Abstract
Objective
Sodium-glucose cotransporter-2 (SGLT2) inhibitors exhibit cardioprotective properties in patients with diabetes. However, SGLT2 is not expressed in the heart, and the underlying molecular mechanisms are not fully understood. We investigated whether the SGLT2 inhibitor luseogliflozin exerts beneficial effects on high glucose-exposed cardiomyocytes via the suppression of sodium-hydrogen exchanger-1 (NHE-1) activity.
Methods
Mouse cardiomyocytes were incubated under normal or high glucose conditions with vehicle, luseogliflozin, or the NHE-1 inhibitor cariporide. NHE-1 activity and gene expression were evaluated by the SNARF assay and real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis, respectively. Six-week-old male db/db mice were treated with vehicle or luseogliflozin for 6 weeks, and the hearts were collected for histological, RT-PCR, and western blot analyses.
Results
High glucose increased NHE-1 activity and transforming growth factor (Tgf)-β2 mRNA levels in cardiomyocytes, both of which were inhibited by luseogliflozin or cariporide, whereas their combination showed no additive suppression of Tgf-β2 mRNA levels. Luseogliflozin attenuated cardiac hypertrophy and fibrosis in db/db mice in association with decreased mRNA and protein levels of TGF-β2.
Conclusions
Luseogliflozin may suppress cardiac hypertrophy in diabetes by reducing Tgf-β2 expression in cardiomyocytes via the suppression of NHE-1 activity.
Keywords: Cariporide, diabetic cardiomyopathy, fibrosis, luseogliflozin, sodium-hydrogen exchanger-1, sodium-glucose cotransporter-2 inhibitor, transforming growth factor-β2
Introduction
Accumulating evidence has shown that diabetes is an independent risk factor and poor prognostic marker of heart failure.1,2 Cardiac hypertrophy and fibrosis are associated with an increased risk of heart failure in patients with diabetes. 3 However, the underlying molecular mechanisms are not fully understood. Therefore, identifying a potential therapeutic target for cardiac hypertrophy and fibrosis is urgently needed in patients with diabetes.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are a new class of oral antidiabetic agents that improve hyperglycemia in patients with diabetes by inhibiting urinary glucose reabsorption at the S1 segment of renal proximal tubules.4,5 Although SGLT2 is not expressed in the heart, 4 several animal studies have shown that SGLT2 inhibitors can prevent cardiac hypertrophy and fibrosis in rodent models of diabetes.6–8 Furthermore, recent cardiovascular outcome trials have consistently shown that compared with placebo, treatment with SGLT2 inhibitors significantly reduces the risk of heart failure in patients with type 2 diabetes. 9 However, the molecular mechanism for this remains to be elucidated.
Sodium-hydrogen exchanger (NHE) is a plasma membrane transporter involved in the regulation of intracellular pH via proton extrusion driven by the transmembrane Na+ gradient. 10 Among the isoforms, NHE-1 is predominantly expressed in the heart. 10 Although NHE-1 plays an essential role in the maintenance of cellular homeostasis under physiological conditions, NHE-1 activation has been reported to be involved in cardiac hypertrophy and fibrosis in the heart of a type 2 diabetic rat model. 11 Recently, SGLT2 inhibitors were shown to inhibit NHE-1 activity by directly binding to NHE-1 protein located on the plasma membrane in rodent cardiomyocytes.12,13 Accordingly, we speculate that the suppression of NHE-1 by SGLT2 inhibitors may contribute to their cardioprotective properties in diabetes.
We previously reported that high glucose and diabetic conditions increase the cardiomyocyte and cardiac tissue expression levels of transforming growth factor (TGF)-β2, respectively, contributing to cardiac hypertrophy and fibrosis in type 2 diabetic mice. 14 However, it remains unclear whether SGLT2 inhibitors attenuate cardiac hypertrophy and fibrosis in diabetic animals by reducing TGF-β2 expression in the heart via the suppression of NHE-1 activity. Therefore, in the present study, we investigated the effects of luseogliflozin, a selective SGLT2 inhibitor, on NHE-1 activity and TGF-β2 expression in high glucose-exposed cultured mouse cardiomyocytes and examined the influence of luseogliflozin on cardiac TGF-β2 expression, hypertrophy, and fibrosis in db/db mice, an animal model of type 2 diabetes with obesity. 15
Materials and methods
Chemical agents
The NHE-1 inhibitor cariporide and nuclear factor of activated T cells (NFAT) inhibitor 11R-VIVIT were purchased from Selleck Biotech (Bunkyo, Tokyo, Japan) and Merck Millipore Japan (Meguro, Tokyo, Japan), respectively. Luseogliflozin was provided by Taisho Pharmaceutical (Toshima, Tokyo, Japan).
Cell culture experiments
Cardiomyocytes isolated from neonatal ICR mice were obtained from Cosmo Bio (Koto, Tokyo, Japan). Cardiomyocytes were seeded onto fibronectin-coated 48-well plates and cultured in Dulbecco’s Modified Eagle Medium (Gibco, Waltham, MA, USA) containing 10% fetal bovine serum and 5.5 mM glucose. 14 For real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis, cells at 60% to 70% confluence were pre-treated with vehicle, cariporide (10 μM), 11R-VIVIT (1 μM), luseogliflozin (1 μM), or their combination for 1 hour and further cultured under normal (5.5 mM) or high (30 mM) glucose conditions for 48 hours.16–19 Experiments were conducted with at least three independent cell cultures.
RT-PCR
Total RNA was extracted from tissues and cells and then used to synthesize cDNA for RT-PCR assays as previously described. 14 Quantitative real-time RT-PCR was performed using the TaqMan gene expression assay and sequence detection system (StepOne Plus; Life Technologies Japan, Minato, Tokyo, Japan). 14 The pre-designed TaqMan probe sets used were as follows: Sglt2, Mm00453831_m1; Nhe-1, Mm00444270_m1; Tgf-β1, Mm 01178820_m1; Tgf-β2, Mm00436955_m1; myosin heavy chain beta (β-Mhc), Mm00600555_m1; and connective tissue growth factor (Ctgf), Mm1192932_g1. The 18S ribosomal RNA probe (18s rna, Mm03928990_g1) was used as an internal control.
Intracellular pH measurement
NHE-1 activity was assessed by intracellular pH recovery after ammonium chloride prepulse treatment in accordance with previously described methods with some modifications.13,20 Briefly, cardiomyocytes at 60% to 70% confluence were cultured under normal (5.5 mM) or high glucose (30 mM) conditions for 48 hours prior to the assay. Dulbecco’s phosphate-buffered saline (Thermo Fisher Scientific Japan [Gibco], Minato, Tokyo, Japan; catalog ID 14040-133; media formulation: 0.9 mM CaCl2, 0.5 mM MgCl2-6H2O, 2.7 mM KCl, 1.5 mM KH2PO4, 137.9 mM NaCl, 8.1 mM Na2HPO4-7H2O, 8 mM HEPES, 5.5 mM [normal] or 30 mM [high] glucose) was used as an assay buffer. 5-(and-6)-Carboxy SNARF-1 acetoxymethyl ester (SNARF-1, Thermo Fisher Scientific Japan) dissolved in the assay buffer at a final concentration of 10 μM was used as a loading buffer. NH4Cl dissolved in assay buffer was used as the stimulation buffer.
First, cell culture medium was replaced with the loading buffer without electrical pacing and incubated for 30 minutes. After five washes with the fresh assay buffer, cells were incubated with the stimulation buffer containing vehicle (0.1% dimethylsulfoxide [DMSO]), cariporide (10 μM), or luseogliflozin (10 μM) for 10 minutes. Then, the stimulation buffer was replaced with the fresh assay buffer containing vehicle (0.1% DMSO), cariporide (10 μM), or luseogliflozin (10 μM), and the ratio of two fluorescence intensities (emission 580 nM, excitation 514 nM; emission 640 nM, excitation 514 nM) was measured from multiple cells located in the central area of each well in 60-second intervals using a microplate reader (TECAN Infinite Pro200; TECAN Japan, Kawasaki, Kanagwa, Japan). At the end of each experiment, the fluorescence intensity was calibrated using an Intracellular pH Calibration Buffer Kit (Thermo Fisher Scientific Japan) in accordance with the manufacturer’s instruction. Cells were maintained at 37°C in a CO2 incubator during SNARF-1 loading and NH4Cl stimulation and room air during the measurement of fluorescence intensity. Δ intracellular pH was calculated between each time point, and Δ intracellular pH/minute was expressed by the average of Δ intracellular pH over the first 6 minutes after stimulation with NH4Cl.
Animal study
The reporting of this study conforms to ARRIVE 2.0 guidelines. 21 The protocol of the animal experiment was approved by the Animal Care Committee of Showa University School of Medicine before the study (approval number: 09059; date of approval: 28 July 2020). All experiments were conducted under adherence to the Guide for the Care and Use of Laboratory Animals (8th Edition). 22 Invasive procedures were conducted under general anesthesia using isoflurane (1.5% to 2.0%, depending on the toe pinch reflex). 23 We minimized the number of animals based on an estimation from our previous studies. 24 We carefully observed the health conditions of mice (at least 6 days a week) to decrease their suffering. Leptin receptor-deficient db/db mice fed a high-fat diet are a widely used mouse model of type 2 diabetes with obesity. 15 Five-week-old male db/db mice (BKS.Cg-Dock7m+/+Leprdb/Jcl, n = 19) purchased from CLEA Japan (Meguro, Tokyo, Japan) were housed in a single cage in the animal care facility of Showa University and maintained on standard rodent chow with free access to water.
After 1 week of acclimatization, mice were switched to a 5.0 g/day high-fat diet (45% of total calories, D12451, Research Diet, New Brunswick, NJ, USA) and then randomly assigned to the vehicle (n = 9) or luseogliflozin group (n = 10) according to their cage numbers (odd number: luseogliflozin, even number: vehicle). Luseogliflozin was given to mice via food, and its dosage (20 mg/kg/day) was determined based on a previous study. 25 After 6 weeks of intervention, all mice were sacrificed by isoflurane overdose. The heart, liver, kidney, and epidydimal fat pad were carefully isolated and weighed to calculate the index for each organ as follows: organ weight (mg)/left tibial length (mm). The apexes of the isolated hearts were excised and snap-frozen for RT-PCR and western blot analyses, and the remaining parts of the hearts were immersed in 4% paraformaldehyde for histological analysis. No unexpected adverse event was observed during the experimental period.
Measurement of plasma levels and blood pressure
Blood samples were collected after 6 hours of fasting at the end of experiments.14,24 Blood levels of glycated hemoglobin (HbA1c) were measured by an immunoassay (Roche Diagnostics, Minato, Tokyo). Plasma levels of glucose, lipids, and insulin were determined with enzyme electrode (Sanwa Kagaku, Nagoya, Aichi, Japan), colorimetric (Fuji Film WAKO, Osaka, Osaka, Japan), and enzyme-linked immunosorbent (ELISA) assays (Ultra-sensitive Mouse Insulin ELISA Kit; Product ID: M1104; Morinaga Institute of Biological Science, Yokohama, Kanagawa, Japan), respectively. Systolic blood pressure and pulse rates were measured 1 week before the end of experiments using a non-invasive tail-cuff method as previously described.14,24
Histological assessment of hearts
Cardiac hypertrophy and fibrosis were evaluated as previously described. 14 Briefly, the hearts were embedded in paraffin blocks, and cross-sections at the papillary muscle level were stained with hematoxylin & eosin for the assessment of cardiomyocyte size and Masson’s trichrome for left ventricular interstitial fibrosis area measurements. The images were analyzed by a researcher blinded to group assignments using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Western blot analysis
Protein expression levels were determined by western blot analysis as previously described. 26 Briefly, 10 μg of proteins extracted from cardiac tissues were electrophoresed in polyacrylamide gels and transferred to polyvinylidene fluoride membranes. After 1-hour incubation with a blocking reagent, the membranes were incubated with primary antibodies overnight at 4°C, followed by secondary antibody for 1 hour at room temperature. The following antibodies were used for western blot analyses: TGF-β2 (Abcam Japan, Chuo, Tokyo, Japan; Product ID: ab36495, RRID: AB_778343; mouse monoclonal antibody; dilution: 1:2000; molecular weight [MW]: 25 kDa [bioactive dimer]), NHE-1 (Santa Cruz Biotechnology, Dallas, TX, USA; Product ID: sc-136239, RRID: AB_2191254; mouse monoclonal antibody; dilution: 1:500; MW: 110 kDa), β-actin (Santa Cruz Biotechnology; Product ID: sc-47778; RRID: AB_2714189; mouse monoclonal antibody; dilution: 1:10,000; MW: 43 kDa), and anti-mouse IgG from sheep (GE Healthcare Japan, Hino, Tokyo, Japan; Product ID: NA931; RRID: AB_ 772212; dilution: 1:20,000). The bands on immunoblots were detected using the Amersham ECL Prime Kit (GE Health Care Japan), digitized using a WSE-6100 LuminoGraph (ATTO, Taito, Tokyo, Japan), and quantified using CS Analyzer 4 software (ATTO). 26
Statistics
Data are expressed as the mean ± standard deviation (SD). Statistical comparisons were performed using JMP software (version 13; SAS Institute Inc., Cary, NC, USA). Comparisons were tested by an unpaired two-sided t-test for two groups and one-way ANOVA followed by Tukey’s test for three or more groups. The number of mice was decided as follows: comparison type, unpaired two-sided t-test; α error, 5%; β error, 20%; estimated mean difference, 15%; and estimated SD of each group, 25%. Correlation was tested by Pearson’s correlation coefficient. The significance level was defined as p < 0.05.
Results
Luseogliflozin inhibited NHE-1 activity and Tgf-β2 gene expression in mouse cardiomyocytes under high glucose conditions
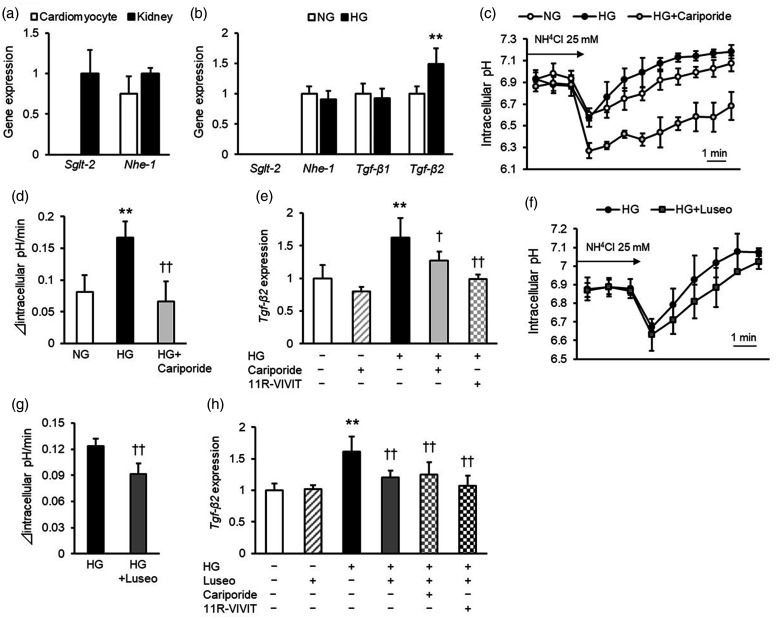
First, we evaluated the effects of luseogliflozin on cultured mouse cardiomyocytes (4–10 samples per group were obtained from at least three independent experiments). In contrast to the levels in positive control mouse kidneys, Sglt2 gene expression was barely detected in cardiomyocytes (Figure 1a). Nhe-1 mRNA levels in cardiomyocytes were comparable to those in mouse kidneys (Figure 1a). Although high glucose did not affect Nhe-1 mRNA levels in cardiomyocytes (Figure 1b), it significantly increased NHE-1 activity, as assessed by intracellular pH recovery after ammonium chloride prepulse treatment (p < 0.01) (Figure 1c, 1d). Furthermore, high glucose significantly increased the mRNA levels of Tgf-β2 in cardiomyocytes (p < 0.01) but did not affect Tgf-β1 gene expression levels (Figure 1b). Cariporide, an inhibitor of NHE-1, inhibited the effects of high glucose on NHE-1 activity (p < 0.01) and Tgf-β2 mRNA levels (p < 0.05) in cardiomyocytes (Figures 1c–1e). Moreover, 11R-VIVIT, an inhibitor of NFAT (essential transcriptional factor downstream of NHE-1 27 ) blocked the high glucose-induced upregulation of Tgf-β2 mRNA levels in cardiomyocytes (p < 0.01) (Figure 1e).
Figure 1.
Effects of cariporide and luseogliflozin on NHE-1 activity and Tgf-β2 gene expression levels in mouse cardiomyocytes. (a) mRNA levels of Sglt2 and Nhe-1 in mouse cardiomyocytes. Data of the kidneys collected from db/db mice were used as a positive control for SGLT2. (b) mRNA levels of Sglt2, Nhe-1, Tgf-β1, and Tgf-β2 in mouse cardiomyocytes under normal and high glucose conditions for 48 hours. (c) Effects of high glucose and cariporide on intracellular pH recovery after ammonium chloride (NH4Cl) prepulse treatment. (d) Quantified data of intracellular pH recovery after ammonium chloride prepulse treatment. Δ intracellular pH was calculated between each time point, and Δ intracellular pH/minute was expressed by the average of Δ intracellular pH over the first 6 minutes after stimulation with NH4Cl. (e) Effect of cariporide and 11R-VIVIT on Tgf-β2 mRNA levels in mouse cardiomyocytes under high glucose conditions. (f) Effects of luseogliflozin on intracellular pH recovery after ammonium chloride prepulse treatment. (g) Quantified data of intracellular pH recovery after ammonium chloride prepulse treatment. (h) Effect of luseogliflozin on Tgf-β2 mRNA levels in mouse cardiomyocytes under high glucose conditions. NHE-1, sodium-hydrogen exchanger-1; TGF, transforming growth factor; SGLT2, sodium-glucose cotransporter-2; NG, normal glucose; HG, high glucose; Luseo, luseogliflozin. Four to ten samples per group were obtained from at least three independent experiments. * and **, p < 0.05 and p < 0.01 vs. normal glucose, respectively. † and ††, p < 0.05 and p < 0.01 vs. high glucose, respectively.
The selective SGLT2 inhibitor luseogliflozin significantly decreased NHE-1 activity in high glucose-exposed cardiomyocytes (p < 0.01) (Figure 1f, 1g). In addition, luseogliflozin inhibited the high glucose-induced increase in Tgf-β2 mRNA levels (p < 0.01), whereas cariporide or 11R-VIVIT did not affect Tgf-β2 gene expression levels in cardiomyocytes exposed to high glucose and luseogliflozin (Figure 1h).
Luseogliflozin prevented cardiac hypertrophy and fibrosis in db/db mice in association with reduced cardiac gene expression levels of Tgf-β2
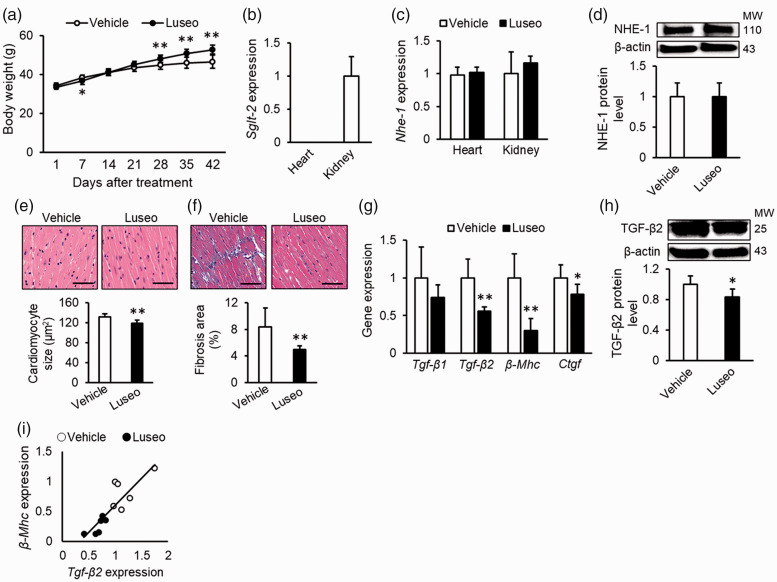
We next evaluated the effect of luseogliflozin on cardiac hypertrophy and fibrosis in high-fat-fed db/db mice, an animal model of type 2 diabetes with obesity. The metabolic parameters of animals are shown in Figure 2a and Table 1. Body weight was significantly decreased by luseogliflozin treatment at 7 days but increased after 28 days (p < 0.01) (Figure 2a). Liver and kidney weight indexes, HbA1c levels, and plasma glucose levels were significantly decreased by treatment with luseogliflozin (all p < 0.01) (Table 1).
Figure 2.
Effects of luseogliflozin treatment on cardiac hypertrophy and fibrosis in diabetic mice with obesity. High-fat-fed db/db mice were treated with vehicle or luseogliflozin for 6 weeks. (a) Body weight changes after treatment. (b) Cardiac mRNA levels of Sglt2. Data of the kidney were used as a positive control for SGLT2. (c) Cardiac mRNA levels of Nhe-1. (d) Cardiac protein levels of NHE-1. Upper panels show the representative immunoblot images. (e) Cardiomyocyte sizes. (F) Interstitial fibrosis area. Upper panels show the representative microscopic images of left ventricle stained with hematoxylin and eosin (E) and Masson’s trichrome (f). Scale bars, 1 mm. (g) Cardiac mRNA levels of Tgf-β1, Tgf-β2, β-Mhc, and Ctgf. (h) Cardiac protein levels of bioactive dimetric TGF-β2. Upper panels show the representative immunoblot images. (i) Correlation between gene expression levels of Tgf-β2 and β-Mhc. (a), (e), (f): Vehicle, n = 9, Luseogliflozin, n = 10; (b), (c): n = 4 per group; (d): n = 5 per group; (g), (i): n = 6 per group; (h): Vehicle, n = 5, Luseogliflozin, n = 6. Gene and protein expression levels of target molecules were normalized to those of the internal controls 18s rna and β-actin, respectively; the data were shown as relative levels to the vehicle. NHE-1, sodium-hydrogen exchanger-1; TGF, transforming growth factor; SGLT2, sodium-glucose cotransporter-2; β-Mhc, myosin heavy chain beta; Ctgf, connective tissue growth factor; MW, molecular weight; Luseo, luseogliflozin. *p < 0.05 vs. vehicle, **p < 0.01 vs. vehicle.
Table 1.
Anthropometric and biochemical parameters of diabetic mice treated with vehicle or luseogliflozin.
| Vehicle | Luseogliflozin | |
|---|---|---|
| Number | 9 | 10 |
| Food intake (g/day) | 5.0 | 5.0 |
| Heart weight index | 8.9 ± 0.5 | 8.6 ± 0.6 |
| Kidney weight index | 18.7 ± 1.4 | 14.7 ± 1.6** |
| Liver weight index | 142.6 ± 11.4 | 108.3 ± 15.9** |
| Visceral fat weight index | 85.7 ± 5.1 | 88.7 ± 5.5 |
| Pulse rate (/minute) | 597 ± 63 | 638 ± 38 |
| Systolic blood pressure (mmHg) | 127 ± 23 | 127 ± 23 |
| HbA1c (%) | 12.4 ± 0.3 | 7.8 ± 0.6** |
| Plasma glucose (mg/dL) | 564 ± 49 | 255 ± 46** |
| Plasma insulin (ng/mL) | 0.3 ± 0.2 | 0.7 ± 0.6 |
| Plasma total cholesterol (mg/dL) | 234 ± 32 | 230 ± 42 |
| Plasma HDL cholesterol (mg/dL) | 139 ± 27 | 140 ± 29 |
| Plasma triglycerides (mg/dL) | 108 ± 38 | 108 ± 58 |
Mean ± standard deviation. HDL, high-density lipoprotein; HbA1c, glycated hemoglobin. *p < 0.05, **p < 0.01 vs. Vehicle.
As shown in Figure 2b and 2c, Sglt2 mRNA was not detected in the hearts of db/db mice, whereas Nhe-1 mRNA was detected at similar levels in the hearts and kidneys. Luseogliflozin treatment did not affect the mRNA or protein levels of NHE-1 in the hearts (Figure 2c and 2d). Luseogliflozin treatment significantly reduced cardiomyocyte size and cardiac interstitial fibrosis area compared with the vehicle (p < 0.01) (Figure 2e and 2f). Furthermore, luseogliflozin treatment significantly decreased Tgf-β2 mRNA levels and bioactive dimetric TGF-β2 protein levels in the hearts of db/db mice (p < 0.01 and p < 0.05, respectively) (Figure 2g and 2h). These changes were accompanied by decreased gene expression levels of β-Mhc, a marker of cardiac hypertrophy 28 (p < 0.01) (Figure 2g), and Ctgf, a fibrogenic growth factor downstream of TGF-β2 signaling 29 (p < 0.05) (Figure 2g). Finally, there was a positive correlation between Tgf-β2 and β-MHC gene expression levels (r = 0.87, p < 0.01) (Figure 2i).
Discussion
In this study, we found for the first time that high glucose increased NHE-1 activity and Tgf-β2 mRNA levels in cultured mouse cardiomyocytes, both of which were inhibited by the selective SGLT2 inhibitor luseogliflozin and NHE-1 inhibitor cariporide. Although the NFAT inhibitor 11R-VIVIT, which suppressed the downstream signaling of NHE-1, also blocked the effects of high glucose on Tgf-β2 gene expression, the combination of cariporide and 11R-VIVIT did not have additive effects on Tgf-β2 gene suppression in high glucose-exposed luseogliflozin-treated cardiomyocytes. Furthermore, luseogliflozin treatment attenuated cardiac hypertrophy and fibrosis in db/db mice in association with decreased gene expression levels of Tgf-β2 and its downstream molecules β-Mhc and Ctgf.28,29 Therefore, our present observations suggest that luseogliflozin may exert anti-hypertrophic and anti-fibrotic effects in the diabetic hearts of db/db mice by reducing cardiac Tgf-β2 gene expression levels, partly via the suppression of NHE-1 activity. This effect may be one of the potential mechanisms underlying the cardioprotective property of SGLT2 inhibitors in patients with diabetes.
Heart failure is a leading cause of death in patients with diabetes.1–3 Cardiac hypertrophy and fibrosis are the characteristic structural changes observed in the hearts of patients with diabetes and are thus predictors of heart failure in these patients. 30 However, the molecular mechanisms underlying cardiac hypertrophy and fibrosis in diabetes are not fully understood. TGF-β is a multifunctional growth factor that has been shown to play a crucial role in the pathogenesis of cardiac hypertrophy and fibrosis by inducing cellular hypertrophy in cardiomyocytes and extracellular matrix production in cardiac fibroblasts. 29 Indeed, we previously reported that cardiac gene expression levels of Tgf-β2 are increased and associated with cardiac hypertrophy and fibrosis in diabetic animals. 14 In this study, we found that luseogliflozin treatment prevented cardiac hypertrophy and fibrosis with a concomitant reduction in TGF-β2 gene and protein expression levels in the hearts of diabetic mice with obesity. We also found that luseogliflozin reduced cardiac gene expression levels of β-Mhc, a marker of cardiac hypertrophy, 28 which were positively correlated with those of Tgf-β2. In addition, luseogliflozin decreased the cardiac gene expression levels of Ctgf, a mediator of TGF-β fibrogenic signaling. 29 These observations suggest that the suppression of cardiac TGF-β2 expression may partly account for the anti-hypertrophic and anti-fibrotic effects of luseogliflozin.
In the present study, we found that luseogliflozin attenuated the high glucose-induced increase in NHE-1 activity and Tgf-β2 gene expression in cardiomyocytes, and the NHE-1 inhibitor cariporide mimicked the effects of luseogliflozin. Because cariporide did not exert additive effects on luseogliflozin-induced downregulation of Tgf-β2 gene expression in high-glucose-exposed cardiomyocytes, luseogliflozin may inhibit Tgf-β2 gene expression in cardiomyocytes via the suppression of NHE-1 activity. Pharmacological inhibition of NHE-1 has been shown to prevent hypoxia-induced TGF-β1 expression and subsequently attenuate cardiac fibrosis in rats, 31 thus supporting our speculation. SGLT2 inhibitors, such as empagliflozin, dapagliflozin, and canagliflozin, are reported to directly bind to NHE-1 protein and inhibit its biological activity. 13 Luseogliflozin may exert the same inhibition against NHE-1 activity. Therefore, the inhibition of cardiac NHE-1 activity by SGLT2 inhibitors may be a novel molecular strategy in the prevention of cardiac hypertrophy and fibrosis in diabetes.
NFAT is a transcriptional factor activated by the calcium-sensitive molecule calmodulin. 32 NHE-1 overexpression causes Na+-induced Ca2+ overload via the compensative activation of Na+-Ca2+ channels and subsequently activates NFAT in cardiomyocytes. 33 Cardiomyocyte-specific overexpression of NFAT in mice induces cardiac hypertrophy and fibrosis, 32 whereas treatment with an NFAT inhibitor prevents pressure overload-induced cardiac hypertrophy and fibrosis. 34 Furthermore, NFAT inhibition has been reported to reduce Tgf-β gene expression in platelet-derived growth factor-exposed vascular smooth muscle cells. 35 Taken together, these findings suggest that the NHE-1/NFAT pathway may play a crucial role in the pathogenesis of cardiac hypertrophy. In this study, we found that 11R-VIVIT suppressed the high glucose-induced Tgf-β2 gene expression in cardiomyocytes but did not affect the gene expression levels of Tgf-β2 in high glucose-exposed luseogliflozin-treated cells. Therefore, our findings suggest that luseogliflozin may attenuate high glucose-induced Tgf-β2 gene expression by suppressing NFAT via the inhibition of NHE-1, as shown in the graphical abstract in Supplemental Figure 1.
There are several limitations to this study. First, we did not evaluate TGF-β2 protein levels in high glucose-exposed cultured cardiomyocytes. Therefore, although luseogliflozin treatment decreased the mRNA and protein levels of TGF-β2 in the hearts of db/db mice, it remains unclear whether luseogliflozin suppresses the translation of Tgf-β2 mRNA in cardiomyocytes exposed to high glucose. Second, consistent with the previous observations showing that SGLT2 inhibitors, such as luseogliflozin, increase body weight in db/db mice, we found that the final body weights were significantly higher in luseogliflozin-treated mice than in vehicle-treated mice.36–39 Although the effects of luseogliflozin on body weight in mice differ from those in humans, the mechanism underlying this difference remains unknown. Third, although luseogliflozin did not affect blood pressure or lipid parameters, it significantly improved hyperglycemia in diabetic mice. However, we did not determine if the glucose-lowering effects of luseogliflozin partly accounted for the suppression of cardiac hypertrophy and fibrosis in our animal model, although a meta-analysis revealed that intensive glycemic control exhibited no impact on the risk of heart failure in patients with type 2 diabetes. 40 Fourth, we could not evaluate cardiac function because of a technical difficulty. Therefore, whether treatment with luseogliflozin improves cardiac function and ultimately reduces the risk of heart failure in db/db mice remains unknown. Finally, we demonstrated direct effects of luseogliflozin on mouse cardiomyocytes in this study. However, our findings need to be further validated by future studies using human cardiomyocytes or heart tissues, which may provide more clinically relevant insight into the molecular mechanisms underlying the cardioprotective property of SGLT2 inhibitors.
Conclusions
Our present findings suggest that luseogliflozin directly inhibits the harmful effect of high glucose on cardiomyocytes by reducing TGF-β2 expression via the suppression of NHE-1 activity, which may be one of potential mechanisms underlying the cardioprotective property of SGLT2 inhibitors in diabetes.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605221097490 for Luseogliflozin inhibits high glucose-induced TGF-β2 expression in mouse cardiomyocytes by suppressing NHE-1 activity by Naoya Osaka, Yusaku Mori, Michishige Terasaki, Munenori Hiromura, Tomomi Saito, Hironori Yashima, Yoshie Shiraga, Raichi Kawakami, Makoto Ohara, Tomoyasu Fukui and Sho-ichi Yamagishi in Journal of International Medical Research
Footnotes
Author contributions: NO performed experiments, analyzed data, interpreted the results of experiments, and drafted and revised manuscript. YM conceived and designed the research, performed experiments, analyzed data, interpreted the results of experiments, prepared figures, drafted manuscript, and edited and revised manuscript. TS performed experiments, interpreted the results of experiments, and edited and revised manuscript. MT, MH, HY, YS, RK, MO, TF, and SY interpreted the results of experiments and edited and revised manuscript. All authors have approved the final version of the manuscript. YM is the guarantor of this work and is responsible for its integrity. Data are available upon reasonable request.
Declaration of conflicting interest: The authors declare that they have no competing interests.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: YM received financial support from Taisho Pharmaceutical, Boehringer Ingelheim JP, and Ono Pharmaceutical. The funders were not involved in the design, analysis, and reporting of the study.
ORCID iD: Yusaku Mori https://orcid.org/0000-0002-1734-0605
References
- 1.Bertoni AG, Hundley WG, Massing MW, et al. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 2004; 27: 699–703. [DOI] [PubMed] [Google Scholar]
- 2.Varela-Roman A, Grigorian Shamagian L, Barge Caballero E, et al. Influence of diabetes on the survival of patients hospitalized with heart failure: a 12-year study. Eur J Heart Fail 2005; 7: 859–864. [DOI] [PubMed] [Google Scholar]
- 3.Gopal K, Chahade JJ, Kim R, et al. The Impact of Antidiabetic Therapies on Diastolic Dysfunction and Diabetic Cardiomyopathy. Front Physiol 2020; 11: 603247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamagishi S, Matsui T. Protective Role of Sodium-Glucose Co-Transporter 2 Inhibition Against Vascular Complications in Diabetes. Rejuvenation Res 2016; 19: 107–114. [DOI] [PubMed] [Google Scholar]
- 5.Yanagisawa K, Sotokawauchi A, Nishino Y, et al. Albuminuria-lowering effect of sodium-glucose cotransporter 2 inhibitors could be partly attributable to the attenuation of tubular damage in type 2 diabetic patients. Diabetes Metab Res Rev 2020: e3327. [DOI] [PubMed] [Google Scholar]
- 6.Joubert M, Jagu B, Montaigne D, et al. The Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Prevents Cardiomyopathy in a Diabetic Lipodystrophic Mouse Model. Diabetes 2017; 66: 1030–1040. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Zhang J, Xue M, et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol 2019; 18: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue M, Li T, Wang Y, et al. Empagliflozin prevents cardiomyopathy via sGC-cGMP-PKG pathway in type 2 diabetes mice. Clin Sci (Lond) 2019; 133: 1705–1720. [DOI] [PubMed] [Google Scholar]
- 9.Lam CSP, Chandramouli C, Ahooja V, et al. SGLT-2 Inhibitors in Heart Failure: Current Management, Unmet Needs, and Therapeutic Prospects. J Am Heart Assoc 2019; 8: e013389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakabayashi S Hisamitsu T, andNakamura TY.. Regulation of the cardiac Na+/H+ exchanger in health and disease. J Mol Cell Cardiol 2013; 61: 68–76. [DOI] [PubMed] [Google Scholar]
- 11.Darmellah A, Baetz D, Prunier F, et al. Enhanced activity of the myocardial Na+/H+ exchanger contributes to left ventricular hypertrophy in the Goto-Kakizaki rat model of type 2 diabetes: critical role of Akt. Diabetologia. 2007; 50: 1335–1344. [DOI] [PubMed] [Google Scholar]
- 12.Baartscheer A, Schumacher CA, Wüst RC, et al. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia 2017; 60: 568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uthman L, Baartscheer A, Bleijlevens B, et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 2018; 61: 722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiromura M, Mori Y, Terasaki M, et al. Glucose-dependent Insulinotropic Polypeptide Inhibits Cardiac Hypertrophy and Fibrosis in Diabetic Mice via Suppression of TGF-β2. Diab Vasc Dis Res 2021; 18: 1479164121999034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burke SJ, Batdorf HM, Burk DH, et al. db/db Mice Exhibit Features of Human Type 2 Diabetes That Are Not Present in Weight-Matched C57BL/6J Mice Fed a Western Diet. J Diabetes Res 2017; 2017: 8503754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teshima Y, Akao M, Jones SP, et al. Cariporide (HOE642), a selective Na+-H+ exchange inhibitor, inhibits the mitochondrial death pathway. Circulation 2003; 108: 2275–2281. [DOI] [PubMed] [Google Scholar]
- 17.Duran J, Oyarce C, Pavez M, et al. GSK-3β/NFAT Signaling Is Involved in Testosterone-Induced Cardiac Myocyte Hypertrophy. PLoS One 2016; 11: e0168255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki T, Seino Y, Fukatsu A, et al. Safety, pharmacokinetics, and pharmacodynamics of single and multiple luseogliflozin dosing in healthy Japanese males: a randomized, single-blind, placebo-controlled trial. Adv Ther 2014; 31: 345–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tahara A, Takasu T, Yokono M, et al. Characterization and comparison of sodium-glucose cotransporter 2 inhibitors in pharmacokinetics, pharmacodynamics, and pharmacologic effects. J Pharmacol Sci 2016; 130: 159–169. [DOI] [PubMed] [Google Scholar]
- 20.Da Costa-Pessoa JM, Damasceno RS, Machado UF, et al. High glucose concentration stimulates NHE-1 activity in distal nephron cells: the role of the Mek/Erk1/2/p90RSK and p38MAPK signaling pathways. Cell Physiol Biochem 2014; 33: 333–343. [DOI] [PubMed] [Google Scholar]
- 21.Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br J Pharmacol 2020; 177: 3617–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington (DC): National Academies Press (US), 2011, pp1–220.
- 23.Navarro KL, Huss M, Smith JC, et al. Mouse Anesthesia: The Art and Science. ILAR J 2021: ilab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori Y, Terasaki M, Hiromura M, et al. Luseogliflozin attenuates neointimal hyperplasia after wire injury in high-fat diet-fed mice via inhibition of perivascular adipose tissue remodeling. Cardiovasc Diabetol 2019; 18: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okauchi S, Shimoda M, Obata A, et al. Protective effects of SGLT2 inhibitor luseogliflozin on pancreatic β-cells in obese type 2 diabetic db/db mice. Biochem Biophys Res Commun 2016; 470: 772–782. [DOI] [PubMed] [Google Scholar]
- 26.Mori Y, Kushima H, Koshibu M, et al. Glucose-Dependent Insulinotropic Polypeptide Suppresses Peripheral Arterial Remodeling in Male Mice. Endocrinology 2018; 159: 2717–2732. [DOI] [PubMed] [Google Scholar]
- 27.Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res 2004; 63: 467–475. [DOI] [PubMed] [Google Scholar]
- 28.Schiaffino S, Samuel JL, Sassoon D, et al. Nonsynchronous accumulation of alpha-skeletal actin and beta-myosin heavy chain mRNAs during early stages of pressure-overload–induced cardiac hypertrophy demonstrated by in situ hybridization. Circ Res 1989; 64: 937–948. [DOI] [PubMed] [Google Scholar]
- 29.Dobaczewski M Chen W, andFrangogiannis NG.. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J Mol Cell Cardiol 2011; 51: 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee M, Gardin JM, Lynch JC, et al. Diabetes mellitus and echocardiographic left ventricular function in free-living elderly men and women: the Cardiovascular Health Study. Am Heart J 1997; 133: 36–43. [DOI] [PubMed] [Google Scholar]
- 31.Chen TI, Tu WC. Exercise Attenuates Intermittent Hypoxia-Induced Cardiac Fibrosis Associated with Sodium-Hydrogen Exchanger-1 in Rats. Front Physiol 2016; 7: 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molkentin JD, Lu JR, Antos CL, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 1998; 93: 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura TY, Iwata Y, Arai Y, et al. Activation of Na+/H+ exchanger 1 is sufficient to generate Ca2+ signals that induce cardiac hypertrophy and heart failure. Circ Res 2008; 103: 891–899. [DOI] [PubMed] [Google Scholar]
- 34.Kuriyama M, Matsushita M, Tateishi A, et al. A cell-permeable NFAT inhibitor peptide prevents pressure-overload cardiac hypertrophy. Chem Biol Drug Des 2006; 67: 238–243. [DOI] [PubMed] [Google Scholar]
- 35.Yu H, Sliedregt-Bol K, Overkleeft H, et al. Therapeutic potential of a synthetic peptide inhibitor of nuclear factor of activated T cells as antirestenotic agent. Arterioscler Thromb Vasc Biol 2006; 26: 1531–1537. [DOI] [PubMed] [Google Scholar]
- 36.Kusakabe T, Yokota S, Shimizu M, et al. Differential effects of sodium-glucose cotransporter 2 inhibitor and low-carbohydrate diet on body composition and metabolic profile in obese diabetic db/db mice. BMJ Open Diabetes Res Care 2020; 8: e001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallo LA, Ward MS, Fotheringham AK, et al. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci Rep 2016; 6: 26428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kern M, Klöting N, Mark M, et al. The SGLT2 inhibitor empagliflozin improves insulin sensitivity in db/db mice both as monotherapy and in combination with linagliptin. Metabolism 2016; 65: 114–123. [DOI] [PubMed] [Google Scholar]
- 39.Terami N, Ogawa D, Tachibana H, et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS One 2014; 9: e100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castagno D, Baird-Gunning J, Jhund PS, et al. Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: evidence from a 37,229 patient meta-analysis. Am Heart J 2011; 162: 938–948.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605221097490 for Luseogliflozin inhibits high glucose-induced TGF-β2 expression in mouse cardiomyocytes by suppressing NHE-1 activity by Naoya Osaka, Yusaku Mori, Michishige Terasaki, Munenori Hiromura, Tomomi Saito, Hironori Yashima, Yoshie Shiraga, Raichi Kawakami, Makoto Ohara, Tomoyasu Fukui and Sho-ichi Yamagishi in Journal of International Medical Research