Abstract
BACKGROUND
In the United States, >1 million adults are living with congenital heart defects (CHDs), but gaps exist in understanding the health care needs of this growing population.
OBJECTIVES
This study assessed the demographics, comorbidities, and health care use of adults ages 20 to 64 years with CHDs.
METHODS
Adults with International Classification of Disease-9th Revision-Clinical Modification CHD-coded health care encounters between January 1, 2008 (January 1, 2009 for Massachusetts) and December 31, 2010 were identified from multiple data sources at 3 U.S. sites: Emory University (EU) in Atlanta, Georgia (5 counties), Massachusetts Department of Public Health (statewide), and New York State Department of Health (11 counties). Demographics, insurance type, comorbidities, and encounter data were collected. CHDs were categorized as severe or not severe, excluding cases with isolated atrial septal defect and/or patent foramen ovale.
RESULTS
CHD severity and comorbidities varied across sites, with up to 20% of adults having severe CHD and >50% having ≥1 additional cardiovascular comorbidity. Most adults had ≥1 outpatient encounters (80% EU, 90% Massachusetts, and 53% New York). Insurance type differed across sites, with Massachusetts having a large proportion of Medicaid (75%) and EU and New York having large proportions of private insurance (44% EU, 67% New York). Estimated proportions of adults with CHD-coded health care encounters varied greatly by location, with 1.2 (EU), 10 (Massachusetts), and 0.6 (New York) per 1,000 adults based on 2010 census data.
CONCLUSIONS
This was the first surveillance effort of adults with CHD-coded inpatient and outpatient health care encounters in 3 U.S. geographic locations using both administrative and clinical data sources. This information will provide a clearer understanding of health care use in this growing population.
Keywords: adult congenital heart disease, epidemiology, surveillance
Congenital heart defects (CHDs) are the most common group of birth defects, affecting nearly 1% of live births in the United States (1). Mortality in affected infants and children has significantly decreased in the past 4 decades with >85% of children born with CHDs now living into adulthood (2–4). There are estimated to be >2 million individuals in the United States living with CHDs, of whom approximately 1.4 million are adults (4). This population includes individuals with all types of CHDs, from mild defects that require only clinical monitoring or medications to highly complex defects that require surgery early in life. Canadian data showed that from 2000 to 2010, the prevalence of CHDs rose nearly 6 times faster among adults than children, and by 2010, adults accounted for nearly 66% of the entire CHD population (5).
Adults with CHDs are at risk for long-term complications from the underlying CHD and/or its treatment, although these individuals often minimize symptoms (6–13). Despite recommendations for lifelong care, individuals with CHDs may cease following up with cardiology providers as early as age 6 years (14). More than 40% of adults with any type of CHD and 25% of adults with complex CHD report having prolonged gaps in cardiology care (14,15). Therefore, individuals lost to follow-up may not present to care until significant morbidity develops, which could partially explain the high rates of heart failure and arrhythmia presentations in the adult population with CHD (16). In addition, this population is at risk of developing other typical chronic conditions of adulthood, such as renal and liver disease, obesity, and diabetes (17,18). True prevalence of adults with CHDs and of their comorbid conditions in the United States is unknown because data are typically limited to patients in specialty adults with CHD clinics, which represents <30% of the estimated CHD population (19).
All of these issues, combined with a lack of national surveillance data, create challenges in the understanding and management of population health for adults with CHDs. Determining national estimates for adults with CHD is particularly difficult because insurance sources, access to health care, and access to specialized health care differ geographically across the United States. This analysis is part of a larger pilot surveillance project of adolescents and adults with CHDs to explore these differences across the United States and to explore the ability to combine data from differing administrative and clinical sources to begin surveillance efforts and determination of prevalence estimates (20). In this analysis, we examined demographics, health care use, and comorbidities in adults with CHD-coded health care encounters age 20 to 64 years in 3 diverse, specified geographic areas. An understanding of the issues in collecting and combining data for inpatient and outpatient data for CHD-coded health care encounters is vital to improving survival, care, and quality of life for this growing population.
METHODS
DATA SOURCES.
This analysis was a component of a 3-site pilot surveillance project of adolescents and adults with CHD-coded health care encounters at 3 U.S. sites. The sites were chosen through a merit-based competitive review process of a Centers for Disease Control and Prevention Funding Opportunity Announcement (CDC-RFA-DD12-1207). The sites were: 1) Emory University (EU) in Atlanta, Georgia, which identified cases from 5 metropolitan Atlanta area counties (Clayton, Cobb, Dekalb, Fulton, and Gwinnett) using Medicaid claims data and administrative and clinical data from 6 pediatric and adult care facilities; 2) Massachusetts Department of Public Health, which identified cases statewide using the Massachusetts All Payer Claims Database, and clinical and administrative data from 4 pediatric and adult care facilities; and 3) the New York State Department of Health, which identified cases from 11 counties (Allegany, Bronx, Cattaraugus, Chautauqua, Erie, Genesee, Monroe, Niagara, Orleans, Westchester, and Wyoming) based on administrative data from 7 pediatric cardiology clinics and hospital-based inpatient and outpatient data from the New York Statewide Planning and Research Cooperative System. Project methodology is detailed in a separate paper (20). For this analysis, cases were adults age 20 to 64 years who resided in the described site-specific geographic areas during 2008 to 2010, were presumed alive as of January 1, 2010, and had a health care encounter that included an eligible CHD International Classification of Disease-9th Revision-Clinical Modification (ICD-9-CM) diagnostic code between January 1, 2008 (January 1, 2009 for Massachusetts) and December 31, 2010. Participating sites used varying linkage methodologies between clinical and administrative data (20). Each site planned collection of the same prespecified data variables, including demographics, type of health care encounter, and codes for CHDs, comorbid conditions, and cardiac procedures. Institutional review board approval was obtained from each site independently, and data use agreements were obtained to share de-identified data with the CDC.
DATA CATEGORIZATION.
As previously described, project-specific groupings were developed for cardiac and noncardiac diagnostic and procedural codes (20). Cases were categorized into 1 of 5 mutually exclusive hierarchical CHD severity groups (severe, valve, shunt, valve+shunt, other) based on those used by Marelli et al. (21). For this analysis, these 5 groups were further collapsed into severe and nonsevere. Those cases with only a 745.5 ICD-9-CM code were excluded from the analysis. The latter group included cases with a 745.5 code and no other CHD codes that were in 1 of the severity groups because the common ICD-9-CM code, 745.5, includes both secundum atrial septal defect and patent foramen ovale. Patent foramen ovale is not considered a CHD and is found in up to 25% of the adult population (22). ICD-9-CM codes unrelated to CHD diagnoses were grouped into 24 comorbidity groups based on project-specific modifications of the Clinical Classification System (23). Cardiac imaging, cardiac procedures, and surgeries, and vascular procedures were determined from ICD-9-CM procedure codes and Current Procedural Terminology codes (20). Imaging procedures included practices such as transthoracic echocardiography and cardiac magnetic resonance. Cardiac procedures included events such as pacemaker placement and intracardiac surgery. Vascular procedures included aortic and vascular surgery, endovascular stents, aneurysm clipping, and peripheral and abdominal venography and/or angiography. Encounter type was determined from available records and collapsed into emergency department (ED), inpatient, outpatient, and other and/or unknown for the purposes of description. For multiple encounters on the same day, the subject was counted as having only 1 encounter, coded using the following hierarchy: 1) inpatient; 2) ED; 3) outpatient; and 4) other and/or unknown. Insurance type, primary or secondary, was determined as any insurance held by the subject during the project period; thus, subjects might have had >1 insurance type.
STATISTICAL ANALYSIS.
Frequencies and proportions of cases are reported by site and demographic characteristics. The denominator for age-specific proportion at each site was estimated using county-level 2010 U.S. Census data. Proportion of cases was defined as the number of cases who were alive as of January 1, 2010 and who had an eligible CHD diagnosis code in the project period (January 1, 2008 [January 1, 2009 for Massachusetts] through December 31, 2010) divided by the total population of the corresponding geographic area according to 2010 census data, reported per 1,000 individuals. As previously discussed, cases with an isolated 745.5 code were excluded from all analyses. All analyses were conducted in SAS version 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
As of December 31, 2010, the estimated proportion of adults age 20 to 64 years with CHD-coded health care encounters at the 3 sites was 1.2 (EU), 10.0 (Massachusetts), and 0.6 (New York) per 1,000 adults. The estimated proportion varied by site for severe (0.3, 1.6, and 0.1, respectively) and nonsevere CHDs (1.0, 9.0, and 0.5, respectively). Figure 1 shows site-specific demographics. By excluding isolated ICD-9-CM code 745.5, the proportions of adults with severe CHDs ranged from 11% in New York to 20% in EU; those with severe CHDs were younger, with approximately 50% to 80% of patients younger than 40 years depending on the site. In addition, women outnumbered men for severe and nonsevere CHDs in both EU and Massachusetts, but not in New York, where sex was more evenly split.
FIGURE 1. Demographics of Adults With CHDs by CHD Severity at 3 U.S. Sites, 2008 to 2010*.
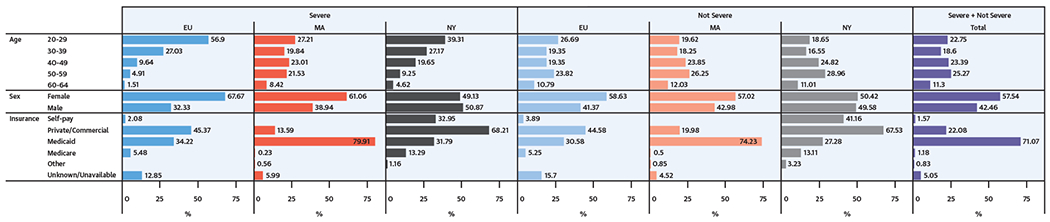
Age, sex, and insurance status of adults with congenital heart defects (CHDs) by location and by CHD severity. *2009 to 2010 for Massachusetts. EU = Emory University: Clayton, Cobb, DeKalb, Fulton, and Gwinnett counties, Georgia; MA = Massachusetts: entire state; NY = New York: Allegany, Bronx, Cattaraugus, Chautauqua, Erie, Genesee, Monroe, Niagara, Orleans, Westchester, and Wyoming counties.
Subjects could have >1 insurance source for health care encounters during the surveillance period; all insurance types are shown in Figure 1. Insurance type varied across sites. Massachusetts had nearly universal coverage statewide (95%) (24) with ≥75% of adults with CHD using Medicaid and <20% using private or commercial insurance. In contrast, in New York, 28% of adults used Medicaid, approximately two-thirds were covered by private or commercial insurance, and a relatively high proportion (40%) were self-pay and/or uninsured. Insurance type among EU adults fell between that of adults in Massachusetts and New York, with approximately 31% using Medicaid and 45% using commercial or private insurance.
Health care use across the surveillance period is presented in Figure 2 as the proportion of adults with each type of encounter. Health care use at each site was strongly influenced by site and by type of data sources used. Overall, across both severity categories, adults in New York used more types of health care than adults in EU or Massachusetts. More than one-half of New York adults had outpatient encounters and approximately 70% had inpatient or ED visits. In EU and Massachusetts, most adults had outpatient encounters (≥79%), with much smaller proportions having inpatient or ED encounters. Figure 2 also shows the variability of cardiac imaging, diagnostic cardiac procedures and/or surgeries, and vascular procedures by site and CHD severity. There was a greater amount of subjects who had imaging tests than diagnostic cardiac procedures and vascular procedures at EU. Adults in Massachusetts had more vascular procedures and much less imaging, whereas adults in New York had a higher proportion of cardiac procedures and surgeries.
FIGURE 2. Selected Encounter Types by CHD Severity at 3 U.S. Sites, 2008 to 2010*.
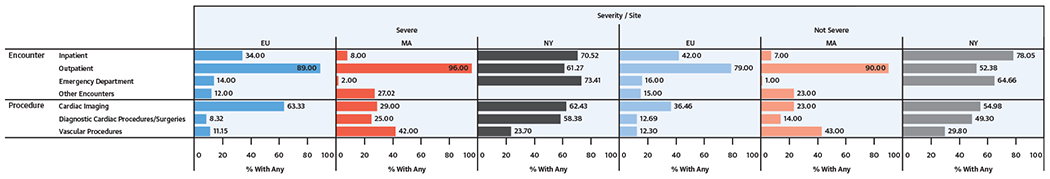
Encounter and procedure types and percentages for adult patients with CHD by location and by CHD severity. *2009 to 2010 for MA. Abbreviations as in Figure 1.
Figure 3 shows that the reported comorbid conditions among adults differed across sites and CHD severity, although, at all sites, the most common condition was stroke and/or thrombosis or other cardiovascular conditions. The cardiac comorbidity patterns showed relatively high proportions of hypertension (15% to 47%), conduction and/or rhythm disorder (26% to 50%), and stroke and/or thrombosis or other cardiovascular conditions (5% to 75%). This last group included multiple types of events, such as stroke, transient ischemic attack, and venous thromboembolism. For noncardiac comorbidities, relatively high proportions of adults with both severe and nonsevere CHD had respiratory and/or pulmonary (33% to 51%), gastrointestinal (21% to 46%), and infectious disease (21% to 38%) issues. A few conditions had more notable differences in proportions across sites and CHD severity, including diabetes and mental health conditions.
FIGURE 3. Selected Comorbidities by CHD Severity at 3 U.S. Sites, 2008 to 2010*.
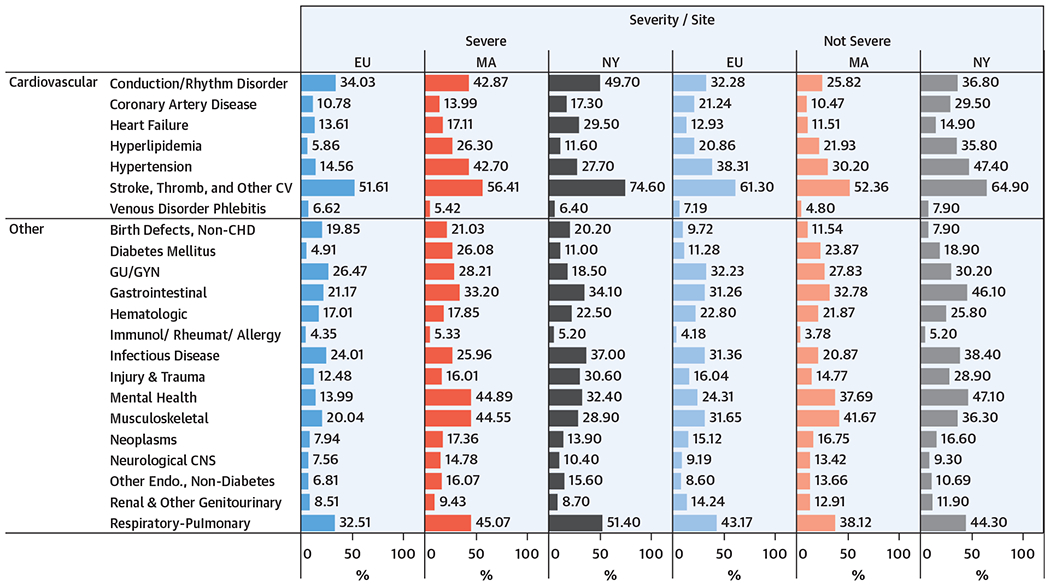
Cardiovascular and noncardiovascular comorbidities for adult patients with CHD by location and by CHD severity.*2009 to 2010 for Massachusetts. Isolated ICD-9-CM code 745.5 excluded. CNS = central nervous system; CV = cardiovascular; endo = endocrine; GU/GYN = genitourinary/gynecological; immunol = immunologic; rheumat = rheumatologic; thromb = thrombotic; other abbreviations as Figure 1.
DISCUSSION
This was the first report to describe and combine diverse cohorts of adults with CHD-coded inpatient and outpatient health care encounters within and outside of specialty centers across 3 U.S. geographic areas (Central Illustration). Previous studies of this type included only inpatient encounters or patients in care at specialty centers. Because most health care encounters usually occurred in the outpatient setting, this analysis was critical in understanding future care and resource needs for adults with CHDs. Nation or province-wide studies were performed using administrative data in Canada and Europe, in countries that have national health care systems (5,21,25). However, addressing these topics in the United States posed significant challenges due to regional differences in care practices, care accessibility, and insurance. This CDC-supported pilot project explored the possibilities of standardizing case definitions and variables across many data sources in the United States to allow consistent data collection, which revealed similarities and differences in results among diverse geographic sites.
CENTRAL ILLUSTRATION. Common Characteristics of Adults With Congenital Heart Disease.
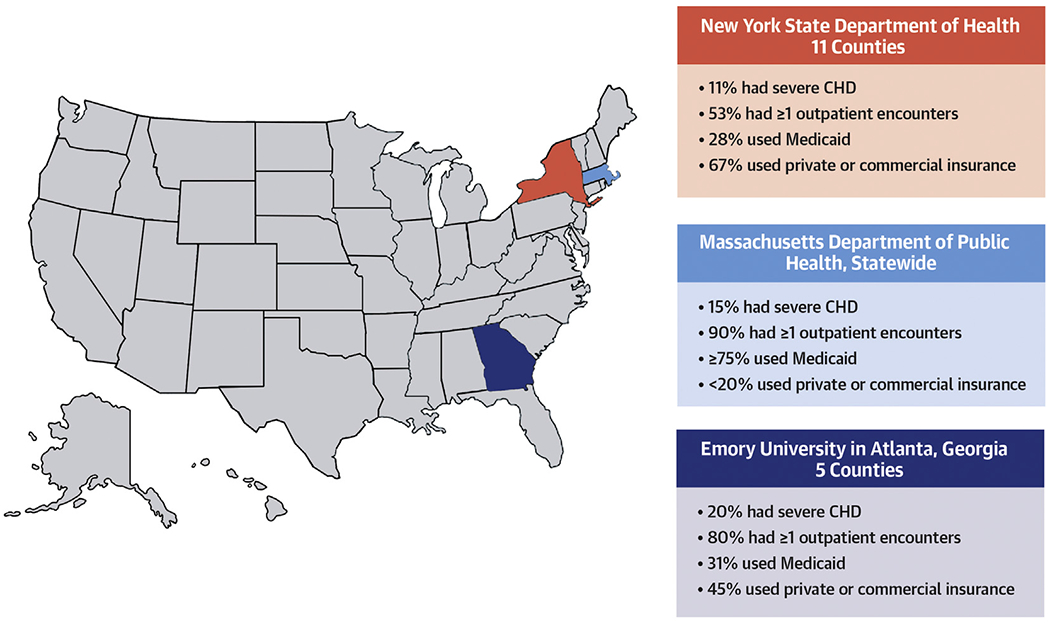
Adults with congenital heart disease who access health care have certain characteristics in common, including being middle age, having outpatient encounters, having multiple cardiac and noncardiac comorbidities, and carrying public insurance.
The local estimates of proportions of subjects with CHD-coded health care encounters during the 3-year project period differed across sites, ranging from 0.5 to 10.0 per 1,000 individuals. This number represented only those who sought health care and did not represent those who might be out of care or who sought care but were not coded as CHD. Many factors might affect these estimates at each site, including data sources, regional care patterns, coding practices, health care access, and insurance availability. These differences likely represented diversity in the health care system and the challenges in doing surveillance activity across the United States. For example, patients who sought complex CHD care might have chosen to live near metropolitan areas with large specialty medical centers for CHDs or with more accessible health insurance. Other patients might have been lost in the transition from pediatric to adult congenital cardiology and were not accessing specialty cardiology care or were not coded correctly for CHD. In addition, the number of health care encounters was greater for those with nonsevere disease than those with severe disease. Because no previous study of adult patients with CHD included outpatient encounters, the typical care patterns of these patients were unknown. The number of health care encounters might be more related to patient age because the nonsevere cohort of patients was older than the severe cohort, and thus, susceptible to non-CHD related illnesses and other comorbidities. Further investigation of characteristics and prevalence of individuals with CHDs in the United States will need to account for such variation and differences.
Site-specific differences in insurance rates, demographic characteristics, and encounter type data were also likely multifactorial. First, because individuals were identified based on CHD-coded health care encounters, the ability for an individual to visit a provider was necessary to enter the dataset; thus, as previously discussed, variation in health care access across geographic regions would have affected the results. There was a higher proportion of women identified overall, specifically in EU and Massachusetts. This was consistent with the general population, because women seek outpatient general health care more regularly than men (26). In New York, this proportion might have been skewed because the foundational dataset was from a hospital-based system with fewer outpatient encounters. In Massachusetts, where health insurance coverage has been nearly universal since 2006 (24), greater access to primary care might result in more outpatient encounters and fewer ED encounters. Overall, in 2010, the uninsured rates by site varied from approximately 5% in Massachusetts to approximately 12% in New York to approximately 19% in the EU catchment area, which would affect identification rates across the sites. In New York, data primarily came from a large hospital-based inpatient and outpatient data source, which likely influenced the larger proportion of inpatient and ED encounters and the high proportion of cardiac procedures and surgeries. These results did not necessarily indicate that adults in New York had higher health care use or were sicker; they were reflective of the data source and the need to account for this in surveillance endeavors. Another source of site-specific data variability might relate to regional practice patterns in cardiac care. For example, there was a large number of echocardiograms among adults in EU, whereas adults at other sites had more vascular procedures or other types of testing.
Comorbid conditions had similar general trends across sites and CHD severity. Adults with both severe and nonsevere CHDs had a high proportion of hypertension, conduction and/or rhythm disorder and stroke and/or thrombosis, or other cardiovascular conditions. From a noncardiac perspective, respiratory-pulmonary and gastrointestinal conditions were prevalent. The most common conditions reported were similar to those noted in population-based studies from other countries and specialty center studies within the United States (18,27–31). However, estimates for the large number of comorbid conditions described here were not previously reported in the United States. Most of the adults at the 3 sites had nonsevere CHDs, a sizable proportion of whom experienced comorbidities and substantial resource use. This might be related to the older age of this population and might or might not be related to the underlying CHD or previous CHD procedures; nonetheless, these results showed that adults with nonsevere CHDs contributed to the overall health burden of this population. In addition, these findings indicated a need for care coordination among primary care, congenital cardiology, and other subspecialties.
STUDY LIMITATIONS.
This report had limitations inherent to the use of administrative data. Specific ICD-9-CM diagnoses, particularly conditions of mild complexity might be inaccurate; however, coding accuracy improved for the more complex defects (22,32–35). Using administrative coding for surveillance might have led to both under-counting, if patients were not in care at all or were coded as “congestive heart failure” or “arrhythmia” rather than as CHDs, or led to over-reporting among conditions that are not always clinically significant, including coronary abnormalities and venous anomalies. Coding practices might also have differed among geographic locations, sites of care (ED, inpatient, or outpatient), types of providers (general or specialty), and the person responsible for coding (administrator or provider) (36). It was unclear if the differences among geographic location or those who accessed health care were reflective of the general CHD population; this will require further investigation. Although findings at the 3 sites were not generalizable across the United States, understanding resource use patterns will inform strategies for further surveillance efforts and improve health care delivery for adults with CHDs. The use of standard definitions and data collection, and assessing data across participating sites, allowed for a clearer understanding of patients with CHDs in the health care system to inform future work.
CONCLUSIONS
This was the first surveillance project of adults with CHD-coded inpatient and outpatient health care encounters in the United States that used multiple clinical and administrative data sources and geographic locations. The data definitions allowed for combining data and comparisons across sites to identify similarities and differences. Although population-based conclusions were limited, the data showed the following important information. The ages in the cohort reflected the growing and aging population of adults with CHDs. The high rates of cardiac and noncardiac comorbid conditions among those with severe and nonsevere CHD, as well as the high number of health care encounters, were notable, particularly in the outpatient realm. Finally, there were insurance differences across sites, which might have affected access to care and overall results. Our findings could inform future surveillance efforts and care provision plans for adults with CHDs.
PERSPECTIVES.
COMPETENCY IN SYSTEMS-BASED PRACTICE:
Adults with CHDs are a growing and aging population with high incidences of cardiac and noncardiac comorbidities and high rates of health care use, regardless of the anatomical complexity of structural heart disease.
TRANSLATIONAL OUTLOOK:
Further studies are needed to understand patterns and Limitations of medical resource use by adults with CHDs to improve outcomes and optimize value.
ACKNOWLEDGMENTS
DCDD Replication Statement: This analysis has undergone replication by Xiaoli Chen.
This study was supported by the Centers for Disease Control and Prevention (Grant/Award Number: CDC-RFA-DD12-1207). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The authors have reported that they have no relationships relevant to the contents of this paper to disclose. Rose Tompkins, MD, served as Guest Associate Editor for this paper. P.K. Shah, MD, served as Guest Editor-in-Chief for this paper.
ABBREVIATIONS AND ACRONYMS
- CDC
Centers for Disease Control and Prevention
- CHD
congenital heart defect
- ED
emergency department
- EU
Emory University
- ICD-9-CM
International Classification of Disease-9th Revision-Clinical Modification
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC author instructions page.
REFERENCES
- 1.Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998-2005. J Pediatr 2008;153:807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warnes CA, Liberthson R, Danielson GK, et al. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol 2001;37:1170–5. [DOI] [PubMed] [Google Scholar]
- 3.Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation 2010;122:2254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilboa SM, Devine OJ, Kucik JE, et al. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation 2016;134:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation 2014;130:749–56. [DOI] [PubMed] [Google Scholar]
- 6.Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:e1–121. [DOI] [PubMed] [Google Scholar]
- 7.Gurvitz MZ, Inkelas M, Lee M, Stout K, Escarce J, Chang RK. Changes in hospitalization patterns among patients with congenital heart disease during the transition from adolescence to adulthood. J Am Coll Cardiol 2007;49:875–82. [DOI] [PubMed] [Google Scholar]
- 8.Afilalo J, Therrien J, Pilote L, Ionescu-Ittu R, Martucci G, Marelli AJ. Geriatric congenital heart disease: burden of disease and predictors of mortality. J Am Coll Cardiol 2011;58:1509–15. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt AB, Rajabali A, He W, Benavidez OJ. High resource use among adult congenital heart surgery admissions in adult hospitals: risk factors and association with death and comorbidities. Congenit Heart Dis 2015;10:13–20. [DOI] [PubMed] [Google Scholar]
- 10.Zomer AC, Vaartjes I, Uiterwaal CS, et al. Social burden and lifestyle in adults with congenital heart disease. Am J Cardiol 2012;109:1657–63. [DOI] [PubMed] [Google Scholar]
- 11.Schoormans D, Mulder BJ, van Melle JP, et al. Illness perceptions of adults with congenital heart disease and their predictive value for quality of life two years later. Eur J Cardiovasc Nursing 2014;13:86–94. [DOI] [PubMed] [Google Scholar]
- 12.Schoormans D, Sprangers MA, Budts W, Mulder BJ, Apers S, Moons P. Perceived health is partially associated with the symptomatological profile in patients with benign and severe conditions: the case of congenital heart disease. Qual Life Res 2013;22:1295–304. [DOI] [PubMed] [Google Scholar]
- 13.Bredy C, Ministeri M, Kempny A, et al. New York Heart Association (NYHA) classification in adults with congenital heart disease: relation to objective measures of exercise and outcome. Eur Heart J Qual Care Clin Outcomes 2018;4:51–8. [DOI] [PubMed] [Google Scholar]
- 14.Mackie AS, Ionescu-Ittu R, Therrien J, Pilote L, Abrahamowicz M, Marelli AJ. Children and adults with congenital heart disease lost to follow-up: who and when? Circulation 2009;120:302–9. [DOI] [PubMed] [Google Scholar]
- 15.Gurvitz M, Valente AM, Broberg C, et al. Prevalence and predictors of gaps in care among adult congenital heart disease patients: HEART-ACHD (The Health, Education, and Access Research Trial). J Am Coll Cardiol 2013;61:2180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Opotowsky AR, Siddiqi OK, Webb GD. Trends in hospitalizations for adults with congenital heart disease in the U.S. J Am Coll Cardiol 2009;54:460–7. [DOI] [PubMed] [Google Scholar]
- 17.Christman MP, Castro-Zarraga M, Defaria Yeh D, Liberthson RR, Bhatt AB. Adequacy of cancer screening in adult women with congenital heart disease. ISRN Cardiol 2013;2013:827696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lui GK, Saidi A, Bhatt AB, et al. Diagnosis and management of noncardiac complications in adults with congenital heart disease: a scientific statement from the American Heart Association. Circulation 2017;136:e348–92. [DOI] [PubMed] [Google Scholar]
- 19.Patel MS, Kogon BE. Care of the adult congenital heart disease patient in the United States: a summary of the current system. Pediatr Cardiol 2010;31:511–4. [DOI] [PubMed] [Google Scholar]
- 20.Glidewell J, Book W, Raskind-Hood C, et al. Population-based surveillance of congenital heart defects among adolescents and adults: surveillance methodology. Birth Defects Res 2018;110:1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation 2007;115:163–72. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez FH 3rd., Ephrem G, Gerardin JF, Raskind-Hood C, Hogue C, Book W. The 745.5 issue in code-based, adult congenital heart disease population studies: relevance to current and future ICD-9-CM and ICD-10-CM studies. Congenit Heart Dis 2018;13:59–64. [DOI] [PubMed] [Google Scholar]
- 23.Elixhauser ASC, Palmer L. Clinical Classification Software. Rockville, MD: U.S. Agency for Healthcare Research and Quality, 2014. [Google Scholar]
- 24.Kaiser Family Foundation. State Health Facts. 2019. Available at: https://www.kff.org/statedata. Accessed January 2020.
- 25.Engelfriet P, Boersma E, Oechslin E, et al. The spectrum of adult congenital heart disease in Europe: morbidity and mortality in a 5 year follow-up period. The Euro Heart Survey on adult congenital heart disease. Eur Heart J 2005;26:2325–33. [DOI] [PubMed] [Google Scholar]
- 26.Thompson AE, Anisimowicz Y, Miedema B, Hogg W, Wodchis WP, Aubrey-Bassler K. The influence of gender and other patient characteristics on health care-seeking behaviour: a QUALICOPC study. BMC Fam Pract 2016;17:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanz J, Brophy JM, Therrien J, Kaouache M, Guo L, Marelli AJ. Stroke in adults with congenital heart disease: incidence, cumulative risk, and predictors. Circulation 2015;132:2385–94. [DOI] [PubMed] [Google Scholar]
- 28.Waldmann V, Laredo M, Abadir S, Mondesert B, Khairy P. Atrial fibrillation in adults with congenital heart disease. Int J Cardiol 2019;287:148–54. [DOI] [PubMed] [Google Scholar]
- 29.Alshawabkeh LI, Opotowsky AR. Burden of heart failure in adults with congenital heart disease. Curr Heart Fail Rep 2016;13:247–54. [DOI] [PubMed] [Google Scholar]
- 30.Opotowsky AR, Webb GD. Population-based data on congenital heart disease and stroke. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajpal S, Alshawabkeh L, Almaddah N, et al. Association of albuminuria with major adverse outcomes in adults with congenital heart disease: results from the Boston Adult Congenital Heart Biobank. JAMA Cardiol 2018;3:308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss JB, Grant A, Marelli A, et al. Assessment of electronic health information system use and need in US adult congenital heart disease centers. Congenit Heart Dis 2011;6:134–8. [DOI] [PubMed] [Google Scholar]
- 33.Broberg C, McLarry J, Mitchell J, et al. Accuracy of administrative data for detection and categorization of adult congenital heart disease patients from an electronic medical record. Pediatr Cardiol 2015;36:719–25. [DOI] [PubMed] [Google Scholar]
- 34.Steiner JM, Kirkpatrick JN, Heckbert SR, et al. Identification of adults with congenital heart disease of moderate or great complexity from administrative data. Congenit Heart Dis 2018;13:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan A, Ramsey K, Ballard C, et al. Limited accuracy of administrative data for the identification and classification of adult congenital heart disease. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorence DP, Ibrahim IA. Benchmarking variation in coding accuracy across the United States. J Health Care Finance 2003;29:29–42. [PubMed] [Google Scholar]


