Abstract
Trisomy 16 is the most common autosomal trisomy in humans which is almost uniformly embryonic lethal. Partial trisomy 16 including a segment of the long arm of chromosome 16 is occasionally compatible with life and has been associated with severe congenital defects, growth retardation, and early lethality. Segmental trisomy of 16q is usually described concomitantly with partial monosomy of another chromosome, often resulting from a parental balanced translocation. Pure partial chromosome 16q trisomy is exceedingly rare, with 16q12→qter and 16q13→qter duplication reported in only nine cases in the literature, almost all described with monosomy of a second chromosome, and highlighting very few long-term survivors. A single report of pure partial distal 16q12.1q23.3 duplication has been reported in an infant, underscoring complexities of genetic counseling and management, especially in view of life-limiting congenital anomalies in rare survivors. Here we present a 12-month old child with pure 16q12.2q24.3 trisomy, having continued morbidity related to pulmonary hypertension and chronic lung disease. The features of intrauterine growth retardation, facial dysmorphism, hypotonia, congenital heart defect, distal contractures, urogenital abnormalities, and hearing loss support the association with 16q partial trisomy, as in previous studies. This report expands our current understanding related to the survival of infants with large segmental aneusomy of the long arm of chromosome 16.
Keywords: Trisomy 16q, congenital anomalies
INTRODUCTION
Trisomy 16 is the most common karyotype abnormality found in spontaneous abortions in the first trimester, and is estimated to occur in 1% of all human conceptions (Gardner & Amor, 2018). Fully trisomic fetuses survive, on average, up to 10th week of gestation (Roberts & Duckett, 1978), and rarely beyond the first trimester, recognized mainly as empty sacs or very disorganized embryos (Yancey, Hardin, Pacheco, Kuslich, & Donlon, 1996). Exceptionally rare reports of survival into the second or third trimester of these conceptuses indicate severe abnormalities that are incompatible with postnatal survival, including complex cardiac defects, small thoracic cavity, skeletal deformity, diaphragmatic defects, imperforate anus, renal dysplasia, and severe growth retardation (Cusick et al., 1995; Yancey et al., 1996). Partial trisomy 16 including a large segment of the long arm of chromosome 16 is often associated with similar severe congenital anomalies and early lethality. It is postulated that trisomy of the long arm is responsible for the lethality associated with trisomy 16 (Garau, Crisponi, Peretti, Vanni, & Zuffardi, 1980), as duplication of the large segment of the long arm of chromosome 16 consistently results in early postnatal death (Schinzel, 2001), as opposed to trisomy of 16p (Jalal, Day, Garcia, Benjamin, & Rogers, 1989; Rochat, Riegel, & Schinzel, 2007). Earlier description of partial trisomy 16q revealed growth retardation, congenital heart defects, and infantile death (Roberts & Duckett, 1978), while more recent reviews added hypotonia, joint contractures, ambiguous genitalia, and anorectal malformations to the spectrum of anomalies (Laus et al., 2012; Mishra et al., 2018). While long-term survival reports assist in prognostication of liveborn infants with duplication of the terminal region of 16q (16q21→qter) to guide management and provide counseling regarding invasive life-prolonging procedures for the severely affected infants, such data are currently lacking for pure partial trisomy 16 involving the large 16q12→qter and 16q13→qter segments, without concurrent partial autosomal monosomy. We are aware of only one pure 16q12.1q23.3 (interstitial) duplication in a surviving 8-month old infant with developmental delay, dysmorphic features, and hearing loss (Türkyılmaz & Yaralı, 2020). Herein, we present an individual with partial trisomy 16q12.2→qter, who is currently alive at 12 months with multiple morbidities. Our patient represents ‘pure’ partial trisomy of a large segment of 16q and provides insight into the postnatal considerations and complexities of genetic counseling related to prognosis of this rare chromosomal abnormality.
CASE REPORT
The fetus was brought to medical attention prenatally at 26 weeks of gestation with abnormal ultrasound scan. The mother was a healthy gravida 2, para 1, 28-year old Hispanic female, with a previously healthy 7-year old daughter. Family history was negative for congenital defects or miscarriages. Prenatal ultrasound demonstrated severe intrauterine growth restriction (IUGR), flattened nose/nasal bridge with effaced nares, mild retromicrognathia, subjective transverse narrowing of the bony palate, cyst along right side of the neck, diffuse cardiomegaly with small left ventricular outflow tract, tortuous umbilical vessels deep in the umbilical insertion, and placentomegaly. Fetal echocardiogram performed at 28 weeks of gestation was within normal limits. Fetal magnetic resonance imaging (MRI) showed a thick placenta, significant dolicocephaly, mild retrognathia, small palate, flattened nasal bridge, deviation of the nasal septum to the right with effacement of the majority of the right nasal cavity, branchial cleft cyst, normal brain parenchyma, cardiomegaly with dysmorphic appearance to the heart with the left ventricular outflow track not well delineated, and severe IUGR (<1%).
The male infant was delivered at 35 weeks of gestation via caesarian section for non-reassuring fetal heart rate. His Apgar scores were 8 and 8 at 1 and 5 minutes of life, respectively. Birthweight was 1765 grams (which corresponded to the 2.5 centile on the Fenton preterm scale, with a Z score of −2.0), length was 42 cm (3.4 centile, Z score of −1.8) and frontal-occipital circumference (FOC) was 30 cm (6.6 centile, Z score of −1.5). Assessment on day of life one revealed a diffusely hypotonic infant, with thin facies, periorbital fullness, narrow downslanting palpebral fissures, large bulbous nose, long philtrum with thin upper lip, small mouth without clefting, excess nuchal skin, small ears, widely spaced nipples, contractures of the distal extremities with limited range of motion (most noticeable in the proximal interphalangeal joints), bilateral clinodactyly of the 4th and 5th digits, overlapping toes, rocker-bottom deformities, scaphoid abdomen, micropenis, and undescended right testis (Figure 1). The infant required continuous positive airway pressure (CPAP) and oxygen supplementation via high flow nasal cannula until he was 5 weeks old (chronologically 40 5/7 weeks). He had feeding difficulties from the beginning and was dependent on nasogastric tube feeding. Swallow study showed silent aspiration and oropharyngeal dysphagia. Additional abnormalities included right choanal stenosis with nasal synechiae and bilateral severe hearing loss. He also had right sided grade III vesicoureteral reflux (VUR) with intrarenal reflux and left cystic dysplasia of the kidney. Renal function studies were normal. Ophthalmological evaluation revealed bilateral hyperopia and intermittent left exotropia. Head ultrasound showed no evidence of intraventricular hemorrhage. Postnatal echocardiogram showed moderately dilated right atrium and hypertrophied right ventricle with large atrial septal defect (ASD), large patent ductus arterioisus (PDA), and moderate hypoplasia of the proximal descending aorta. Palliative care service was involved for support since his birth. The parents opted to pursue aggressive intervention for his care. At 7 months of age, he underwent cardiac catheterization which indicated right ventricular pressure to be 80–86% systemic pulmonary hypertension. Stenosis of the common left pulmonary vein was seen, which was stented. The moderate ASD was also closed, with a small residual shunt post ASD closure. The inferior vena cava was noted to be diffusely hypoplastic throughout its course. Serial chest x-rays showed pulmonary edema which was treated with furosemide. Gastrotomy tube was placed at 8 months of age which resulted in improved weight gain (5th centile for age), but his length and head circumference continued to be below the first centile. Over the course of one year, he had several other surgical procedures including placement of pressure equalizing tubes (PETs), repair of inguinal hernia, and orchiopexy, and had multiple hospitalizations due to respiratory failure, pulmonary hypertension, and urinary tract infections.
Figure 1: Dysmorphic features associated with 16q12.2q24.3 partial trisomy, observed at 1 day (A) and 5 months of age (B).
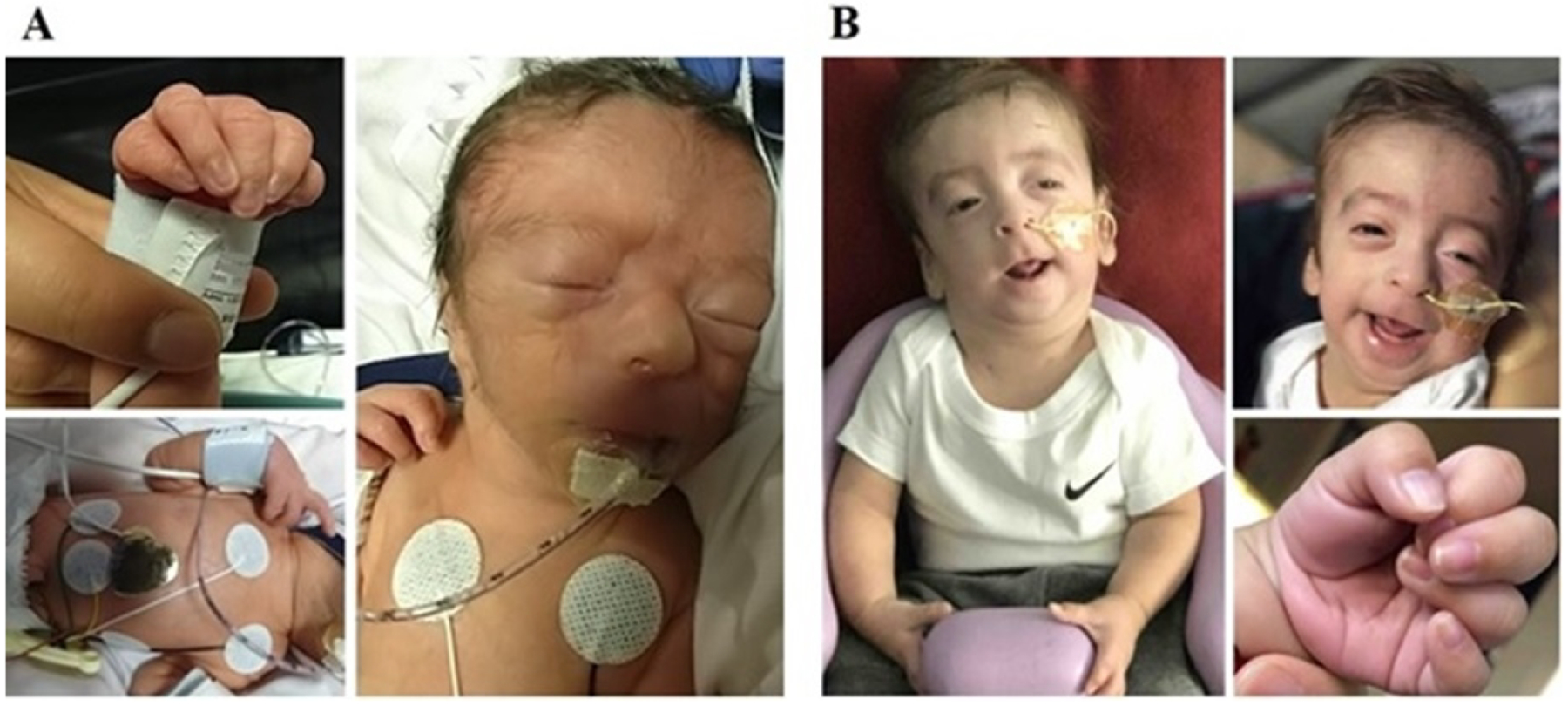
A – Large inset shows periorbital fullness, narrow palpebral fissure, bulbous nose, long philtrum, thin upper lip, and flattened mildly low set ears. Upper small inset shows overlapping of the 4th finger over the 2nd and 3rd, lower inset shows scaphoid abdomen along with wide neck. B – Large inset shows myopathic facies with narrow downslanting palpebral fissures, long philtrum and thin upper lip, along with high forehead, and wide neck; lower inset shows persisting camptodactyly.
From the developmental perspective, he was able to smile at 1 month of age and coo at 4 months of age. By 5 months, he was able to lift his head when prone and move from side to side when lying supine at 5 months. He was not able to roll in either direction. On direct developmental assessment at chronological age of 5 months (corrected age, 4 months), his gross motor skills were at about 1 month old level (severe-to-profound delay), his visual-motor problem solving skills appeared most secure at a 1.3 month old level (severe delay), and his overall language skills were at a 1.5 month old level (severe delay). At 10 months of age, his motor developmental assessment showed ability to partially roll, grab toys, and sit without assistance for few seconds.
METHODOLOGY
Editorial Policies and Ethical Considerations
Written informed consent was obtained from the patient’s mother and the child was enrolled in a research study approved by an institutional review board (IRB). This study conforms to all ethical standards as put forthby our institution and international.
Data acquisition and analysis
Amniocentesis was performed and cytogenetic studies using G-banding at a resolution level of 500 bands were completed. A total of 20 metaphases were analyzed. In addition, the fetal DNA was studied by V8.3 chromosomal microarray analysis (CMA) designed by Baylor Genetics Laboratories and manufactured by Agilent Technology (Santa Clara, CA, USA). The 400k array targeted over 1,900 genes at the exon level and included 60,000 probes used for SNP analysis for the detection of uniparental disomy (UPD) and absence of heterozygosity (AOH). DNA was extracted by the Puregene DNA Blood Kit (Gentra) according to the manufacturer’s instructions. The procedures for DNA digestion, labeling, and hybridization for the oligo arrays were performed according to the manufacturers’ instructions (Cheung et al., 2005). Data analyses were performed using an in-house developed pipeline (Gambin et al., 2017).
RESULTS
Prenatal karyotype study showed 46,XY,der(22)t(16;22)(q13;p11.2), indicating a derivative chromosome 22 arising from an unbalanced translocation between the long arm of chromosome 16 and the short arm of chromosome 22, resulting in trisomy for the 16q13-16q24.3 region (Figure 1). CMA indicated a large copy number gain of 33.5 million base pairs (Mb) on 16q12.2q24.3 encompassing ~350 Refseq genes without any loss of euchromatin on chromosome 22. In addition, the infant was found to have a paternally inherited 10 kb loss on 11q22.3, including multiple exons of ATM. Maternal karyotype analysis showed 46,XX,t(16;22)(q13;p11.2), with a reciprocal translocation between chromosomes 16 and 22 at bands 16q13 and 22p11.2.
DISCUSSION
It has long been considered that duplication of the long arm of chromosome 16 is responsible for the embryonic lethality associated with trisomy 16. Despite being a common cause of fetal loss, very little is known about the cause of early developmental failure of embryos and the critical genes on 16q11.2q24.3 that regulate growth and cellular processes. Compared to trisomy 21, 18 and 13, there is evidently less tolerance for surplus gene dosage in trisomy 16, but the molecular basis of failure of the developmental processes in the most common autosomal trisomy underlying spontaneous abortions remains unknown. Increased protein expression particularly for genes that are dosage sensitive may play a role, along with several other factors such as DNA damage and proteotoxic stress in aneuploid cells (Passerini et al., 2016). Chromosome 16 is ~90 million base pairs in length with an estimated 880 protein-coding genes. Our patient’s 16q duplication comprises of ~33 Mb encompassing ~350 genes, some of which are ascertained to be dosage sensitive, including FOXF1 (MIM #601089) (Sen et al., 2013), CTCF (MIM #604167) (Konrad et al., 2019), and CBFB (MIM #121360) (Talebian et al., 2007). It remains to be seen if overexpression of such dosage sensitive genes critically disrupts embryogenesis or major developmental processes in partial trisomy 16q.
The general prognosis for survivors of larger partial trisomy 16 remains poor, however, exceptions exist. The longest reported survival with a trisomy of similar length (16q13→qter) as in our patient was 31 months (see table 1). Survival to 3½ years (Balestrazzi, Giovannelli, Landucci Rubini, & Dallapiccola, 1979) and 7 years (de Carvalho et al., 2010) have been reported in patients with more distal 16q21→qter trisomy. Survival in long segment partial trisomy 16 is inevitably dependent upon close follow up by multidisciplinary team, assisted respiratory support, enteral/parenteral feeding devices, intensive rehabilitation therapy, and invasive procedures intense therapies. Our patient is currently 12 months old, with multiple morbidities including atrial septal defect s/p device closure, hypoplastic aortic arch, pulmonary vein stenosis, pulmonary hypertension, chronic kidney disease with cystic dysplasia of right kidney, oropharyngeal dysphagia, gastric tube dependence, and global developmental delay. He has had recurrent hospitalizations (at a frequency of about once a month) secondary to respiratory and urinary tract infections. At 10 months, we estimated his motor development to be at 4 months, with profound delays in fine motor, language and visual-motor problem solving skills. He is closely followed by a complex care team and receives home nursing services. Our patient’s ASD was closed by patched delivered by a catheter. Poor cardiopulmonary reserve may limit cardiac intervention options for more complex lesions. Congenital heart defects are unfortunately observed in over half of all children reported with variable segmental trisomy of 16q. Septal defects, coarctation of the aorta (CoA), hypoplastic aortic arch, hypoplastic left heart, dysplastic valves, and double outlet right ventricle have all been observed. Given that there are minimal reports of long term survival for 16q12.2→16qter trisomy, genetic counseling regarding prognosis remains challenging. Similar to trisomy 18 and 13 (Kaulfus et al., 2019), parents, neonatologists, cardiologists, genetic counselors, geneticists, and palliative care team should all be involved in decisions pertaining to surgical intervention in these children for long-term survival.
Table 1. Phenotypic summary of reprted cases of partial 16q trisomy resulting in liveborns.
The table includes only liveborn deliveries. Interstitial duplications were not included in this table to account only for the more common trisomic births resulting from parental translocation. The monosomic segment is not indicated to highlight the common syndromic features of this partial trisomy. Developmental delay was uniformly reported in patients surviving long enough for high quality assessment. Due to the high perinatal mortailtiy resulting in incomplete developmental assessment for the majority of cases, developmental delay is not included in the table.
| 2/2 | 3/6 | 2/2 | 3/6 | 6/8 | 5/7 | 4/6 | 2/6 | 3/3 | ||
| Dolichocephaly (15%) | 2/6 | 1/2 | 1/6 | 2/8 | 1/7 | |||||
| Prominent forehead (61%) | 5/6 | 2/2 | 4/6 | 6/8 | 5/7 | 3/6 | 1/6 | 2/3 | ||
| Other reported features | Craniosynostosis | + | + | + (metopic) | ++ | |||||
| Triangular facies | + | ++ | ++ | + | ||||||
| Small fontanelles | + | ++ | ||||||||
| Microcephaly | ++ | ++ | ||||||||
| Trigonocephaly | + | + | ||||||||
| Low hairline | + | + | + | |||||||
| Brachycephaly | + | + | ||||||||
| Mid-face hypoplasia | + | + | + | + | ||||||
| Other | scalp cutis aplasia | temporal hirsutism abnormal ossification of the skull | Coarse facial features | High frontal hairline | Bitemporal narrowing large anterior fontanelle | |||||
| Downslanting palpebral fissure (46%) | 2/2 | 6/6 | 3/6 | 4/8 | 2/7 | 2/6 | 1/6 | 1/3 | ||
| Telecanthus/Hypertelorism (39%) | 1/2 | 1/6 | 2/2 | 4/8 (2,2) | 3/7 (1,2) | 5/6 (1,5) | 1/6 | 1/3 | ||
| Small palpebral fissures | 1/2 | 4/8 | 3/7 | 2/6 | 1/3 | |||||
| Other reported features | Periorbital edema | + | + | + | ||||||
| Epicanthal folds | + | + | ++ | ++ | +++ | |||||
| Strabismus | ++ | + | ++ | + | ||||||
| Upslanting palpebral fissures | + | + | + | ++ | ||||||
| Prominent/arched eyebrows | + | ++ | + | + | ||||||
| Coloboma | + | + | ||||||||
| Ptosis | + | + | ||||||||
| Other | Exophthalmos infraorbital creases | narrow palpebral fissure long eyelashes | Megalocornea optic nerve atrophy | retinal hypopigmentation | hypermyopia | Rieger’s anomaly congenital glaucoma | ||||
| Low set/dysplastic (83%) | 2/2 | 6/6 | 2/2 | 5/6 | 8/8 | 4/7 | 5/6 | 4/6 | 2/3 | |
| Preauricular pits | + | + | + | + (tragus) | ||||||
| Large ears | + | + | ||||||||
| Smooth long philtrum (43%) | 2/2 | 2/6 | 4/6 | 3/8 | 4/7 | 2/6 | 3/6 | |||
| Thin upper lip (50%) | 1/2 | 2/6 | 1/2 | 4/6 | 4/8 | 6/7 | 2/6 | 1/6 | 1/6 | |
| Depressed nasal bridge (28%) | 1/2 | 2/2 | 2/6 | 3/8 | 2/6 | 2/6 | 1/3 | |||
| Micrognathia (54%) | 1/2 | 6/6 | 2/2 | 2/6 | 5/8 | 3/7 | 3/6 | 2/6 | 1/3 | |
| High arched | 3/6 | 2/6 | 2/8 | 3/7 | 1/6 | 2/6 | ||||
| Cleft palate | 1/6 | 2/6 | 2/8 | 1/6 | ||||||
| Other features | Bulbous nose/tip | ++ | + | + | + | |||||
| Prominent nasal bridge | +++ | +++ | ||||||||
| Beaked nose | + | ++ | + | |||||||
| Anteverted nares | + | + | ++ | |||||||
| Choanal stenosis/atresia | + | + | + | |||||||
| Other | hypoplasia of the glottis | hypoplastic lower lip mount open at rest ankyloglossia | thick columella gingival hypertrophy down-turned corner of mouth | thick everted lips short philtrum widely spaced teeth | Partial bifid tongue | |||||
| Wide set nipples (17%) | 1/2 | 2/6 | 3/6 | 2/8 | ||||||
| Short/webbed neck (37%) | 3/6 | 1/2 | 2/6 | 4/8 | 3/7 | 1/6 | 2/6 | 1/2 | ||
| Other features | Long and narrow thorax | ++ | + | + | ||||||
| Pulmonary hypoplasia | + | + | ||||||||
| Other | Pneumothorax pectus excavatum small thorax | |||||||||
| Contractures of large joints (28%) | 1/2 | 3/6 | 3/6 | 2/8 | 2/7 | 1/6 | 1/6 | |||
| Joints | Elbow | + | + | + | + | |||||
| Knee | ++ | ++ | + | |||||||
| Wrists | + | ++ | + | |||||||
| Hip | + | ++ | + | |||||||
| Ankle | + | ++ | + | + | ||||||
| Clinodactyly (52%) | 1/2 | 3/6 | 2/2 | 6/6 | 4/8 | 4/7 | 2/6 | 2/3 | ||
| Overlying fingers / toes (20%) | 1/2 | 3/6 | 4/6 | 1/7 | ||||||
| Other reported features | Hypopl. phalanges/metacarpals | + | ++ | + | ||||||
| Scoliosis/kyphosis | + | ++ | ||||||||
| Rocker-bottom feet | + | + | ||||||||
| Talipes equinovarus | ++ | |||||||||
| Cubitus/Genu/pes valgus | + | ++ | + | |||||||
| Camptodactyly/syndactyly | + | ++ | + | + | ||||||
| Proximally placed thumb | + | + | ||||||||
| Single palmar crease | + | + | ||||||||
| Platyspondyly/vertebral hypoplasia | + | + | + | |||||||
| Other | nail hypoplasia | Brachydactyly delayed bone age brittle teeth spina bifida occulta | sacral dimple abducted hips | C7-T1 spinal canal anomaly short 5th finger persistence of fetal pat-pad | Hyperextensible joints broad hands with tapering fingers arachnodactyly diastasis recti umbilical hernia diaphragmatic hernia enlarged sternal heads costal flaring large iliac wings osteochondroma | |||||
| 2/2 | 4/6 | 1/2 | 6/6 | 4/8§ | 3/7 | 3/6 | 3/6 | 2/3 | ||
| Types | ASD | + | + | ++ | + | + | ||||
| VSD | + | + | ++ | + | ||||||
| PDA | +++ | +++ | +++ | + | + | |||||
| Coarctation/hypoplastic arch | TAPVR | + | + | + | ++ | |||||
| Hypoplastic left heart | + | |||||||||
| PFO | + | |||||||||
| Dysplastic pulmonic/aortic valve | ++ | + | ||||||||
| Other | AV block | Double-outlet right ventricle anomalous origin of the subclavian artery | TGA | |||||||
| Anteriorly displaced anus (15%) | 1/2 | 2/6 | 2/8 | 1/7 | 1/3 | |||||
| OtherΔ | Malrotation | + | + | |||||||
| Gallbladder hypoplasia | ++ | + | + | |||||||
| Other biliary anomalies | + (jaundice) | + (fibrosis) | + (paucity) | |||||||
| Liver anomalies | + | + | ||||||||
| Hernia | + | ++ | + | + | ||||||
| Other features | short intestine | Imperforate anus | severe GERD | Anal stenosis chronic constipation | NEC | |||||
| Cryptorchidism/ambiguous genitalia (57%) | 1/1 | 3/4 | 1/2 | 2/4 | 4/5 | 2/4 | 1/4 | |||
| Micropenis (58%) [N=19] | 1/1 | 4/4 | 4/4 | 2/4 | ||||||
| Hypoplastic/dysplastic labia majora (17%) [N=18] | 1/2 | 1/2 | 1/2 | |||||||
| Hydronephrosis (15%) | 1/2 | 1/8 | 1/6 | |||||||
| other | Renal | cortical and medul. hemorrhages megacystis | Vesicoureteral reflux | Vesicoureteral reflux | ||||||
| GU | Ovarian hypoplasia, bicornuate uterus | |||||||||
| Hypotonia¶ (61%) | 1/2 | 3/6 | 3/6 | 6/8 | 4/7 | 4/6 | 5/6 | 2/3 | ||
| Hypertonia | 1/2 | 1/8 | 1/6 | |||||||
| CNS malformations (24%)Δ | 1/2 | 1/6 | 2/6 | 1/8 | 2/7 | 3/6 | 1/6 | |||
| Types | Ventriculomegaly | + | + | + | ||||||
| Third ventricle anomalies | + | + | ||||||||
| Hypomyelination# | + | ++ | + | |||||||
| Other | frontal lobe cyst | Rhomboencephalosynapsis absence of olfactory nerve | occipital lobe hypoplasia | Periventricular leukodystrophy | ||||||
| Recurrent sinopulmonary infections | + | ++ | + | |||||||
| Recurrent otitis media | + | ++ | ||||||||
| Meningitis | + | + | ||||||||
| Other | Hypoplastic thymus Macrophages infiltration of myocardium & lung Recurrent E coli infections | Persistent diarrhea | Persistent diarrhea | Severe asthma | ||||||
| Sensorineural hearing loss | + | + | ++ | |||||||
| Blindness | ++ | |||||||||
| Seizures | + | + | ||||||||
| Reduced subcutaneous fat tissue | + | + | ||||||||
| Other | cutaneous appendages | Meconium aspiration Hypoglycemia large hydrocele lanugo of upper body ascites diminished primitive reflexes diffuse hypopigmentation obstructive sleep apnea torticollis laryngomalacia erythroblastosis fetalis | IQ of 51 ADHD Laryngomalacia 4 limb dystonia | |||||||
| 7 weeks to 7 months | 6 days - 4 months | 10 days | 8 days - 31 months | 18 days – alive at 7 years | 9 days – Alive at 9 months | 3 months– Alive at 10.5 years | Alive at 15 month – Alive at 19 years | 14 days, 3 months, and 1 year | ||
Reference (Bacino et al., 1999) is not included as the mild phenotype is associated with X inactivation of the translocated chromosome.
(Tsien, Morava, Talarski, & Marble, 2005) Report includes a translocation involving the Y chromosome with only a significantly milder phenotype.
(Garau et al., 1980) Reports a systolic murmur was auscultated but autopsy was denied. No echocardiogram was available.
in some cases, poor suck and feeding difficulties are reported without the mentioning of general muscular tone.
hypomyelination includes dysgenesis of corpus callosum
The majority of case reports predate advanced brain and GI imaging and postmortem studies and/or barium series were not performed on all affected babies.
References for cases in the table.
q11→qter: (Eggermann, Kolin-Gerresheim, Gerresheim, & Schwanitz, 1998; Hahm, Chitayat, Iqbal, Cho, & Nitowsky, 1987; Nevin, Coffey, Nevin, & Reid, 1983; Ridler & McKeown, 1979)
q12→qter: (Chen et al., 2003; Paladini et al., 1999)
q13→qter: (Buckton & Barr, 1981; Davison & Beesley, 1984; Dowman, Lockwood, & Allanson, 1989; Hatanaka, Ozaki, Suzuki, Murata, & Fujita, 1984; Luberda-Zapaśnik, Midro, & Szwałkiewicz-Warowicka, 1995)
q21→qter: (Balestrazzi et al., 1979; Calva, Frias, Carnevale, & Reyes, 1984; de Carvalho et al., 2010; Garau et al., 1980; Lessick, Israel, Wong, & Szego, 1989; Maher, Willatt, Cuthbert, Chapman, & Hodgson, 1991; Mishra et al., 2018; Yue et al., 2019)
q22→qter: (Basinko et al., 2011; Houlston, Renshaw, James, Ironton, & Temple, 1994; Nyhan, Mascarello, Barshop, Doroski, & Hirschhorn, 1989; Rethoré et al., 1982; Sousa et al., 2004; Tsien et al., 2005)
q23→qter: (Basinko et al., 2011; Maher et al., 1991; Papadopoulou et al., 2017; Savary et al., 1991)
q24→qter: (Baker et al., 2002; Brisset et al., 2002; Ferrero et al., 2006; Giardino et al., 2001; Maher et al., 1991; Zahn et al., 2005; Zhou et al., 2013)
Unknown: (Eriksson et al., 1971; Ferguson & Hicks, 1987; Francke, 1972) These publications did not utilize modern chromosome analysis techniques however the phenotypic description is most consistent with involvement of large segement of 16q; for reference (Francke, 1972), the relevant case is #10.
The clinical features in the patient presented here are comparable with the previously reported findings for large segment 16q trisomies. Our data, similarly to earlier reports (Bacino, Lee, Spikes, & Shaffer, 1999; Chen et al., 2016; de Carvalho et al., 2010; Dikmetas et al., 2012; Laus et al., 2012; Mishra et al., 2018; Papadopoulou et al., 2017; Pérez-Castillo, Martin-Lucas, & Abrisqueta, 1990; Yamada et al., 2009; Yue et al., 2019), show a near universal presence of hypotonia, IUGR, characteristic facial dysmorphism (small palpebral fissures, periorbital fullness, bulbous nose with flat nasal bridge, retrognathia), dysplastic ears, and high incidence of limb contractures, genitourinary anomalies, and congenital heart defects (table 1). Comparing longer partial trisomies (table 2, 16q11→qter and 16q21→qter) to more distal partial trisomies (16q22→qter and 16q24→qter), we found similar frequency of IUGR, hypotonia (both 50–70%), ear dysplasia (70 to 90%) and facial characteristics (smooth philtrum, thin upper lip and microgranthia) (table 1). The distal trisomies have relatively lower frequency of cryptorchidism and limb contractures (30% compared to 65%), and slightly reduced frequency of congenital heart defects (50% compared to 70%). Brisset et al. (Brisset et al., 2002) reviewing 25 patients with partial trisomy 16q and 3 individuals with interstitial 16q duplications, found abnormal behavior (self-destructive) in patients sharing duplication of the 16q11 ‐ 16q13 region, also corroborated by Fryns et al (Fryns, Kleczkowska, Decock, & Van den Berghe, 1990), showing no dysmorphism associated with “proximal” interstitial duplication. However, more recent reports describe intellectual disability, abnormal behavior, and severe neurological manifestations in an “intermediate” (16q21) (Lonardo et al., 2011) and two “distal” (16q24.1) (Gardner & Amor, 2018) interstitial duplications. Gall bladder agenesis and gut malrotation that were initially reported only in partial trisomy of 16q11-21→qter (Brisset et al., 2002; Laus et al., 2012; Mishra et al., 2018), were both reported also with interstitial duplication of 16q24.1 (Dharmadhikari et al., 2014), thus confounding more than a few attempts at mapping loci-specific phenotype associations.
Table 2. Prevalence of common features found in partial trisomy 16 involving most of the long chromosomal arm.
Cases include trisomy of 16q11→qter to 16q21→qter. References per table 1.
| Feature | prevalence |
|---|---|
| Dysplastic/low set ears | 93% |
| IUGR/LBW | 70% |
| Prominent forehead | 70% |
| Congenital heart defect | 70% |
| Contractures | 67% |
| Clinodactyly | 67% |
| Cryptorchidism / ambiguous genitalia | 65% |
| Micrognathia | 63% |
| Downslanting palpebral fissures | 59% |
| Hypotonia | 56% |
| Thin upper lip | 48% |
| High arched palate | 48% |
| Short/webbed neck | 48% |
| Smooth philtrum | 41% |
| Micropenis | 33% |
| Overlying fingers | 30% |
| Anteriorly placed anus | 19% |
| CNS malformations | 19% |
| Gallbladder agenesis | 15% |
To conclude, we present here a second infant with pure trisomy 16q12.2→qter without concurrent monosomy of a second chromosome, with a phenotype that resembles the previously reported features and survival up to 12 months with continued medical and surgical intervention. This report broadens the existing data regarding long-term survival for partial trisomy16 of this size and provides a useful source for genetic counseling and management of survivors of this rare chromosomal abnormality.
Figure 2. Chromosomal microarray analysis (CMA) showing gain of long arm of chromosome 16 consistent with 16q duplication.
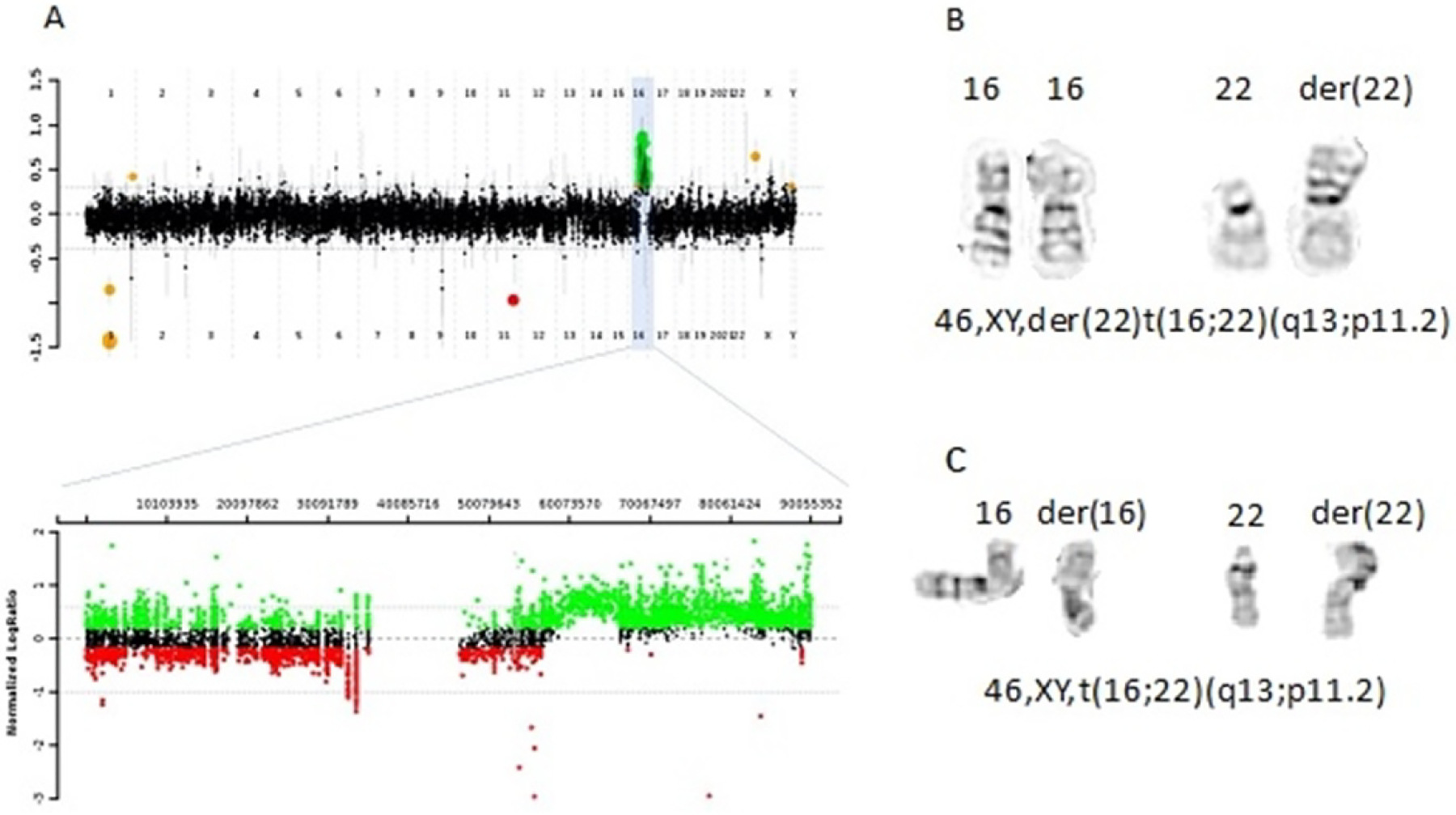
(A) Array CGH plot of the proband showing the copy number gain of 16q12.2q24.3 with magnified view of copy number gain on chromosome 16. (B) depicts partial high resolution chromosome analysis, with derivative chromosome 22, with an extra copy of 16q12.2→qter attached to 22p11.2. (C) Maternal chromosome analysis showing the reciprocal translocation between chromosomes 16 and 22.
Acknowledgment
Sincere appreciation is extended to the patient’s family for their willingness to participate in this study. We also wish to acknowledge their advocy efforts to bring this case to publication for the benefit of the medical community. Support for this work was provided in part by 3UM1HG006348-10S2 to Dr. Seema R. Lalani.
References
- Bacino CA, Lee B, Spikes AS, & Shaffer LG (1999). Trisomy 16q in a female newborn with a de novo X;16 translocation and hypoplastic left heart. Am J Med Genet, 82(2), 128–131. doi: [DOI] [PubMed] [Google Scholar]
- Baker E, Hinton L, Callen DF, Altree M, Dobbie A, Eyre HJ, … Haan E (2002). Study of 250 children with idiopathic mental retardation reveals nine cryptic and diverse subtelomeric chromosome anomalies. Am J Med Genet, 107(4), 285–293. doi: 10.1002/ajmg.10159 [DOI] [PubMed] [Google Scholar]
- Balestrazzi P, Giovannelli G, Landucci Rubini L, & Dallapiccola B (1979). Partial trisomy 16q resulting from maternal translocation. Hum Genet, 49(2), 229–235. doi: 10.1007/bf00277648 [DOI] [PubMed] [Google Scholar]
- Basinko A, Audebert-Bellanger S, Douet-Guilbert N, Le Franc J, Parent P, Quemener S, … De Braekeleer M (2011). Subtelomeric monosomy 11q and trisomy 16q in siblings and an unrelated child: molecular characterization of two der(11)t(11;16). Am J Med Genet A, 155a(9), 2281–2287. doi: 10.1002/ajmg.a.34162 [DOI] [PubMed] [Google Scholar]
- Brisset S, Joly G, Ozilou C, Lapierre JM, Gosset P, LeLorc’h M, … Romana SP (2002). Molecular characterization of partial trisomy 16q24.1-qter: clinical report and review of the literature. Am J Med Genet, 113(4), 339–345. doi: 10.1002/ajmg.b.10740 [DOI] [PubMed] [Google Scholar]
- Buckton KE, & Barr DG (1981). Partial trisomy for long arm of chromosome 16. J Med Genet, 18(6), 483. doi: 10.1136/jmg.18.6.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calva P, Frias S, Carnevale A, & Reyes P (1984). Partial trisomy 16q resulting from maternal translocation 11p/16q. Ann Genet, 27(2), 122–125. [PubMed] [Google Scholar]
- Chen CP, Hung FY, Chern SR, Wu PS, Chen YN, Chen SW, … Wang W (2016). Prenatal diagnosis and molecular cytogenetic characterization of de novo partial monosomy 3p (3p26.3→pter) and partial trisomy 16q (16q23.1→qter). Taiwan J Obstet Gynecol, 55(2), 288–292. doi: 10.1016/j.tjog.2016.02.015 [DOI] [PubMed] [Google Scholar]
- Chen CP, Lin SP, Chern SR, Shih SL, Lee CC, Wang W, & Liao YW (2003). Perinatal findings and molecular cytogenetic analysis of trisomy 16q and 22q13.3 deletion. Prenat Diagn, 23(6), 504–508. doi: 10.1002/pd.629 [DOI] [PubMed] [Google Scholar]
- Cheung SW, Shaw CA, Yu W, Li J, Ou Z, Patel A, … Beaudet AL (2005). Development and validation of a CGH microarray for clinical cytogenetic diagnosis. Genet Med, 7(6), 422–432. doi: 10.1097/01.gim.0000170992.63691.32 [DOI] [PubMed] [Google Scholar]
- Cusick W, Bork M, Fabri B, Benn P, Rodis JF, & Buttino L Jr. (1995). Trisomy 16 fetus surviving into the second trimester. Prenat Diagn, 15(11), 1078–1081. doi: 10.1002/pd.1970151115 [DOI] [PubMed] [Google Scholar]
- Davison EV, & Beesley JR (1984). Partial trisomy 16 as a result of familial 16;20 translocation. J Med Genet, 21(5), 384–386. doi: 10.1136/jmg.21.5.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho AF, da Silva Bellucco FT, dos Santos NP, Pellegrino R, de Azevedo Moreira LM, Toralles MB, … Melaragno MI (2010). Trisomy 16q21 --> qter: Seven-year follow-up of a girl with unusually long survival. Am J Med Genet A, 152a(8), 2074–2078. doi: 10.1002/ajmg.a.33524 [DOI] [PubMed] [Google Scholar]
- Dharmadhikari AV, Gambin T, Szafranski P, Cao W, Probst FJ, Jin W, … Stankiewicz P (2014). Molecular and clinical analyses of 16q24.1 duplications involving FOXF1 identify an evolutionarily unstable large minisatellite. BMC Med Genet, 15, 128. doi: 10.1186/s12881-014-0128-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikmetas O, Simsek Kiper PO, Mocan MC, Utine EG, Boduroglu K, & Irkec M (2012). Bilateral anterior segment dysgenesis in an infant with partial trisomy 16q and partial monosomy 3p. J aapos, 16(5), 473–475. doi: 10.1016/j.jaapos.2012.05.008 [DOI] [PubMed] [Google Scholar]
- Dowman C, Lockwood D, & Allanson J (1989). Familial translocation t(9;16). J Med Genet, 26(8), 525–528. doi: 10.1136/jmg.26.8.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann T, Kolin-Gerresheim I, Gerresheim F, & Schwanitz G (1998). A case of de novo translocation 16;21: trisomy 16q phenotype and origin of the aberration. Ann Genet, 41(4), 205–208. [PubMed] [Google Scholar]
- Eriksson B, Fraccaro M, Hultén M, Lindsten J, Thorén C, & Tiepolo L (1971). Structural abnormalities of chromosome 18. II. Two familial translocations, B-18 and 16–18, ascertained through unbalanced forms. Ann Genet, 14(4), 281–290. [PubMed] [Google Scholar]
- Ferguson JG Jr., & Hicks EL (1987). Rieger’s anomaly and glaucoma associated with partial trisomy 16q. Case report. Arch Ophthalmol, 105(3), 323. doi: 10.1001/archopht.1987.01060030037015 [DOI] [PubMed] [Google Scholar]
- Ferrero GB, Belligni E, Sorasio L, Delmonaco AG, Oggero R, Faravelli F, … Silengo M (2006). Phenotype resembling Donnai-Barrow syndrome in a patient with 9qter;16qter unbalanced translocation. Am J Med Genet A, 140(8), 892–894. doi: 10.1002/ajmg.a.31188 [DOI] [PubMed] [Google Scholar]
- Francke U (1972). Quinacrine mustard fluorescence of human chromosomes: characterization of unusual translocations. Am J Hum Genet, 24(2), 189–213. [PMC free article] [PubMed] [Google Scholar]
- Fryns JP, Kleczkowska A, Decock P, & Van den Berghe H (1990). Direct duplication 16q11.1----16q13 is not associated with a typical dysmorphic syndrome. Ann Genet, 33(1), 46–48. [PubMed] [Google Scholar]
- Gambin T, Yuan B, Bi W, Liu P, Rosenfeld JA, Coban-Akdemir Z, … Stankiewicz P (2017). Identification of novel candidate disease genes from de novo exonic copy number variants. Genome Med, 9(1), 83. doi: 10.1186/s13073-017-0472-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garau A, Crisponi G, Peretti D, Vanni R, & Zuffardi O (1980). Trisomy 16q21 = to qter. Hum Genet, 53(2), 165–167. doi: 10.1007/bf00273489 [DOI] [PubMed] [Google Scholar]
- Gardner RJM, & Amor DJ (2018). Chromosomal Abnormalities and Genetic Counseling (Fifth ed.): Oxford University Press. [Google Scholar]
- Giardino D, Finelli P, Gottardi G, Clerici D, Mosca F, Briscioli V, & Larizza L (2001). Cryptic subtelomeric translocation t(2;16)(q37;q24) segregating in a family with unexplained stillbirths and a dysmorphic, slightly retarded child. Eur J Hum Genet, 9(12), 881–886. doi: 10.1038/sj.ejhg.5200730 [DOI] [PubMed] [Google Scholar]
- Hahm SY, Chitayat D, Iqbal MA, Cho S, & Nitowsky HM (1987). Partial duplication 16q: report of two affected siblings resulting from a maternal translocation and literature review. Clin Genet, 31(5), 343–348. doi: 10.1111/j.1399-0004.1987.tb02819.x [DOI] [PubMed] [Google Scholar]
- Hatanaka K, Ozaki M, Suzuki M, Murata R, & Fujita H (1984). Trisomy 16q13----qter in a infant from a t(11;16)(q25;q13) translocation-carrier father. Hum Genet, 65(3), 311–315. doi: 10.1007/bf00286525 [DOI] [PubMed] [Google Scholar]
- Houlston RS, Renshaw RM, James RS, Ironton R, & Temple IK (1994). Duplication of 16q22-->qter confirmed by fluorescence in situ hybridisation and molecular analysis. J Med Genet, 31(11), 884–887. doi: 10.1136/jmg.31.11.884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal SM, Day DW, Garcia M, Benjamin T, & Rogers J (1989). Familial transmission of 16p trisomy in an infant. Hum Genet, 81(2), 196–198. doi: 10.1007/bf00293904 [DOI] [PubMed] [Google Scholar]
- Kaulfus ME, Gardiner H, Hashmi SS, Mendez-Figueroa H, Miller VJ, Stevens B, & Carter R (2019). Attitudes of clinicians toward cardiac surgery and trisomy 18. J Genet Couns, 28(3), 654–663. doi: 10.1002/jgc4.1089 [DOI] [PubMed] [Google Scholar]
- Konrad EDH, Nardini N, Caliebe A, Nagel I, Young D, Horvath G, … Zweier C (2019). CTCF variants in 39 individuals with a variable neurodevelopmental disorder broaden the mutational and clinical spectrum. Genet Med, 21(12), 2723–2733. doi: 10.1038/s41436-019-0585-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laus AC, Baratela WA, Laureano LA, Santos SA, Huber J, Ramos ES, … Martelli L (2012). Karyotype/phenotype correlation in partial trisomies of the long arm of chromosome 16: case report and review of literature. Am J Med Genet A, 158a(4), 821–827. doi: 10.1002/ajmg.a.32988 [DOI] [PubMed] [Google Scholar]
- Lessick ML, Israel J, Wong PW, & Szego K (1989). Partial trisomy 16q secondary to a maternal 9;16 translocation. J Med Genet, 26(1), 63–64. doi: 10.1136/jmg.26.1.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonardo F, Perone L, Maioli M, Ciavarella M, Ciccone R, Monica MD, … Scarano F (2011). Clinical, cytogenetic and molecular-cytogenetic characterization of a patient with a de novo tandem proximal-intermediate duplication of 16q and review of the literature. Am J Med Genet A, 155a(4), 769–777. doi: 10.1002/ajmg.a.33852 [DOI] [PubMed] [Google Scholar]
- Luberda-Zapaśnik J, Midro AT, & Szwałkiewicz-Warowicka E (1995). [Syndrome of congenital malformations and dysmorphic features in a newborn with partial trisomy 16q due to maternal translocation t(9;16)(p24;q13)]. Pediatr Pol, 70(9), 769–773. [PubMed] [Google Scholar]
- Maher ER, Willatt L, Cuthbert G, Chapman C, & Hodgson SV (1991). Three cases of 16q duplication. J Med Genet, 28(11), 801–802. doi: 10.1136/jmg.28.11.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R, Paththinige CS, Sirisena ND, Nanayakkara S, Kariyawasam U, & Dissanayake VHW (2018). Partial trisomy 16q21→qter due to an unbalanced segregation of a maternally inherited balanced translocation 46,XX,t(15;16)(p13;q21): a case report and review of literature. BMC Pediatr, 18(1), 4. doi: 10.1186/s12887-017-0980-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin NC, Coffey WW, Nevin J, & Reid MM (1983). Partial trisomy 16q in two boys resulting from a maternal translocation, t(15;16)(p12;q11). Clin Genet, 24(5), 375–379. doi: 10.1111/j.1399-0004.1983.tb00088.x [DOI] [PubMed] [Google Scholar]
- Nyhan WL, Mascarello J, Barshop B, Doroski D, & Hirschhorn K (1989). Duplication of 16q and deletion of 15q. Am J Med Genet, 34(2), 183–186. doi: 10.1002/ajmg.1320340210 [DOI] [PubMed] [Google Scholar]
- Paladini D, D’Agostino A, Liguori M, Teodoro A, Tartaglione A, Colombari S, & Martinelli P (1999). Prenatal findings in trisomy 16q of paternal origin. Prenat Diagn, 19(5), 472–475. doi: [DOI] [PubMed] [Google Scholar]
- Papadopoulou Z, Papoulidis I, Sifakis S, Markopoulos G, Vetro A, Vlaikou AM, … Manolakos E (2017). Partial monosomy 8p and trisomy 16q in two children with developmental delay detected by array comparative genomic hybridization. Mol Med Rep, 16(6), 8808–8818. doi: 10.3892/mmr.2017.7760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passerini V, Ozeri-Galai E, de Pagter MS, Donnelly N, Schmalbrock S, Kloosterman WP, … Storchova Z (2016). The presence of extra chromosomes leads to genomic instability. Nat Commun, 7, 10754. doi: 10.1038/ncomms10754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Castillo A, Martin-Lucas MA, & Abrisqueta JA (1990). Duplication 16q12----qter arising from 3:1 segregation in a 46,XX,t(13;16) (q12;q12) mother. Ann Genet, 33(2), 121–123. [PubMed] [Google Scholar]
- Rethoré MO, Lafourcade J, Couturier J, Harpey JP, Hamet M, Engler R, … Lejeune J (1982). [Increased activity of adenine phosphoribosyl transferase in a child trisomic for 16q22.2 to 16qter due to malsegregation of a t(16;21) (q22.2;q22;2)pat]. Sem Hop, 58(45), 2639–2645. [PubMed] [Google Scholar]
- Ridler MA, & McKeown JA (1979). Trisomy 16q arising from a maternal 15p;16q translocation. J Med Genet, 16(4), 317–320. doi: 10.1136/jmg.16.4.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SH, & Duckett DP (1978). Trisomy 16p in a liveborn infant and a review of partial and full trisomy 16. J Med Genet, 15(5), 375–381. doi: 10.1136/jmg.15.5.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochat MK, Riegel M, & Schinzel AA (2007). Long-term follow-up of a 26-year-old male with duplication of 16p: clinical report and review. Am J Med Genet A, 143(4), 399–408. doi: 10.1002/ajmg.a.31605 [DOI] [PubMed] [Google Scholar]
- Savary JB, Vasseur F, Manouvrier S, Daudignon A, Lemaire O, Thieuleux M, … Deminatti MM (1991). Trisomy 16q23----qter arising from a maternal t(13;16)(p12;q23): case report and evidence of the reciprocal balanced maternal rearrangement by the Ag-NOR technique. Hum Genet, 88(1), 115–118. doi: 10.1007/bf00204941 [DOI] [PubMed] [Google Scholar]
- Schinzel A (2001). Catalogue of Unbalanced Chromosone Aberrations in Man (2nd ed.). Berlin, New York: De Gruyter. [Google Scholar]
- Sen P, Yang Y, Navarro C, Silva I, Szafranski P, Kolodziejska KE, … Stankiewicz P (2013). Novel FOXF1 mutations in sporadic and familial cases of alveolar capillary dysplasia with misaligned pulmonary veins imply a role for its DNA binding domain. Hum Mutat, 34(6), 801–811. doi: 10.1002/humu.22313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa B, Rocha G, Doria S, Alves JR, Guedes B, & Guimarães H (2004). New findings in partial trisomy 16q: clinical report. Acta Paediatr, 93(6), 852–854. doi: 10.1111/j.1651-2227.2004.tb03032.x [DOI] [PubMed] [Google Scholar]
- Talebian L, Li Z, Guo Y, Gaudet J, Speck ME, Sugiyama D, … Speck NA (2007). T-lymphoid, megakaryocyte, and granulocyte development are sensitive to decreases in CBFbeta dosage. Blood, 109(1), 11–21. doi: 10.1182/blood-2006-05-021188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien F, Morava E, Talarski A, & Marble M (2005). Phenotypic features of a boy with trisomy of 16q22-->qter due to paternal Y; 16 translocation. Clin Dysmorphol, 14(4), 177–181. doi: 10.1097/00019605-200510000-00002 [DOI] [PubMed] [Google Scholar]
- Türkyılmaz A, & Yaralı O (2020). A Very Rare Partial Trisomy Syndrome: De Novo Duplication of 16q12.1q23.3 in a Turkish Girl with Developmental Delay and Facial Dysmorphic Features. Balkan J Med Genet, 23(1), 103–108. doi: 10.2478/bjmg-2020-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Uchiyama A, Arai M, Kubodera K, Yamamoto Y, Orii KO, … Kohno Y (2009). Severe upper airway stenosis in a boy with partial monosomy 16p13.3pter and partial trisomy 16q22qter. Congenit Anom (Kyoto), 49(2), 85–88. doi: 10.1111/j.1741-4520.2009.00228.x [DOI] [PubMed] [Google Scholar]
- Yancey MK, Hardin EL, Pacheco C, Kuslich CD, & Donlon TA (1996). Non-mosaic trisomy 16 in a third-trimester fetus. Obstet Gynecol, 87(5 Pt 2), 856–860. [PubMed] [Google Scholar]
- Yue F, Jiang Y, Pan Y, Li L, Li L, Liu R, & Wang R (2019). Molecular cytogenetic characterization of partial monosomy 2p and trisomy 16q in a newborn: A case report. Exp Ther Med, 18(2), 1267–1275. doi: 10.3892/etm.2019.7695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn S, Ehrbrecht A, Bosse K, Kalscheuer V, Propping P, Schwanitz G, … Engels H (2005). Further delineation of the phenotype maps for partial trisomy 16q24 and Jacobsen syndrome by a subtle familial translocation t(11;16)(q24.2;q24.1). Am J Med Genet A, 139(1), 19–24. doi: 10.1002/ajmg.a.30995 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yao Q, Cui YX, Yao B, Fan K, Xia XY, … Li XJ (2013). Clinical and cytogenetic characterization of a boy with a de novo pure partial trisomy 16q24.1q24.3 and complex chromosome rearrangement. Am J Med Genet A, 161a(4), 897–900. doi: 10.1002/ajmg.a.35782 [DOI] [PubMed] [Google Scholar]


