Abstract
Microelements represent an emerging resource for medicine and its preventive branch. Zinc is the second most abundant element in our organism with peculiar physiologic functions and pathophysiologic implications in systemic and gastrointestinal (GI) diseases. It interacts very often with gut microbiota (GM) and can affect natural course of GI diseases through a bidirectional relationship with intestinal bugs. We aimed to review literature data regarding zinc chemistry, role in health, and GI diseases in man with a special focus on its interaction with GM. We conducted a search on the main medical databases for original articles, reviews, meta‐analyses, randomized clinical trials and case series using the following keywords and acronyms and their associations: zinc, microelements, gut microbiota, gut health, and COVID‐19. Zinc has a rapid and simple metabolism and limited storage within our body. Its efficacy on immune system modulation reflects on improved response to pathogens, reduced inflammatory response, and improved atopic/allergic reactions. Zinc is also involved in cell cycle regulation (namely, apoptosis) with potential anti‐cancerogenic effects. All these effects are in a “symbiotic” relationship with GM. Finally, zinc shows preliminary viral antireplicative effects. Zinc seems to gain more and more evidences on its efficacy in allergic, atopic and infectious diseases treatment, and prevention. COVID‐19 can be the booster for research on future applications of zinc as perfect “postbiotic” in medicine.
Keywords: COVID‐19, gut health, gut microbiota, microelements, zinc
Abbreviations
- A2M
alpha‐2‐macroglobulin
- ACE‐2
acetyl cholinesterase 2
- AdcABC, YcdHI‐YceA or YciABC
zinc transport systems
- cAMP
cyclic adenosine monophosphate
- CD
Crohn's disease
- CNS
central nervous system
- COX
cyclo‐oxygenase
- E. coli
Escherichia coli
- ETEC
enterotoxigenic E. coli
- FDA
Food and Drug Administration
- GERD
gastroesophageal reflux disease
- GH
growth hormone
- GI
gastrointestinal
- GM
gut microbiota
- IBD
inflammatory bowel disease
- IGF‐1
insulin‐like growth factor 1
- MT
metallothionein
- PPI
proton pump inhibitors
- RDA
recommended dietary allowance
- SIRT1
Sirtuin 1
- TG‐2
transglutaminase‐2
- TJ
tight junctions
- TPN
total parenteral nutrition
- UC
ulcerative colitis
- WHO
World Health Organization
- ZnO
zinc oxide
- ZnuABC
zinc uptake system proteins
- Zo‐1
zonulin‐1
1. INTRODUCTION
Microelements play a wide and variegate role in animals' and humans' physiology and pathophysiology.
Similarly to A, D, E vitamins and the most abundant iron, zinc is a divalent, nonredox active cation without reducing or oxidizing properties in mammalians cells. 1 Interestingly, the tiny zinc is an integral component of almost 10% of the human “proteome” (e.g., several key enzymes and transcription factors). Consequently, zinc is an essential modulator of mammalians' “epigenome.”
In human body, zinc is mainly present in bone and skin. Extracellulary, zinc is predominantly bound to several proteins (albumin, alpha‐2‐macroglobulin [A2M], and transferrin). 2
Intracellular zinc levels reflect in real time its metabolism and functioning. Indeed, intracellular zinc content is regulated via the modulation of the zinc‐sequestering proteins, namely “metallothioneins” (MT). The latter are able to trigger cytokines secretion from macrophages. This is the first hint of the multitasking properties of zinc in our immune‐system modulation. 3 Further, there are also intracellular zinc transporter proteins. 3
Thus, zinc is a cofactor of several enzymes and strictly interacts with gut microbiota (GM) that is a complex ecosystem that harbors the human GI tract: it includes over 100 trillion microbes, mainly bacteria, 4 divided into phyla, classes, orders, families, genera, and species. 5 Main gut microbial phyla are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fuso‐bacteria, and Verrucomicrobia. The entire genome of the gut microbiota is called “microbiome,” about 150‐fold bigger than that of human cells. 6
One‐tenth of the total colonizing bacterial species per individual constitutes a dynamic “microbial fingerprint,” potentially modulated by aging, dietary changes, and use of antibiotics, prebiotics, and probiotics. 7 Indeed, there is an intestinal microbial “core” that includes 66 species conserved in over 50% of the general population. 8
Gut microbiota is crucial for nutrients absorption and fermentation, regulation of intestinal permeability (IP), host metabolism (e.g., carbohydrates absorption and processing, proteins putrefaction, bile acids formation, insulin sensitivity), and modulation of mucosal/systemic immunity. The latter is crucial for maintenance of antigen tolerance and helps containing pathogens expansion. 9
Intra‐ and extracellular zinc concentrations can affect gut microbiota behavior and vice versa. This can contribute to alteration of qualitative and quantitative gut microbiota composition, namely “dysbiosis,” starting from initial “eubiosis.” 10 In the middle of the co‐existence of intra‐ and extracellular zinc content, diet, prebiotics, and probiotics play a variegate role.
Thus, we aimed to review literature data on zinc biochemistry, metabolism, and functions in health and main GI diseases, with a special focus on its relationship with GM at the time of SARS‐CoV 2 pandemic.
2. MATERIALS AND METHODS
We conducted a PubMed and Medline search for original articles, reviews, meta‐analyses, and case series using the following keywords, their acronyms, and their associations: zinc, microelements, gut microbiota, gut health, and COVID‐19. When appropriate, preliminary evidences from abstracts belonging to main national and international gastroenterological meetings (e.g., United European Gastroenterology Week, Digestive Disease Week) were also included. The papers found from the above‐mentioned sources were reviewed by two of the authors (E.S. and V.M.) according to PRISMA guidelines. 11 The last MEDLINE search was dated November 30, 2021.
3. RESULTS
3.1. Zinc sources, metabolism, and functions in health
Beside iron, zinc is the second most abundant biometal found in human body (e.g., tissues and fluids). It is estimated a total amount of zinc in adults' body of 1.4–2.3 g. 12 Interestingly, 85% of zinc is found in skeletal muscle and bone, 11% in skin and liver, and the remaining in all the other tissues, with high concentrations in prostate and eye. 13 On the other hand, plasma zinc represents about 0.1% of the total zinc amount. 14
Zinc is stored in cellular vesicles (namely, “zincosomes”) and released in a controlled way. 15 Of interest, zinc content is not regulated by a precise and solid storage system like iron, which emphasizes the need for a regular dietary supply. 16 Dietary sources of zinc are red meat and poultry, seafood (e.g., oysters, crab, lobster) and, in smaller quantities, dairy products, beans, nuts, whole grains, fortified breakfast cereals. Of relevance, the presence of phytates in cereals, legumes, and grains is able to bind zinc and inhibits its absorption versus those derived from animal foods. 17 , 18
Zinc absorption is mainly a process of the duodenum and proximal jejunum. It is increased by citric acid and decreased by iron, copper, calcium, fibers, and phytate. Further, a portion of zinc is bound to MT and stored in enterocytes (replacing GI loss due to mucosal shedding); the rest enters the blood circle and is redistributed to tissues. The excess amount of zinc is excreted by urine. 2
Therefore, zinc homeostasis is regulated at a plasmatic concentration cut‐off value of 12–15 μmol/L. 19 The latter is affected by intestinal absorption, gastrointestinal/urinary excretion, and cellular retention.
In humans, recommended dietary allowance (RDA) has been established at 8–11 mg/day. Requirements increase in infants, children, pregnant, lactating women, and, last but not least, elderly. The tolerable upper limit of zinc intake to avoid toxicity is 40 mg/day. 20
Zinc was recognized to be essential for human health in 1963. Since 1978, it is mandatory to include zinc in the total parenteral nutrition composition according to Food and Drug Administration (FDA). 21
Zinc has several functions: It has a structural role in proteins, nucleic acid, ribosomes, and cell membranes composition 22 ; it has a “catalytic” function as a cofactor of more than 300 enzymes (e.g., carbonic anhydrase, alkaline phosphatase, alcohol dehydrogenase, superoxide dismutase) 23 ; and it has regulatory role in apoptosis, gene transcription, neurotransmission, 24 hormones release (especially growth hormone [GH] secretion and insulin‐like growth factor 1 [IGF‐1]). 25 Finally, zinc also regulates directly and indirectly oxidative cascade and immune system. 15
Clinically, zinc is crucial in wound healing processes, immune response to pathogens, coagulation, cognitive functions development and regulation, reproductive male system development, pregnancy, and infants growth. 15
According to World Health Organization (WHO), nearly 2 billion subjects in the developing world can present with zinc nutritional deficiency. The latter can result from decreased intake (malnutrition, anorexia, parenteral nutrition) or bioavailability (strict vegetarian diets, iron, copper or calcium over‐intake), decreased absorption (celiac sprue, inflammatory bowel disease, chronic pancreatic disease, portal hypertension), increased losses (renal disease, diuretics use, burns, hemodialysis, exudative skin disease, chronic blood loss or gastrointestinal loss), increased requirement (pregnancy, lactation, neoplasia, chronic infections or inflammation) 26 (Table 1).
TABLE 1.
Main causes of zinc deficiency
| Mechanism | Individual causes |
|---|---|
| Reduced/inadequate intake | GI disease: Crohn's disease, jejunoileal bypass, previous bariatric surgery, small bowel resection, acrodermatitis enteropathica, alcoholic pancreatitis, cystic fibrosis |
| Reduced absorption | Low dietary zinc, inadequately supplemented nutrition, diet rich in phytate, sodium polyphosphate or EDTA |
| Increased zinc losses | Inflammatory bowel disease, diarrhea, steatorrhea, enterostomy, fistula, chyle leaks: skin burns; urine losses: burns, trauma, sepsis, renal disease, alcoholism, drugs (e.g., thiazides, penicillamine, diethylenetriamine pentacetate, valproate, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, EDTA‐containing propofol and chelating agents, cysteine, cisplatin, estrogen, oral contraceptive pill, corticosteroids), hemodialysis (namely, hemofiltration) |
| Increased zinc demand | Systemic illness: inflammatory response (e.g., sepsis, ARDS, COVID‐19); pregnancy, lactation |
Interestingly, no single test reflects whole body zinc status in humans. Plasma or serum zinc dosages are the most widely used as they describe dietary supplementation in patients with low or moderate loss. Nevertheless, these tests are affected by significant variability, namely low sensitivity and specificity. 16 In urinary zinc excretion assessment, hair zinc dosage appears to describe zinc supplementation with a limited use in case of suspected deficiency. 27
Zinc deficiency is suspected in case of subjects suffering from anorexia, dysgeusia, and dysosmia; diarrhea can occur and worsen zinc losses. Skin is affected by rash, alopecia, nonhealing ulcers, and delayed wound healing. Patients also show recurrent infections. Impaired cognitive function, visual loss, poor growth, and hypogonadism have also been reported (Table 2).
TABLE 2.
Clinical manifestations of zinc deficiency
| Organ/System | Clinical manifestation |
|---|---|
| Skin | Skin rash, alopecia, nonhealing ulcers, delayed wound healing |
| GI tract | Dysgeusia, diarrhea, malabsorption, infections development |
| CNS | Impaired cognitive function, dysosmia |
| Immune system | Recurrent infections, cancer(s) |
| Bone(s) | Poor growth |
| Reproductive system | Hypogonadism, low birth weight, congenital abnormalities |
Abbreviations: CNS, central nervous system; GI, gastrointestinal.
3.2. Zinc and gut microbiota: Animal and human studies evidences
3.2.1. Animal studies
In general, bacteria are able to store zinc via zinc‐binding proteins. They range from 5% to 6% of total proteins in prokaryotic organisms. 28 These proteins exert different actions such as bacterial gene expression, metabolic, antioxidant, and, last but not least, affect virulence.
On the other side of the wall, zinc contributes to the regulation of immune system with protection against bacterial infections. This can be explained by its inhibition of manganese import used by pathogenic bacteria. 29
From an animal evidence‐based point of view, zinc is commonly used as nutraceutical in animal feeding to reduce infections prevalence. In fact, zinc oxide (ZnO) is commonly used in swine feeding at a dose that greatly exceeds the dietary requirements (50–125 mg/kg of feed) 30 to ameliorate diarrhea incidence in postweaning phase. This results in a bigger swine growth and lower gut proliferation of pathogens. From a mechanistic point of view, zinc has mainly three effects: reduction of intestinal damage, anti‐inflammatory, and modulation of mucosal integrity through gut microbiota composition reshuffling. In particular, zinc decreases enterobacteria and clostridial cluster XIV and increases acetate and butyrate levels. 31 Zinc also reduces coliforms and Escherichia coli (E. coli) concentrations within the colon. 32 Functionally, zinc can inhibit E. coli pathogenic strain influencing a reduced expression of alpha‐hemolysin, 33 with attenuated alpha‐hemolysin‐induced barrier dysfunction and consequent “leaky gut.” 34
The other side of the story tells that the observed reduction of E. coli intestinal concentration is not necessarily associated with increased abundance of beneficial bacteria. Indeed, zinc is able to reduce lactobacilli and bifidobacteria concentrations in swine gut. This would suppose that zinc has this pathogens lowering effects due to alternative mechanisms than competition or through different gut bugs upregulation. Perhaps, zinc regulates directly the innate immune response and cytokine production in man. 35
The wall dividing different features of zinc influence on gut microbiota has several wings as different sources of the microelement can have different degrees of effects in the animal systems. For example, coated ZnO nanoparticles protect the intestinal mucosal barrier and increase the expression of occludin and zonula occludens in the small intestine, with improved gut permeability to antigens and pathogens or their fragments. 36 Moreover, these nanoparticles can increase Ruminococcus flavefaciens abundance versus uncoated ones. In another report from the literature, hot‐melt extruded ZnO nanoparticles significantly decrease Clostridium spp. and coliforms concentration with a consensual improved gut integrity versus control group and pigs administered with ZnO only. 37
At the opposite of the wall dividing zinc concentration in animals' body, there is evidence that the excessive use of zinc increases the expression of antibiotic‐resistance genes within bacteria in the pig gut. 38 For example, zinc usage is positively correlated with the isolation of methicillin‐resistant Staphylococcus aureus in pigs. 39
From a mechanistic point of view, zinc modulates bacterial survival through several mechanisms: modulating host defense and the antioxidant response, gene expression/inhibition (e.g., virulence factors, growth‐promoting factors). 32 More in detail, zinc is required for functioning of proteins crucial for cell survival such as superoxide dismutase, proteases, DNA polymerases, and ribosomal proteins. 40 Thus, bacteria developed at least two transport systems for zinc import: high‐affinity zinc uptake system proteins (ZnuABC) and zinc transport systems (AdcABC, YcdHI‐YceA or YciABC). More interesting, in E. coli, zinc transporters expression is dependent on zinc uptake regulator. 41 Furthermore, different species and strains of Lactobacillus spp. show a variegate affinity for zinc import from the environment. These evidences suggest species‐ and strain‐specific mechanisms for zinc uptake among bacteria. 40
Finally, once zinc is uptaken by the bug, it is able to guarantee its survival or disrupt key processes for survival (e.g., cell stress response, carbon metabolism, motility, pathogenicity factors such as toxin production 42 (Figure 1).
FIGURE 1.
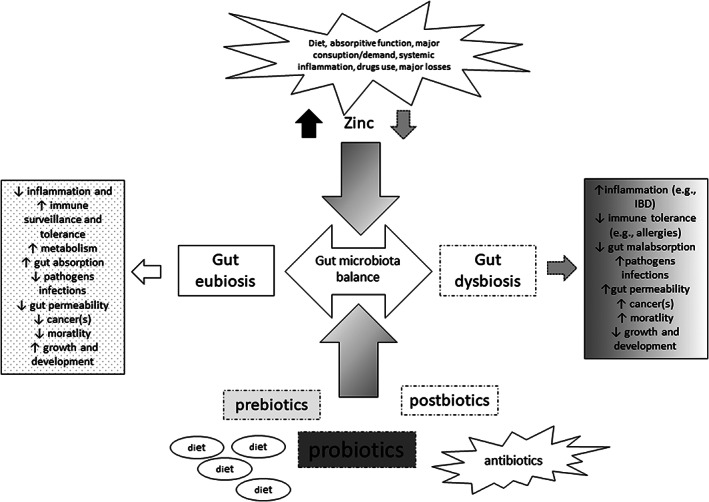
Zinc role in the interaction with the gut microbiota: Zinc variable concentration (namely, affected by several factors) is able to affect GM composition balance leading to “dysbiosis” “or maintaining” “eubiosis.” This interaction is reciprocal. Other factors play a role in maintaining this balance (e.g., diet, use of pre‐, pro‐, postbiotics, and antibiotics). GM dysbiosis is associated with several pathophysiologic manifestations. GM eubiosis is essential to human health (e.g., maintaining immune system surveillance and tolerance function, regulating metabolism, inflammation, cell proliferation)
3.2.2. Human studies
A few evidences describe the at least bidirectional interaction between zinc and gut microbiota in humans.
Among the zinc transporters, Irt‐related protein 4 is crucial for the uptake of zinc from diet at the apical membrane in enterocites. In fact, the SLC39A4 gene mutation is associated with zinc deficiency and results in a rare recessive disease, namely acrodermatitis enteropathica. 43 On the other hand, zinc extracellular efflux from enterocytes is operated by ZnT1 transporter (namely, SLC30A1). 44 According to these evidences, zinc uptake from cells would be regulated by these transporters and its dietary intake only. However, when an insufficient zinc dietary intake happens, the gut microbiota is shown being able to affect zinc absorption. 45 Interestingly, in vitro studies confirm the bioaccessibility of zinc from vegetables being mainly influenced by colonic microbiota. 46 Indeed, the bioaccessibility of zinc from vegetables seems to be more affected by small bowel microbiota. 44 , 45 Thus, lettuce seems to be the vegetable with the highest zinc content and the best micro‐mineral bioavailability in the small intestine in man. However, 300 g of lettuce only meets 2.7% and 4.5% of zinc daily demands by males and females, respectively. 45 Subsequently, the unabsorbed zinc in the small bowel reaches the colon and is available from both colonocytes and commensal bacteria. The latter are likely to improve zinc bioavailability for the host. 47 This process can be largely affected by inflammation, pathogenic bacteria, and other food components. For example, 4 weeks of Arabic gum consumption, a dried exudate of Acacia, a prebiotic fiber, significantly increased the amount of Bifidobacteria and Lactobacilli in human stool samples, with a likely consensual increase of absorbed zinc. 48
In summary, a few in vitro and in vivo studies characterize interactions between gut microbiota and zinc in man: gut microflora is able to bind mineral particles; pathogens (e.g., Salmonella, Shighella, and E. coli) compete with commensals and enterocytes for zinc absorption; zinc can move the balance of pathogenic versus commensal strains development; modification of bacterial biochemical processes by zinc (e.g., metabolism of nucleic acids and signal transduction); modification of host's biochemical pathways both by microorganisms and zinc; alterations of host's synthesis, secretion, and absorption of peptides, hormones, and cytokines; and modifications of disease intensity by microorganisms, in turn affected by zinc uptake. Pathogens, probiotics, and prebiotics can affect this balance in a multidirectional way, modulated by diet. Thus, such a small micro‐element is able to modulate several “axis” such as “gut‐brain,” “–liver,” “–endocrine,” and “‐immune” 49 , 50 (Figure 1).
3.3. Zinc and GI diseases
3.3.1. Zinc and gastrointestinal cancers
Zinc deficiency has been significantly associated with carcinogenesis increase in animal models. In fact, intragastric administration of 2 mg/kg body weight of the carcinogen methylbenzylnitrosamine leads to higher frequency of esophageal tumor in zinc‐deficient rats versus controls. 51 In addition, it has been observed that this is not a phenomenon limited to squamous cell cancers. Indeed, similarly to C vitamin and aspirin, zinc supplementation results in a dramatic reduction of colon tumors in rodents. 52 From a pathophysiological point of view, zinc deficiency allows a pro‐carcinogenetic environment as it induces cell proliferation. Going deeper, microelement deficiency also alters gene expression. In detail, Liu et al. identified 33 genes differentially expressed in a model of hyperplastic esophagus with dietary associated zinc deficiency. 53 Intriguingly, treating these rats with a cyclo‐oxygenase (COX)‐2 inhibitor, there was only a reduction in cell proliferation but not an anti‐carcinogenetic effect. 54
Deficit of zinc from diet and other co‐factors (e.g., p53 deficiency and cyclin D1 overexpression) strongly up‐regulate the progression toward cancer. 55 , 56 On the other hand, zinc replenishment would be supposed to contribute to cancer prevention. In fact, zinc dietary reload is able to stimulate apoptosis through increased expression of the proapoptotic Bax protein 57 with decreased cell proliferation. 58 Consequently, this results in the reverse of pre‐cancerous phenotype condition. Altogether, these produce a reduced incidence of tumors and tumor progression induced by both low and high doses of chemical carcinogen in cancer animal models. 59 However, the value of zinc supplementation in cancer chemo‐prevention in humans has to be further investigated.
Mechanisms responsible for zinc‐induced anti‐cancerogenic effect are: induction of cell proliferation, selective switch‐on/off of gene expression, and promotion of inflammatory response with prevention of oxidative stress that causes DNA damage 60 (Table 3).
TABLE 3.
GI diseases associated with zinc homeostasis demodulation
| GI disease | Experimental model used | References |
|---|---|---|
| GI tract cancer (s) | Animal model: rodent | 50, 51, 52, 53, 54, 55, 56, 57, 58 |
| Malabsorption and GI infections | Animal and human evidences | 53, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74 |
| IBD | Human: ex vivo, in vitro, animal: porcine | 77 |
| Celiac sprue | Human model | 67, 75, 76, 77, 78, 79, 80 |
| GERD | Animal models | 81, 82, 83, 84, 85, 86, 87 |
| Alcoholic liver disease | Human: in vitro; animal: mice, rats | 88, 89, 90 |
Abbreviations: GERD, gastroesophageal reflux disease; GI, gastrointestinal; IBD, inflammatory bowel disease(s).
3.3.2. Zinc and gastrointestinal absorptive function and dysfunction
Acute zinc deficiency can be found in case of diarrhea. Acrodermatitis enteropathica is a perfect model of example of systemic disease due to a hereditary disorder of zinc metabolism. Infants with skin lesions presented with diarrhea in the early 1970s. 61 Later, a similar syndrome was found in adults on total parenteral nutrition (TPN). Typical symptoms were diarrhea, depression, and dermatitis. 62
Widening the view, very recent meta‐analyses show decreased diarrhea duration after zinc supplementation. 63 This field of action involves also infectious diarrhea. 64 This anti‐diarrhoic effect of zinc administration can be explained by enhancement of GI barrier function of GI mucosa. 53 In fact, zinc deficiency is associated with increased intestinal permeability (namely, “leaky gut”). 65 Rats with zinc deficiency show up‐regulated expression of intestinal uroguanylin (a peptide that stimulate Cl secretion and consequent intestinal water secretion), 66 decreased absorption of triglycerides due to reduced chylomicrons formation, 67 and protein‐dispersion entheropahy due to decreased enterocyte peptidase activity. 68
On the contrary, chronic diarrhea can cause and perpetuate zinc deficiency, especially in developing countries where children malnutrition is efficaciously treated with microelement supplementation. 69
Infectious diarrhea is another example of detrimental results of zinc deficiency on human health due to altered water secretion in the bowel and depressed immune function. For example, zinc deficiency can impair diarrhea caused by Vibrio cholera. 70 V. cholera causes diarrhea through increased cyclic adenosine monophosphate (cAMP) production, increasing intestinal secretion of water and chloride, and through the inhibition of sodium absorption. 71 Interestingly, zinc supplementation significantly counteracts chloride secretion regulated by cAMP with reduction of cholera‐induced diarrhea duration. This effect can be explained by the re‐modulatory zinc action on basal‐lateral membrane K+ channels. 72 At the genetic level, zinc supplementation down‐regulates the expression of genes involved in the detrimental inflammatory reaction to enterotoxigenic E. coli (ETEC). In detail, zinc down‐regulates MUC4 expression, functioning as an ETEC K88 receptor. 73 From a clinical point of view, way of zinc delivery significantly affects the success of diarrhea treatment. 74 In addition, zinc shows also a direct antimicrobial effect on other intestinal pathogens in vitro (namely, E. coli, Shigella, and several strains of Salmonella) 91 (Table 3).
3.3.3. Zinc and inflammatory bowel diseases
Crohn's disease
Deficiency of zinc is a consolidated data in Crohn's disease (CD) and is due to its poor absorption in the small intestine and lower intake because of food intolerances. 92 It has been estimated that the prevalence of low serum microelement levels is of about 29% in Crohn's patients and it is also present in the remission phase. 93 Interestingly, CD patients on total parenteral nutrition can easily develop zinc deficiency and present with acrodermatitis enteropathica and impaired vision. 94 Children with CD often show stunted growth, decreased taste sensation, visual acuity, and immune function. 95
In vitro studies on human intestinal CACO‐2/T7 cell line showed how zinc deprivation is able to increase apoptosis, compromising tight junctions integrity, and intestinal permeability. 96 Therefore, zinc supplementation seems to decrease trans‐mucosal leak in Crohn's patients. 97
This finding is crucial as intestinal epithelial barrier dysfunction can allow the passage of leukocytes with exposure to a “storm” of luminal antigens, an hallmark of this inflammatory bowel disease (IBD) activity (e.g., high frequency of neutrophils and crypt abscesses in the intestinal mucosa). 98 Thus, zinc supplementation is beneficial in decreasing inflammatory response and maintains remission in CD. However, these empiric evidences do not find yet specific guidelines regulating its supplementation. 99
Ulcerative colitis
As zinc absorption happens mainly in distal duodenum and proximal jejunum, its deficiency is very unfrequent in ulcerative colitis (UC) patients. 100 Conversely, in active UC flares, there are high serum zinc concentrations that significantly correlate with increased C3c levels (a feature of up‐regulation of innate immune response) and antinuclear antibodies, a well‐known predictor steroid dependence. 101 This finding can be explained by the zinc local release in response to the inflammatory storm typical of active UC. However, these evidences are not uniform.
UC patients with zinc deficiency have decreased concentrations of MT in their colonic mucosa and increased concentrations of reactive oxygen species. This has been confirmed by animal models of UC resulting in increased disease activity index (DAI) and serum TNFα levels. 102 Therefore, in active UC patients, zinc supplementation has controversial effects on inflammatory cascade. Animal experiments showed the improvement of diarrhea and weight loss despite no effect on neutrophils' infiltration and mucosal inflammation. 103 In fact, human investigations showed no change in inflammatory markers and intestinal biopsies beside zinc supplementation.60 However, intrarectal zinc administration has been shown to be beneficial at the microscopic level, reducing the inflammation in rats and mice but for a short period of time. 104 Thus, in line with UC pathophysiology and histology, mucosal inflammation is not significantly associated with zin deficiency but, if malnutrition is present, zinc can have low levels that contribute to reduced colonic anti‐inflammatory balance (Table 3).
Zinc and celiac disease
Celiac sprue has several nutritional deficits due to the localization of disease activity (namely, second portion of duodenum) such as vitamins and minerals, including zinc. 75 Indeed, decreased plasmatic zinc concentration has been described in both un‐treated and under gluten‐free diet patients. 76 In addition, microelement deficiency is significantly correlated with villous atrophy and its degrees. 77
Therefore, zinc levels normalize after gluten‐free diet. 67 However, zinc homeostasis impairment is the constant finding in celiac disease as both gluten‐free diet and its supplementation do not affect its blood levels. 78 , 79 Supportive evidences from Stenberg et al. suggest that zinc deficiency is a localized feature of celiac sprue (namely, mucosal), perhaps due to recruitment of zinc in the inflamed tissue. This microelement usage activates the enzyme transglutaminase‐2 (TG2) in the intestine. More interestingly, the TG2‐thioester intermediate‐deamidated gliadin complex could act as a “neo‐antigen” and activate T‐cells in “gluten sensitive and/or genetically predisposed” subjects. 80
Finally, zinc remains an interesting therapeutic option in celiac sprue according to its ability to up‐regulate TJs functioning and improve intestinal permeability to gliadin and other intraluminal antigens 81 (Table 3).
3.3.4. Zinc and gastric acidity: A gastroesophageal reflux disease treatment hope
Zinc significantly protects gastric mucosa. 82 Animal studies reported orally administered zinc protection against chemically induced ulcerative gastritis. This effect was presumably due to inhibition of gastric acid secretion. 83 Several historical reports confirmed this mechanism and resulting ulcers healing. 84 More recently, Kirchhoff et al. reported the low dose of zinc being as effective as proton pump inhibitors (PPI) to stop secretagogue‐induced gastric acid secretion. 85 It remains unclear whether orally administered zinc can be safer than PPI in treatment of GERD, without their potential side effects (e.g., liver cytochrome P450 inhibition). At a cellular point of view, these therapeutic effects of zinc may be explained by its stabilizing properties on secretory cells lysosomal organelles. 86
To make the issue more complicated, there is some evidence that PPI‐induced inhibition of gastric acid production and consequent elevation of gastro‐duodenal pH can reduce small bowel microelement absorption. 87
This controversy can be explained by multiple and not‐exclusive sites of zinc absorption: duodenum but also cecum and colon contribute to its absorption, especially if small bowel is not perfectly functional. 105
3.3.5. Zinc and alcohol: Foes separated by intestinal permeability
Alcohol‐induced liver disease has as cornerstone of its pathophysiology the entrance of gut luminal bacterial endotoxins through the impaired epithelial barrier into the bloodstream because of altered paracellular intestinal permeability. 88 The latter has been shown being affected by direct toxic alcohol damage and perpetuating “gut‐liver” inflammatory axis both in animal and human studies.
Intestinal permeability is defined as “the paracellular capability of the intestinal barrier to let pass the macromolecules into the blood stream.” This process is regulated by the opening of the intercellular tight junctions (TJ), structures with a complex composition, where zonulin‐1 (Zo‐1), caludin (Cl) and occludin are the main components. 89 Their functioning can be affected by several physiologic and pathophysiologic factors. 90
Alcohol‐fed mice have impaired intestinal permeability (measured as increased plasma endotoxin levels and assessed histologically as leak of fluorescein‐labeled dextrans) and consequent decreased levels of TJ proteins (namely, occludin and ZO‐1). 97 Interestingly, zinc supplementation is able to improve until complete reverse of alcohol‐induced epithelial barrier and liver damages. Furthermore, zinc pretreatment upon alcohol “binge” consumption maintains serum levels of endotoxin to a normal level, protecting liver parenchyma in mice. 106 In an original in vitro model of zinc depletion, obtained in CACO‐2 cells by a zinc chelator, namely TPEN, it was observed significant downregulation of claudin‐1, occludin, and ZO‐1 expression and functioning. More interestingly, combination of mild zinc depletion and alcohol exposure in CACO‐2 cells results in more significant intestinal permeability impairment due to decreased expression of such constitutive proteins. 107
On the other hand, when supplementation with zinc acetate is made in alcohol‐consuming rats, there is significant improvement of alveolar epithelial cell permeability to sucrose and significant increase of TJs proteins expression on alveolar epithelium 108 (Table 3).
3.4. Zinc, gut microbiota, and COVID‐19
SARS‐CoV 2 has been responsible for the multisystemic hyper‐inflammatory disease, namely COVID‐19, spread all around the world as tremendous pandemic. 109
Preliminary evidences showed that acetyl cholinesterase 2 (ACE‐2) expression is regulated by Sirtuin 1 (SIRT1). As zinc is able to down‐regulate SIRT1 activity, this could result in decreased ACE‐2 expression and reduced SARS‐CoV 2 entry into the cell. 110
Some other observations support a role for zinc in COVID‐19 natural history. Physiological concentrations of zinc increase ciliary beat frequency, preventing lung infections by SARS‐CoV 2. 111 Moreover, persistent low serum zinc has been documented in critically ill COVID‐19 patients and inversely correlated with mortality from sepsis. 112
Interestingly, Prasad et al. reported zinc supplementation could lead to a milder form of COVID‐19 because this metal inhibits pH‐dependent steps of SARS‐CoV 2 replication within the cells, increasing pH of intracellular vesicles. 113
Starting from the consolidated report of zinc being a brick for immune activity in man physiology (e.g., involving both CD4+ and CD8+ cells activation and maturation), this microelement could be used as a natural “booster” for immune‐system activation against SARS‐CoV 2 such as the available vaccine booster dose for the prevention of infection in man. 107
Finally, the exaggerated cytokines production (namely, “cytokines storm”) typical of COVID‐19, able to impact on multiple organs, seems to be significantly correlated with transient zinc deficiency. 114
All these evidences are in favor of zinc supplementation in COVID‐19 patients together with C, D‐vitamins and direct antivirals, already available and to come.
4. CONCLUSIONS
Zinc is the second most studied and abundant microelement in our body. Due to its rapid and simple metabolism and limited storage, its body concentration can be easily modulated by food intake and nutraceuticals. Moreover, its variegate and strong effects on immune response to pathogens, cell cycle regulation and hormones synthesis and functioning make zinc the optimal candidate for pharmaceutical industry work‐up.
Beside these evidences, there animal and human studies on the interactions between different zinc concentrations and GM species in a dose‐dependent manner. Avoiding over‐dosage of this microelement, it is possible to beneficially modulate GM in animals and humans. All these evidences make of zinc a valid “postbiotic” candidate for the nutraceuticals industry to come.
For instance, the long‐lasting SARS‐CoV‐2 pandemic has shed light on the immune‐modulatory and direct/indirect antiviral properties of zinc versus this novel coronavirus responsible for COVID‐19 endurance all around the world.
Zinc use as probiotics' add‐on remedy and interactive actor in gut microbiota remodulation in GI diseases is one promising scenario for the next years of clinical and pharmacological research.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: Emidio Scarpellini and Ludovico Abenavoli had the original idea of this manuscript; Methodology: Valentina Maurizi, Lukas M. Balsiger, Emanuele Rinninella, Carlo Rasetti and Pierangelo Santori performed the review of literature; Validation: Emidio Scarpellini, Ludovico Abenavoli and Antonio Gasbarrini revised and validated the literature findings; Formal Analysis: Emidio Scarpellini, Emanuele Rinninella; Investigation: Emidio Scarpellini, Lukas M. Balsiger, Valentina Maurizi and Nena Giostra; Data Curation: Emidio Scarpellini and Ludovico Abenavoli; Writing‐Original Draft Preparation: Emidio Scarpellini, Ludovico Abenavoli, Lukas M. Balsiger and Valentina Maurizi; Writing‐Review & Editing: Emidio Scarpellini, Ludovico Abenavoli and Antonio Gasbarrini; Visualization: Emanuele Rinninella, Lukas M. Balsiger and Valentina Maurizi; Supervision: Emidio Scarpellini, Ludovico Abenavoli and Carlo Rasetti; Project Administration: Emidio Scarpellini and Ludovico Abenavoli. All authors read and approved the final version of the manuscript.
Scarpellini E, Balsiger LM, Maurizi V, Rinninella E, Gasbarrini A, Giostra N, et al. Zinc and gut microbiota in health and gastrointestinal disease under the COVID‐19 suggestion. BioFactors. 2022;48:294–306. 10.1002/biof.1829
DATA AVAILABILITY STATEMENT
The data reported in this article are available on Pubmed, Medilne and main National and International gastroenterology congresses websites.
REFERENCES
- 1.Smith JC Jr, Morris ER, Ellis R. Zinc: requirements, bioavailabilities and recommended dietary allowances. Prog Clin Biol Res. 1983;129:147‐69. PMID: 6318226. [PubMed] [Google Scholar]
- 2. Livingstone C. Zinc: physiology, deficiency, and parenteral nutrition. Nutr Clin Pract. 2015;30:371–82. [DOI] [PubMed] [Google Scholar]
- 3. Hojyo S, Fukada T. Zinc transporters and signaling in physiology and pathogenesis. Arch Biochem Biophys. 2016;611:43–50. [DOI] [PubMed] [Google Scholar]
- 4. Scarpellini E, Ianiro G, Attili F, Bassanelli C, De Santis A, Gasbarrini A. The human gut microbiota and virome: potential therapeutic implications. Dig Liver Dis. 2015;47:1007–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Mende DR, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tap J, Mondot S, Levenez F, Pelletier E, Caron C, Furet JP, et al. Owards the human intestinal microbiota phylogenetic core. Environ Microbiol. 2009;11:2574–84. [DOI] [PubMed] [Google Scholar]
- 8. Zhuang L, Chen H, Zhang S, Zhuang J, Li Q, Feng Z. Intestinal microbiota in early life and its implications on childhood health. Genomics Proteomics Bioinformatics. 2019;17:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489(7415):231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zheng Z, Wang B. The gut‐liver Axis in health and disease: the role of gut microbiota‐derived signals in liver injury and regeneration. Front Immunol. 2021;10(12):775526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferreira CR, Gahl WA. Disorders of metal metabolism. Transl Sci Rare Dis. 2017;2(3–4):101–39. 10.3233/TRD-170015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. Zinc and human health: an update. Arch Toxicol. 2012;86(4):521–34. 10.1007/s00204-011-0775-1 [DOI] [PubMed] [Google Scholar]
- 14. Vitamin and Mineral Requirements in Human Nutrition FAO/WHO expert consultation on human vitamin and mineral requirements; 2001.
- 15. Maret W. Zinc and human disease. In: Sigel A, Sigel H, Sigel R, editors. Interrelations between essential metal ions and human diseases. Metal ions in life sciences. Volume 13. Dordrecht: Springer; 2013. 10.1007/978-94-007-7500-8_12 [DOI] [PubMed] [Google Scholar]
- 16. King JC, Shames DM, Lowe NM, Woodhouse LR, Sutherland B, Abrams SA, et al. Effect of acute zinc depletion on zinc homeostasis and plasma zinc kinetics in men. Am J Clin Nutr. 2001;74:116–24. [DOI] [PubMed] [Google Scholar]
- 17. Institute of Medicine, Food and Nutrition Board . Dietary reference intakes for Vitamin a, Vitamin K, Arsenic, boron, Chromium, copper, Iodine, iron, Manganese, molybdenum, Nickel, silicon, Vanadium, and Zincexternal link disclaimer. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 18. U.S. Department of Agriculture, Agricultural Research Service . FoodData Centralexternal link disclaimer; 2019.
- 19. Hess SY, Peerson JM, King JC, Brown KH. Use of serum zinc concentration as an indicator of population zinc status. Food Nutr Bull. 2007;28(3 Suppl):S403–29. 10.1177/15648265070283S303 [DOI] [PubMed] [Google Scholar]
- 20. Institute of Medicine (US) Panel on Micronutrients . Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academies Press (US); 2001. [PubMed] [Google Scholar]
- 21.Prasad AS. Discovery of zinc for human health and biomarkers of zinc deficiency. In: Collins JF, editors. Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals. Academic Press; 2017. pp. 241–60. 10.1016/B978-0-12-802168-2.00020-8 [DOI]
- 22. Cummings JE, Kovacic JP. The ubiquitous role of zinc in health and disease. J Vet Emerg Crit Care (San Antonio). 2009;19(3):215–40. 10.1111/j.1476-4431.2009.00418.x PMID: 19691507. [DOI] [PubMed] [Google Scholar]
- 23. McCall KA, Huang C, Fierke CA. Function and mechanism of zinc metalloenzymes. J Nutr. 2000;130(5S Suppl):1437S–46S. 10.1093/jn/130.5.1437S [DOI] [PubMed] [Google Scholar]
- 24. Beyersmann D. Homeostasis and cellular functions of zinc. Materwiss Werksttech. 2002;33(12):764–9. 10.1002/mawe.200290008 [DOI] [Google Scholar]
- 25. Rocha ÉD, de Brito NJ, Dantas MM, Silva Ade A, Almeida M, Brandão‐Neto J. Effect of Zinc supplementation on GH, IGF1, IGFBP3, OCN, and ALP in non‐zinc‐deficient children. J Am Coll Nutr. 2015;34(4):290–9. 10.1080/07315724.2014.929511 [DOI] [PubMed] [Google Scholar]
- 26. Maxfield L, Shukla S, Crane JS. Zinc deficiency. Treasure Island, FL: StatPearls [Internet]; 2021. [Google Scholar]
- 27. Lowe NM, Fekete K, Decsi T. Methods of assessment of zinc status in humans: a systematic review. Am J Clin Nutr. 2009;89:2040S–51S. 10.3945/ajcn.2009.27230G [DOI] [PubMed] [Google Scholar]
- 28. Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM. Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem. 2008;13:1205–18. [DOI] [PubMed] [Google Scholar]
- 29. Couñago RM, Ween MP, Begg SL, Bajaj M, Zuegg J, O'Mara ML, et al. Imperfect coordination chemistry facilitates metal ion release in the Psa permease. Nat Chem Biol. 2014;10:35–41. [DOI] [PubMed] [Google Scholar]
- 30. Lopez‐Alonso M. Trace minerals and livestock: not too much not too little. ISRN Vet Sci. 2012;2012:704825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pieper R, VahjenW NK, Van Kessel AG, Zentek J. Dose‐dependent effects of dietary zinc oxide on bacterial communities and metabolic profiles in the ileum of weaned pigs. J Anim Physiol Anim Nutr (Berl). 2012;96:825–33. [DOI] [PubMed] [Google Scholar]
- 32. Kociova S, Dolezelikova K, Horky P, Skalickova S, Baholet D, Bozdechova L, et al. Zinc phosphate‐based nanoparticles as alternatives to zinc oxide in diet of weaned piglets. J Anim Sci Biotechnol. 2020;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Velasco E, Wang S, Sanet M, Fernández‐Vázquez J, Jové D, Glaría E, et al. A new role for Zinc limitation in bacterial pathogenicity: modulation of alpha‐hemolysin from uropathogenic Escherichia coli . Sci Rep. 2018;8:6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weigand E, Egenolf J. A moderate zinc deficiency does not alter lipid and fatty acid composition in the liver of weanling rats fed diets rich in cocoa butter or safflower oil. J Nutr Metab. 2017;2017:4798963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Subramanian Vignesh K, Deepe GS Jr. Immunological orchestration of zinc homeostasis: the battle between host mechanisms and pathogen defenses. Arch Biochem Biophys. 2016;611:66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu H, Bai M, Xu K, Zhou J, Zhang X, Yu R, et al. Effects of different concentrations of coated nano zinc oxide material on fecal bacterial composition and intestinal barrier in weaned piglets. J Sci Food Agric. 2021;101(2):735–45. doi: 10.1002/jsfa.10686. Epub 2020 Sep 23. PMID: 32706118. [DOI] [PubMed] [Google Scholar]
- 37. Oh SM, Kim MJ, Hosseindoust A, Kim KY, Choi YH, Ham HB, et al. Hot melt extruded‐based nano zinc as an alternative to the pharmacological dose of ZnO in weanling piglets. Asian‐Australas. J Anim Sci. 2020;33:992e–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bednorz C, Oelgeschläger K, Kinnemann B, Hartmann S, Neumann K, Pieper R, et al. The broader context of antibiotic resistance: zinc feed supplementation of piglets increases the proportion of multi‐resistant Escherichia coli in vivo. Int J Med Microbiol. 2013;303(6e7):396e–403. [DOI] [PubMed] [Google Scholar]
- 39. Slifierz MJ, Friendship RM, Weese JS. Methicillin‐resistant Staphylococcus aureus in commercial swine herds is associated with disinfectant and zinc usage. Appl Environ Microbiol. 2015;81(8):2690e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hantke K. Bacterial zinc uptake and regulators. Curr Opin Microbiol. 2005;8(2):196–202. [DOI] [PubMed] [Google Scholar]
- 41. Patzer SI, Hantke K. The zinc‐responsive regulator Zur and its control of the znu gene cluster encoding the ZnuABC zinc uptake system in Escherichia coli. J Biol Chem. 2000;275(32):24321–32. [DOI] [PubMed] [Google Scholar]
- 42. Panina EM, Mironov AA, Gelfand MS. Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc Natl Acad Sci U S A. 2003;100(17):9912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schmitt S, Küry S, Giraud M, Dréno B, Kharfi M, Bézieau S. An update on mutations of theSLC39A4gene in acrodermatitis enteropathica. Hum Mutat. 2009;30:926–33. [DOI] [PubMed] [Google Scholar]
- 44. Shusterman E, Beharier O, Shiri L, Zarivach R, Etzion Y, Campbell CR, et al. ZnT‐1 extrudes zinc from mammalian cells functioning as a Zn2+/H+exchanger. Metallomics. 2014;6:1656–63. [DOI] [PubMed] [Google Scholar]
- 45. Bielik V, Kolisek M. Bioaccessibility and bioavailability of minerals in relation to a healthy gut microbiome. Int J Mol Sci. 2021;22:6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cai X, Chen X, Yin N, Du H, Sun G, Wang L. Estimation of the bioaccessibility and bioavailability of Fe, Mn, cu, and Zn in Chinese vegetables using the in vitro digestion/Caco‐2 cell model: the influence of gut microbiota. Food Funct. 2017;8:4592–600. [DOI] [PubMed] [Google Scholar]
- 47. Celis AI, Relman DA. Competitors versus collaborators: micronutrient processing by pathogenic and commensal human associated gut bacteria. Mol Cell. 2020;78:570–6. [DOI] [PubMed] [Google Scholar]
- 48. Calame W, Weseler AR, Viebke C, Flynn C, Siemensma AD. Gum arabic establishes prebiotic functionality in healthy human volunteers in a dose‐dependent manner. Br J Nutr. 2008;100:1269–75. [DOI] [PubMed] [Google Scholar]
- 49. Ma N, Tian Y, Wu Y, Ma X. Contributions of the interaction between dietary protein and gut microbiota to intestinal health. Curr Protein Pept Sci. 2017;18:795–808. [DOI] [PubMed] [Google Scholar]
- 50. Chen J, Li Y, Tian Y, Huang C, Li D, Zhong Q, et al. Interaction between microbes and host intestinal health: modulation by dietary nutrients and gut‐brain‐endocrine‐immune axis. Curr Protein Pept Sci. 2015;16:592–603. [DOI] [PubMed] [Google Scholar]
- 51. Fong LY, Sivak A, Newberne PM. Zinc deficiency and methylbenzylnitrosamine‐induced esophageal cancer in rats. J Natl Cancer Inst. 1978;61:145–50. [DOI] [PubMed] [Google Scholar]
- 52. Christudoss P, Selvakumar R, Pulimood AB, Fleming JJ, Mathew G. Protective role of aspirin, vitamin C, and zinc and their effects on zinc status in the DMH‐induced colon carcinoma model. Asian Pac J Cancer Prev. 2013;14:4627–34. [DOI] [PubMed] [Google Scholar]
- 53. Liu P, Pieper R, Rieger J, Vahjen W, Davin R, Plendl J, et al. Effect of dietary zinc oxide on morphological characteristics, mucin composition and gene expression in the colon of weaned piglets. PLoS One. 2014;9:e91091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fong LY, Jiang Y, Riley M, Liu X, Smalley KJ, Guttridge DC, et al. Prevention of upper aerodigestive tract cancer in zinc‐deficient rodents: inefficacy of genetic or pharmacological disruption of COX‐2. Int J Cancer. 2008;122:978–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fong LY, Ishii H, Nguyen VT, Vecchione A, Farber JL, Croce CM, et al. p53 deficiency accelerates induction and progression of esophageal and forestomach tumors in zinc deficient mice. Cancer Res. 2003;63:186–95. [PubMed] [Google Scholar]
- 56. Fong LY, Mancini R, Nakagawa H, Rustgi AK, Huebner K. Combined cyclin D1 overexpression and zinc deficiency disrupts cell cycle and accelerates mouse forestomach carcinogenesis. Cancer Res. 2003;63:4244–52. [PubMed] [Google Scholar]
- 57. Fong LY, Nguyen VT, Farber JL. Esophageal cancer prevention in zinc‐deficient rats: rapid induction of apoptosis by replenishing zinc. J Natl Cancer Inst. 2001;93:1525–33. [DOI] [PubMed] [Google Scholar]
- 58. Fong LY, Farber JL, Magee PN. Zinc replenishment reduces esophageal cell proliferation and N‐nitrosomethylbenzylamine (NMBA)‐induced esophageal tumor incidence in zinc‐deficient rats. Carcinogenesis. 1998;19:1591–6. [DOI] [PubMed] [Google Scholar]
- 59. Fong LY, Jiang Y, Rawahneh ML, Smalley KJ, Croce CM, Farber JL, et al. Zinc supplementation suppresses 4‐nitroquinoline 1‐oxide‐induced rat oral carcinogenesis. Carcinogenesis. 2011;32:554–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Skrovanek S, Di Guilio K, Bailey R, Huntington W, Urbas R, Mayilvaganan B, et al. Zinc and gastrointestinal disease. World J Gastrointest Pathophysiol. 2014;15(5):496–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Barnes PM, Moynahan EJ. Zinc deficiency in acrodermatitis enteropathica: multiple dietary intolerance treated with synthetic diet. Proc R Soc Med. 1973;66:327–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kay RG, Tasman‐Jones C, Pybus J, Whiting R, Black H. A syndrome of acute zinc deficiency during total parenteral alimentation in man. Ann Surg. 1976;183:331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lamberti LM, Walker CL, Chan KY, Jian WY, Black RE. Oral zinc supplementation for the treatment of acute diarrhea in children: a systematic review and meta‐analysis. Nutrients. 2013;5:4715–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yazar AS, Güven Ş, Dinleyici EÇ. Effects of zinc or synbiotic on the duration of diarrhea in children with acute infectious diarrhea. Turk J Gastroenterol. 2016;27:537–40. [DOI] [PubMed] [Google Scholar]
- 65. Rodriguez P, Darmon N, Chappuis P, Candalh C, Blaton MA, Bouchaud C, et al. Intestinal paracellular permeability during malnutrition in Guinea pigs: effect of high dietary zinc. Gut. 1996;39:416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Blanchard RK, Cousins RJ. Upregulation of rat intestinal uroguanylin mRNA by dietary zinc restriction. Am J Physiol. 1997;272:G972–8. [DOI] [PubMed] [Google Scholar]
- 67. Koo SI, Turk DE. Effect of zinc deficiency on intestinal transport triglyceride in the rat. J Nutr. 1977;107:909–19. [DOI] [PubMed] [Google Scholar]
- 68. Moran JR, Lyerly A. The effects of severe zinc deficiency on intestinal amino acid losses in the rat. Life Sci. 1985;36:2515–21. [DOI] [PubMed] [Google Scholar]
- 69. Fischer Walker CL, Black RE. Micronutrients and diarrheal disease. Clin Infect Dis. 2007;45(Suppl 1):S73–7. [DOI] [PubMed] [Google Scholar]
- 70. Roy SK, Tomkins AM, Ara G, Jolly SP, Khatun W, Chowdhury R, et al. Impact of zinc deficiency on vibriocholerae enterotoxin‐stimulated water and electrolyte transport in animal model. J Health Popul Nutr. 2006;24:42–7. [PubMed] [Google Scholar]
- 71. Qadir MI, Arshad A, Ahmad B. Zinc: role in the management of diarrhea and cholera. World J Clin Cases. 2013;16(1):140–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hoque KM, Rajendran VM, Binder HJ. Zinc inhibits cAMPstimulated Cl secretion via basolateral K‐channel blockade in rat ileum. Am J Physiol Gastrointest Liver Physiol. 2005;288:G956–63. [DOI] [PubMed] [Google Scholar]
- 73. Sargeant HR, McDowall KJ, Miller HM, Shaw MA. Dietary zinc oxide affects the expression of genes associated with inflammation: transcriptome analysis in piglets challenged with ETEC K88. Vet Immunol Immunopathol. 2010;137:120–9. [DOI] [PubMed] [Google Scholar]
- 74. Kwon CH, Lee CY, Han SJ, Kim SJ, Park BC, Jang I, et al. Effects of dietary supplementation of lipid‐encapsulated zinc oxide on colibacillosis, growth and intestinal morphology in weaned piglets challenged with enterotoxigenic Escherichia coli. Anim Sci J. 2014;85:805–13. [DOI] [PubMed] [Google Scholar]
- 75. Wierdsma NJ, van der Schueren MA, Berkenpas M, Mulder CJ, van Bodegraven AA. Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients. 2013;5:3975–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McGrogan L, Mackinder M, Stefanowicz F, Aroutiounova M, Catchpole A, Wadsworth J, et al. Micronutrient deficiencies in children with coeliac disease; a double‐edged sword of both untreated disease and treatment with gluten‐free diet. Clin Nutr. 2021;40:2784–90. [DOI] [PubMed] [Google Scholar]
- 77. Singhal N, Alam S, Sherwani R, Musarrat J. Serum zinc levels in celiac disease. Indian Pediatr. 2008;45:319–21. [PubMed] [Google Scholar]
- 78. Crofton RW, Aggett PJ, Gvozdanovic S, Gvozdanovic D, Mowat NA, Brunt PW. Zinc metabolism in celiac disease. Am J Clin Nutr. 1990;52:379–82. [DOI] [PubMed] [Google Scholar]
- 79. Tran CD, Katsikeros R, Manton N, Krebs NF, Hambidge KM, Butler RN, et al. Zinc homeostasis and gut function in children with celiac disease. Am J Clin Nutr. 2011;94:1026–32. [DOI] [PubMed] [Google Scholar]
- 80. Stenberg P, Roth EB, Sjöberg K. Transglutaminase and the pathogenesis of coeliac disease. Eur J Intern Med. 2008;19:83–91. [DOI] [PubMed] [Google Scholar]
- 81. Stenberg P, Roth B, Ohlsson B. Zinc as a modulator of transglutaminase activity ‐ laboratory and pathophysiological aspects. J Transl Autoimmun. 2021;17(4):100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bandyopadhyay B, Bandyopadhyay SK. Protective effect of zinc gluconate on chemically induced gastric ulcer. Indian J Med Res. 1997;106:27–32. [PubMed] [Google Scholar]
- 83. Escolar G, Bulbena O. Zinc compounds, a new treatment in peptic ulcer. Drugs Exp Clin Res. 1989;15:83–9. [PubMed] [Google Scholar]
- 84. Orr KB. Letter: healing of gastric ulcers by zinc sulphate. Med J Aust. 1976;1(8):244. [PubMed] [Google Scholar]
- 85. Kirchhoff P, Socrates T, Sidani S, Duffy A, Breidthardt T, Grob C, et al. Zinc salts provide a novel, prolonged and rapid inhibition of gastric acid secretion. Am J Gastroenterol. 2011;106:62–70. [DOI] [PubMed] [Google Scholar]
- 86. Pfeiffer CJ, Cho CH, Cheema A, Saltman D. Reserpineinduced gastric ulcers: protection by lysosomal stabilization due to zinc. Eur J Pharmacol. 1980;61:347–53. [DOI] [PubMed] [Google Scholar]
- 87. Joshaghani H, Amiriani T, Vaghari G, Besharat S, Molana A, Badeleh M, et al. Effects of omeprazole consumption on serum levels of trace elements. J Trace Elem Med Biol. 2012;26:234–7. [DOI] [PubMed] [Google Scholar]
- 88.Scarpellini E, Forlino M, Lupo M, Rasetti C, Fava G, Abenavoli L, De Santis A. Gut Microbiota and Alcoholic Liver Disease. Rev Recent Clin Trials. 2016;11(3):213–9. doi: 10.2174/1574887111666160810100538. PMID: 27515958. [DOI] [PubMed] [Google Scholar]
- 89. Scarpellini E, Lupo M, Iegri C, Gasbarrini A, De Santis A, Tack J. Intestinal permeability in non‐alcoholic fatty liver disease: the gut‐liver axis. Rev Recent Clin Trials. 2014;9(3):141–7. [DOI] [PubMed] [Google Scholar]
- 90. Fasano A. Physiological, pathological, and therapeutic implications of zonulin‐mediated intestinal barrier modulation: living life on the edge of the wall. Am J Pathol. 2008;173:1243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Surjawidjaja JE, Hidayat A, Lesmana M. Growth inhibition of enteric pathogens by zinc sulfate: an in vitro study. Med Princ Pract. 2010;13:286–9. [DOI] [PubMed] [Google Scholar]
- 92. Matsui T. Zinc deficiency in Crohn's disease. J Gastroenterol. 1998;33:924–5. [DOI] [PubMed] [Google Scholar]
- 93. Filippi J, Al‐Jaouni R, Wiroth JB, Hébuterne X, Schneider SM. Nutritional deficiencies in patients with Crohn's disease in remission. Inflamm Bowel Dis. 2006;12:185–91. [DOI] [PubMed] [Google Scholar]
- 94. Myung SJ, Yang SK, Jung HY, Jung SA, Kang GH, Ha HK, et al. Zinc deficiency manifested by dermatitis and visual dysfunction in a patient with Crohn's disease. J Gastroenterol. 1998;33:876–9. [DOI] [PubMed] [Google Scholar]
- 95. Ishihara J, Arai K, Kudo T, Nambu R, Tajiri H, Aomatsu T, et al. Serum Zinc and Selenium in Children with Inflammatory Bowel Disease: A Multicenter Study in Japan. Dig Dis Sci. 2021 Jun 8. doi: 10.1007/s10620‐021‐07078‐z. Epub ahead of print. PMID: 34101059. [DOI] [PubMed] [Google Scholar]
- 96. Ranaldi G, Ferruzza S, Canali R, Leoni G, Zalewski PD, Sambuy Y, et al. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNFα. J Nutr Biochem. 2013;24:967–76. [DOI] [PubMed] [Google Scholar]
- 97. Sturniolo GC, Di Leo V, Ferronato A, D'Odorico A, D'Incà R. Zinc supplementation tightens “leaky gut” in Crohn's disease. Inflamm Bowel Dis. 2001;7:94–8. [DOI] [PubMed] [Google Scholar]
- 98. Finamore A, Massimi M, Conti Devirgiliis L, Mengheri E. Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in Caco‐2 cells. J Nutr. 2008;138:1664–70. [DOI] [PubMed] [Google Scholar]
- 99. Mulder TP, van der Sluys VA, Verspaget HW, Griffioen G, Peña AS, Janssens AR, et al. Effect of oral zinc supplementation on metallothionein and superoxide dismutase concentrations in patients with inflammatory bowel disease. J Gastroenterol Hepatol. 1994;9:472–7. [DOI] [PubMed] [Google Scholar]
- 100. Ringstad J, Kildebo S, Thomassen Y. Serum selenium, copper, and zinc concentrations in Crohn's disease and ulcerative colitis. Scand J Gastroenterol. 1993;28:605–8. [DOI] [PubMed] [Google Scholar]
- 101. Dalekos GN, Ringstad J, Savaidis I, Seferiadis KI, Tsianos EV. Zinc, copper and immunological markers in the circulation of well nourished patients with ulcerative colitis. Eur J Gastroenterol Hepatol. 1998;10:331–7. [DOI] [PubMed] [Google Scholar]
- 102. Suwendi E, Iwaya H, Lee JS, Hara H, Ishizuka S. Zinc deficiency induces dysregulation of cytokine productions in an experimental colitis of rats. Biomed Res. 2012;33:329–36. [DOI] [PubMed] [Google Scholar]
- 103. Di Leo V, D'Incà R, Barollo M, Tropea A, Fries W, Mazzon E, et al. Effect of zinc supplementation on trace elements and intestinal metallothionein concentrations in experimental colitis in the rat. Dig Liver Dis. 2001;33:135–9. [DOI] [PubMed] [Google Scholar]
- 104. Luk HH, Ko JK, Fung HS, Cho CH. Delineation of the protective action of zinc sulfate on ulcerative colitis in rats. Eur J Pharmacol. 2002;443:197–204. [DOI] [PubMed] [Google Scholar]
- 105. Hara H, Konishi A, Kasai T. Contribution of the cecum and colon to zinc absorption in rats. J Nutr. 2000;130:83–9. [DOI] [PubMed] [Google Scholar]
- 106. Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ, Kang YJ. Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol‐induced liver damage in mice. J Pharmacol Exp Ther. 2003;305:880–6. [DOI] [PubMed] [Google Scholar]
- 107. Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol‐induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol. 2010;298:G625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bao S, Knoell DL. Zinc modulates cytokine‐induced lung epithelial cell barrier permeability. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1132–41. [DOI] [PubMed] [Google Scholar]
- 109. Ravindra K, Singh T, Vardhan S, Shrivastava A, Singh S, Kumar P, et al. COVID‐19 pandemic: what can we learn for better air quality and human health? J Infect Public Health. 2022;15(2):187–198. doi: 10.1016/j.jiph.2021.12.001. Epub 2021 Dec 4. PMID: 34979337; PMCID: PMC8642828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Oyagbemi AA, Ajibade TO, Aboua YG, Gbadamosi IT, Adedapo ADA, Aro AO, et al. Potential health benefits of zinc supplementation for the management of COVID‐19 pandemic. J Food Biochem. 2021;45:e13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Woodworth BA, Zhang S, Tamashiro E, Bhargave G, Palmer JN, Cohen NA. Zinc increases ciliary beat frequency in a calcium‐dependent manner. Am J Rhinol Allergy. 2010;24:6–10. [DOI] [PubMed] [Google Scholar]
- 112. Hoeger J, Simon TP, Beeker T, Marx G, Haase H, Schuerholz T. Persistent low serum zinc is associated with recurrent sepsis in critically ill patients—a pilot study. PLoS One. 2017;12:e0176069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rahman MT, Idid SZ. Can Zn be a critical element in COVID‐19 treatment? Biol Trace Elem Res. 2021;199(2):550–558. doi: 10.1007/s12011‐020‐02194‐9. Epub 2020 May 26. PMID: 32458149; PMCID: PMC7250542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Stratton CW, Tang YW, Lu H. Pathogenesis‐directed therapy of 2019 novel coronavirus disease. J Med Virol. 2021;93(3):1320–1342. doi: 10.1002/jmv.26610. Epub 2020 Nov 10. PMID: 33073355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data reported in this article are available on Pubmed, Medilne and main National and International gastroenterology congresses websites.


