients, which results in prolonged ICU stay with increased morbidity and mortality. 1 , 2 ICUAW comprises critical illness myopathy (CIM) and/or critical illness polyneuropathy (CIP). It has been noted that approximately 50% of critically ill patients receiving mechanical ventilation for more than 7 days develop CIM and/or CIP. 3
The diagnosis of CIM is based on clinical, electrodiagnostic, and muscle biopsy findings. 2 , 4 The latter is characterized by severe muscle atrophy, loss of thick filaments, and electron microscopy (EM) findings of extensive loss of A bands (thick filaments) with retained I bands (thin filaments) and Z lines. Muscle fiber necrosis has also been reported as “acute necrotizing myopathy of intensive care.” 4 , 5 Although the pathogenesis of CIM/ICUAW remains incompletely understood, one critical mechanism of CIM/ICUAW involves mitochondrial dysfunction with compromised energy production due to reduced mitochondrial generation of ATP. 4 , 5 , 6 , 7 The mitochondrial dysfunction in critical illness may be attributed to direct mitochondrial damage further aggravated by inflammation, hyperglycemia, and free radicals. It is unknown whether the COVID‐19‐associated CIM differs from its non‐COVID‐19 counterpart, 8 and whether direct viral infection plays a role in COVID‐19‐associated myopathy. 9 This study examined COVID‐19‐associated CIM with a focus on its pathological changes.
Case Presentation and Muscle Pathology
Written informed consent was obtained from the patients/families for publication of this case series. A copy of the written consent is available for review by the Editor of this journal. In all patients, systemic SARS‐CoV‐2 infection was detected by reverse transcription‐polymerase chain reaction (PCR) assay of nasopharyngeal swabs. All the patients underwent an open skeletal muscle biopsy.
Muscle biopsy examination
The muscle biopsy frozen tissue was processed and examined as described previously. 10 Additional immunohistochemistry (IHC) was performed on formalin‐fixed, paraffin‐embedded tissue of the same biopsies at the IHC laboratory of University of California – San Francisco (UCSF) Health, San Francisco. The following antibodies were used: p62/SQSTM1 (Progen Biotechnik; 1:100 dilution following antigen retrieval), LC3 (clone 5F10, Enzo; 1:200 dilution following antigen retrieval), 11 and SARS‐CoV nucleoprotein (SARS‐CoV, Sino Biological; NB100‐56576, raised by a synthetic peptide corresponding to amino acids 399 to 411 [ADMDDFSRQLQNS‐C] of the human SARS coronavirus nucleocapsid protein; 1:5000 dilution following antigen retrieval). After deparaffination, rehydration, and antigen retrieval, automated IHC was performed on 5‐μm sections using the Ventana Ultra autostainer (Ventana Medical Systems; for p62) or Leica BOND III autostainer (Leica Biosystems Inc.; for LC3 and SARS‐CoV). The positive and negative controls for SARS‐CoV IHC are shown in Supplementary Figure S1. EM was performed in a small portion of the muscle tissue that was fixed in 2% glutaraldehyde, postfixed in 1% osmium tetroxide, dehydrated, and embedded in resin. Ultrathin sections were stained with uranyl acetate followed by lead citrate, and examined with JEOL 1230 transmission electron microscope. 10
Case 1
A 67‐year‐old woman was admitted with idiopathic thrombocytopenic purpura treated with high‐dose corticosteroids and intravenous immunoglobulin. Other medical history included hypertension, dyslipidemia, and type 2 diabetes. On admission day 15, she developed pneumonia and hypoxemia requiring intubation and prone ventilation; SARS‐CoV‐2 testing was positive. ICU course was complicated by Klebsiella bacteremia, elevated serum ammonia, metabolic encephalopathy, seizures, hemolytic anemia, liver cirrhosis, pleural effusion, and severe weakness with difficulty weaning off the respirator. Neurological examination revealed bilateral facial weakness, limb edema, and diffuse muscle atrophy with power graded 1/5 (Medical Research Council [MRC] scale) in deltoid muscles and 0/5 in all the other muscles. Nerve conduction studies (NCSs) showed unobtainable motor and sensory responses from facial, median, tibial, peroneal, and sural nerves; concentric needle electromyography (EMG) demonstrated increased insertional activity and presence of positive sharp waves (PSWs) and fibrillation potentials (FPs) in multiple distal and proximal muscles, with occasional myopathic motor units in deltoid muscle and no volitional activity in any other muscles. Serum creatine kinase (CK) levels were 35 to 175 IU/L (normal <220); plasma lactate levels were persistently elevated at 3.4 to 26.8 mmol/L (normal 0.5–2.0). A muscle biopsy of the left vastus lateralis, performed on day 92, revealed severe myopathic changes including frequent atrophic and degenerative fibers with loss of histological stainings, vacuoles containing cellular debris, increased adipose tissue, and scattered fibers with increased reactivity for nicotinamide adenine dinucleotide (NADH) and succinate dehydrogenase (SDH). IHC demonstrated focally scattered CD68+ macrophages, CD8+ T‐cells, rare CD4+ T‐cells, sarcolemmal and sarcoplasmic MHC‐1 staining in necrotic and nonnecrotic fibers, occasional p62+ and LC3+ puncta/vacuoles, and SARS‐CoV+ fibers showing granular staining (Fig 1A–I). EM showed extensive loss of A bands with retained I bands and Z lines, numerous abnormal mitochondria with focal to extensive loss of cristae and other degenerative changes, crystalline inclusions, and virus‐like particles (some of which had characteristic surface projections/spikes, consistent with SARS‐CoV‐2; Fig 1J–R). The patient had 3 follow‐up SARS‐CoV‐2 tests that remained positive; she died of sepsis and multi‐organ failure on day 101.
FIGURE 1.
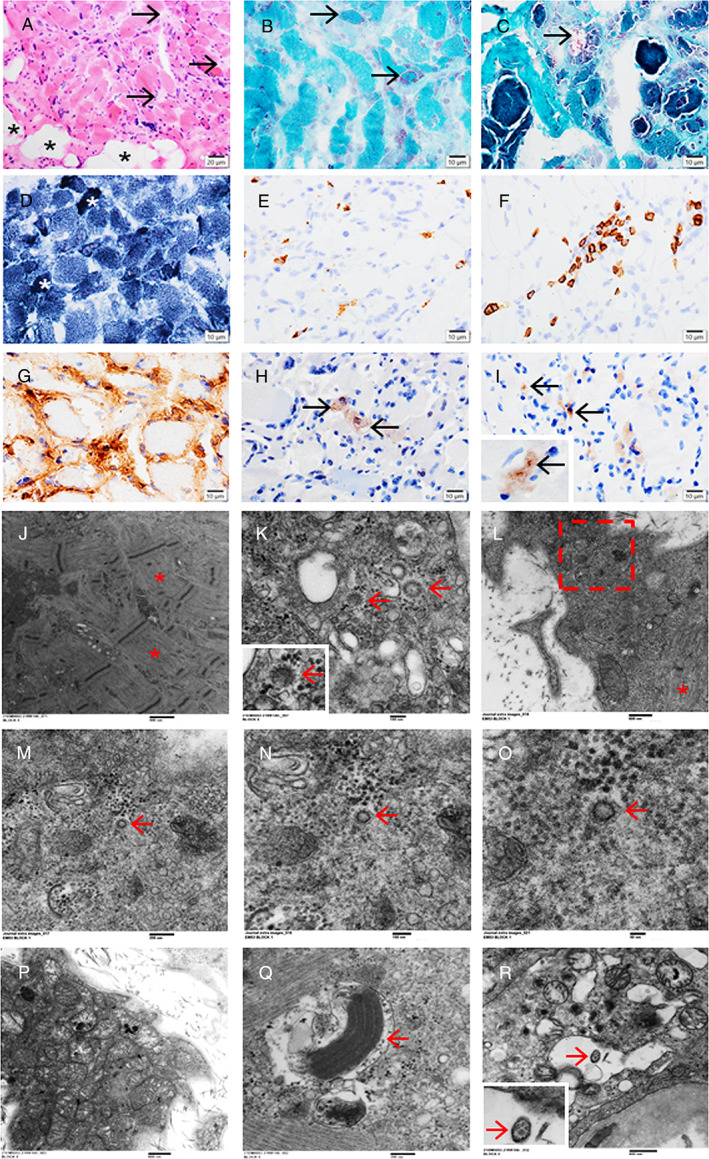
Case 1. A vastus lateralis muscle biopsy showing severe myopathic changes with atrophic fibers, degenerative/necrotic fibers with loss of normal staining (A, hematoxylin and eosin; arrows), increased adipose tissue (A,*), fibers with mitochondrial proliferation (B, Gomori trichrome staining; arrows), vacuoles containing debris (C, Gomori trichrome staining; arrow), and fibers with increased reactivity for NADH (D,*). Immunohistochemistry revealing focally scattered CD68+ macrophages (E), CD8+ T‐cells (F), MHC‐1 sarcolemmal and sarcoplasmic staining in necrotic and non‐necrotic fibers (G), occasional p62+ puncta/vacuoles (H, arrows), and SARS‐CoV+ fibers containing strongly positive granules (I, arrows). Electron microscopy demonstrating extensive loss of A bands with retained Z lines and I bands (J,*), virus‐like particles in necrotic fibers (K, arrows, with characteristic surface projections/spikes; an inset of the left arrowed area), and degenerative fibers (L‐O, a series of magnifications focusing on a virus‐like particle, arrows; L, with identifiable myofibrils,*; M, the rectangle in L; N, higher magnification of the arrowed area in M; O, higher magnification of the arrowed area in N), numerous abnormal mitochondria with electron‐dense granules (P–R), focal to extensive loss of cristae, crystalline inclusions (Q, arrow), and virus‐like particles (R, arrow, with an inset of the arrowed area). Scale bars: 20 μm (A), 10 μm (B–I), 800 nm (J), 100 nm (K, N), 600 nm (L, P), 200 nm (M, Q), 50 nm (O), and 500 nm (R). NADH = nicotinamide adenine dinucleotide; SARS‐CoV = severe acute respiratory syndrome‐coronavirus.
Case 2
A 25‐year‐old previously healthy man was admitted with a 1‐month history of myalgia, fever, cough, and shortness of breath. He tested positive for SARS‐CoV‐2 and was treated for pneumonia with secondary hypoxemia; he was admitted to the ICU requiring prone ventilation and treated with tocilizumab and high‐dose dexamethasone for 10 days. On ICU day 11, he was extubated but found to have profound symmetrical weakness of the upper and lower extremities. Neurological examination revealed an overweight man with diffuse erythematous rash (drug‐related), and symmetrical weakness that was MRC grade 1 to 2/5 in the proximal and 4/5 in the distal muscles of the upper and lower extremities. NCS demonstrated absent sensory responses for the right sural and superficial peroneal nerves. The compound motor action potentials (CMAPs) were diminished in the right peroneal and tibial nerves with no prolongation of distal motor latencies, CMAP durations, or slowing of the motor velocities; EMG demonstrated increased insertional activity with presence of PSW and FP in multiple muscles, and early recruitment in keeping with a myopathic process. CK was elevated >22,000 IU/L; however, after receiving intravenous immunoglobulin (2 g/kg), it decreased to 503 IU/L 7 days later and normalized a month later. Venous plasma lactate levels were 1.6 to 1.8 mmol/L (normal 0.0–1.4). SARS‐CoV‐2 testing repeated on day 33 was positive, and a biopsy of the vastus lateralis muscle was performed on day 36. The biopsy showed marked myopathic changes with a few necrotic and regenerating fibers, uneven NADH and SDH staining, p62+ and LC3+ autophagic puncta/vacuoles, moderate inflammatory cell infiltrates (mostly CD68+ macrophages with rare CD8+/CD4+ T‐cells), focal MHC‐1 staining, and SARS‐CoV immunostaining + fibers showing granular staining (Fig 2A–G). EM demonstrated loss of muscle fiber A bands with retained I bands and Z lines, abnormal mitochondria, and vacuoles containing virus‐like particles (Fig 2H–O). The patient's clinical course was complicated by staphylococcal bacteremia and acute kidney injury, but he responded well to treatment and regained motor function; follow‐up assessment 2 months after the discharge revealed no residual weakness.
FIGURE 2.
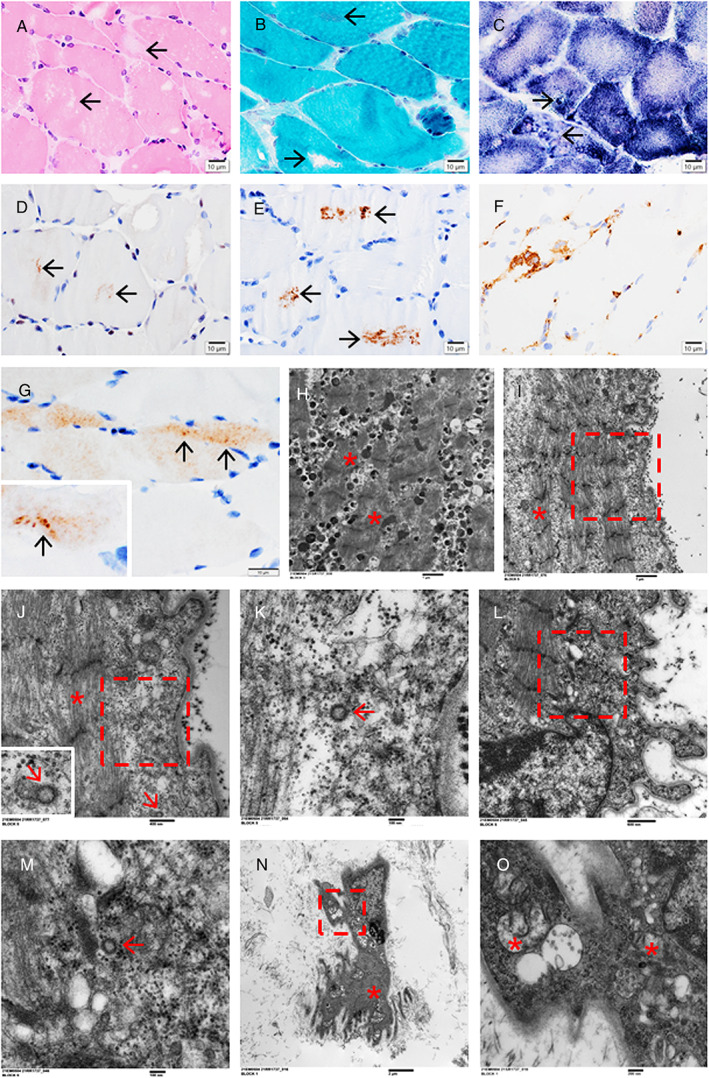
Case 2. A vastus lateralis muscle biopsy showing marked myopathic changes with vacuoles (A, loss of hematoxylin and eosin staining; B, lost or increased Gomori trichrome staining; arrows), rubbed‐out centers on NADH staining with granular/vacuolar positivity preferentially at the periphery (C, arrows), punctate immunostaining for p62 (D, arrows) and LC3 (E, arrows) in the fiber centers, focal MHC‐1 immunostaining (F), SARS‐CoV+ fibers containing strongly positive granules (G, arrows), and electron microscopy findings of fibers with loss of A bands and retained Z lines (H–J, *), vacuoles containing granules/particles (H), virus‐like particles in the subsarcolemmal areas (I–K, a series of magnifications focusing on a virus‐like particle; J, the rectangle in I, with an inset of the arrowed virus‐like particle; K, the rectangle in J, containing the arrowed virus‐like particle), and disrupted myofibrils (L; M, the rectangle in L, containing the arrowed virus‐like particle), and abnormal mitochondria in an atrophic/degenerative fiber (N, with focally identifiable myofibrils and Z lines, *; O, the rectangle in L, containing abnormal mitochondria, *). Scale bars: 10 μm (A–G), 1 μm (H, I), 400 nm (J), 100 nm (K, M), 600 nm (L), 2 μm (N), and 200 nm (O). NADH = nicotinamide adenine dinucleotide; SARS‐CoV = severe acute respiratory syndrome‐coronavirus.
Case 3
A 61‐year‐old, fully ambulatory woman presented with a 1‐month history of respiratory distress and fever, and SARS‐CoV‐2+ testing. Her medical history was remarkable for interstitial lung disease, inflammatory myositis (with positive anti‐SSA/Ro60, anti‐Jo‐1, and anti‐Ro52/TRIMS21 autoantibodies; treated with mycophenylate and corticosteroids), insulin‐dependent diabetes, and dyslipidemia. She required ICU admission with prolonged mechanical ventilation and difficulty weaning off the ventilator; she was treated with high‐dose pulse intravenous methylprednisolone. Neurological examination revealed an alert and conscious woman with significant peripheral edema, atrophy of the extremity muscles, and inability to voluntarily move her limbs (MRC grade 0/5), but light touch and vibration sensations were grossly intact. NCS showed no obtainable motor responses from the bilateral median, ulnar, and left peroneal nerves. The CMAPs were diminished for the left tibial nerve with no prolongation of distal motor latency, CMAP durations, or slowing of the motor velocity. The amplitudes of sensory nerve action potentials were diminished for the bilateral median and ulnar nerves. Palmar and sensory latencies were only mildly prolonged for the bilateral median nerves. Sensory responses were not obtainable for the left sural and superficial peroneal nerves (potentially technical issue and attributable to marked peripheral edema); EMG demonstrated PSW and FP in biceps, triceps, and vastus lateralis muscles with absent volitional activity. Serum CK levels were persistently low (23–41 U/L). Plasma lactate levels were elevated at 2.5 to 14.5 mmol/L (normal 0.5–2.0). A muscle biopsy of the vastus lateralis (severely atrophic at operation, on ICU day 53) exhibited fibro‐adipose tissue with rare residual atrophic fibers, occasional vacuoles containing muscle debris, focally scattered CD68+ macrophages, occasional CD8+ T‐cells, and ultrastructurally abnormal mitochondria (Fig 3). Although her atrophic muscle biopsy was SARS‐CoV IHC negative, systemic SARS‐CoV‐2 tests 15 days before the biopsy (ICU day 38) and 11 days after the biopsy were positive. She was discharged from the ICU with significant residual weakness on day 74.
FIGURE 3.
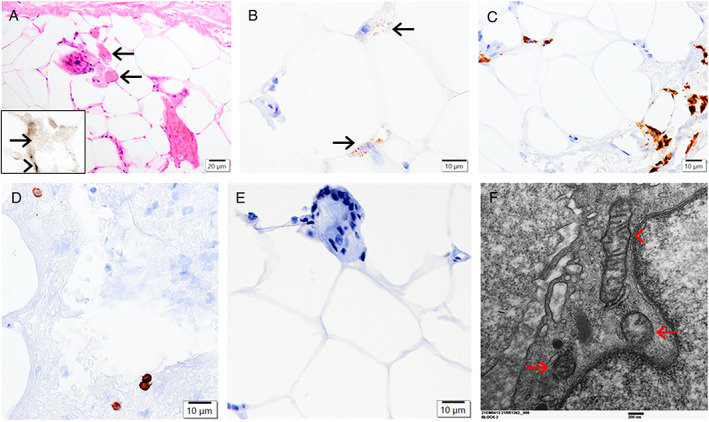
Case 3. A biopsy of the vastus lateralis muscle showing fibro‐adipose tissue with rare residual atrophic fibers (A, hematoxylin and eosin; arrows; inset in A, ATPase 4.3 histochemical staining: negative atrophic fibers compared to the arrowheaded positive granules), occasional vacuoles containing muscle debris positive for myoglobin immunostaining (B, arrows), focally scattered CD68+ macrophages (C) and CD8+ T‐cells (D), but negative SARS‐CoV immunostaining (E). Electron microscopy demonstrating abnormal mitochondria with focal degenerative changes (F; arrows, compared to a relatively preserved mitochondrion, arrowhead). Scale bars: 20 μm (A), 10 μm (B–E), and 200 nm (F). SARS‐CoV = severe acute respiratory syndrome‐coronavirus.
Discussion
SARS‐CoV‐2 infection‐associated myopathy may be an immune‐mediated necrotizing myopathy, unspecified necrotizing myopathy or myositis. 12 , 13 , 14 SARS‐CoV immunostaining of skeletal muscle was performed in 2 recently reported autopsy studies, and negative possibly due to postmortem changes with a postmortem interval of up to 6 days, 13 , 14 although positive SARS‐CoV‐2 testing by reverse transcription quantitative PCR (RT‐qPCR) was found in at least 16% (7/41) of quadriceps and 5% (2/42) of deltoid muscles. 14 Our present study (with the muscle biopsy tissue processed in 0.5 day) is the first to demonstrate SARS‐CoV+ myofibers with granular staining and EM finding of virus‐like particles consistent with SARS‐CoV‐2, which suggests direct viral infection of the muscle in some patients with COVID‐19.
Our present cases are clinically and pathologically consistent with CIM. The muscle biopsies in our study showed not only typical CIM pathological features (reported in non‐COVID‐19 patients), such as abnormal mitochondria and selective loss of thick filaments, but also additional features, such as variable inflammation with autophagic puncta/vacuoles as demonstrated by p62 and LC3 IHC, 11 and SARS‐CoV+ myofibers with EM finding of virus‐like particles. The presence of moderate inflammatory cell infiltrates with predominantly CD68+ macrophages and variable CD8+ T‐cells (but rare CD4+ T‐cells) is compatible with an inflammatory/infectious or necrotizing process, which may be associated with secondary mitochondrial abnormalities in critically ill patients. 15 In our study, the mitochondrial abnormalities in muscle biopsies were congruent with elevated blood lactate levels in those patients. The additional pathological features are presumably related to COVID‐19, in keeping with prolonged systemic SARS‐CoV‐2 infection in our patients.
There have been increasing reports of patients with COVID‐19‐associated CIM, but few studies with muscle biopsies. 2 , 16 A study of 12 patients who were diagnosed COVID‐19 positive with CIM/CIP had 3 muscle biopsies showing scattered necrotic and regenerative myofibers in one patient, and nonspecific myopathic features in 2 other patients, but no EM information. 2 On EM, SARS‐CoV‐2 particles are typically aggregated in single‐membrane‐bound vacuoles or isolated singly with surface projections (spikes) in well preserved tissue. 17 , 18 , 19 Our EM finding of coronavirus‐like particles (with some characteristic surface projections/spikes, as shown in Figs 1K–O and 2I–M) in the muscle is similar to that demonstrated previously in the other organs, such as the lungs, kidneys, and olfactory mucosa, 17 , 18 , 19 which is consistent with the IHC finding of SARS‐CoV+ granules and prolonged systemic SARS‐CoV‐2 infection with repeat PCR+ results in our patients.
Long‐term COVID‐19 (“long COVID”) has been increasingly recognized, with a range of persistent symptoms, including fatigue or muscle weakness in most patients following COVID‐19. 20 , 21 An EMG NCS study has identified myopathic changes in 55% of patients with long‐term fatigue after COVID‐19. 21 Although the underlying mechanisms of long‐term COVID‐19 remain unknown, the direct effects of viral infection have been considered along with a few other parameters, such as immunological response and ICU stay. 20 The rapid loss of muscle mass following severe COVID‐19 has been termed “post‐COVID‐19 associated sarcopenia” and mitochondrial dysfunction has been proposed to be one of the underlying mechanisms, as established for sarcopenia of aging. 22 Our present findings of the direct viral infection of skeletal muscle and associated mitochondrial abnormalities may help elucidate the pathogenesis of muscle weakness and myopathic changes in patients with long‐term COVID‐19.
In conclusion, our case series demonstrates myopathic changes that are consistent with CIM associated with SARS‐CoV‐2 muscle infection. We speculate that SARS‐CoV‐2 muscle infection contributes to the CIM‐like pathology in critically ill patients with COVID‐19. The COVID‐19‐associated CIM may show not only conventional CIM features described in non‐COVID‐19 patients, but also COVID‐19‐associated myopathic changes, such as autophagic vacuoles, SARS‐CoV immunostaining+ granules, variable inflammation, and additional mitochondrial abnormalities.
Author Contributions
D.D. and J.Q.L. contributed to the conception and design of the case series. D.D., M.M., K.G. and J.Q.L. contributed to the acquisition of data. All authors contributed to interpretation of the data. D.D., M.A.T., M.M., M.J.F., and J.Q.L. contributed to the writing and revision of the manuscript.
Potential Conflict of Interests
The authors declared no conflict of interest.
Supporting information
Supplementary Fig S1 Positive and negative controls for SARS‐CoV immunohistochemistry. (A) SARS‐CoV + hyaline membranes and alveolar macrophages containing strongly positive granules (arrows) in autopsy lung specimen from a patient who died of COVID‐19 pneumonia complicated by diffuse alveolar damage 1 week after testing positive for SARS‐CoV‐2 by PCR. (B) Negative SARS‐CoV immunostaining in the muscle biopsied from a patient with critical illness myopathy and no history of COVID‐19. A fiber in the center of the image shows nonspecific background staining but no strongly positive granules. (C) Negative SARS‐CoV immunostaining in the autopsy muscle specimen from a patient who died from COVID‐19 following a 3 week‐long ICU stay; at autopsy, he was found to have COVID‐19‐associated immune‐mediated necrotizing myopathy, severe type 2 fiber atrophy, and loss of myosin staining consistent with critical illness myopathy, as well as a subacute infarct in the left middle cerebral artery territory and pulmonary sequelae of COVID‐19. (D) Negative SARS‐CoV immunostaining in the muscle biopsied from a patient with post‐COVID‐19 seronegative immune‐mediated necrotizing myopathy. Scale bars: 50 μm A–D. All images were captured from the whole slide images acquired by Philips ultrafast bright field slide scanner. COVID‐19 = coronavirus disease 2019; ICU = intensive care unit; PCR = polymerase chain reaction; SARS‐CoV = severe acute respiratory syndrome‐coronavirus.
Contributor Information
Dubravka Dodig, Email: dubravka.dodig@uhn.ca.
Jian‐Qiang Lu, Email: luj85@mcmaster.ca.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol 2011;10:931–941. [DOI] [PubMed] [Google Scholar]
- 2. Cabañes‐Martínez L, Villadóniga M, González‐Rodríguez L, et al. Neuromuscular involvement in COVID‐19 critically ill patients. Clin Neurophysiol 2020;131:2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Z'Graggen WJ, Tankisi H. Critical illness myopathy. J Clin Neurophysiol 2020;37:200–204. [DOI] [PubMed] [Google Scholar]
- 4. Swash M, de Carvalho M. Intensive care unit‐acquired weakness: neuropathology. J Clin Neurophysiol 2020;37:197–199. [DOI] [PubMed] [Google Scholar]
- 5. Dubowitz V, Sewry C, Oldfors A. Muscle biopsy: a practical approach. 5th ed. Philadelphia, PA: Saunders Elsevier, 2020. [Google Scholar]
- 6. Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002;360:219–223. [DOI] [PubMed] [Google Scholar]
- 7. Friedrich O, Reid MB, Van den Berghe G, et al. The sick and the weak: neuropathies/myopathies in the critically ill. Physiol Rev 2015;95:1025–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tankisi H. Critical illness myopathy and polyneuropathy in Covid‐19: is it a distinct entity? Clin Neurophysiol 2021;132:1716–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suh J, Amato AA. Neuromuscular complications of coronavirus disease‐19. Curr Opin Neurol 2021;21:669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu JQ, Monaco CMF, Hawke TJ, et al. Increased intra‐mitochondrial lipofuscin aggregates with spherical dense body formation in mitochondrial myopathy. J Neurol Sci 2020;413:116816. [DOI] [PubMed] [Google Scholar]
- 11. Hiniker A, Daniels BH, Lee HS, Margeta M. Comparative utility of LC3, p62 and TDP‐43 immunohistochemistry in differentiation of inclusion body myositis from polymyositis and related inflammatory myopathies. Acta Neuropathol Commun 2013;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Veyseh M, Koyoda S, Ayesha B. COVID‐19 IgG‐related autoimmune inflammatory necrotizing myositis. BMJ Case Rep 2021;14:e239457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suh J, Mukerji SS, Collens SI, et al. Skeletal muscle and peripheral nerve histopathology in COVID‐19. Neurology 2021;97:e849–e858. [DOI] [PubMed] [Google Scholar]
- 14. Aschman T, Schneider J, Greuel S, et al. Association between SARS‐CoV‐2 infection and immune‐mediated myopathy in patients who have died. JAMA Neurol 2021;78:948–960. [DOI] [PubMed] [Google Scholar]
- 15. McKenna HT, Murray AJ. Reconsidering critical illness as an uncharacterised acquired mitochondrial disorder. J Intensive Care Soc 2020;21:102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frithiof R, Rostami E, Kumlien E, et al. Critical illness polyneuropathy, myopathy and neuronal biomarkers in COVID‐19 patients: a prospective study. Clin Neurophysiol 2021;132:1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Falasca L, Nardacci R, Colombo D, et al. Postmortem findings in Italian patients with COVID‐19: a descriptive full autopsy study of cases with and without comorbidities. J Infect Dis 2020;222:1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Menter T, Haslbauer JD, Nienhold R, et al. Postmortem examination of COVID‐19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020;77:198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meinhardt J, Radke J, Dittmayer C, et al. Olfactory transmucosal SARS‐CoV‐2 invasion as a port of central nervous system entry in individuals with COVID‐19. Nat Neurosci 2021;24:168–175. [DOI] [PubMed] [Google Scholar]
- 20. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet 2021;397:220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agergaard J, Leth S, Pedersen TH, et al. Myopathic changes in patients with long‐term fatigue after COVID‐19. Clin Neurophysiol 2021;132:1974–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Piotrowicz K, Gąsowski J, Michel JP, Veronese N. Post‐COVID‐19 acute sarcopenia: physiopathology and management. Aging Clin Exp Res 2021;33:2887–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig S1 Positive and negative controls for SARS‐CoV immunohistochemistry. (A) SARS‐CoV + hyaline membranes and alveolar macrophages containing strongly positive granules (arrows) in autopsy lung specimen from a patient who died of COVID‐19 pneumonia complicated by diffuse alveolar damage 1 week after testing positive for SARS‐CoV‐2 by PCR. (B) Negative SARS‐CoV immunostaining in the muscle biopsied from a patient with critical illness myopathy and no history of COVID‐19. A fiber in the center of the image shows nonspecific background staining but no strongly positive granules. (C) Negative SARS‐CoV immunostaining in the autopsy muscle specimen from a patient who died from COVID‐19 following a 3 week‐long ICU stay; at autopsy, he was found to have COVID‐19‐associated immune‐mediated necrotizing myopathy, severe type 2 fiber atrophy, and loss of myosin staining consistent with critical illness myopathy, as well as a subacute infarct in the left middle cerebral artery territory and pulmonary sequelae of COVID‐19. (D) Negative SARS‐CoV immunostaining in the muscle biopsied from a patient with post‐COVID‐19 seronegative immune‐mediated necrotizing myopathy. Scale bars: 50 μm A–D. All images were captured from the whole slide images acquired by Philips ultrafast bright field slide scanner. COVID‐19 = coronavirus disease 2019; ICU = intensive care unit; PCR = polymerase chain reaction; SARS‐CoV = severe acute respiratory syndrome‐coronavirus.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


