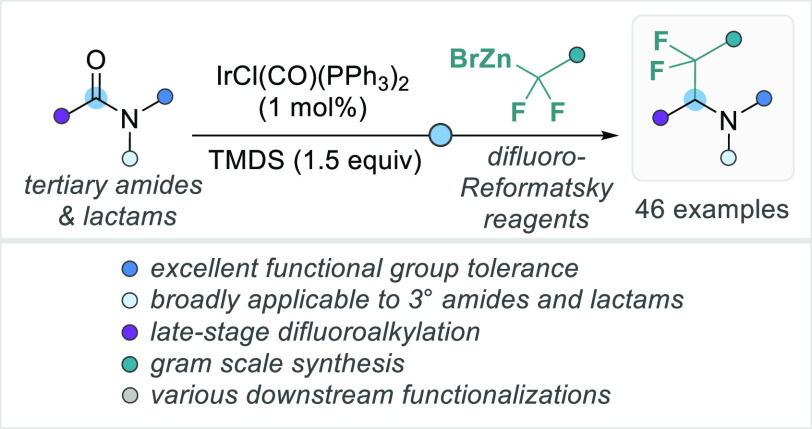
Abstract
An iridium-catalyzed, reductive alkylation of abundant tertiary lactams and amides using 1–2 mol % of Vaska’s complex (IrCl(CO)(PPh3)2), tetramethyldisiloxane (TMDS), and difluoro-Reformatsky reagents (BrZnCF2R) for the general synthesis of medicinally relevant α-difluoroalkylated tertiary amines is described. A broad scope (46 examples), including N-aryl- and N-heteroaryl-substituted lactams, demonstrated an excellent functional group tolerance. Furthermore, late-stage drug functionalizations, a gram-scale synthesis, and common downstream transformations proved the potential synthetic relevance of this new methodology.
The incorporation of the gem-difluoromethylene (−CF2−) group, an oxygen bioisostere,1 into organic molecules has gained considerable attention in pharmaceutical and agrochemical research as well as in materials science, due to the unique influence of fluorine atoms on physical, chemical, and biological properties.2 More specifically, the β,β-difluoro-α-amino motif represents a key building block in many bioactive molecules, owing to the electronic influence of the fluorine atoms on the neighboring nitrogen center. The strong electron-withdrawing character of β-fluorine substitution on amines or nitrogen-containing heterocycles significantly lowers their basicity and pKa, which in turn influence critical parameters in medicinal lead optimization, such as physicochemical properties, binding affinities and absorption, distribution, metabolism, and excretion (ADME).3 The relevance of this structural motif in drug discovery is further exemplified by the large variety of β,β-difluoro-α-amino-containing pharmaceutical compounds such as gemcitabine,4 cedazuridine,5 eflornithine,6 GDC-0077,7 and glecaprevir8 (Scheme 1A). Therefore, the development of new concise and selective methods for the late-stage introduction of gem-difluoromethylene units onto nitrogen-containing scaffolds remains an attractive goal in synthetic chemistry.9
Scheme 1. (A) Drug Molecules Containing the gem-Difluoro Motif and (B) Reductive Functionalization of Amides and Lactams by an Iridium-Catalyzed Reformatsky Reaction.
In the past decade, several research groups have become involved in the challenging late-stage reductive C–C coupling of amides with organometallic reagents for the synthesis of α-functionalized amines.10 Stoichiometric approaches for the reductive functionalization of different amide classes, including lactams with various organometallic reagents, have been reported by Huang,11 Sato and Chida,12 and Chiba and our group.13 These methods employ DIBAL-H, Schwartz’s reagent (Cp2ZrHCl), triflic anhydride/metal hydride, or a NaH/NaI composite as the stoichiometric reductants. A highly chemoselective reductive functionalization of amides can be achieved by a transition-metal-catalyzed approach, as demonstrated by our group14 and others.15 Using catalytic amounts of Vaska’s complex (IrCl(CO)(PPh3)2) and 1,1,3,3-tetramethyldisiloxane (TMDS) led to the formation of metastable O-silylated hemiaminal intermediates, which are precursors to reactive iminium ions that can undergo subsequent nucleophilic functionalization.
Continuing our group’s ongoing efforts on reductive iridium-catalyzed C–C bond-forming reactions, we envisioned combining amide functionalization with commonly known difluoromethylene sources to form highly desirable and medicinally relevant α-difluoroalkylated amines (Scheme 1B). The ethoxycarbonyl-difluoromethyl (−CF2CO2Et) moiety is a versatile difluoromethylene source, due to its potential as a handle for further modifications into various functional groups.16 In addition to cross coupling,17 C–H functionalization,16,17a,18 and radical addition,18a,19 this difluoro-methylene-containing unit is traditionally introduced via nucleophilic attack of the corresponding difluoro-Reformatsky reagent (BrZnCF2CO2Et) on carbonyl groups, imines, or azodicarboxylates.20 This long-serving reagent with its efficacious reactivity toward various electrophiles caught our attention for its potential unprecedented deployment in a general late-stage amide functionalization approach, and herein we wish to report our findings.
N,N-Dimethyl-1-naphthamide 1a was chosen as a model substrate for the reductive functionalization with difluoro-organozinc reagent 2a′, which was freshly prepared from the corresponding ethyl bromodifluoroacetate (2a) and zinc in THF. We were very pleased that staged treatment of a toluene solution of 1a with 1 mol % of Vaska’s complex, 2.0 equiv of TMDS, and 1.1 equiv of difluoro-organozinc reagent 2a′ gave the desired tertiary amine 3a in promising 53% yield, alongside minor amounts of secondary alcohol 4 and overreduction product 5 (Scheme 2, entry 1). Increasing the equivalents of organozinc reagent 2a′ improved the yield of desired product 3a slightly (Scheme 2, entry 2). More significantly, lowering the amount of TMDS to 1.5 equiv drastically reduced the rate of overreduction and allowed access to synthetically useful yields of functionalization product 3a (Scheme 2, entry 3). Finally, changing the concentration of organozinc reagent 2a′ by dilution provided a 75% isolated yield (Scheme 2, entry 4). Further changes to the reaction conditions, such as using different solvent combinations, temperatures, or reaction times, did not have a positive effect on the reaction outcome (see SI for full optimization details).
Scheme 2. Reaction Optimization.
NMR yield using 1,3,5-trimethoxybenzene as an internal standard; isolated yield in parentheses.
With optimized conditions in hand, we then examined the reaction scope with respect to tertiary amides and lactams 1 (Scheme 3). Satisfyingly, several N,N-dimethyl-benzamides 1a–1f with electron-deficient and electron-rich substituents in ortho or para positions, as well as furan substrate 1g, could be successfully converted into the corresponding difluoromethylated tertiary amines 3b–3g in good isolated yields (62–84%). Pyrrolidine-, piperidine-, morpholine-, azepane-, and azocane-derived amides 3h–3o were reductively functionalized in good to excellent yields (69–98%) while demonstrating tolerance to various substituents such as boronic ester, acetal, iodo, or nitro groups. N,N-Dibenzylamide 1p, N,N-benzylethylamide 1q, and anilide 1r were successfully employed to furnish the desired products 3p–3r in 64–87% yields. However, increased amounts of TMDS (2.5 equiv) and Vaska’s complex (2 mol %) were used to force the slow reduction step of these more challenging substrates to full conversion. Anilide 1s, bearing an ethyl ester moiety, was converted into amine 3s in the same way, albeit in a diminished 56% yield. Weinreb amide 1t reacted smoothly to product 3t in 91% yield, while α,β-unsaturated amides gave difluoro products 3u and 3v in moderate 34% and 58% yields, which is due to competing conjugate addition. Furthermore, aliphatic amides 1w and 1x underwent reductive functionalization in 73% and 68% yields. Encouraged by these results, we also envisioned including lactams in the substrate scope. Five- and six-membered lactams 1y and 1z gave the corresponding difluoroalkylated pyrrolidine 3y and piperidine 3z in moderate 60% and 42% yields, despite slightly reoptimized reaction conditions. For these products, we observed significantly higher yields by reducing the time between the addition of TMDS and the organozinc bromides and by changing the solvent from toluene to THF or 2-methyl-THF.21 Very pleasingly, N-benzyl-, N-phenyl-, N-pyridyl- and N-pyrimidyl-substituted difluoroalkylated azepanes 3aa–3ae were obtained in overall good yields (57–81%) under the standard reaction conditions. This method was also successfully applied to the late-stage functionalization of the active pharmaceutical ingredients (APIs) piperine (1af), napropamide (1ag), acetyletamivan (1ah), and CX-546 (1ai). The corresponding difluorinated drug derivates 3af–3ai were isolated in good yields (56–80%), highlighting the potential application of this method for pharmaceutical drug discovery and lead structure optimization. No C–C coupling was observed using secondary amides, and mainly aldehyde formation was witnessed after aqueous workup.
Scheme 3. Reaction Scope of Tertiary Amides and Lactams.
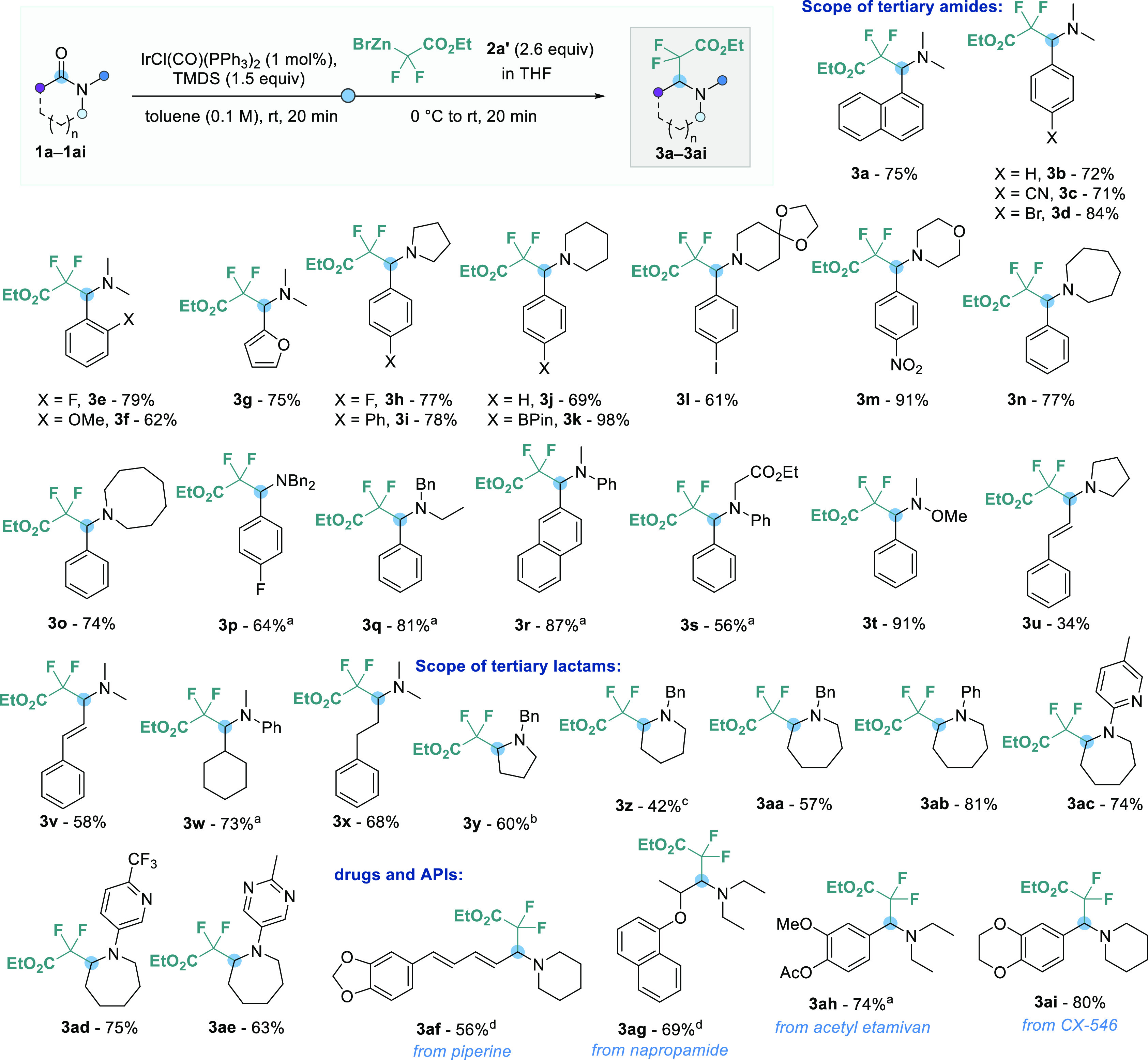
2.5 equiv of TMDS and 2 mol % of IrCl(CO)(PPh3)2 were used.
2-Methyl-THF was used as the solvent in the first step and stirred for 2 min.
THF was used as the solvent in the first step.
2.5 equiv of TMDS and 2 mol % of IrCl(CO)(PPh3)2 were used, and the first step was stirred for 1 h.
Standard conditions: amide or lactam 1 (0.15 mmol), IrCl(CO)(PPh3)2 (1 mol %), TMDS (0.23 mmol), toluene (1.50 mL), and 2a′ (0.40 mmol) in THF (1.63 mL); isolated yields are given.
Next, we assessed the scope of the difluoro-organozinc reagents 2′ and were again pleased to find that azepan-1-yl(phenyl)methanone (1n) could be readily functionalized with benzyl, trimethylsilyethyl, and isopropyl difluoroacetates 2b′–2d′ to form 3aj–3al in good yields (63–85%) (Scheme 4). Vaska’s complex (2 mol %) and 2.5 equiv of TMDS were used to ensure that starting amide 1n was fully converted into the silylated hemiaminal intermediate before adding the nucleophile. Employing l-menthol- and glycerol-derived difluoroacetates 2e′ and 2f′, products 3am and 3an were isolated in 49% and 64% yields as 1.2:1 and 1:1 mixtures of diastereomers, respectively. Sterically demanding benzhydryl difluoroacetate 2g′ could be introduced efficiently in 74% yield to give tertiary amine 3ao. Notably, difluoroacetamide-containing zinc bromides 2h′ and 2i′ could also be used under the same reaction conditions to furnish amines 3ap and 3aq in near quantitative yields. Using morpholine-derived difluoroacetamide 2j′, 3ar was obtained in good yield (63%). Further reduction of the difluoroacetamide moiety in these products was not observed under the reported reaction conditions, which can be explained by the active iridium catalyst being quenched by the organozinc bromides upon addition. Highlighting lactams as suitable feedstock compounds, the reductive functionalization of 1ab with benzyl difluoroacetate 2b′ and difluoroacetamide 2h′ gave the C2-difluoroalkylated saturated nitrogen-containing heterocyclic amines 3as and 3at in 42% and 53% yields, respectively.
Scheme 4. Reaction Scope of Difluoro-Organozinc Reagents.
Lactam 1ab (0.15 mmol), 1.5 equiv of TMDS, and 1 mol % of IrCl(CO)(PPh3)2 were used.
Standard conditions: amide 1n (0.15 mmol), IrCl(CO)(PPh3)2 (2 mol %), TMDS (0.38 mmol), toluene (1.50 mL), 2′ (0.40 mmol) in THF; isolated yields are given.
To showcase the synthetic utility of this methodology, we performed a gram-scale reductive difluoroalkylation of amide 1b, generating tertiary amine 3b in a 67% (1.15 g, 4.47 mmol) yield (Scheme 5), which was comparable to the small-scale reaction. Identifying the ester moiety in 3b as a useful handle for downstream derivatizations, we synthesized several CF2-containing compounds 6–10 by standard organic procedures. Primary alcohol 6 was obtained in 81% yield by reduction with sodium borohydride. Addition of a methanolic ammonia solution gave corresponding primary amide 7 in 85% yield. Tertiary alcohol 8 was formed in 61% yield, using 2.1 equiv of Grignard reagent. Saponification and subsequent acidification furnished carboxylic acid 9 in quantitative yield. Finally, enol ether 10 was installed in 42% yield by employing the Tebbe reagent under basic reaction conditions.
Scheme 5. Gram-Scale Reaction and Downstream Functionalization.
Isolated yields are given.
In conclusion, a broadly applicable and efficient method for the synthesis of acyclic and cyclic α-difluoroalkylated tertiary amines with good overall yields has been developed. The mild iridium-catalyzed reductive difluoroalkylation shows excellent functional group tolerance with respect to both coupling partners: amides/lactams and organozinc reagents, which are among other things highlighted by the late-stage derivatization of four drug molecules. Furthermore, the reaction was readily performed on a gram scale without a significant loss in yield, and several CF2-containing derivates were made by common downstream transformations, altogether demonstrating the potential utility of the method developed herein as a useful tool in current and future drug discovery programs.
Acknowledgments
The authors thank Daniel Matheau-Raven, Yaseen Almehmadi, Daniel Rozsar, and Andrew Maitland, as well as former group members (Chemistry Research Laboratory, Department of Chemistry, University of Oxford), for starting material synthesis and enriching discussions. This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No. 892540.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.2c00438.
General information, full optimization details, experimental procedures, compound characterization, and NMR spectra. (PDF)
Author Contributions
All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- a Blackburn G. M.; Kent D. E.; Kolkmann F. Three new β,γ-methylene analogues of adenosine triphosphate. J. Chem. Soc. Chem. Commun. 1981, 0, 1188–1190. 10.1039/C39810001188. [DOI] [Google Scholar]; b Blackburn G. M.; Kent D. E.; Kolkmann F. The synthesis and metal binding characteristics of novel, isopolar phosphonate analogues of nucleotides. J. Chem. Soc. Perkin Trans. 1 1984, 1119–1125. 10.1039/p19840001119. [DOI] [Google Scholar]; c Motherwell W. B.; Tozer M. J.; Ross B. C. A convenient method for replacement of the anomeric hydroxy group in carbohydrates by difluoromethyl functionality. J. Chem. Soc. Chem. Commun. 1989, 1437–1439. 10.1039/c39890001437. [DOI] [Google Scholar]
- a Müller K.; Faeh C.; Diederich F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886. 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]; b Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]; c O’Hagan D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. 10.1039/B711844A. [DOI] [PubMed] [Google Scholar]; d Yerien D. E.; Barata-Vallejo S.; Postigo A. Difluoromethylation Reactions of Organic Compounds. Chem. Eur. J. 2017, 23, 14676–14701. 10.1002/chem.201702311. [DOI] [PubMed] [Google Scholar]; e Britton R.; Gouverneur V.; Lin J.-H.; Meanwell M.; Ni C.; Pupo G.; Xiao J.-C.; Hu J. Contemporary synthetic strategies in organofluorine chemistry. Nat. Rev. Methods Primers 2021, 47, 1–22. 10.1038/s43586-021-00042-1. [DOI] [Google Scholar]
- a Peng Q.; Yan B.; Li F.; Lang M.; Zhang B.; Guo D.; Bierer D.; Wang J. Biomimetic enantioselective synthesis of β,β-difluoro-α-amino acid derivatives. Commun. Chem. 2021, 4 (148), 1–7. 10.1038/s42004-021-00586-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Braun M. The Asymmetric Difluoro-Reformatsky Reaction. Eur. J. Org. Chem. 2021, 2021, 1825–1836. 10.1002/ejoc.202100004. [DOI] [Google Scholar]; c Fadeyi O. O.; Lindsley C. W. Rapid, General Access to Chiral β-Fluoroamines and β,β-DifluoroaminesviaOrganocatalysis. Org. Lett. 2009, 11 (4), 943–946. 10.1021/ol802930q. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Böhm H.-J.; Banner D.; Bendels S.; Kansy M.; Kuhn B.; Müller K.; Obst-Sander U.; Stahl M. Fluorine in Medicinal Chemistry. ChemBioChem. 2004, 5, 637–643. 10.1002/cbic.200301023. [DOI] [PubMed] [Google Scholar]; e Hagmann W. K. J. The Many Roles for Fluorine in Medicinal Chemistry. Med. Chem. 2008, 51 (15), 4359–4369. 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]; f Jeschke P. The Unique Role of Fluorine in the Design of Active Ingredients for Modern Crop Protection. ChemBioChem. 2004, 5, 570–589. 10.1002/cbic.200300833. [DOI] [PubMed] [Google Scholar]; g Morgenthaler M.; Schweizer E.; Hoffmann-Röder A.; Benini F.; Martin R. E.; Jaeschke G.; Wagner B.; Fischer H.; Bendels S.; Zimmerli D.; Schneider J.; Diederich F.; Kansy M.; Müller K. Predicting and Tuning Physicochemical Properties in Lead Optimization: Amine Basicities. ChemMedChem. 2007, 2, 1100–1115. 10.1002/cmdc.200700059. [DOI] [PubMed] [Google Scholar]
- Noble S.; Goa K. L. Gemcitabine. A review of its pharmacology and clinical potential in non-small cell lung cancer and pancreatic cancer. Drugs 1997, 54 (3), 447–472. 10.2165/00003495-199754030-00009. [DOI] [PubMed] [Google Scholar]
- Ferraris D.; Duvall B.; Delahanty G.; Mistry B.; Alt J.; Rojas C.; Rowbottom C.; Sanders K.; Schuck E.; Huang K.-C.; Redkar S.; Slusher B. B.; Tsukamoto T. Design, Synthesis, and Pharmacological Evaluation of Fluorinated Tetrahydrouridine Derivatives as Inhibitors of Cytidine Deaminase. J. Med. Chem. 2014, 57 (6), 2582–2588. 10.1021/jm401856k. [DOI] [PubMed] [Google Scholar]
- Barman Balfour J. A.; McClellan K. Topical eflornithine. Am. J. Clin. Dermatol. 2001, 2 (3), 197–201. 10.2165/00128071-200102030-00009. [DOI] [PubMed] [Google Scholar]
- Hong R.; Edgar K.; Song K.; Steven S.; Young A.; Hamilton P.; Arrazate A.; De La Cruz C.; Chan C.; Pang J.; Salphati L.; Belvin M.; Nannini M.; Staben S.; Friedman L.; Sampath D. Abstract PD4–14: GDC-0077 is a selective PI3Kalpha inhibitor that demonstrates robust efficacy in PIK3CA mutant breast cancer models as a single agent and in combination with standard of care therapies. Cancer Res. 2018, 78, 4–14. 10.1158/1538-7445.SABCS17-PD4-14. [DOI] [Google Scholar]
- Salam K. A.; Akimitsu N. Hepatitis C Virus NS3 Inhibitors: Current and Future Perspectives. BioMed. Research International 2013, 2013, 1–9. 10.1155/2013/467869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J. B. I.; Meyer C. F.; Straathof N. J. W.; Iwumene N.; Am Ende C. W.; Trabanco A. A.; Gouverneur V. Late-stage difluoromethylation: concepts, developments and perspective. Chem. Soc. Rev. 2021, 50, 8214–8247. 10.1039/D1CS00360G. [DOI] [PubMed] [Google Scholar]
- a Czerwinski P. J.; Furman B. ;Reductive Functionalization of Amides in Synthesis and for Modification of Bioactive Compounds. Front. Chem. 2021, 9, 655849. 10.3389/fchem.2021.655849. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Matheau-Raven D.; Gabriel P.; Leitch J. A.; Almehmadi Y. A.; Yamazaki K.; Dixon D. J. Catalytic Reductive Functionalization of Tertiary Amides using Vaska’s Complex: Synthesis of Complex Tertiary Amine Building Blocks and Natural Products. ACS Catal. 2020, 10 (15), 8880–8897. 10.1021/acscatal.0c02377. [DOI] [Google Scholar]; c Ong D. Y.; Chen J.-H.; Chiba S. Reductive Functionalization of Carboxamides: A Recent Update. Bull. Chem. Soc. Jpn. 2020, 93, 1339–1349. 10.1246/bcsj.20200182. [DOI] [Google Scholar]; d Pace V.; Holzer W.; Olofsson B. Increasing the Reactivity of Amides towards Organometallic Reagents: An Overview. Adv. Synth. Catal. 2014, 356, 3697–3736. 10.1002/adsc.201400630. [DOI] [Google Scholar]; e Sato T.; Yoritate M.; Tajima H.; Chida N. Total synthesis of complex alkaloids by nucleophilic addition to amides. Org. Biomol. Chem. 2018, 16, 3864–3875. 10.1039/C8OB00733K. [DOI] [PubMed] [Google Scholar]; f Kaiser D.; Bauer A.; Lemmerer M.; Maulide N. Amide activation: an emerging tool for chemoselective synthesis. Chem. Soc. Rev. 2018, 47, 7899–7925. 10.1039/C8CS00335A. [DOI] [PubMed] [Google Scholar]
- a Huang P.-Q.; Huang Y.-H.; Xiao K.-J.; Wang Y.; Xia X.-E. A General Method for the One-Pot Reductive Functionalization of Secondary Amides. J. Org. Chem. 2015, 80, 2861–2868. 10.1021/jo502929x. [DOI] [PubMed] [Google Scholar]; b Xiao K.-J.; Wang A.-E.; Huang P.-Q. Direct Transformation of Secondary Amides into Secondary Amines: Triflic Anhydride Activated Reductive Alkylation. Angew. Chem., Int. Ed. 2012, 51, 8314–8317. 10.1002/anie.201204098. [DOI] [PubMed] [Google Scholar]; c Xiao K.-J.; Luo J.-M.; Ye K.-Y.; Wang Y.; Huang P.-Q. Direct, One-pot Sequential Reductive Alkylation of Lactams/Amides with Grignard and Organolithium Reagents through Lactam/Amide Activation. Angew. Chem., Int. Ed. 2010, 49, 3037–3040. 10.1002/anie.201000652. [DOI] [PubMed] [Google Scholar]
- a Vincent G.; Guillot R.; Kouklovsky C. Stereodivergent Synthesis of Substituted N, O-Containing Bicyclic Compounds by Sequential Addition of Nucleophiles to N-Alkoxybicyclolactams. Angew. Chem., Int. Ed. 2011, 50, 1350–1353. 10.1002/anie.201006590. [DOI] [PubMed] [Google Scholar]; b Shirokane K.; Kurosaki Y.; Sato T.; Chida N. A Direct Entry to Substituted N-Methoxyamines from N-Methoxyamidesvia N-Oxyiminium Ions. Angew. Chem., Int. Ed. 2010, 49, 6369–6372. 10.1002/anie.201001127. [DOI] [PubMed] [Google Scholar]; c Nakajima M.; Oda Y.; Wada T.; Minamikawa R.; Shirokane K.; Sato T.; Chida N. Chemoselective Reductive Nucleophilic Addition to Tertiary Amides, Secondary Amides, and N-Methoxyamides. Chem. Eur. J. 2014, 20, 17565–17571. 10.1002/chem.201404648. [DOI] [PubMed] [Google Scholar]
- Ong D. Y.; Fan D.; Dixon D. J.; Chiba S. Transition-Metal-Free Reductive Functionalization of Tertiary Carboxamides and Lactams for α-Branched Amine Synthesis. Angew. Chem., Int. Ed. 2020, 59, 11903–11907. 10.1002/anie.202004272. [DOI] [PubMed] [Google Scholar]
- a Xie L.-G.; Dixon D. J. Iridium-catalyzed reductive Ugi-type reactions of tertiary amides. Nat. Commun. 2018, 9, 2841–2849. 10.1038/s41467-018-05192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Fuentes de Arriba Á. L.; Lenci E.; Sonawane M.; Formery O.; Dixon D. J. Iridium-Catalyzed Reductive Strecker Reaction for Late-Stage Amide and Lactam Cyanation. Angew. Chem., Int. Ed. 2017, 56, 3655–3659. 10.1002/anie.201612367. [DOI] [PubMed] [Google Scholar]; c Tan P. W.; Seayad J.; Dixon D. J. Expeditious and Divergent Total Syntheses of Aspidosperma Alkaloids Exploiting Iridium(I)-Catalyzed Generation of Reactive Enamine Intermediates. Angew. Chem., Int. Ed. 2016, 55, 13436–13638. 10.1002/anie.201605503. [DOI] [PubMed] [Google Scholar]; d Rogova T.; Gabriel P.; Zavitsanou S.; Leitch J. A.; Duarte F.; Dixon D. J. Reverse Polarity Reductive Functionalization of Tertiary Amides via a Dual Iridium-Catalyzed Hydrosilylation and Single Electron Transfer Strategy. ACS Catal. 2020, 10, 11438–11447. 10.1021/acscatal.0c03089. [DOI] [Google Scholar]; e Yamazaki K.; Gabriel P.; Di Carmine G.; Pedroni J.; Farizyan M.; Hamlin T. A.; Dixon D. J. General Pyrrolidine Synthesis via Iridium-Catalyzed Reductive Azomethine Ylide Generation from Tertiary Amides and Lactams. ACS Catal. 2021, 11, 7489–7497. 10.1021/acscatal.1c01589. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Matheau-Raven D.; Dixon D. J. General α-Amino 1,3,4-Oxadiazole Synthesis via Late-Stage Reductive Functionalization of Tertiary Amides and Lactams. Angew. Chem., Int. Ed. 2021, 60, 19725–19729. 10.1002/anie.202107536. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Gabriel P.; Almehmadi Y. A.; Wong Z. R.; Dixon D. J. A General Iridium-Catalyzed Reductive Dienamine Synthesis Allows a Five-Step Synthesis of Catharanthine via the Elusive Dehydrosecodine. J. Am. Chem. Soc. 2021, 143 (29), 10828–10835. 10.1021/jacs.1c04980. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Xie L.-G.; Dixon D. J. Tertiary amine synthesis via reductive coupling of amides with Grignard reagents. Chem. Sci. 2017, 8, 7492–7497. 10.1039/C7SC03613B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Takahashi Y.; Sato T.; Chida N. Iridium-catalyzed Reductive Nucleophilic Addition to Tertiary Amides. Chem. Lett. 2019, 48, 1138–1141. 10.1246/cl.190467. [DOI] [PubMed] [Google Scholar]; b Katahara S.; Kobayashi S.; Fujita K.; Matsumoto T.; Sato T.; Chida N. An Iridium-Catalyzed Reductive Approach to Nitrones from N-Hydroxyamides. J. Am. Chem. Soc. 2016, 138, 5246–5249. 10.1021/jacs.6b02324. [DOI] [PubMed] [Google Scholar]; c Nakajima M.; Sato T.; Chida N. Iridium-Catalyzed Chemoselective Reductive Nucleophilic Addition to N-Methoxyamides. Org. Lett. 2015, 17, 1696–1699. 10.1021/acs.orglett.5b00664. [DOI] [PubMed] [Google Scholar]; d Katahara S.; Sugiyama Y.; Yamane M.; Komiya Y.; Sato T.; Chida N. Five-Step Total Synthesis of (±)-Aspidospermidine by a Lactam Strategy via an Azomethine Ylide. Org. Lett. 2021, 23, 3058–3063. 10.1021/acs.orglett.1c00735. [DOI] [PubMed] [Google Scholar]; e Ou W.; Han F.; Hu X.-H.; Chen H.; Huang P.-Q. Iridium-Catalyzed Reductive Alkylations of Secondary Amides. Angew. Chem., Int. Ed. 2018, 57, 11354–11358. 10.1002/anie.201806747. [DOI] [PubMed] [Google Scholar]; f Huang P.-Q.; Ou W.; Han F. Chemoselective reductive alkynylation of tertiary amides by Ir and Cu(I) bis-metal sequential catalysis. Chem. Commun. 2016, 52, 11967–11970. 10.1039/C6CC05318A. [DOI] [PubMed] [Google Scholar]; g Li Z.; Zhao F.; Ou W.; Huang P.-Q.; Wang X. Asymmetric Deoxygenative Alkynylation of Tertiary Amides Enabled by Iridium/Copper Bimetallic Relay Catalysis. Angew. Chem., Int. Ed. 2021, 60, 26604–26609. 10.1002/anie.202111029. [DOI] [PubMed] [Google Scholar]; h Chen D. H.; Sun W. T.; Zhu C. J.; Lu G. S.; Wu D. P.; Wang A. E.; Huang P.-Q. Enantioselective Reductive Cyanation and Phosphonylation of Secondary Amides by Iridium and Chiral Thiourea Sequential Catalysis. Angew. Chem., Int. Ed. 2021, 60, 8827–8831. 10.1002/anie.202015898. [DOI] [PubMed] [Google Scholar]
- a Mizuta S.; Stenhagen I. S. R.; O’Duill M.; Wolstenhulme J.; Kirjavainen A. K.; Forsback S. J.; Tredwell M.; Sandford G.; Moore P. R.; Huiban M.; Luthra S. K.; Passchier J.; Solin O.; Gouverneur V. Catalytic Decarboxylative Fluorination for the Synthesis of Tri- and Difluoromethyl Arenes. Org. Lett. 2013, 15, 2648–2651. 10.1021/ol4009377. [DOI] [PubMed] [Google Scholar]; b Yang Y.-Y.; Xu J.; You Z.-W.; Xu X.-H.; Qiu X.-L.; Qing F.-L. Synthesis of 3′,3′-Difluoro-2′- hydroxymethyl-4′,5′-Unsaturated Carbocyclic Nucleosides. Org. Lett. 2007, 9, 5437–5440. 10.1021/ol7023955. [DOI] [PubMed] [Google Scholar]; c Fujikawa K.; Fujioka Y.; Kobayashi A.; Amii H. A New Method for Aromatic Difluoromethylation: Copper-Catalyzed Cross-Coupling and Decarboxylation Sequence from Aryl Iodides. Org. Lett. 2011, 13, 5560–5563. 10.1021/ol202289z. [DOI] [PubMed] [Google Scholar]; d Otsuka T.; Ishii A.; Dub P. A.; Ikariya T. Practical Selective Hydrogenation of α-Fluorinated Esters with Bifunctional Pincer-Type Ruthenium(II) Catalysts Leading to Fluorinated Alcohols or Fluoral Hemiacetals. J. Am. Chem. Soc. 2013, 135, 9600–9603. 10.1021/ja403852e. [DOI] [PubMed] [Google Scholar]; e Geraschenko O. V.; Solomin V. V.; Vashchenko B. V.; Khodakivskyi P.; Tolmachev A. A.; Grygorenko O. O. Synthesis and chemical transformations of diazolyl α,α-difluoroacetates. J. Fluorine Chem. 2020, 229, 109407–109415. 10.1016/j.jfluchem.2019.109407. [DOI] [Google Scholar]; f Ruan Z.; Zhang S.-K.; Zhu C.; Ruth P. N.; Stalke D.; Ackermann L. Ruthenium(II)-Catalyzed meta C-H Mono- and Difluoromethylations by Phosphine/Carboxylate Cooperation. Angew. Chem., Int. Ed. 2017, 56, 2045–2049. 10.1002/anie.201611595. [DOI] [PubMed] [Google Scholar]; g Fan W.-T.; Li Y.; Wang D.; Ji S.-J.; Zhao Y. Iron-Catalyzed Highly para-Selective Difluoromethylation of Arenes. J. Am. Chem. Soc. 2020, 142, 20524–20530. 10.1021/jacs.0c09545. [DOI] [PubMed] [Google Scholar]
- a Belhomme M.-C.; Besset T.; Poisson T.; Pannecoucke X. Recent Progress toward the Introduction of Functionalized Difluoromethylated Building Blocks onto C(sp2) and C(sp) Centers. Chem. Eur. J. 2015, 21, 12836–12865. 10.1002/chem.201501475. [DOI] [PubMed] [Google Scholar]; b Feng Z.; Xiao Y.-L.; Zhang X. Transition-Metal (Cu, Pd, Ni)-Catalyzed Difluoroalkylation via Cross-Coupling with Difluoroalkyl Halides. Acc. Chem. Res. 2018, 51, 2264–2278. 10.1021/acs.accounts.8b00230. [DOI] [PubMed] [Google Scholar]
- a Dong D.-Q.; Yang H.; Shi J.-L.; Si W.-J.; Wang Z.-L.; Xu X.-M. Promising reagents for difluoroalkylation. Org. Chem. Front. 2020, 7, 2538–2575. 10.1039/D0QO00567C. [DOI] [Google Scholar]; b Qu C.-H.; Song G.-T.; Xu J.; Yan W.; Zhou C.-H.; Li H.-Y.; Chen Z.-Z.; Xu Z.-G. Merging Visible Light with Cross-Coupling: The Photochemical Direct C–H Difluoroalkylation of Imidazopyridines. Org. Lett. 2019, 21, 8169–8173. 10.1021/acs.orglett.9b02487. [DOI] [PubMed] [Google Scholar]; c Fan W.-T.; Li Y.; Wang D.; Ji S.-J.; Zhao Y. Iron-Catalyzed Highly para-Selective Difluoromethylation of Arenes. J. Am. Chem. Soc. 2020, 142, 20524–20530. 10.1021/jacs.0c09545. [DOI] [PubMed] [Google Scholar]
- a Besset T.; Poisson T.; Pannecoucke X. Direct Vicinal Difunctionalization of Alkynes: An Efficient Approach Towards the Synthesis of Highly Functionalized Fluorinated Alkenes. Eur. J. Org. Chem. 2015, 2015, 2765–2789. 10.1002/ejoc.201403507. [DOI] [Google Scholar]; b Xu C.; Cheng R.; Luo Y.-C.; Wang M.-K.; Zhang X. trans-Selective Aryldifluoroalkylation of Endocyclic Enecarbamates and Enamides by Nickel Catalysis. Angew. Chem., Int. Ed. 2020, 59, 18741–18747. 10.1002/anie.202008498. [DOI] [PubMed] [Google Scholar]; c Zhou M.; Zhao H.-Y.; Zhang S.; Zhang Y.; Zhang X. Nickel-Catalyzed Four-Component Carbocarbonylation of Alkenes under 1 atm of CO. J. Am. Chem. Soc. 2020, 142, 18191–18199. 10.1021/jacs.0c08708. [DOI] [PubMed] [Google Scholar]; d Zhang Z.; Li X.; Shi D. Visible-Light-Promoted Oxy-difluoroalkylation of Aryl Alkynes for the Synthesis of β-Fluoroenones and α-Difluoroalkyl Ketones. Adv. Synth. Catal. 2021, 363, 3348–3353. 10.1002/adsc.202100289. [DOI] [Google Scholar]; e Zhang M.; Lin J.-H.; Jin C.-M.; Xiao J.-C. Difluorocarbene-based cyanodifluoromethylation of alkenes induced by a dual-functional Cu-catalyst. Chem. Commun. 2021, 57, 2649–2652. 10.1039/D1CC00160D. [DOI] [PubMed] [Google Scholar]; f Li X.; He S.; Song Q. Diethylzinc-Mediated Radical 1,2-Addition of Alkenes and Alkynes. Org. Lett. 2021, 23, 2994–2999. 10.1021/acs.orglett.1c00669. [DOI] [PubMed] [Google Scholar]; g Cheng X.; Liu X.; Wang S.; Hu Y.; Hu B.; Lei A.; Li J. Organozinc pivalates for cobalt-catalyzed difluoroalkylarylation of alkenes. Nat. Commun. 2021, 12, 4366–4376. 10.1038/s41467-021-24596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Otaka A.; Watanabe H.; Mitsuyama E.; Yukimasa A.; Tamamura H.; Fujii N. Synthesis of (Z)-fluoroalkene dipeptide isosteres utilizing organocopper-mediated reduction of γ,γ-difluoro-α,β-enoates. Tetrahedron Lett. 2001, 42, 285–287. 10.1016/S0040-4039(00)01924-9. [DOI] [Google Scholar]; b Peng W.; He P.; Zhu S.; Li Z. Structural determination of a zinc reagent from ethyl 3-bromodifluoromethyl-3-benzyloxyacrylate and its reactions with aldehydes. Tetrahedron Lett. 2004, 45, 3677–3680. 10.1016/j.tetlet.2004.02.160. [DOI] [Google Scholar]; c Pedrosa R.; Sayalero S.; Vicente M. A direct efficient diastereoselective synthesis of enantiopure 3-substituted-isobenzofuranones. Tetrahedron 2006, 62, 10400–10407. 10.1016/j.tet.2006.08.058. [DOI] [Google Scholar]; d Vidal A.; Nefzi A.; Houghten R. A. Solid-Phase Synthesis of α,α-Difluoro-β-amino Acids via the Reformatsky Reaction. J. Org. Chem. 2001, 66, 8268–8272. 10.1021/jo010872z. [DOI] [PubMed] [Google Scholar]; e Sorochinsky A.; Voloshin N.; Markovsky A.; Belik M.; Yasuda N.; Uekusa H.; Ono T.; Berbasov D. O.; Soloshonok V. A. Convenient Asymmetric Synthesis of β-Substituted α,α-Difluoro-β-amino Acids via Reformatsky Reaction between Davis’ N-Sulfinylimines and Ethyl Bromodifluoroacetate. J. Org. Chem. 2003, 68, 7448–7454. 10.1021/jo030082k. [DOI] [PubMed] [Google Scholar]; f March T. L.; Johnston M. R.; Duggan P. J. Diastereoselective Synthesis of Aliphatic α,α-Difluoro-β3-Amino Esters via a Sonocatalyzed Reformatsky Reaction. Org. Lett. 2012, 14 (1), 182–185. 10.1021/ol202969w. [DOI] [PubMed] [Google Scholar]; g Mamone M.; Morvan E.; Milcent T.; Ongeri S.; Crousse B. Electrophilic Amination of Fluoroalkyl Groups on Azodicarboxylate Derivatives. J. Org. Chem. 2015, 80, 1964–1971. 10.1021/jo502638y. [DOI] [PubMed] [Google Scholar]
- Presumably, reduced yields of products 3y and 3z are linked to a relatively short lifetime of their corresponding in situ generated silylated hemiaminal intermediates.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.