Abstract
Objective
As SARS‐CoV‐2 is known to invade neural cell mitochondria, a plasma system for quantifying central nervous system proteins in living humans was used to investigate neuropathogenic mechanisms of long‐COVID‐19.
Methods
SARS‐CoV‐2 proteins and mitochondrial proteins (MPs) in enriched plasma neuron‐derived extracellular vesicles (NDEVs) and astrocyte‐derived EVs (ADEVs) were quantified in resolved acute COVID‐19 without post‐acute sequelae of SARS‐CoV‐2 (PASC), PASC without neuropsychiatric manifestations (NP), PASC with NP and healthy controls.
Results
NDEV and ADEV mean levels of SARS‐CoV‐2 S1 and nucleocapsid (N) proteins were higher in all PASC sub‐groups than controls, but only N levels were higher in PASC with than without NP. Exosome marker CD81‐normalized NDEV mean levels of subunit 6 of MP respiratory chain complex I and subunit 10 of complex III, and neuroprotective MPs Humanin and mitochondrial open‐reading frame of the 12S rRNA‐c (MOTS‐c) all were decreased significantly in PASC with NP but not in PASC without NP relative to controls. NDEV levels of MPs voltage‐dependent anion‐selective channel protein 1 (VDAC1) and N‐methyl‐D‐aspartate receptor 1 (NMDAR1) were decreased in PASC without and with NP, whereas those of calcium channel MPs mitochondrial calcium uniporter (MCU), sodium/calcium exchanger (NCLX) and leucine zipper EF‐hand containing transmembrane 1 protein (LETM1) were decreased only in PASC with NP. ADEV levels of MCU and NCLX only were increased in PASC without and with NP.
Interpretation
Abnormal NDEV and ADEV levels of SARS‐CoV‐2 N and S1 protein and MPs correlate with NP and may be biomarkers for long‐COVID prognostics and therapeutic trials. ANN NEUROL 2022;91:772–781
Introduction
A significant set of individuals infected with the Severe Acute Respiratory Syndrome coronavirus‐2 (SARS‐CoV‐2), who develop Coronavirus Disease‐2019 (COVID‐19), will experience continuing or new symptoms after a 4‐week acute phase and often for many months. 1 , 2 The most disabling components of post‐acute sequelae of SARS‐CoV‐2 (PASC) or long‐COVID are respiratory, including dyspnea, chest pain, cough and wheezing, and neurological or psychiatric, including attention deficit, memory loss, depression, confusion, anxiety, obsessive–compulsive disorders and special sensory impairments. The incidence of associated psychoneurological conditions during acute COVID‐19 is directly related to disease severity, but the pathophysiology of such conditions in long‐COVID and useful predictive biomarkers are unknown. 3
In acute encephalopathic syndromes at the initial presentation of COVID‐19, viral particles were detected within cytoplasmic vacuoles of brain capillary endothelial cells in the frontal lobes and RT‐PCR confirmed the presence of SARS‐CoV‐2 in numerous regions of the brain at autopsy. 4 , 5 The angiotensin‐converting enzyme 2 (ACE2) receptor for SARS‐CoV‐2 also is widespread in numerous regions of the human brain, which likely promotes its neurotropic distribution. 6 SARS‐CoV‐2 RNAs are enriched in host neural cell mitochondria and nucleoli, and both 5′‐ and 3′‐untranslated regions contain mitochondrial localization signals. 7 , 8 Numerous SARS‐CoV‐2 proteins interact meaningfully with host mitochondrial proteins (MPs). 9 , 10 For example, SARS‐CoV‐2 ORF9c and Nsp7 interact, respectively, with mitochondrial NDUFAF1 and NDUFAF2, which both are involved in the assembly of complex I of the mitochondrial electron transport chain. It thus tentatively appears that both SARS‐CoV‐2 RNA‐ and protein‐dependent mechanisms may diminish effectiveness of neural mitochondrial pathways of defense against this virus. Mitochondrial localization of coronaviruses and mitochondrial dependence of their replication and pathways of cellular damage have been confirmed in domestic animal infections. 11 , 12 Coronavirus evasion of immunity also is strongly dependent on modulation of mitochondrial mechanisms. 13
There have been no reliable approaches in living patients for determining the extent or mechanisms of mitochondrial involvement in the effects of central nervous system (CNS) infection by SARS‐CoV‐2. Our recently established platform permitted separate enrichment of sets of exosome‐containing plasma extracellular vesicles derived from neurons, astrocytes, microglia and cerebrovascular endothelial cells in living humans. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 We focused initially on quantifying plasma neuron‐derived extracellular vesicle (NDEV) levels of functional proteins, that had been added to exosomal cargo in neuronal endosomal pathways and reflected neuronal levels in situ. Clinically relevant findings were prediction of a high risk of developing Alzheimer's disease from abnormal NDEV levels of pathogenic proteins 15 and correction of abnormal NDEV levels of some mitochondrial proteins (MPs) after successful responses to anti‐depressant therapy. 18 , 23 , 24 , 25
Our initial hypothesis based on the neural mitochondrial tropism and effects of SARS‐CoV‐2 was that delineation of the NDEV and astrocyte‐derived EV (ADEV) profiles of functional neural MPs in COVID‐19 might elucidate some aspects of pathogenesis of its diverse CNS effects. Here, we report both the presence of SARS‐CoV‐2 proteins in EVs and distinctively abnormal patterns of EV levels of some MPs during long‐COVID‐19 with neurological and psychiatric symptoms.
Methods
Participant Selection and Evaluation
All participants were enrolled in the San Francisco‐based Long‐term Impact of Infection with Novel Coronavirus (LIINC) COVID‐19 recovery cohort (NCT04362150; www.liincstudy.org) in the period March, 2020 to February, 2021 through physician‐ or self‐referral after a positive test result from a nasal or throat swab sample. 26 , 27 , 28 Their initial evaluation included confirmation of prior SARS‐CoV‐2 RNA positivity, a complete range of basic clinical laboratory studies, and detailed assessment of acute and post‐acute COVID‐19‐attributable symptoms by a clinical research coordinator and infectious disease specialist physician. This interview checklist encompassed questions about current symptoms, preexisting co‐morbidities and disabilities due to the present illness. At the initial and all subsequent visits, participants completed a general Patient Health Questionnaire (PHQ) and those with any neuropsychiatric (NP) issues also completed several specific questionnaires designed to further define NP manifestations. Relevant NP manifestations tabulated were irritability or agitation determined by the PHQ and anxiety or depression delineated by the EuroQuol, PHQ‐8 and General Anxiety Disorder‐7 (GAD‐7) questionnaires. 29 , 30 , 31 Exclusion criteria were transfusion‐dependent anemia, HIV‐AIDS, other known immunodeficiency, or inability to provide informed consent. Abnormal levels of selected plasma immune‐inflammatory cytokines and NDEV neurodegeneration‐associated proteins of some of these patients have been reported.28, 32
Four COVID‐19 groups were assembled for the study, in addition to age‐ and sex‐matched controls (Ctls) who donated plasma before the current pandemic: a) convalescence after acute COVID‐19 with elapse of at least 4 weeks since onset of SARS‐CoV‐2 infection, no fever and overall improvement in symptoms for at least 2 weeks, and no evidence of PASC, defined as continued manifestations of their acute COVID‐19 and/or new symptoms attributable to COVID‐19 that had appeared beyond 4 weeks after onset of acute COVID‐19, b) PASC without (w/o) neuropsychiatric (NP) symptoms, c) PASC with (w/) NP symptoms (≤7 symptoms) and d) PASC w/severe NP symptoms (>7 symptoms). Study blood samples were donated between 04‐24‐2020 and 02‐11‐2021.
This research was approved by the UCSF Institutional Review Board (IRB) and all participants provided written Informed Consent.
Isolation of Neuron‐Derived Extracellular Vesicles (NDEVs) and Astrocyte‐Derived EVs (ADEVs)
Portions of 0.25 ml of thawed EDTA‐plasma from the PASC period of each group (Table 1) were incubated with thromboplastin D (ThermoFisher Scientific, Waltham, MA) and treated with protease inhibitor cocktail (Roche, Indianapolis, IN) and phosphatase inhibitor cocktail (Thermo Fisher Scientific; DBS++) as described. 18 After centrifugation at 3000 g for 30 minutes at 4°C, total extracellular vesicles (EVs) were harvested from resultant supernatants by precipitation with 126 μL per tube of ExoQuick (System Biosciences, Mountain View, CA) and centrifugation at 1500 g for 30 minutes at 4°C.
TABLE 1.
Demographic and Clinical Characteristics of Participant Groups
| Control | COVID‐19 w/o PASC | PASC w/o NP | PASC w/ NP | PASC w/ severe NP | |
|---|---|---|---|---|---|
| Number | 12 | 8 | 15 | 15 | 8 |
| Mean age (range) | 48(26–67) | 42(30–54) | 46(34–56) | 34(31–46) | 49(44–52) |
| Female/male/TG male | 9/3/0 | 8/0/0 | 9/6/0 | 9/6/0 | 7/0/1 |
| Race, ethnicity H/W/B/A/O | 4/3/1/4/0 | 2/5/0/1/0 | 5/7/0/2/1 | 3/10/1/0/1 | 3/5/0/0/0 |
| Co‐Morbidities | |||||
| Autoimmune disease | 3 | 0 | 3 | 2 | 1 |
| Cancer | 1 | 1 | 0 | 0 | 1 |
| Diabetes | 1 | 0 | 0 | 0 | 0 |
| Cardiovascular disease | 2 | 0 | 1 | 0 | 1 |
| HIV/AIDS | 0 | 0 | 0 | 0 | 0 |
| Lung disease | 0 | 1 | 0 | 4 | 2 |
| Kidney disease | 1 | 0 | 0 | 0 | 0 |
| Days from symptom onset to blood sampling, mean(range) | N/A | 57(35–64) | 62(35–81) | 60(37–80) | 60(44–84) |
| Manifestations of PASC | |||||
| Total symptoms at time of blood sampling | 0 | 0 | 0–9 | 0–7 | 8–13 |
| Feeling fatigue or low energy | 0 | 0 | 5 | 3 | 8 |
| Trouble w/ smell or taste | 0 | 0 | 4 | 5 | 3 |
| Muscle aches | 0 | 0 | 2 | 4 | 6 |
| Loss of appetite | 0 | 0 | 0 | 2 | 4 |
| Trouble concentrating or thinking | 0 | 0 | 0 | 6 | 8 |
| Trouble w/ vision | 0 | 0 | 1 | 1 | 3 |
| Trouble w/ balance | 0 | 0 | 1 | 2 | 5 |
| Trouble w/ sleeping | 0 | 0 | 1 | 6 | 5 |
| Cough | 0 | 0 | 1 | 2 | 1 |
| Shortness of breath | 0 | 0 | 5 | 3 | 6 |
| Chest pain | 0 | 0 | 1 | 2 | 3 |
H, Hispanic; W, white; B, black; A, Asian; O, other; TG, transgender; np, neuropsychiatric manifestations; w/, with; w/o, without.
To enrich NDEVs including neuron‐derived exosomes (NDEs), replicate preparations of total plasma EVs were resuspended in 0.35 mL of DBS++ with 2.0 μg of mouse anti‐human CD171 (L1CAM neural adhesion protein) biotinylated antibody (clone 5G3; eBiosciences, San Diego, CA) in 50 μL of 3% bovine serum albumin (BSA; 1:3.33 dilution of Blocker BSA 10% solution in DBS; ThermoFisher Scientific) per tube. After this incubation, 10 μl of streptavidin‐agarose Ultralink resin (ThermoFisher Scientific) in 40 μl of 3% BSA were added to each tube followed by a second incubation as described. 19 After centrifugation at 800 g for 10 minutes at 4°C and removal of the supernatant, each pellet with NDEVs was suspended in 100 μl of cold 0.05 M glycine‐HCl (pH 3.0) for 5 minutes and centrifuged at 4000 g for 10 minutes, all at 4°C. Glycine‐HCl supernatants then were transferred to clean tubes containing 25 μl of 10% BSA and 10 μl of 1 M Tris–HCl (pH 8.0) and mixed gently. An aliquot of 5 μl was removed from each tube for NDEV counts before addition of 370 μl of mammalian protein extraction reagent (M‐PER; ThermoFisher Scientific). Astrocyte‐derived EVs (ADEVs) were enriched from total plasma EVs by immunoabsorption with mouse anti‐human glutamine aspartate transporter (GLAST) (ACSA‐1) biotinylated antibody (Miltenyi Biotec, Inc., Auburn, CA, USA), that yielded numbers, sizes, and contents of exosome markers, glial fibrillary acidic protein (GFAP) and glial‐derived neurotrophic factor (GDNF) identical to those described. 16 , 19 Resultant 0.5 ml lysates of NDEVs and ADEVs were frozen and thawed twice, and then stored at −80°C.
For counting and sizing of extracellular vesicles, each suspension was diluted 1:50 in PBS. The mean diameter (nanometers) and concentration (particles per milliliter) of EVs in each suspension were determined by nanoparticle tracking analysis (NTA) using the Nanosight NS500 system with a G532nm laser module and NTA 3.1 nanoparticle tracking software (Malvern Instruments, Malvern, United Kingdom) as described. 14
Quantification of NDEV and ADEV Proteins
Human NDEV and ADEV proteins were quantified by enzyme‐linked immunosorbent assay (ELISA) kits for tetraspanning exosome marker CD81, subunit 6 of NADH–ubiquinone oxidoreductase (respiratory chain complex I) (CI‐6), 16S rRNA‐encoded Humanin (CUSABIO by American Research Products, Waltham, MA), N‐methyl‐d‐aspartate receptor 1 (NMDAR1) (Novus Biologicals, LLC, Centennial, CO), myosin VI (MY06), subunit 10 of cytochrome b‐c1 oxidase (respiratory chain complex III) (CIII‐10) (Abbkine Scientific by American Research Products), mitochondrial open‐reading frame of the 12S rRNA‐c (MOTS‐c) (Cloud‐Clone Corp. by American Research Products), SARS‐CoV‐2 protein S1 (RBD), SARS‐CoV‐2 protein N (Abcam, Inc., Waltham, MA and Raybiotech Life, Inc., Peachtree Corners, GA), syntaphilin (SNPH), translocator protein (TSPO), leucine zipper EF‐hand containing transmembrane 1 protein (LETM1), voltage‐dependent anion‐selective channel protein 1 (VDAC1), mitochondrial calcium uniporter protein (MCU), and solute carrier family 8 member B1 (SLC24A6) or mitochondrial Na+/Ca++ exchanger (NCLX) (Wuhan FineTest Biotech Co. by American Research Products and Abbexa, Ltd., Cambridge, UK). The mean value for all determinations of CD81 in each assay group was set at 1.00, and relative values of CD81 for each sample were used to normalize their recovery. All ELISAs were performed by one investigator (EJG) without knowledge of the identity of any subject.
Statistics
After establishing that data were normally distributed, the significance of differences between COVID‐19 patient levels and control levels were calculated by an unpaired t test (Graphpad Holdings, LLC, San Diego, CA). Pearson Correlation Coefficient analysis was performed to assess relationships between NDEV counts and NDEV levels of CD81 (Graphpad Prism 9). There were no adjustments for potential confounding variables.
Results
Participant Characteristics
The participants in each sub‐group were predominantly early middle age, female and of Hispanic and European Caucasian ethnicity (Table 1). None of the participants with convalescent acute COVID‐19 w/o post‐acute sequelae of SARS‐CoV‐2 infection (PASC) had respiratory or neuropsychiatric (NP) abnormalities. Of the subjects with PASC, 30 had mild to moderate disease and eight had severe disease. There was a higher prevalence of pre‐existing lung disease in the participants who had PASC with NP (PASC w/ NP) and respectively lower and higher aggregate incidences of co‐morbidities in the convalescent COVID‐19 w/o PASC and healthy control (Ctl) sub‐groups. Healthy Ctls gave plasma in 2015 before the current pandemic. No participant had HIV/AIDS and none had received any SARS‐CoV‐2 vaccine. The prevalence and severity of respiratory‐type abnormalities were consistent with similarly mild to moderate involvement in the PASC w/o NP manifestations (PASC w/o NP) and PASC w/ NP sub‐groups (Table 1). None of the PASC w/o NP participants had trouble concentrating or thinking and none had new problems with memory, vision, balance, sleeping or appetite.
EV Properties
Counts of NDEVs in preparations from the PASC w/o NP sub‐group (15.2 ± 1.35 × 1010/ml) and the PASC with severe NP sub‐group (6.25 ± 0.94 × 1010/ml) were respectively significantly higher (p = 0.0002) and lower (p = 0.0235) than in Ctls (8.51 ± 0.41 × 1010/ml) and paralleled the levels for the exosome marker CD81 (Fig 1A). Levels of CD9 and CD63 markers for exosomes were as previously seen without significant differences among the subgroups. Counts of NDEVs in preparations from the PASC w/o NP sub‐group were significantly higher (p = 0.0078) than those of the PASC w/ NP subgroup (10.7 ± 0.86 × 1010/ml), as were the levels of CD81 (Fig 1A). The Pearson Correlation Coefficient for the comparison of NDEV counts with CD81 levels of the total group was 0.9879 (p < 0.0001). CD81 levels thus were used to normalize levels of all analytes as has been described. 14 All counts of ADEVs were in the range of 6.72 ± 0.40 × 1010/ml to 8.86 ± 0.59 × 1010/ml with no significant differences among sub‐groups. ADEV levels of the astroglial markers glial fibrillary acidic protein and glutamine synthetase were similar to those reported earlier. 16
FIGURE 1.
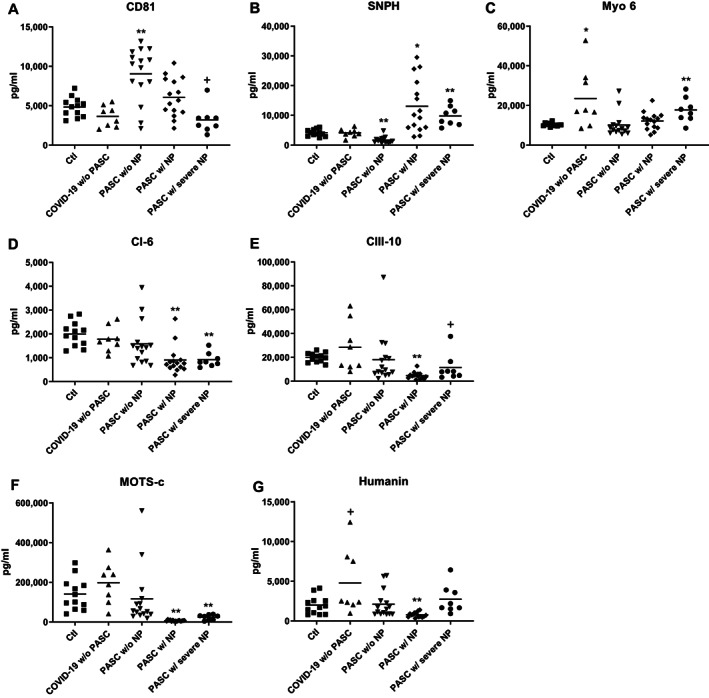
COVID‐19‐associated alterations in NDEV levels of proteins involved in mitochondrial dynamics, energy generation, metabolism, and maintenance of neuronal survival. Each point represents the value for one study participant after CD81 (A) normalization (B‐G). The horizontal line in each column of points depicts mean value. Statistical significance of the difference in level for each group was calculated relative to Ctl values by an unpaired t test; NS, not significant; +p <0.05; *p <0.01; **p <0.001. The significance (p values) of differences between participants who had PASC w/ neuropsychiatric (NP) findings and those who had PASC w/o NP and between participants who had PASC w/ NP and those who had PASC w/ severe NP, respectively, were: <0.01 and <0.01 for CD81 (A); <0.0001 and NS for syntaphilin (SNPH) (B); NS and <0.05 for myosin 6 (Myo 6) (C); <0.05 and NS for subunit 6 of NADH–ubiquinone oxidoreductase (respiratory chain complex I) (CI‐6) (D); <0.05 and <0.05 for subunit 10 of cytochrome b‐c1 oxidase (respiratory chain complex III) (CIII‐10) (E); <0.01 and <0.001 for mitochondrial open‐reading frame of the 12S rRNA‐c (MOTS‐c) (F); and <0.01 and <0.001 for 16S rRNA‐encoded Humanin (G). NP = neuropsychiatric abnormalities.
NDEV and ADEV Contents of SARS‐CoV‐2 Proteins
NDEV and ADEV levels of SARS‐CoV‐2 proteins spike S1 with the cellular receptor binding domain (RBD) and nucleocapsid (N) were significantly higher in all affected sub‐groups than in the healthy controls (Table 2). Comparing protein S1 levels among non‐control groups revealed a significant difference only between convalescent COVID‐19 w/o PASC and PASC w/ NP (p = 0.0172) for NDEVs, whereas there was a significant difference between COVID‐19 w/o PASC and all three PASC sub‐groups (p < 0.01) for ADEVs. Comparing protein N levels among non‐control groups revealed significant differences between PASC w/ NP and PASC w/o NP (p = 0.0298 for NDEVs and p = 0.0228 for ADEVs), and between COVID‐19 w/o PASC and both PASC w/ NP (p = 0.0109 for NDEVs and p = 0.0029 for ADEVs) and PASC w/severe NP (p = 0.0212 for NDEVs and p = 0.0109 for ADEVs). The mean levels of both S1 (RBD) and N proteins were significantly higher in NDEVs than ADEVs of the PASC w/ NP subgroup, which implied higher intracellular and possibly intramitochondrial levels in neurons than astrocytes. These differences suggested the importance of determining levels of NDEV and ADEV mitochondrial proteins (MPs) known to be abnormal in several central nervous system diseases.
Table 2.
SARS‐CoV‐2 Proteins in ADEVs and NDEVs of Participant Groups
| Source of EVs | SARS‐CoV‐2 protein | Control (n = 12) | COVID‐19 w/o PASC (n = 8) | PASC w/o NP (n = 15) | PASC w/ NP (n = 15) | PASC w/ severe NP (n = 8) |
|---|---|---|---|---|---|---|
| NDEVs | S1 (RBD) | 197 ± 11.2 | 628 ± 80.8** | 839 ± 217 † | 1,128 ± 133** | 994 ± 154** |
| N | 936 ± 90.9 | 11,265 ± 2495** | 15,283 ± 3308** | 26,838 ± 3814** | 23,560 ± 4027** | |
| ADEVs | S1 (RBD) | 84.2 ± 10.1 | 371 ± 50.9** | 727 ± 80.2** | 714 ± 60.9** | 645 ± 66.6** |
| N | 371 ± 13.8 | 8,576 ± 2332** | 12,452 ± 1627** | 17,979 ± 1618** | 17,241 ± 1810** |
N = nucleocapsid protein, NP = neuropsychiatric manifestations, RBD = receptor‐binding domain, w/= with, w/o = without. Each value is the mean pg/ml ± SEM. Statistical significance of differences between each value and the control value were determined by an unpaired Student's t test: †, p <0.05; **, p <0.001.
NDEV and ADEV Levels of Mitochondrial Proteins (MPs)
Levels of three pairs of MPs in plasma NDEV extracts have been shown to be abnormal relative to those of matched healthy controls in subjects with Alzheimer's disease (AD), major depressive disorders (MDDs) and first episode of psychosis (FP) in early schizophrenia. 23 , 24 , 33 These MPs were quantified initially in the NDEVs of current participants to identify effects of SARS‐CoV‐2 invasion of neurons. The NDEV level of tethering protein SNPH, which anchors mitochondria to neuron axonal microtubules, was statistically significantly decreased relative to that of Ctls in PASC w/o NP as in MDD, increased in PASC w/ NP and PASC w/ severe NP as in FP, but unaffected in convalescent COVID‐19 w/o PASC (Fig 1B). The NDEV level of tethering protein Myo 6, which anchors mitochondria to synaptic microfilaments, was significantly increased relative to that of Ctls only in PASC w/ severe NP and convalescent COVID‐19 w/o PASC (Fig 1C). NDEV levels of constituent proteins CI‐6 (Fig 1D) and CIII‐10 (Fig 1E) of the inner mitochondrial membrane electron transfer chain both were significantly decreased relative to Ctls in PASC w/ NP and PASC w/ severe NP as in MDDs and FP, but unchanged in PASC w/o NP and convalescent COVID‐19 w/o PASC. The NDEV level of metabolic regulatory peptide MOTS‐c, which is encoded by a mitochondrial ribosomal RNA, also was decreased significantly relative to Ctls in PASC w/ NP and PASC w/severe NP as in MDDs and FP, but unchanged in PASC w/o NP and convalescent COVID‐19 w/o PASC (Fig 1F). The NDEV level of neuroprotective MP Humanin, which is encoded by another mitochondrial ribosomal RNA, was decreased significantly relative to Ctls in PASC w/NP as in MDDs and FP, but unchanged in PASC w/o NP and increased marginally relative to Ctls in convalescent COVID‐19 w/o PASC (Fig 1G).
NDEV levels of six neuronal mitochondrial membrane ion channel/exchanger or transporter proteins, that have not been examined previously and of which five are involved in Ca++ movement across the mitochondrial inner (IMM) or outer (OMM) membranes, were quantified for the same four groups of patients and Ctls (Fig 2). VDAC1, the quantitatively predominant protein in OMM, is a voltage‐gated channel for Ca++, other ions, diverse metabolites and nucleotides. 34 NMDAR1 in the IMM of synaptic and extrasynaptic mitchondria, as well as in neuronal plasma membranes, is a ligand‐gated Ca++ channel. 35 VDAC1 and NMDAR1 levels in NDEV extracts both were significantly depressed below those of Ctls in PASC w/o NP and PASC w/ NP, but not in convalescent COVID‐19 w/o PASC (Fig 2A, E). In contrast, NDEV levels of the mechanistically distinct MCU, NCLX and LETM1 IMM channels for Ca++ all were decreased very significantly below those of Ctls in PASC w/NP, but not in PASC w/o NP or convalescent COVID‐19 w/o PASC (Fig 2B, C and D). The NDEV levels of OMM cholesterol translocator TSOP showed no differences from those of Ctls except for a marginal increase in PASC w/severe NP (Fig 2F).
FIGURE 2.
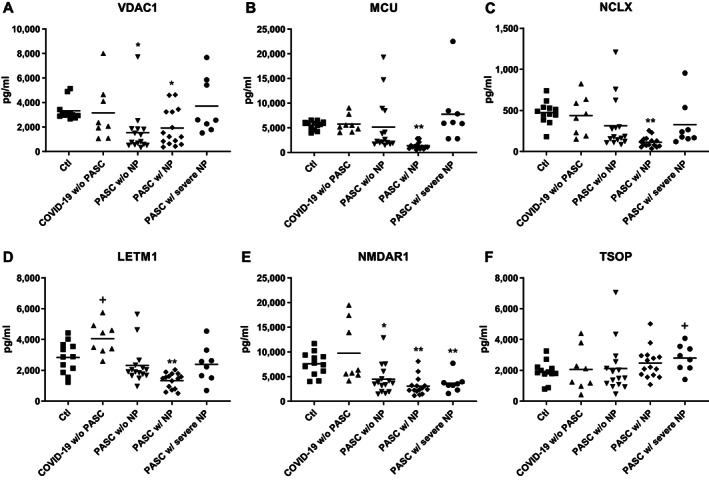
COVID‐19‐associated alterations in NDEV levels of proteins in mitochondrial ion channels and translocators. Each point represents the value for one study participant after CD81 normalization. The horizontal line depicts mean value. Statistical significance of the difference in level for each group was calculated relative to Ctl values by an unpaired t test; NS, not significant; +, p <0.05; *, p <0.01; **, p <0.001. The significance (p values) of differences between participants who had PASC w/ NP and those who had PASC w/o NP and between participants who had PASC w/ NP and those who had PASC w/ severe NP, respectively, were: NS and <0.05 for voltage‐dependent anion‐selective channel protein 1 (VDAC1) (A); <0.05 and <0.001 for mitochondrial calcium uniporter protein (MCU) (B); <0.05 and <0.05 for mitochondrial Na+/Ca++ exchanger (NCLX) (C); <0.01 and <0.01 for leucine zipper EF‐hand containing transmembrane 1 protein (LETM1) (D); NS and NS for N‐methyl‐D‐aspartate receptor 1 (NMDAR1) (E); and NS and NS for translocator protein (TSOP) (F). NP = neuropsychiatric abnormalities.
The ADEV levels of several types of MPs in PASC without or with neuropsychiatric manifestations were different from those of controls only for the IMM calcium channels MCU and NCLX (Table 3). There were no differences for CIII‐10, SNPH, Humanin, MOTS‐c (Table 3), VDAC1 or LETM1 (not shown). Further, the significant differences observed for MCU and NCLX were modest increases above control levels rather than the predominant decreases observed in NDEVs.
Table 3.
Abnormalities in Plasma ADEV Levels of Mitochondrial Functional Proteins in PASC Groups Limited to Calcium Channels
| Mitochondrial protein | Control | COVID‐19 w/o PASC | PASC w/o NP | PASC w/ NP | PASC w/ severe NP |
|---|---|---|---|---|---|
| CIII‐10 | 2,281 ± 149 | 3,026 ± 449 | 2,410 ± 277 | 2,415 ± 146 | 2,388 ± 203 |
| SNPH | 3,310 ± 523 | 3,208 ± 978 | 3,673 ± 445 | 2,663 ± 270 | 3,256 ± 643 |
| NCLX | 58.2 ± 2.70 | 85.5 ± 16.3 | 110 ± 13.8* | 109 ± 11.9** | 95.0 ± 13.8* |
| MCU | 4,796 ± 320 | 4,742 ± 229 | 6,067 ± 301* | 6,172 ± 300* | 4,673 ± 253 |
| Humanin | 381 ± 35.6 | 396 ± 54.6 | 430 ± 54.0 | 432 ± 40.8 | 428 ± 52.3 |
| MOTS‐C | 47,541 ± 6,237 | 50,502 ± 11,174 | 70,932 ± 11,715 | 73,194 ± 10,101 | 70,018 ± 12,203 |
NP = neuropsychiatric manifestations, w/= with, w/o = without. Each value is the mean pg/ml ± SEM. Statistical significance of differences between each value and the control value were determined by an unpaired Student's t test: *p <0.01; **p <0.001.
Discussion
The major assumption underlying our interpretation of these initial results is that levels of SARS‐CoV‐2 proteins and diverse MPs in ADEVs and NDEVs respectively reflect those in astrocytes and neurons, as well as in their mitochondria in situ. For other neuronal surface and intracellular membrane proteins, there is evidence that NDEV levels do represent accurately their CNS neuronal levels in steady states such as chronic neurodegenerative diseases. 36 However, less is known about MPs that enter NDEVs at least in part through mitophagic pathways and even less is understood about MPs in ADEVs. Further, there is no direct proof in human COVID‐19 that such changes in levels of neuronal MPs account for neuropsychiatric abnormalities in PASC. Nonetheless, abnormal neuronal physiology and diminished survival have been documented in cultured cells and animal models of human diseases when critical neuronal MP levels were altered proportionally to those found here in NDEV extracts. 37 , 38 Similarly abnormal NDEV levels of MPs have been documented in some human neurodegenerative diseases and mental illnesses with symptoms related to those of PASC. 23 , 24 , 25 , 33 NDEV levels of CI‐6 and CIII‐10 were decreased approximately 60% in MDDs, FP of early schizophrenia and AD, as in PASC w/ NP (Fig 1). SNPH was decreased approximately 60% in MDDs but increased nearly three‐fold in FP with changes of the same magnitude as in PASC w/o NP and PASC w/NP, respectively (Fig 1). Decreases in NDEV levels of MOTS‐c and Humanin in PASC w/NP resembled the changes in MDDs and FP (Fig 1).
Ca++ concentration [Ca++] in the mitochondrial matrix is a vital determinant of mitochondrial dynamic distribution, energy generation and other metabolic functions in neurons, as well as of neuronal survival. These roles of neuronal mitochondrial [Ca++] are in part coupled to their capacity to buffer functionally critical increases in neuronal cytoplasmic [Ca++]. Five neuronal MPs, that are involved in Ca++ homeostasis, were at abnormally low NDEV levels in PASC relative to healthy Ctls (Fig 2). Of these, only the ADEV levels of MCU and NCLX differed in PASC from those of Ctls with increases rather than their decreases in NDEVs (Table 3). MCU and NCLX are the principal channels for moving Ca++ in and out of the mitochondrial matrix, respectively, and the Na++/Ca++ exchanger LETM1 is one of many alternative channels to MCU. The importance of normal Ca++ efflux from mitochondria for neuronal function was demonstrated by the metabolic abnormalities, including increased levels of ROS, that resulted when expression of NCLX was genetically reduced. 39 A similar reduction in the expression of NCLX in neurons increased the rate of CNS accumulation of amyloid and altered behavior in a mouse model of AD. 40
It is interesting to find meaningful levels of two functionally critical SARS‐CoV‐2 proteins in NDEVs and that their NDEV levels differ significantly among the subgroups of participants (Table 2). Another laboratory has detected S1 (RBD) on the surface of intact plasma exosomes of COVID‐19 patients, that were anchored to a surface by anti‐exosome antibodies. 41 As for the mitochondrial proteins, it is not possible yet to assume that NDEV levels of SARS‐CoV‐2 proteins accurately reflect those in the central nervous system. Nonetheless, the mean levels of both S1 and N were significantly higher in ADEVs and NDEVs of all affected subgroups than background levels in controls. Further, mean ADEV and NDEV levels of N distinguished PASC w/NP from PASC w/o NP and from COVID‐19 w/o PASC.
Levels of mitochondrial proteins and viral proteins in plasma NDEVs may be valuable biomarkers for the diagnosis of PASC and for evaluating effects of new treatment modalities. However, the most useful clinical extensions of our current findings will be identification of any NDEV or ADEV mitochondrial protein or viral protein abnormalities that appear during acute COVID‐19 and that may accurately predict the risk of developing PASC and its likely severity and/or duration. This will require longitudinal studies of a larger number of patients at multiple times from acute COVID‐19 into PASC with many different manifestations and through complete resolution without and with treatments. With much larger numbers of plasmas to study, it may be necessary to introduce a faster and more efficient hybrid technology between our techniques and those of Pesce, et al. 41 This would involve anchoring all plasma EVs to wells by an anti‐EV surface antibody and then double labeling with a neuron‐specific antibody and an analyte‐directed antibody. Some additional mitochondrial analytes should be included in these analyses for parallel assessment of inflammation with neural cell dysfunction and identification of potential drug targets.
Author Contributions
EJG, MJP, DK, SGD, JDK and TJH contributed to the conception and design of the study; EJG, MJP, MM, SL, SG, RH, JC, EM and JM contributed to the acquisition and analysis of data; EJG and MJP contributed to drafting the text or preparing the figures.
Potential Conflicts of Interest
No authors declare any conflict‐of‐interest except for Dr. Goetzl who has filed an application covering this lab methodology with the US Patent and Trademark Office.
Acknowledgments
We thank personnel of the UCSF AIDS Specimen Bank for processing plasmas and maintaining the LIINC Biospecimen Repository. We are grateful to Judith H. Goetzl for preparing publication illustrations. Support for the research came from the UCSF Resource Allocation Program, the Intramural Program of the National Institute on Aging (MM and DK) and NIH grants NIAID3RO1AI141003‐03S1 (TJH) and NIAIDK23AI157875 (MJP).
References
- 1. Lopez‐Leon S, Wegman‐Ostrosky T, Perelman C, et al. More than 50 long‐term effects of COVID‐19: a systematic review and meta‐analysis. Sci Rep 2021;11:16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Michelen M, Manoharan L, Elkheir N, et al. Characterising long COVID: a living systematic review. BMJ glob Health 2021;6:e005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taquet M, Geddes JR, Husain M, et al. 6‐month neurological and psychiatric outcomes in 236 379 survivors of COVID‐19: a retrospective cohort study using electronic health records. Lancet Psychiatry 2021;8:416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paniz‐Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). J Med Virol 2020;92:699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guerrero JI, Barragan LA, Martinez JD, et al. Central and peripheral nervous system involvement by COVID‐19: a systematic review of the pathophysiology, clinical manifestations, neuropathology, neuroimaging, electrophysiology, and cerebrospinal fluid findings. BMC Infect Dis 2021;21:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen R, Wang K, Yu J, et al. The spatial and cell‐type distribution of SARS‐CoV‐2 receptor ACE2 in the human and mouse brains. Front Neurol 2020;11:573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020;579:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu K, Zou J, Chang HY. RNA‐GPS predicts SARS‐CoV‐2 RNA residency to host mitochondria and nucleolus. Cell Syst 2020;11(1):102–108 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gordon DE, Jang GM, Bouhaddou M, et al. A SARS‐CoV‐2 protein interaction map reveals targets for drug repurposing. Nature 2020;583:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu R, Zhao X, Li J, et al. Genomic characterisation andepidemiology of 2019 novel coronavirus: implications for virus origins andreceptor binding. Lancet 2020;395(10224):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee SM, Kleiboeker SB. Porcine reproductive and respiratory syndrome virus induces apoptosis through a mitochondria‐mediated pathway. Virology 2007;365:419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang J, Han Y, Shi H, et al. Swine acute diarrhea syndrome coronavirus‐induced apoptosis is caspase‐ and cyclophilin D‐ dependent. Emerg Microbes Infect 2020;9:439–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gatti P, Ilamathi HS, Todkar K, Germain M. Mitochondria targeted viral replication and survival strategies‐prospective on SARS‐CoV‐2. Front Pharmacol 2020;11:578599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mustapic M, Eitan E, Werner JK Jr, et al. Plasma extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes. Front Neurosci 2017;11:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fiandaca MS, Kapogiannis D, Mapstone M, et al. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case‐control study. Alzheimers Dement 2015;11:600–7 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goetzl EJ, Mustapic M, Kapogiannis D, et al. Cargo proteins of plasma astrocyte‐derived exosomes in Alzheimer's disease. FASEB J 2016;30:3853–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Winston CN, Goetzl EJ, Akers JC, et al. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement (Amst) 2016;3:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goetzl EJ, Abner EL, Jicha GA, et al. Declining levels of functionally specialized synaptic proteins in plasma neuronal exosomes with progression of Alzheimer's disease. FASEB J 2018;32:888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goetzl EJ, Schwartz JB, Abner EL, et al. High complement levels in astrocyte‐derived exosomes of Alzheimer disease. Ann Neurol 2018;83:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goetzl EJ, Elahi FM, Mustapic M, et al. Altered levels of plasma neuron‐derived exosomes and their cargo proteins characterize acute and chronic mild traumatic brain injury. FASEB J 2019;33:5082–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goetzl EJ, Ledreux A, Granholm AC, et al. Neuron‐derived exosome proteins may contribute to progression from repetitive mild traumatic brain injuries to chronic traumatic encephalopathy. Front Neurosci 2019;13:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kapogiannis D, Mustapic M, Shardell MD, et al. Association of Extracellular Vesicle Biomarkers with Alzheimer Disease in the Baltimore longitudinal study of aging. JAMA Neurol 2019;76:1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goetzl EJ, Srihari VH, Guloksuz S, et al. Decreased mitochondrial electron transport proteins and increased complement mediators in plasma neural‐derived exosomes of early psychosis. Transl Psychiatry 2020;10:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goetzl EJ, Wolkowitz OM, Srihari VH, et al. Abnormal levels of mitochondrial proteins in plasma neuronal extracellular vesicles in major depressive disorder. Mol Psychiatry 2021;26:7355–7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goetzl EJ, Srihari VH, Guloksuz S, et al. Neural cell‐derived plasma exosome protein abnormalities implicate mitochondrial impairment in first episodes of psychosis. FASEB J 2021;35:e21339. [DOI] [PubMed] [Google Scholar]
- 26. Peluso MJ, Kelly JD, Lu S, et al. Persistence, magnitude, and patterns of postacute symptoms and quality of life following onset of SARS‐CoV‐2 infection: Cohort description and approaches for measurement. Open Forum Infect Dis 2022;9(2):ofab640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peluso MJ, Deitchman AN, Torres L, et al. Long‐term SARS‐CoV‐2‐specific immune and inflammatory responses in individuals recovering from COVID19 with and without postacute symptoms. Cell Rep 2021;36(6):109518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peluso MJ, Takahashi S, Hakim J, et al. SARS‐CoV‐2 antibody magnitude and detectability are driven by disease severity, timing, and assay. Sci Adv 2021;7(31). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rabin R, de Charro F. EQ‐5D: a measure of health status from the EuroQol group. Ann Med 2001;33:337–343. [DOI] [PubMed] [Google Scholar]
- 30. Kroenke K, Strine TW, Spitzer RL, et al. The PHQ‐8 as a measure of current depression in the general population. J Affect Disord 2009;114:163–173. [DOI] [PubMed] [Google Scholar]
- 31. Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD‐7. Arch Intern Med 2006;166:1092–1097. [DOI] [PubMed] [Google Scholar]
- 32. Sun B, Tang N, Peluso MJ, et al. Characterization and biomarker analyses of post‐COVID‐19 complications and neurological manifestations. Cell 2021;10:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yao PJ, Eren E, Goetzl EJ, Kapogiannis D. Mitochondrial electron transport chain protein abnormalities detected in plasma extracellular vesicles in Alzheimer's disease. Biomedicine 2021;9:1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Camara AKS, Zhou Y, Wen PC, et al. Mitochondrial VDAC1: a key gatekeeper as potential therapeutic target. Front Physiol 2017;8:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Korde AS, Maragos WF. Identification of an N‐methyl‐D‐aspartate receptor in isolated nervous system mitochondria. J Biol Chem 2012;287:35192–35200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Delgado‐Peraza F, Nogueras‐Ortiz CJ, Volpert O, et al. Neuronal and astrocytic extracellular vesicle biomarkers in blood reflect brain pathology in mouse models of Alzheimer's disease. Cell 2021;10:993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kwok WM, Tajkhorshid E, Camara AKS. Editorial: mitochondrial exchangers and transporters in cell survival and death. Front Physiol 2021;12:745353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Urbani A, Prosdocimi E, Carrer A, et al. Mitochondrial ion channels of the inner membrane and their regulation in cell death signaling. Front Cell Dev Biol 2020;8:620081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Palty R, Silverman WF, Hershfinkel M, et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci U S A 2010;107:436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jadiya P, Kolmetzky DW, Tomar D, et al. Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer's disease. Nat Commun 2019;10:3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pesce E, Manfrini N, Cordiglieri C, et al. Exosomes recovered from the plasma of COVID‐19 patients expose SARS‐CoV‐2 spike‐derived fragments and contribute to the adaptive immune response. Front Immunol 2021;12:785941. [DOI] [PMC free article] [PubMed] [Google Scholar]


