Abstract
Introduction:
Following resection of pancreatic acinar cell carcinoma (PACC) distant recurrence remains high. We utilized the national cancer database (NCDB) to evaluate the role of systemic therapy in early-stage resected PACC.
Methods:
We queried the NCDB registry from 2004 to 2015 for patients with pathologic stage I-IIB PACC. For each stage, patients who underwent surgery alone (SA) were compared to patients who received systemic and/or radiation therapy in addition to surgery (surgery + therapy [S + T]).
Results:
A total of 271 patients (101 pI, 81 pIIA, and 89 pIIB) were analyzed. Of all clinically node positive patients (n = 41), the majority (n = 32, 78%) had node-positive disease at resection (pIIB). SA was performed in 112 patients (41.3%), whereas 159 (58.7%) patients received S + T. There was no difference in overall survival (OS) between S + T and SA with respect to pI or pIIA disease. In pIIB disease, S + T was associated with improved OS compared to SA (34.9 vs. 16.9 months, p = 0.031). Single-agent chemotherapy was associated with improved OS for pIIB disease when compared to SA (hazard ratio: 0.38, 95% confidence interval: 0.16, 0.83).
Conclusion:
In resectable PACC, the survival benefit of adjuvant therapy is limited to pathologic stage IIB disease. This benefit is evident even in patients treated with single-agent chemotherapy.
Keywords: chemotherapy, pancreas, surgical oncology
1 |. INTRODUCTION
Pancreatic acinar cell carcinomas (PACC) represent 1%–2% of adult exocrine pancreatic neoplasms with peak incidence in the 6th decade of life.1 These neoplasms are distinguished from pancreatic ductal adenocarcinoma (PDAC) both clinically and pathologically.2,3 In contrast to PDAC, patients with PACC typically present with abdominal pain, rather than biliary obstruction, and lesions more frequently located in the body/tail of the pancreas (32.8% vs. 16.4%).2,4 Following diagnosis, nearly 40% of PACC patients undergo surgical resection of the primary tumor, as compared with approximately 20% of patients diagnosed with PDAC.3,5 The 5-year overall survival (OS) for resected PACC is 72% compared to 16.3% for patients with PDAC. Despite a more favorable survival following resection, distant recurrence of PACC still approaches 72%.1 Adjuvant therapy use for PACC is increasing and current strategies are largely based on existing literature pertaining to PDAC.
Currently, adjuvant chemotherapy with modified FOLFIRINOX is standard for appropriately fit patients6,7; however, 40% do not complete adjuvant therapy due to postoperative morbidity, poor performance status, and/or early disease progression.8–10 As such, the role of neoadjuvant therapy is gaining interest and is now standard of care for borderline resectable PDAC, while many institutions prefer neoadjuvant therapy for resectable disease as well.11
In the context of PACC, evidence is limited to case series and retrospective analyses.1–4,12–14 Recent evidence for resectable PACC suggests improved survival with adjuvant therapy compared to resection alone, particularly in node positive disease.13,14 The role of neoadjuvant therapy, however, is unclear. We utilized the national cancer database (NCDB) to evaluate the role of systemic therapy in early-stage resected PACC.
2 |. METHODS
2.1 |. Patient selection
We queried the NCDB registry between 2004 and 2015 for patients with pathologic stage I-IIB (pI-IIB) PACC. The International Classification of Diseases for Oncology (edition 3.2) site and histology codes were used to identify patients with acinar cell carcinoma or acinar cell cystadenocarcinoma of the pancreas (ICD-O-3.2 code 8550/3 and 8551/3, respectively). Patients with non-metastatic disease who underwent curative intent surgical resection were included. Patients with pathologic stage ≥III disease or those who underwent treatment with palliative intent, were excluded from the analysis.
2.2 |. Patient characteristics and treatment cohorts
Patient characteristics included age, sex, race, Charlson–Deyo (CD) score, facility type and location. Clinicopathologic variables included tumor grade, tumor size, clinical and pathologic T and N stages as defined by the American Joint Committee on Cancer (AJCC) staging system (6th and 7th edition), lymphovascular invasion (LVI), and margin positivity. The 6th and 7th edition of the AJCC staging system are equivalent for pancreatic cancer.15,16 For each stage group, outcomes for patients who underwent surgery alone were compared with outcomes of patients who received systemic and/or radiation therapy in addition to surgery (neoadjuvant, perioperative, or adjuvant).
2.3 |. Statistical analysis
Statistical analysis was conducted using SAS 9.4 (SAS Institute Inc.) and SAS macros developed at the Biostatistics Shared Resource at the Winship Cancer Institute.17 Statistical significance was set at a p value of 0.05. Descriptive analyses were performed for each variable. Patient characteristics were compared across treatment groups using chi-squared tests or Fisher’s exact tests for categorical covariates and by the analysis of variance or Kruskal–Wallis tests for numerical covariates, where appropriate. The primary outcome was OS, calculated from the time of diagnosis to the time of death. Kaplan–Meier method was used to generate OS curves and the log-rank test was used for comparison of survival curves between cohorts. Univariate and multivariable Cox proportional hazard analysis for OS were performed using Firth’s bias-reduced, penalized likelihood approach to account the rarity of event.18,19
2.4 |. Data source
The NCDB is a hospital-based registry maintained by the American Cancer Society and American College of Surgeons that captures 70% of newly diagnosed cancers from more than 1500 participating facilities across the United States.20 The NCDB has not verified and is not responsible for the statistical analysis employed here, or the conclusions drawn by the investigators.
3 |. RESULTS
3.1 |. Patient demographics and treatment characteristics
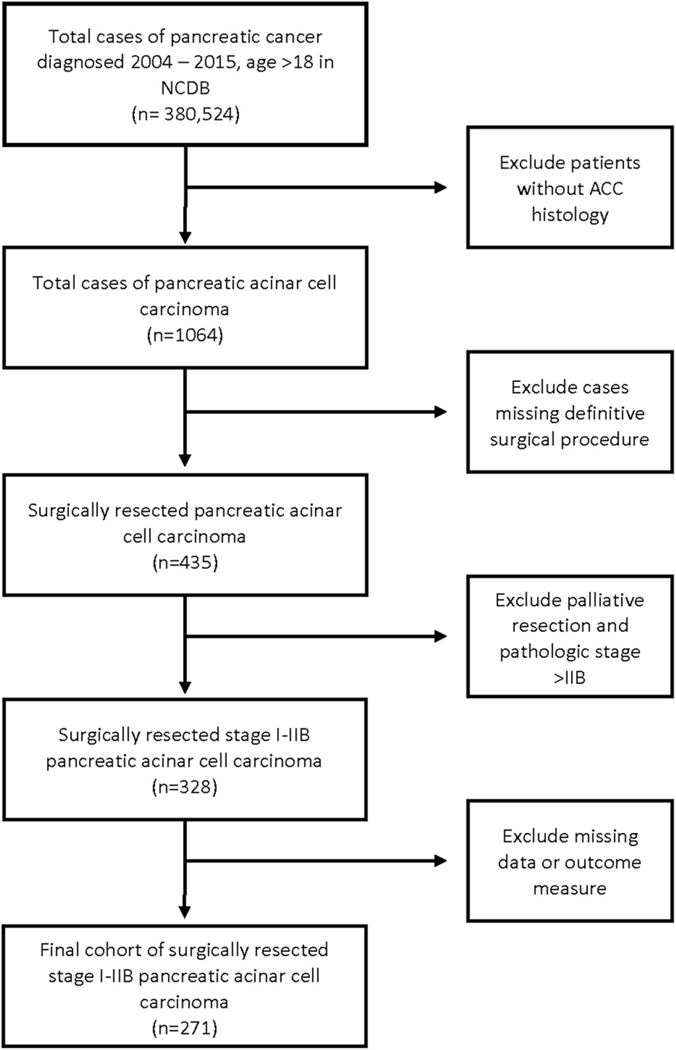
Among 380 524 pancreatic cancer patients identified in the NCDB participant user file from 2004 to 2015, there were 1064 cases of PACC, of which 271 met our inclusion criteria (Figure 1). Patient characteristics are listed in Table 1. Within the cohort, median age was 64 years with the majority being male patients (n = 189, 69.7%) with CD score of 0 (n = 189, 69.7%). Mean primary tumor size was 59 mm (SD ± 50) and was most often located in the pancreatic head (n = 114, 42.1%). Surgical resection alone (SA) was performed in 112 patients (41.3%), whereas 159 (58.7%) patients received systemic and/or radiation therapy in addition to surgery (surgery + therapy [S + T]). Within the S + T cohort, 14 patients received neoadjuvant therapy, 3 received perioperative therapy, and 142 received adjuvant therapy (data not shown). The node positive rate was 32.8% with margin positive resection observed in 14% of all cases. The 30- and 90-day postoperative mortality was 1.5% and 2.9%, respectively (data not shown).
FIGURE 1.
Flow diagram of selection criteria
TABLE 1.
Descriptive statistics by pathologic stage for resected PACC
| Covariate | Level | Stage I |
p value | Stage IIA |
p value | Stage IIB |
p value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| S + T N = 48 | SA N = 53 | S + T N = 52 | SA N = 29 | S + T N = 59 | SA N = 30 | |||||
|
| ||||||||||
| Age | <65 | 26 (54.17) | 24 (45.28) | 0.373 | 25 (48.08) | 12 (41.38) | 0.562 | 33 (55.93) | 10 (33.33) | 0.044 |
| ≥65 | 22 (45.83) | 29 (54.72) | 27 (51.92) | 17 (58.62) | 26 (44.07) | 20 (66.67) | ||||
|
| ||||||||||
| Sex | Male | 29 (60.42) | 29 (54.72) | 0.563 | 37 (71.15) | 26 (89.66) | 0.092 | 46 (77.97) | 21 (70) | 0.41 |
| Female | 19 (39.58) | 24 (45.28) | 15 (28.85) | 3 (10.34) | 13 (22.03) | 9 (30) | ||||
|
| ||||||||||
| Race | White | 40 (83.33) | 40 (75.47) | 0.331 | 45 (86.54) | 26 (89.66) | 1 | 53 (89.83) | 27 (90) | 1 |
| Others | 8 (16.67) | 13 (24.53) | 7 (13.46) | 3 (10.34) | 6 (10.17) | 3 (10) | ||||
|
| ||||||||||
| Facility type | Others | 17 (37.78) | 25 (49.02) | 0.268 | 27 (55.1) | 10 (35.71) | 0.101 | 20 (35.09) | 11 (36.67) | 0.884 |
| Academics | 28 (62.22) | 26 (50.98) | 22 (44.9) | 18 (64.29) | 37 (64.91) | 19 (63.33) | ||||
|
| ||||||||||
| Charlson-Deyo Score |
0 | 32 (66.67) | 37 (69.81) | 0.896 | 42 (80.77) | 15 (51.72) | 0.016 | 42 (71.19) | 20 (66.67) | 0.677 |
| 1 | 12 (25) | 13 (24.53) | 7 (13.46) | 11 (37.93) | 13 (22.03) | 9 (30) | ||||
| 2+ | 4 (8.33) | 3 (5.66) | 3 (5.77) | 3 (10.34) | 4 (6.78) | 1 (3.33) | ||||
|
| ||||||||||
| Primary payor | Others | 27 (56.25) | 31 (58.49) | 0.82 | 31 (59.62) | 8 (27.59) | 0.006 | 34 (57.63) | 24 (80) | 0.036 |
| Private | 21 (43.75) | 22 (41.51) | 21 (40.38) | 21 (72.41) | 25 (42.37) | 6 (20) | ||||
|
| ||||||||||
| Grade | Well differentiated | 7 (30.43) | 9 (36) | 0.92 | 1 (4.55) | 4 (26.67) | 0.212 | 2 (4.88) | 2 (9.52) | 0.334 |
| Moderately differentiated | 11 (47.83) | 11 (44) | 14 (63.64) | 7 (46.67) | 19 (46.34) | 6 (28.57) | ||||
| Poorly differentiated/ undifferentiated | 5 (21.74) | 5 (20) | 7 (31.82) | 4 (26.67) | 20 (48.78) | 13 (61.9) | ||||
|
| ||||||||||
| AJCC clinical T | c0 | 1 (2.13) | 0 (0) | 0.392 | 1 (1.92) | 3 (10.34) | 0.498 | 2 (3.45) | 1 (3.45) | 0.271 |
| c1 | 9 (19.15) | 6 (11.76) | 15 (28.85) | 8 (27.59) | 27 (46.55) | 7 (24.14) | ||||
| c2 | 19 (40.43) | 26 (50.98) | 21 (40.38) | 10 (34.48) | 17 (29.31) | 12 (41.38) | ||||
| c3 | 6 (12.77) | 3 (5.88) | 2 (3.85) | 0 (0) | 2 (3.45) | 1 (3.45) | ||||
| cX | 12 (25.53) | 16 (31.37) | 13 (25) | 8 (27.59) | 10 (17.24) | 8 (27.59) | ||||
|
| ||||||||||
| AJCC clinical N | c0 | 33 (70.21) | 38 (73.08) | 0.651 | 34 (65.38) | 23 (79.31) | 0.484 | 25 (43.1) | 14 (50) | 0.783 |
| c1 | 3 (6.38) | 1 (1.92) | 4 (7.69) | 1 (3.45) | 23 (39.66) | 9 (32.14) | ||||
| cX | 11 (23.4) | 13 (25) | 14 (26.92) | 5 (17.24) | 10 (17.24) | 5 (17.86) | ||||
|
| ||||||||||
| Lymph vascular invasion, 2010 | Not present | 14 (53.85) | 24 (85.71) | 0.03 | 18 (51.43) | 8 (42.11) | 0.584 | 9 (20.93) | 3 (17.65) | 1 |
| Present | 6 (23.08) | 3 (10.71) | 13 (37.14) | 10 (52.63) | 29 (67.44) | 12 (70.59) | ||||
| Unknown | 6 (23.08) | 1 (3.57) | 4 (11.43) | 1 (5.26) | 5 (11.63) | 2 (11.76) | ||||
|
| ||||||||||
| Primary site | Others | 31 (64.58) | 36 (67.92) | 0.723 | 33 (63.46) | 19 (65.52) | 0.853 | 27 (45.76) | 12 (40) | 0.604 |
| Head | 17 (35.42) | 17 (32.08) | 19 (36.54) | 10 (34.48) | 32 (54.24) | 18 (60) | ||||
|
| ||||||||||
| Surgical margin | Negative | 44 (93.62) | 53 (100) | 0.062 | 38 (76) | 23 (92) | 0.122 | 44 (74.58) | 23 (79.31) | 0.624 |
| Positive | 3 (6.38) | 0 (0) | 12 (24) | 2 (8) | 15 (25.42) | 6 (20.69) | ||||
|
| ||||||||||
| Tumor size | >4 | 18 (37.5) | 23 (43.4) | 0.547 | 36 (69.23 | 18 (62.07) | 0.512 | 32 (54.24) | 19 (63.33) | 0.412 |
| ≤4 | 30 (62.5) | 30 (56.6) | 16 (30.77) | 11 (37.93) | 27 (45.76) | 11 (36.67) | ||||
Abbreviations: PACC, pancreatic acinar cell carcinoma; SA, surgery alone; S + T, surgery + therapy.
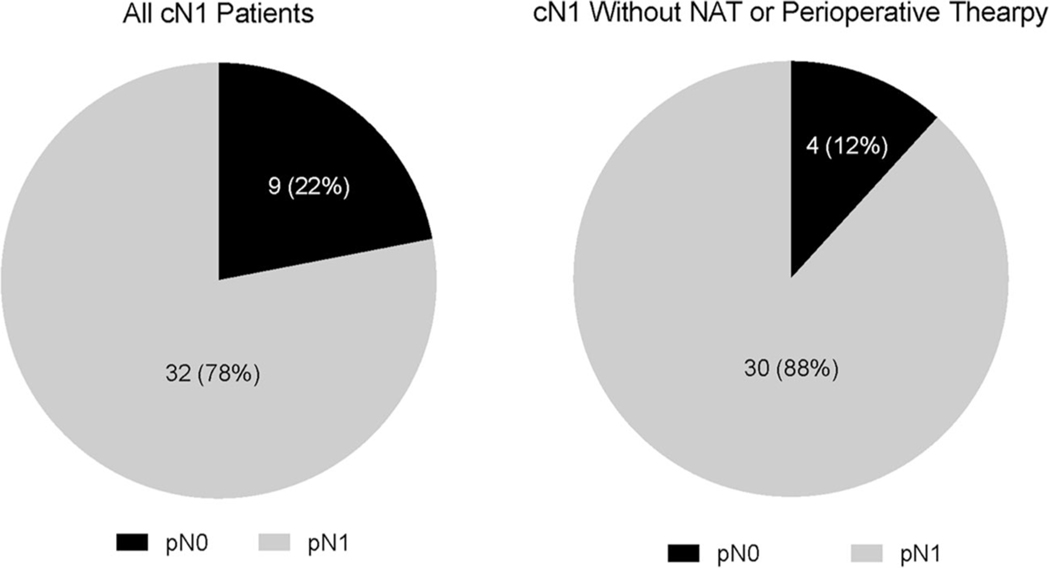
The cohort consisted of 101 pathologic stage I (pI = pT1-2N0), 81 pathologic stage IIA (pIIA = pT3N0), and 89 pathologic stage IIB (pIIB = pT1-3N1) patients. Patients with pI and pIIA disease presented most often (70.3%) with clinically node negative (cN0) disease. Clinically, node positive (cN1) disease was suspected in 4%, 6%, and 36% of patients pI, pIIA, and pIIB disease, respectively. Of all cN1 patients (n = 41), the majority (n = 32, 78%) had node-positive disease at resection (pIIB), with a concordance rate of 88% in patients who underwent surgery first (no neoadjuvant or perioperative therapy) (Figure 2). Of the 9 patients presenting with cN1 disease who were pathologically node negative (pI and pIIA), 5 patients had received neoadjuvant (n = 4) or perioperative therapy (n = 1) (data not shown).
FIGURE 2.
Pie charts of cN1 positive patients with pN0 and pN1 disease at resection
S + T was performed in 48/101 (47.5%), 52/81 (64%), and 59/89 (66%) of patients with pI, pIIA, and pIIB disease, respectively. Patients with pI disease were more likely to undergo S + T when LVI was present (23% vs. 10.7%, p = 0.028). Amongst pIIA patients, those with lower CD score (p = 0.016) and non-private insurance (p = 0.006) were more likely to undergo S + T when compared to SA. Patients with pIIB disease were more likely to undergo S + T if they were under age 65 (56% vs 33%, p = 0.044) and had private insurance (43% vs. 20%, p = 0.036).
3.2 |. Kaplan–Meier and univariate analysis (UVA)
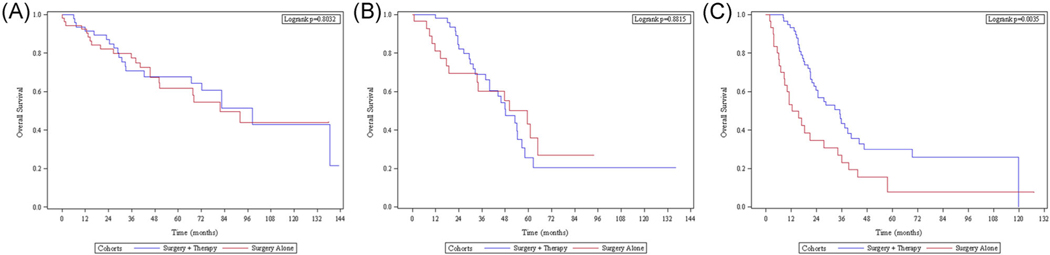
In patients with pI disease, median OS was 92 months with no difference in OS between the S + T group and SA group (median OS = 98.4 months vs. 81.6 months; hazard ratio [HR]: 0.92, p = 0.803) (Figure 3A). These results were consistent when pI was sub-grouped into pathologic stage IA and pathologic stage IB (p = 0.729 and p = 0.497, respectively) (data not shown). Patients with pIIA disease had a median OS of 50 months with no OS difference between the S + T group and SA group (median OS = 48.2 months vs. 59.4 months; HR: 0.94, p = 0.871) (Figure 3B). In patients with pIIB disease, the median OS was 24.6 months and the S + T group was associated with significant improvement in OS compared to the SA group (median OS = 34.9 months vs. 16.9 months; HR: 0.54, p = 0.031) (Figure 3C).
FIGURE 3.
Kaplan–Meier analysis of surgery + therapy versus surgery alone for resected (A) pI, (B) pIIA, and (C) pIIB PACC. PACC, pancreatic acinar cell carcinoma
UVA of patients with pIIB disease demonstrated additional improved OS for margin-negative resection (HR: 0.46, p = 0.008) and private insurance payers (HR: 0.53, p = 0.027). Additionally, patients with pIIB disease undergoing resection at a nonacademic center predicted worse OS compared to academic centers (HR: 1.82, p = 0.023) (Table 2). Only three patients with pIIB disease received either neoadjuvant or perioperative therapy, which did not allow for optimal analysis in this subgroup.
TABLE 2.
Univariate analysis of resected pathologic stage IIB PACC
| Univariate analysis of pathologic stage IIB | ||||
|---|---|---|---|---|
| Covariate | Level | N | OS |
|
| Hazard ratio (95% CI) | p value | |||
|
| ||||
| Cohorts | Surgery + therapy | 59 | 0.47 (0.28–0.79) | 0.004 |
| Surgery alone | 30 | − | − | |
|
| ||||
| Age | ≥65 | 46 | 1.51 (0.91–2.50) | 0.111 |
| <65 | 43 | − | − | |
|
| ||||
| Sex | Female | 22 | 1.16 (0.66–2.04) | 0.597 |
| Male | 67 | − | − | |
|
| ||||
| Race | Others | 9 | 0.89 (0.38–2.06) | 0.78 |
| White | 80 | − | − | |
|
| ||||
| Facility type | Others | 31 | 1.82 (1.08–3.05) | 0.023 |
| Academics | 56 | − | − | |
|
| ||||
| Charlson-Deyo | 1 | 22 | 1.23 (0.69–2.19) | 0.487 |
| Score | 2+ | 5 | 2.84 (0.98–8.23) | 0.054 |
| 0 | 62 | − | − | |
|
| ||||
| Primary payor | Private | 31 | 0.53 (0.30–0.93) | 0.027 |
| Others | 58 | − | − | |
|
| ||||
| Grade | Moderately differentiated |
25 | 0.96 (0.27–3.39) | 0.944 |
| Poorly | 33 | 1.51 (0.44–5.18) | 0.513 | |
| differentiated/ | ||||
| undifferentiated | ||||
| Well differentiated | 4 | − | − | |
|
| ||||
| LVI, 2010 | Present | 41 | 0.57 (0.26–1.22) | 0.146 |
| Unknown | 7 | 0.88 (0.29–2.63) | 0.818 | |
| Not present | 12 | − | − | |
|
| ||||
| Primary site | Others | 39 | 1.15 (0.69–1.92) | 0.581 |
| Head | 50 | − | − | |
|
| ||||
| Surgical margin | Negative | 67 | 0.46 (0.26–0.82) | 0.008 |
| Positive | 21 | − | − | |
|
| ||||
| Tumor size | >4 | 51 | 1.30 (0.78–2.18) | 0.312 |
| ≤4 | − | − | ||
Abbreviations: CI, confidence interval; OS, overall survival; PACC, pancreatic acinar cell carcinoma.
3.3 |. Multivariable analysis (MVA) of pIIB disease
MVA of patients with pIIB disease continued to demonstrate significantly improved OS from S + T when compared to SA (HR: 0.39, p < 0.001), and margin negative resection compared to margin positive resection (HR: 0.39, p = 0.003). Treatment at a nonacademic center was again associated with significantly worse OS compared to academic centers (HR: 2.02, p = 0.014) (Table 3).
TABLE 3.
Multivariable analysis of resected pathologic stage IIB PACC
| Multivariable analysis of pathologic stage IIB | |||
|---|---|---|---|
| Covariate | Level | OS |
|
| Hazard ratio (95% CI) | p value | ||
|
| |||
| Cohorts | Surgery + therapy | 0.39 (0.23–0.68) | <0.001 |
| Surgery alone | − | − | |
|
| |||
| Race | Others | 0.77 (0.29–2.05) | 0.699 |
| White | − | − | |
|
| |||
| Primary payor | Others | 1.60 (0.89–2.88) | 0.12 |
| Private | − | − | |
|
| |||
| Facility type | Others | 2.02 (1.15–3.55) | 0.014 |
| Academics | − | − | |
|
| |||
| Charlson-Deyo Score | 1 | 1.08 (0.59–1.99) | 0.805 |
| 2+ | 2.75 (0.90–8.37) | 0.075 | |
| 0 | − | − | |
|
| |||
| Primary site | Others | 1.30 (0.73–2.32) | 0.378 |
| Head | − | − | |
|
| |||
| Surgical margin | Negative | 0.39 (0.21–0.72) | 0.003 |
| Positive | − | − | |
|
| |||
| Tumor size | >4 | 1.08 (0.61–1.92) | 0.781 |
| ≤4 | − | − | |
Abbreviations: CI, confidence interval; OS, overall survival; PACC, pancreatic acinar cell carcinoma.
3.4 |. UVA of therapy in pIIB disease
We subsequently performed UVA of pIIB patients with respect to OS for each additional therapy. Two patients who received additional therapy did not have an identified type of therapy and were therefore excluded. Using the SA group as our reference standard, we identified significantly improved OS for chemotherapy (HR: 0.39, 95% CI: 0.19, 0.75) and radiation therapy + chemotherapy (HR: 0.54, 95% CI: 0.3, 0.96). Subset analysis of these therapies demonstrated significantly improved OS for singleagent chemotherapy (HR: 0.38, 95% CI: 0.16, 0.83) when compared to SA (Table 4).
TABLE 4.
Univariate analysis of additional therapy for resected pIIB PACC
| Cohort | Treatment | N | OS |
||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | HR p value | Log-rank p value | |||
|
| |||||
| Grouped | Chemotherapy | 22 | 0.39 (0.19–0.75) | 0.007 | 0.010 |
| Radiation +che- motherapy | 33 | 0.54 (0.30–0.96) | 0.038 | ||
| Surgery alone | 30 | − | − | ||
|
| |||||
| Subset | Single-agent chemotherapy | 13 | 0.38 (0.16–0.83) | 0.025 | 0.099 |
| Multiagent chemotherapy | 9 | 0.47 (0.17–1.09) | 0.114 | ||
| Radiation + single-agent chemotherapy | 19 | 0.64 (0.32–1.22) | 0.193 | ||
| Radiation + multiagent chemotherapy | 14 | 0.50 (0.22–1.02) | 0.075 | ||
| Radiation only | 2 | 0.54 (0.11–1.70) | 0.372 | ||
| Surgery alone | 30 | − | − | ||
Note: Firth’s penalized maximum likelihood estimation was used.
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival.
4 |. DISCUSSION
We utilized the NCDB to evaluate the role of systemic therapy in early-stage resected PACC. Our study delineates stage-specific OS for patients with resected pI-IIB PACC. The OS of patients with pI-IIA disease did not differ between S + T and SA groups. In contrast, patients with pIIB disease demonstrated significantly improved OS with S + T compared to SA. Furthermore, patients with pIIB disease who received S + T had significantly improved OS with single-agent chemotherapy when compared to SA. These findings suggest that patients with pI-IIA PACC may have limited benefit from adjuvant therapy. In addition, patients with pIIB disease appear to derive benefit from at least a single-agent chemotherapeutic regimen.
Surgical resection remains the standard of care for resectable disease and has demonstrated benefit for early-stage PACC across a variety of retrospective studies and case reports.2,3,13,14,21–23 For patients with resected pI disease, median OS in our study was 92 months. Previous literature has not defined median OS for resected pI patients, however, 5-year survival rates are reported from 46% to 53%.14,22 Median OS for resected pIIA and pIIB patients in our study was 50 and 24.6 months, respectively. This demonstrates a large difference in OS within pII disease and highlights the significance of nodal positivity on patient outcomes following resection.
Our findings both support and contrast those of prior NCDB studies. Similar to studies by Schmidt et al. and Patel et al.,14 we identified margin-positive resection associated with increased risk of death in pIIB disease. In contrast, utilizing a patient cohort across all stages of PACC from 1985 to 2005, Schmidt et al.22 found no difference in survival from adjuvant therapy on multivariable analysis.22 More recently, however, Patel et al.14 conducted an analysis through the NCDB of clinical stage I-II PACC from 2004 to 2015 where they identified survival benefit for adjuvant therapy over surgery alone (p = 0.015), particularly in the context of positive lymph nodes (p < 0.001).14 While our study corroborates these findings for pIIB disease, we found limited benefit from additional therapy beyond resection in pI-IIA. This difference is likely a result of grouping patients by pathologic rather than clinical stage. Through early pathologic stage analysis, we are able to include all clinical stages that undergo resection, increasing our patient cohort. Furthermore, we exclude those patients who were upstaged to pathologic stage III or greater, who are more likely to benefit from adjuvant therapy. Additionally, we have included both neoadjuvant and perioperative therapy to broaden our inclusion criteria, which allows for more complete analysis of this patient population.
The literature is scarce with regards to additional systemic and locoregional therapies for resectable PACC. Most reviews of the topic are limited by sample size and encompass both resectable and unresectable disease. A systematic review identified disease control rates (DCR) for gemcitabine-based (0%–50%)13,24–26 or fluoropyrimidine-based (33%–67%)13,26,27 chemotherapy regimens, while DCR for surgical resection with concurrent chemoradiation neared 100% for up to 80 months.2,13,27,28 In regards to surgically resected disease, a case series of 14 patients by Glazer et al.13 demonstrated significantly improved survival with adjuvant radiation therapy compared to surgery alone (HR: 0.05, p = 0.03). However, the small sample size of this study limits its clinical applicability. In our analysis of resected patients with pIIB disease, those who received chemotherapy as well as radiation therapy + chemotherapy, demonstrated significantly improved OS compared to SA. Further stratification identified benefit from at least single-agent chemotherapy when compared to SA. This is an important finding as it demonstrates that if patients are unable to receive multiagent chemotherapy, administration of single-agent chemotherapy does predict improved OS compared to SA in patients with pIIB disease.
Pancreatic resection carries a high morbidity rate and in the context of PDAC, approximately 40% of patients do not receive adjuvant therapy.29 Neoadjuvant therapy provides potential benefit of decreased tumor size, improved margin negative resection rate, targeting of micrometastatic disease and ensuring patients receive systemic therapy as part of their management due to a large proportion of patients being unable to receive therapy after surgery. As such, neoadjuvant therapy has been proposed for resectable PDAC and has shown promise in recent phase II/III randomized controlled trials.30,31 Few studies have investigated the effect of neoadjuvant therapy in resectable PACC.12 Our S + T cohort included patients who underwent neoadjuvant, perioperative, and adjuvant therapy. Due to sample size, we were unable to separate these groups for analysis. Our study demonstrates that the majority (n = 32/41, 78%) of clinically node positive patients have node positive disease at resection. After removing patients who underwent neoadjuvant or perioperative therapy, this correlation increased to 88% (30/34). Based on these results we would propose that the presence of clinically node positive disease on initial staging imaging could be a useful tool to guide patient selection for neoadjuvant therapy in PACC patients.
Our study has several limitations that should be recognized. First, this is a retrospective design that can be subject to selection bias. We combined neoadjuvant, perioperative, and adjuvant therapies into a single cohort due to very small numbers of patients within each of these treatment groups, which would make subsequent analysis suboptimal. In addition, the agents used, dosing and duration of systemic therapy is not delineated in the NCDB. As such, we cannot make any definite conclusion in relation to the impact of single-agent or multiagent chemotherapy. Furthermore, there may be significant selection bias between patients who did and did not receive chemotherapy. Patients who did not receive adjuvant chemotherapy may have had a more complicated postoperative course or other comorbidities that would independently limit their survival. Despite these limitations, our study is the first large, comprehensive pathologic stage-specific analysis of resectable PACC and provides detailed insight into patient populations that will benefit from additional therapy.
5 |. CONCLUSION
In patients undergoing surgical resection for pancreatic acinar cell carcinoma, the association of adjuvant therapy with improved OS is limited to patients with pathologic stage IIB disease. Furthermore, the possible benefit of adjuvant chemotherapy is evident even in patients treated with singleagent chemotherapy, which may be useful as there are a number of patients who cannot tolerate multiagent chemotherapy after resection. Most patients who present with clinically node positive disease have pathologically positive nodes at resection, and this may be useful in patient selection for neoadjuvant therapy. Future studies should focus on defining the role of neoadjuvant therapy in patients with pancreatic acinar cell carcinoma with clinically node positive disease.
Acknowledgments
Funding information
National Institutes of Health, Grant/Award Number: P30CA138292
FUNDING INFORMATION
Dr. Carpizo is funded by the following grants NIH R01 CA20080. Research reported in this publication was supported in part by the Biostatistics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
REFERENCES
- 1.Chaudhary P Acinar cell carcinoma of the pancreas: a literature review and update. Indian J Surg. 2015;77(3):226–231. doi: 10.1007/s12262-014-1049-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seth AK, Argani P, Campbell KA, et al. Acinar cell carcinoma of the pancreas: an institutional series of resected patients and review of the current literature. J Gastrointest Surg. 2008;12(6):1061–1067. doi: 10.1007/s11605-007-0338-1 [DOI] [PubMed] [Google Scholar]
- 3.Wisnoski NC, Townsend CM, Nealon WH, Freeman JL, Riall TS. 672 patients with acinar cell carcinoma of the pancreas: a populationbased comparison to pancreatic adenocarcinoma. Surgery. 2008; 144(2):141–148. doi: 10.1016/j.surg.2008.03.006 [DOI] [PubMed] [Google Scholar]
- 4.Klimstra DS, Heffess CS, Oertel JE, Rosai J. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol. 1992;16(9):815–837. doi: 10.1097/00000478-199209000-00001 [DOI] [PubMed] [Google Scholar]
- 5.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004; 363(9414):1049–1057. doi: 10.1016/S0140-6736(04)15841-8 [DOI] [PubMed] [Google Scholar]
- 6.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310(14):1473–1481. doi: 10.1001/jama.2013.279201 [DOI] [PubMed] [Google Scholar]
- 7.Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018; 379(25):2395–2406. doi: 10.1056/NEJMoa1809775 [DOI] [PubMed] [Google Scholar]
- 8.Oba A, Ho F, Bao QR, Al-Musawi MH, Schulick RD, Del Chiaro M. Neoadjuvant treatment in pancreatic cancer. Front Oncol. 2020;10: 245. doi: 10.3389/fonc.2020.00245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayo SC, Gilson MM, Herman JM, et al. Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg. 2012;214(1):33–45. doi: 10.1016/j.jamcollsurg.2011.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260(2):372–377. doi: 10.1097/SLA.0000000000000378 [DOI] [PubMed] [Google Scholar]
- 11.Network NCC. Pancreatic Adenocarcinoma (Version 2.2021).
- 12.Distler M, Rückert F, Dittert DD, et al. Curative resection of a primarily unresectable acinar cell carcinoma of the pancreas after chemotherapy. World J Surg Oncol. 2009;7:22. doi: 10.1186/14777819-7-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glazer ES, Neill KG, Frakes JM, et al. Systematic Review and Case Series Report of Acinar Cell Carcinoma of the Pancreas. Cancer Control. 2016;23(4):446–454. doi: 10.1177/107327481602300417 [DOI] [PubMed] [Google Scholar]
- 14.Patel DJ, Lutfi W, Sweigert P, et al. Clinically resectable acinar cell carcinoma of the pancreas: is there a benefit to adjuvant systemic therapy? Am J Surg. 2020;219(3):522–526. doi: 10.1016/j.amjsurg.2019.10.013 [DOI] [PubMed] [Google Scholar]
- 15.Bilimoria KY, Bentrem DJ, Ko CY, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110(4):738–744. doi: 10.1002/cncr.22852 [DOI] [PubMed] [Google Scholar]
- 16.Allen PJ, Kuk D, Castillo CF, et al. Multi-institutional validation study of the American Joint Commission on Cancer (8th Edition) Changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg. 2017;265(1):185–191. doi: 10.1097/SLA.0000000000001763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Nickleach DC, Zhang C, Switchenko JM, Kowalski J. Carrying out streamlined routine data analyses with reports for observational studies: introduction to a series of generic SAS. F1000Res. 2018;7: 1955. doi: 10.12688/f1000research.16866.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Firth D Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 19.Heinze G, Schemper M. A solution to the problem of monotone likelihood in Cox regression. Biometrics. 2001;57(1):114–119. doi: 10.1111/j.0006-341x.2001.00114.x [DOI] [PubMed] [Google Scholar]
- 20.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690. doi: 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitagami H, Kondo S, Hirano S, Kawakami H, Egawa S, Tanaka M. Acinar cell carcinoma of the pancreas: clinical analysis of 115 patients from Pancreatic Cancer Registry of Japan Pancreas Society. Pancreas. 2007;35(1):42–46. doi: 10.1097/mpa.0b013e31804bfbd3 [DOI] [PubMed] [Google Scholar]
- 22.Schmidt CM, Matos JM, Bentrem DJ, Talamonti MS, Lillemoe KD, Bilimoria KY. Acinar cell carcinoma of the pancreas in the United States: prognostic factors and comparison to ductal adenocarcinoma. J Gastrointest Surg. 2008;12(12):2078–2086. doi: 10.1007/s11605-008-0705-6 [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Lee KG, Park HK, Lee KS. [Acinar cell carcinoma of the pancreas in Korea--clinicopathologic analysis of 27 patients from korean literature and 2 cases from our hospital--]. Korean J Gastroenterol. 2010;55(4):245–251. doi: 10.4166/kjg.2010.55.4.245 [DOI] [PubMed] [Google Scholar]
- 24.Butturini G, Pisano M, Scarpa A, D’Onofrio M, Auriemma A, Bassi C. Aggressive approach to acinar cell carcinoma of the pancreas: a single-institution experience and a literature review. Langenbecks Arch Surg. 2011;396(3):363–369. doi: 10.1007/s00423-010-0706-2 [DOI] [PubMed] [Google Scholar]
- 25.Lowery MA, Klimstra DS, Shia J, et al. Acinar cell carcinoma of the pancreas: new genetic and treatment insights into a rare malignancy. Oncologist. 2011;16(12):1714–1720. doi: 10.1634/theoncologist.2011-0231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seki Y, Okusaka T, Ikeda M, Morizane C, Ueno H. Four cases of pancreatic acinar cell carcinoma treated with gemcitabine or S-1 as a single agent. Jpn J Clin Oncol. 2009;39(11):751–755. doi: 10.1093/jjco/hyp085 [DOI] [PubMed] [Google Scholar]
- 27.Holen KD, Klimstra DS, Hummer A, et al. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol. 2002;20(24):4673–4678. doi: 10.1200/JCO.2002.02.005 [DOI] [PubMed] [Google Scholar]
- 28.Matos JM, Schmidt CM, Turrini O, et al. Pancreatic acinar cell carcinoma: a multi-institutional study. J Gastrointest Surg. 2009;13(8): 1495–1502. doi: 10.1007/s11605-009-0938-z [DOI] [PubMed] [Google Scholar]
- 29.Ahmad SA, Duong M, Sohal DPS, et al. Surgical outcome results from SWOG S1505: a randomized clinical trial of mFOLFIRINOX versus gemcitabine/Nab-paclitaxel for perioperative treatment of resectable pancreatic ductal adenocarcinoma. Ann Surg. 2020; 272(3):481–486. doi: 10.1097/SLA.0000000000004155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motoi F, Kosuge T, Ueno H, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol. 2019;49(2):190–194. doi: 10.1093/jjco/hyy190 [DOI] [PubMed] [Google Scholar]
- 31.Versteijne E, Suker M, Groothuis K, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the dutch randomized phase III PREOPANC Trial. J Clin Oncol. 2020;38(16):1763–1773. doi: 10.1200/JCO.19.02274 [DOI] [PMC free article] [PubMed] [Google Scholar]