Abstract
Natural products offer an important avenue to novel therapeutics against drug-resistant bacteria, viruses, fungi, parasites, and cancer. However, there are numerous hurdles and challenges in discovering such molecules, including antimicrobial peptides (AMPs). While a thorough characterization of AMPs is limited by the amount of material, existing technology, and researcher’s expertise, peptide classification is complicated by incomplete information as well as different methods proposed for AMPs from bacteria, plants, and animals. This article describes unified classification schemes for natural AMPs on a common platform: the Antimicrobial Peptide Database (APD; https://aps.unmc.edu). The various criteria for these unified classifications include peptide biological source, biosynthesis machinery, biological activity, amino acid sequence, mechanism of action, and three-dimensional structure. To overcome the problem with a limited number of known 3D structures, a universal peptide classification has also been refined and executed in the APD database. This universal method, based on the spatial connection patterns of polypeptide chains, is independent of peptide source, size, activity, 3D structure, or mechanism of action. It facilitates information registration, naming, exchange, decoding, prediction, and design of novel antimicrobial peptides.
Keywords: Antibiotics, antimicrobial peptides, classification, database, hydrophobic content, net charge, peptide discovery, peptide length, structure
1. INTRODUCTION
Many drugs are derived from natural products, including peptides and small molecules. After years of exploration of small molecule drugs, there is now an increased interest in peptide therapeutics. One important reason is high specificity, making them important candidates for future precise medicine. Nature produces a variety of peptide scaffolds, ranging from linear to cyclic structures. These peptides vary in half-life, allowing them to play important roles in the biosphere such as growth factor, hormone, and anti-infection. At present, 80 peptides have been approved and hundreds more are under development (Craik & Kan, 2021).
Antimicrobial peptides (AMPs) are a special class of natural products that protect the host from microbial infection. This observation stimulated the development of AMPs into novel antibiotics. However, the isolation of novel peptides remains a challenging task. Frequently, the search is limited by the material available for purification and characterization. As a consequence, many peptides do not have known sequences and/or antimicrobial activity. Fleming noticed lysozyme from a droplet of his nose, but large-scale production only became possible from egg white. Boman had to find a “larger” insect organism for cecropin isolation (Faye and Lindberg, 2016). Peptide characterization can also be challenging, especially for those with extensive post-translationally modifications. Nisin was initially found in 1928. Its structure was established as a lantibiotic only after decades of work (Gross and Morell, 1971). Gassericin A and reutericin 6 were initially thought to differ in the number of D-amino acids. A follow-up study established that these two peptides are identical (Arakawa et al., 2010). Sublancin 168 was originally reported as an unusual lantibiotic where both lanthionine and disulfide bridges confer stability to the peptide (Paik et al., 1998). However, there is no Lan enzyme in the genome to facilitate a thioether bond formation. Rather, a glycosyltransferase is encoded, leading to an S-linked glycopeptide (Stepper et al., 2011). Microcin J25 was first proposed to be a head-to-tail circular structure (Blond et al., 1999). It was later found to have a lasso structure where the sidechain carboxylic group of Glu8 forms a peptide bond with the N-terminal amine of the peptide, allowing the N-terminal region to enter and lock in this cyclic structure (Wilson et al., 2003; Rosengren et al., 2003).
Peptide classification poses another challenge. On one hand, only a limited number of peptides have been characterized today, leading to incomplete information. On the other hand, there are different classification approaches for AMPs from different life kingdoms (Section 2). A unified classification of AMPs from a variety of sources is needed. This chapter describes a data platform for AMPs that enables a detailed classification of natural AMPs (Section 3). We then present unified classification methods for natural AMPs, including a refined universal peptide classification system (Section 4). We also compare the universal peptide classification with those proposed for bacteria, plants, insects, and amphibians (Section 5). Our systematic classifications of natural AMPs lay the foundation for effective information registration, storage, exchange, and use.
2. THE PROBLEM OF PEPTIDE CLASSIFICATION
There are different classification schemes for antimicrobial peptides from different kingdoms. In animals, AMPs are separated into families such as defensins, cathelicidins, and histatins. Each family has a recognizable trait. Many defensins comprise three disulfide bonds (Schneider et al., 2005). While the precursor sequences of cathelicidins are highly conserved, the mature AMP portions in this family are rather diverse (Scocchi et al., 1997). In amphibians, different family names were also introduced based on peptide sources and sequence patterns. Examples of some commonly accepted family names are aureins, maganins, dermaseptins, brevinins, esculentins, temporins, ranatuerins, and phylloseptins (Amiche et al., 2008; Conlon, 2008; Wang, 2020b). Unlike amphibians, insect AMPs are grouped into three categories: disulfide bond stabilized hairpin-like β-sheet or α-helical-β-sheet mixed structures, α-helical amphipathic peptides, and proline or glycine-rich peptides (Bulet et al., 1999). In plant AMPs, disulfide bonds frequently occur and are utilized as a basis to classify them into several families, including defensins, thionins, lipid transfer proteins, hevein-like peptides, knottin-type peptides, glycine-rich peptides, hairpin-like peptides, cyclotides, and snakins (Egorov et al., 2005).
The classification of bacteriocins has been evolving with time. Initially, Klaenhammer (1993) classified them into four classes: (I) Lantibiotics, (II) Small heat-stable non-lanthionine containing membrane-active peptides, (III) large heat-labile proteins, and (IV) complex bacteriocins containing lipid or carbohydrate. Class II contains three subclasses: (IIa) Listeria-active peptides with a consensus N-terminal sequence YGNGVXC such as pediocin-like peptides, (IIb) poration complexes consisting of two proteinaceous peptides for activity, and (IIc) thiol-activated peptides. Since thioether bonds are characteristic of bacterial lantibiotics, Cotter et al. (2005) recasted the above classification into two major classes: (I) Lanthionine-containing bacteriocins/lantibiotics and (II) Non-lanthionine-containing bacteriocins. Class II was further classified into four subclasses. Class IIa contains heterogeneous small peptides. Class IIb includes two peptides. While cyclic peptides belong to class IIc, non-pediocin single linear peptides form class IId. Also, class III is assigned to non-bacteriocin lytic proteins such as lysostaphin. In a comment article, Heng and Tagg (2006) proposed a “universal” bacteriocin classification scheme. Class I contains lantibiotics, which can be linear (Ia), globular (Ib), and multi-component (Ic). Class II consists of unmodified peptides (IIa: pediocin-like, IIb: miscellaneous, and IIc: multi-component), whereas class III is also reserved for large proteins (IIIa: bacteriolytic and IIIb: non-lytic). However, cyclic peptides became a new class IV. A more recent article puts bacteriocins into two classes: modified and unmodified (Alvarez-Sieiro et al., 2016). Class I (modified) includes lantipeptides (Ia), head-to-tail cyclized peptides (Ib), sactibiotics (Ic), linear azol(in)e-containing peptides (LAPs) (Id), glycocins (Ie), and lasso peptides (If). Class II (unmodified) comprises pediocin-like bacteriocins (IIa), two-peptide bacteriocins (IIb), leaderless bacteriocins (IIc), and non-pediocin-like, single-peptide bacteriocins (IId). Class III is again reserved for heat-labile large antimicrobial proteins. It is evident that these classification schemes for different kingdoms are diverse and have not been unified under the same umbrella.
3. CLASSIFICATION PLATFORM
The information on antimicrobial peptides from different kingdoms is scattered in the literature. To unify peptide classification, it is necessary to register these peptides from various sources into a common platform. For this purpose, we created the APD (Wang and Wang, 2004). The first version contained 525 AMPs. The peptide number increased to 1280 in the second version (APD2) (Wang et al., 2009). In the third version with 2619 peptides (Wang et al., 2016), the following set of criteria was applied to data registration: (1) natural peptides, (2) known antimicrobial activity, (3) known amino acid sequences, and (4) a peptide size less than 100 amino acids. Based on these criteria, over 50 such peptides were reported in 1993. The annual total passed 100 AMPs in 2000. A record high of 273 peptides was documented in 2011 (Figure 1A). Due to continued updates (Wang, 2020a), 3273 such peptides have been registered into the APD as of August 2021 (available at https://aps.unmc.edu).
Figure 1.
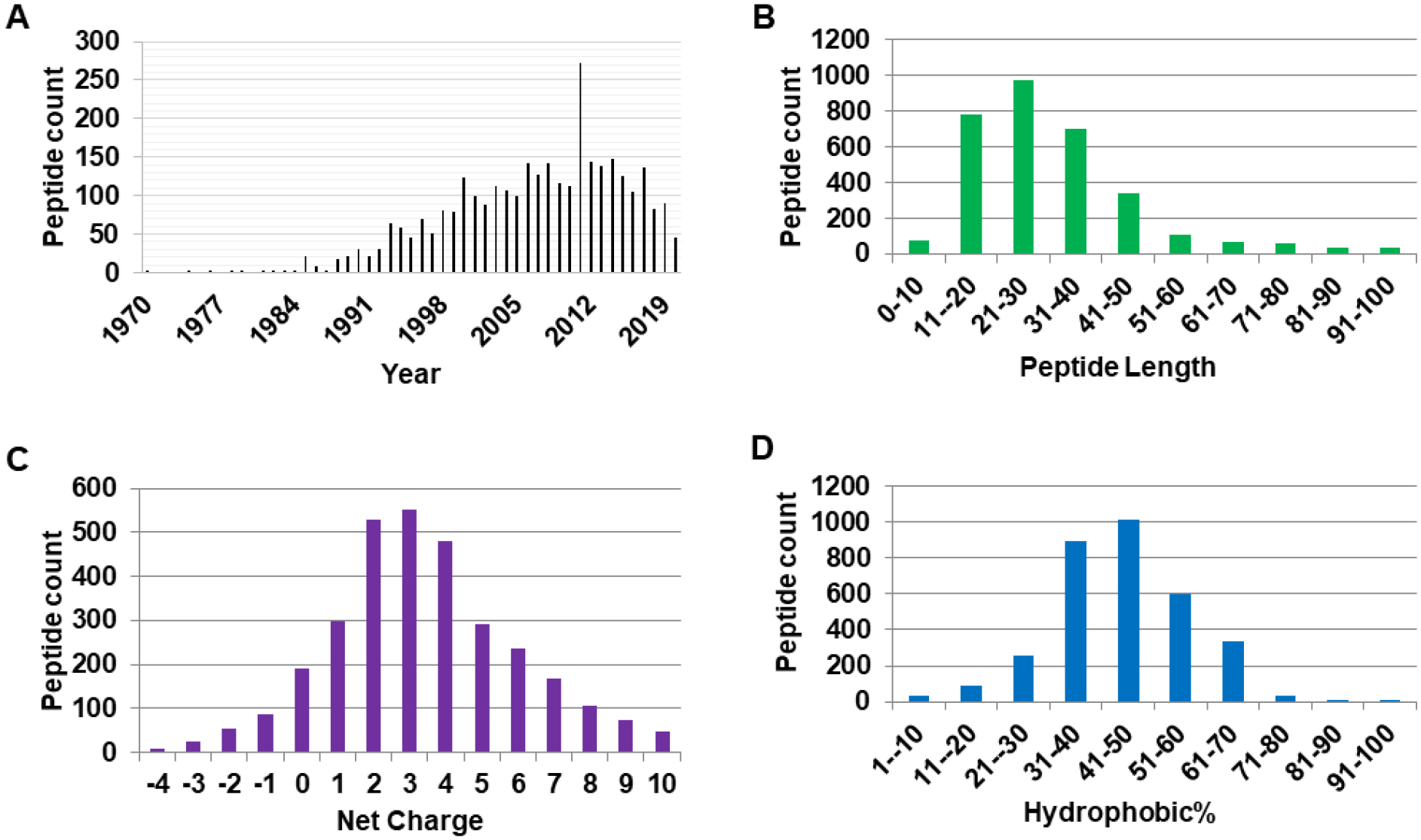
Antimicrobial peptide discovery and parameter space defined in the antimicrobial peptide database: (A) peptide reported per year; (B) peptide count as a function of length; (C) peptide count as a function of net charge; and (D) Peptide count as a function of peptide hydrophobic content.
4. UNIFIED CLASSIFICATION OF ANTIMICROBIAL PEPTIDES
In the following, we describe unified peptide classification methods, each based on a single peptide trait (source, activity, charge, length, and so on).
4.1. Biological sources
AMPs have been discovered in a variety of biological sources, ranging from single-cell prokaryotes to multicellular eukaryotes. The source of each polypeptide is usually unambiguous and can be validated in the genome at the DNA level. However, there are also cases where the peptide source became complicated. For example, cecropin P1 (AP00134) was originally thought to be isolated from pigs. A revisit uncovered its source as roundworms living within pigs (Sipos et al., 1992; Andersson et al., 2003). The peptide source classification in the APD3 has been merged into six life kingdoms: bacteria, archaea, protists, fungi, plants, and animals (Wang et al., 2016). Some major sources for natural AMPs are bacteria (369 peptides), plants (361 peptides), and animals (2424 peptides). Most of the animal peptides originate from amphibians (1127 peptides) and insects (325 peptides).
4.2. Peptide machinery
In nature, AMPs are biosynthesized in two mechanisms: ribosomally and non-ribosomally. Such information is annotated in the APD. Ribosomally synthesized peptides dominate (97%). Non-ribosomally made peptides have been discovered in bacteria and fungi. Frequently, they contain non-standard amino acids and special chemical bonds. These natural peptides are excellent templates for chemists to make synthetic or recombinant peptides in laboratories to understand the structure-activity relationships.
4.3. Biological activity
AMPs share a common denominator of being able to inhibit the growth of microbes (viruses, bacteria, fungi, and parasites). They are labeled as such when the minimal inhibitory concentration (MIC) is below 100 μM, or at least inhibition is observed in vitro under laboratory conditions. AMPs may have varying antimicrobial activity spectra. Magainins, defensins, and LL-37 are able to eliminate numerous pathogens (Selsted et al., 1985; Zasloff, 1987; Wang et al., 2019). However, plant defensins mainly kill fungi (Egorov et al., 2005), while bacteriocins may only inhibit the growth of competing strains (Cotter et al., 2005).
AMPs may also act on the host cells by binding to cell receptors (usually below MIC) to trigger signal transduction and gene transcription, leading to an alteration in the level of released cytokines for instance. Depending on the type of diseases that the peptide has an impact on, AMPs may be anticancer, anti-infectious, anti-diabetics, and wound healing. The APD has annotated over 20 peptide activities/functions (Wang et al., 2016).
4.4. Amino acid sequence
The APD enables the calculations of peptide length, net charge, hydrophobic content, and amino acid composition, which form the basis for peptide classification.
Peptide length:
The size distribution of natural AMPs in the APD is shown in Figure 1B. The shortest peptide comprises two amino acids (aa) plus chemical modifications. The upper limit is defined by the peptides collected into the APD (currently less than 200 amino acids). Based on peptide length, they may be further grouped: ultrashort (2–9 aa), short (10–24 aa), medium (25–50 aa), and long (50–100 aa). The majority of AMPs (88%) are small with less than 50 amino acids (with a peak at 21–30 aa in Figure 1B). Approximately 10% contain 50–100 amino acids. AMPs with greater than 100 aa are antimicrobial proteins.
Net charge:
The classic view is that AMPs are cationic. However, some peptides are anionic (Wang and Wang, 2004). The net charge distribution of the AMPs in the APD is shown in Figure 1C. 88% of AMPs are cationic (net charge > 0) with the maximum at +3. Only 6% are neutral (net charge = 0) and the remaining 6% are anionic (net charge < 0). It is important to note that the net charge calculation in the APD has considered the effect of post-translational modifications.
Hydrophobic, hydrophilic, and amphipathic:
AMPs are typically amphipathic, consisting both cationic and hydrophobic amino acids. Figure 1D shows the Pho plot. In the APD, Pho represents the hydrophobic content of a peptide, which is defined as the sum of A, C, F, I, L, M, V, and W divided by the total of amino acids in the peptide. In Figure 1D, the majority of AMPs (87%) have hydrophobic contents in the range of 31–70% with the peak at 41–50%. However, there are exceptions to the classic amphipathic concept. Surfactant-associated anionic peptide SAAP lacks hydrophobic amino acids and consists of merely a string of hydrophilic aspartates (Brogden et al., 1996). In contrast, two AMPs (e.g., baceridin and lugdunin) are composed of entirely hydrophobic amino acids (Niggemann et al., 2014; Zipperer et al., 2016).
Amino acid composition:
Some AMPs are recognized as “amino acid-rich”. The classic examples are His-rich, Trp-rich, Pro-rich, Arg-rich, and Gly-rich AMPs. This was judged based on the recurring of a certain amino acid in the sequence. However, there was no consensus definition. The rich amino acid in nearly all the above rich families is greater than 27% except for His-rich (23.3%). The APD defined the word “rich” to be at least >25% (Wang et al., 2016). Such a whole sequence-based definition, however, has a shortcoming since it may miss some AMPs that contain an amino acid-rich motif or domain. Therefore, it may be useful to also define the sequence regions that are rich in certain amino acid for multiple domain peptides (>25%).
4.5. Peptide targets
Many cationic, amphipathic AMPs recognize anionic bacterial membranes. However, the molecular targets of AMPs are not restricted to membranes. While nisins are known to target cell walls (van Heusden et al., 2002), Pro-rich peptides can inhibit protein synthesis by targeting ribosomes (Krizsan et al., 2014). Additional molecular mechanisms of AMPs are summarized elsewhere (Wang et al., 2015). However, such mechanistic information is limited, making it impractical to do a full classification of existing AMPs.
4.6. Peptide three-dimensional structure
It is logical to classify AMPs based on 3D structure. Boman classified AMPs into three types: α-helix, β-sheet, and rich (Boman, 2003). This is a mixed scheme that considers both secondary structure (α and β) and amino acid composition (rich or not). However, both His-rich and Trp-rich peptides may form an α-helical structure, causing overlap between classes (Brewer et al., 1998; Lakshmaiah Narayana et al., 2020). Another classification scheme is also mixed: α-helix, β-sheet, extended structure, and loop (Schneider et al., 2005). The APD proposed and executed a self-consistent structure-based classification scheme: α, β, αβ, and non-αβ (Wang, 2010). While α-helix and β-sheet are self-explanatory, AMPs in the αβ family consist of both α-helix and β-sheet structures. It is worthwhile to point out that the non-αβ family is broad. It can include extended structures, random coiled, turns, spiral structures, and so on. However, structure-based classification suffers from a limited number of known structures determined physically (13.6% in the APD). This is because both multidimensional multinuclear NMR spectroscopy and X-ray crystallography are specialized technologies requiring years of training and experience. Another difficulty is a lack of mechanistic information for many AMPs, making it impractical to determine a meaningful structure in complex with a proper molecular target.
4.7. A universal peptide classification protocol
A universal classification should consider the key aspects of peptides. In our opinion, the peptide connection pattern is a key feature as each chemical bond restricts the folding space of a polypeptide chain substantially, thereby determining both structure and activity. Here we describe a refined universal classification (UC) originally proposed by Wang (2015). In this classification system, peptides are separated into four large classes based on the polypeptide chain bonding types: class O, class P, class S, and class L (Figure 2). Class O peptides are circular due to a chemical bond between the N-terminal and C-terminal backbone atoms (i.e., UCBB). Class P peptides (also called UCSB) resemble the letter in shape where a chemical bond forms between the sidechain of one amino acid and the backbone of another amino acid in the chain. The third class (Class S) contains a chemical bond between different sidechains (UCSS). Finally, all linear peptides (one or multiple chains) are included in class L (i.e., UCLL). The longer class names (UCBB, UCSB, UCSS, and UCLL) are all unique and searchable in the APD. In the following, we define the classification criteria and priority for this universal classification system.
Figure 2.
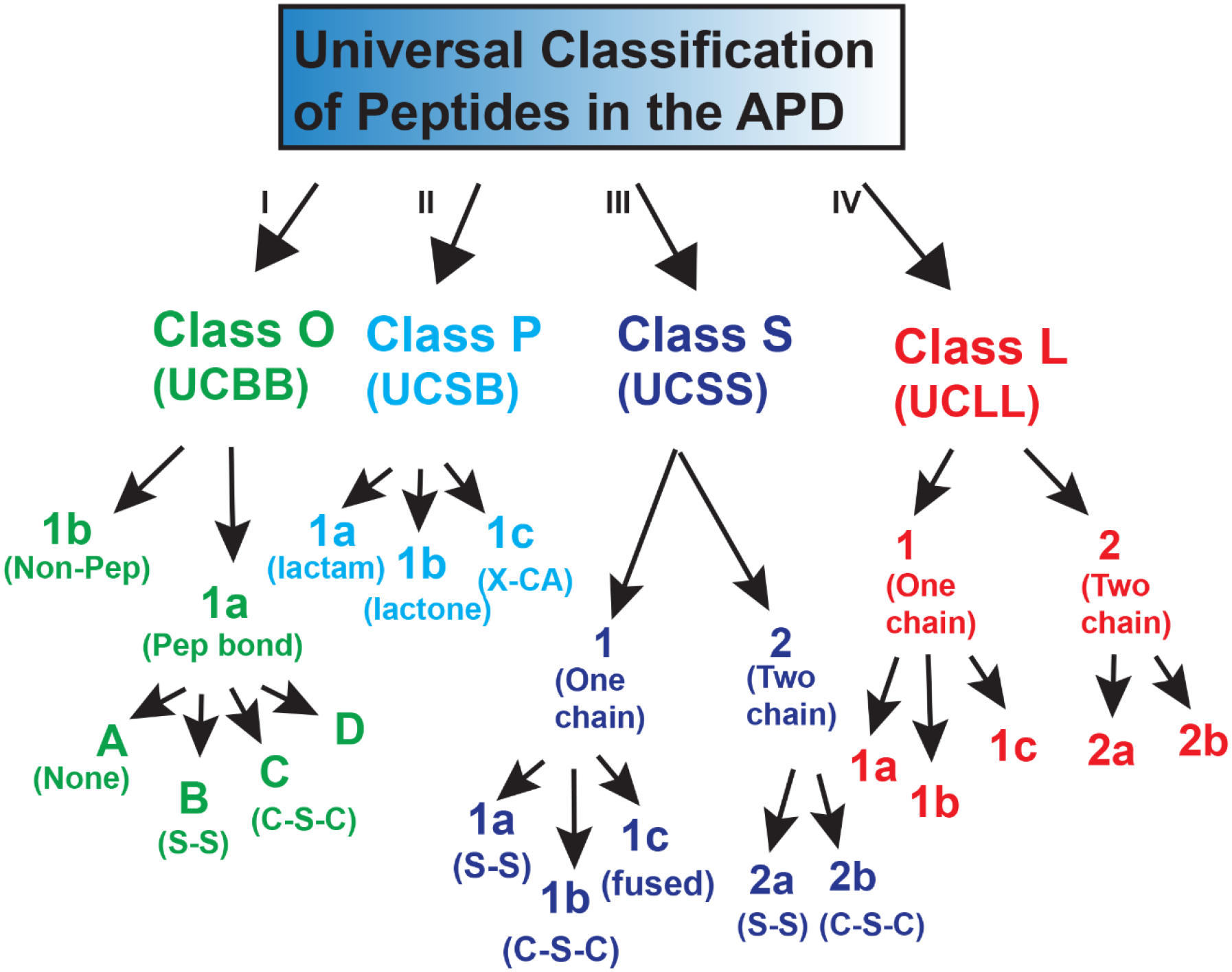
A universal peptide classification scheme for antimicrobial peptides where the ppetpides are classified into four classes. For simplicity, a single-letter class name is also introduced. For further details and examples, see the text.
Key classification criteria:
Peptide chain connection patterns (UCBB, UCSB, UCSS, and UCLL);
Peptide chain number (unlinked and covalently linked);
Each class can be further separated based on the type and number of additional chemical modifications, which are annotated most comprehensively in the APD.
Classification priority:
The first step is to determine the major class (UCBB, UCSB, UCSS, and UCLL) for the peptide based on the chain connection patterns. The classification priority is outlined in Figure 3 and described below:
If the peptide is circular (i.e., head-to-tail connection into a circle), it is assigned as UCBB (e.g., AS-48). This is the highest level (Class I in Figure 2) in this classification system.
Next, the peptide is classified as UCSB if there is a chemical bond from sidechain to backbone between different amino acids (e.g., daptomycin). This class has the second priority (Class II in Figure 2).
If the peptide consists of one or more covalent bonds between side chains (e.g., disulfide bonds in defensins and thioether bonds in lantibiotics), they belong to the UCSS class. Such a bond is usually within a single peptide chain, but can also occur between two different peptide chains. This class takes the third priority (Class III in Figure 2).
If the peptide is linear and does not have any chemical bond between two different amino acids, it belongs to UCLL (e.g., LL-37 and magainin). This class has the lowest level in this universal classification scheme (Class IV).
When the peptide sequence is incomplete, it will be labeled as “unclassified”.
A peptide may be re-classified once additional information becomes available.
Figure 3.
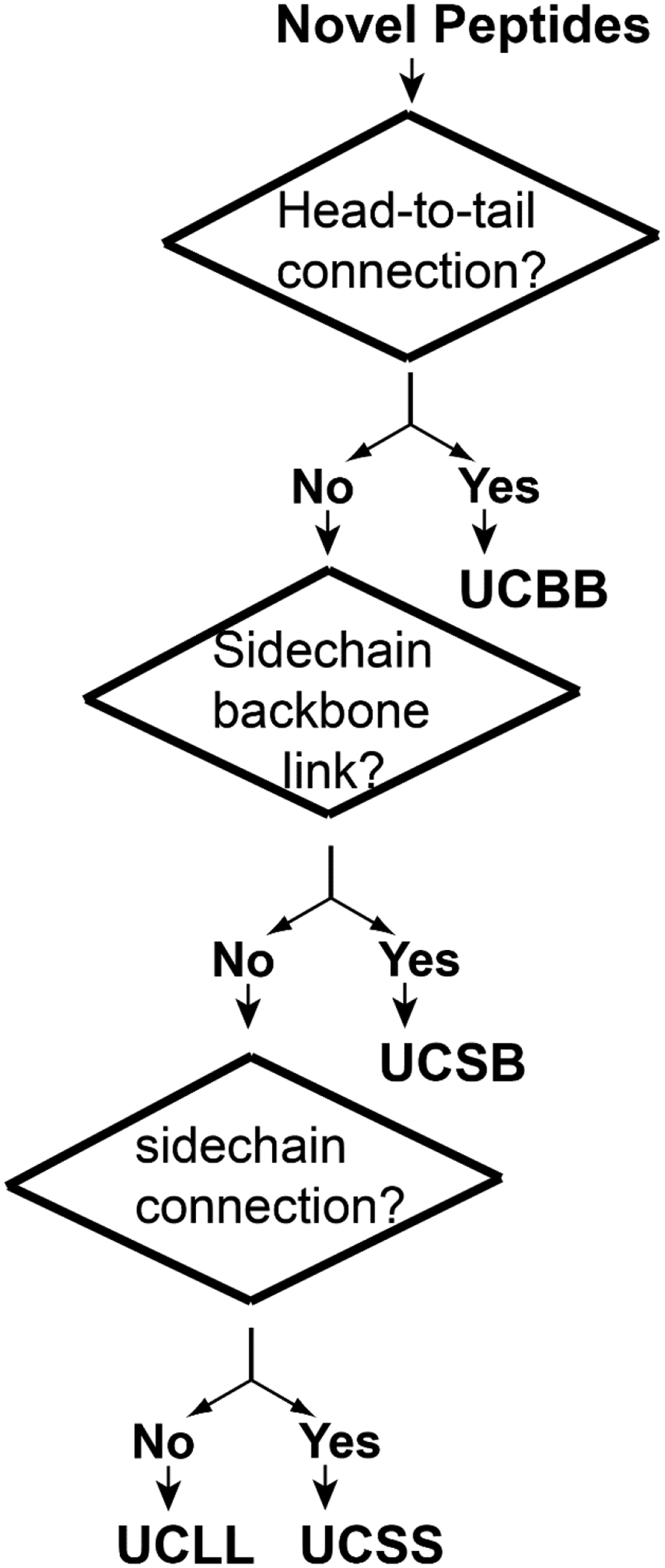
The classification priority of antimicrobial peptides from cyclic to linear sequences.
Basis for further classification
After assigning the major class for a peptide, one can then further classify it into subclasses based on chain number and additional types of chemical modification (Figure 2). UCBB peptides normally become circular by forming a peptide bond (UCBB1a). This class can be split into four groups. Group A does not have any additional chemical modification (e.g., AS-48). The B group possesses disulfide bridges (S-S). Both plant cyclotides and monkey ɵ-defensins form three pairs of disulfide bonds that further stabilize the circular structure. The third group C has a thioether bond (C-S-C) in the ring structure (e.g., subtilosin A). We also define a D group here where D-amino acids have been found (searchable in the APD using UCBB1aD).
UCBB peptides may also become circular via non-peptide bonds (UCBB1b). Lugdunin is a member of the UCBB1b subclass where the two termini cyclization is achieved via a multiple enzyme system (nonribosomally). The N-terminal amine of L-Cys and C-terminal aldehyde (L-Val) first form a macrocyclic imine/Schiff base. This is followed by a nucleophilic attack of the cysteine thiol group to generate a five-membered thiazolidine heterocycle (Zipperer et al., 2016). This peptide, discovered from human nasal microbiota, is effective against methicillin-resistant Staphylococcus aureus (MRSA).
UCSB can also be further classified: (1) UCSB1a with a NH-CO peptide bond (lactam, e.g., lassos MccJ25), (2) UCSB1b with a CO-O ester bond (lactone, e.g., daptomycin), and (3) UCSB1c with a sidechain atom-Cα (X-CA) bond (e.g., thurincin H and tryglysin). Both UCSB1a (17 entries) and UCSB1b (16 entries) peptides are classic members in this class. The UCSB1c peptides (eight members) were first characterized during 2009–2010 and most of the members were reported during 2018 and 2021. These peptides, called sactipeptides (Flühe et al., 2013), contain a thioether bond from the Cys sidechain to peptide backbone Cα of a different amino acid (S-CA). During this study, a new member in the UCSB1c subclass has been reported (Rued et al., 2021). Tryglysin contains a C-Cα (C-CA) bond between aromatic sidechain carbon and Cα. Meanwhile, one additional bond is established between the sidechains of lysine and tryptophan to produce sidechain fusion.
UCSS peptides can be further classified based on the number of chains (UCSS1 and UCSS2 in Figure 2). Each class can be further classified based on the chemical bond type and number. The known interchain bond types are disulfide bonds S-S (UCSS1a and UCSS2a) and thioether bonds C-S-C between two beta carbons (UCSS1b and UCSS2b). For two-chain peptides, the interchain number of S-S bonds can vary from one (distinctin) to five (rattusin) (Resende et al., 2009; Min et al., 2017). For single-chain peptides, the intrachain S-S bonds in 1092 UCSS1a peptides can vary from one to eight, while the intrachain thioether bonds in 71 UCSS1b lantibiotics can vary from two to seven. We also define a third subclass here reported in 2019 (UCSS1c). Darobactin has an unusual structure with two fused rings formed post-translationally between sidechains. There is an unusual ether bond that links C6 of aromatic W1 and β-carbon of W3. A second carbon-carbon bond connects C7 of aromatic W3 and β-carbon of basic K5 (Imai et al., 2019).
Linear UCLL peptides can be further classified based on the peptide chain number. Each can then be further separated based on localized chemical modifications limited to certain amino acid. The UCLL1a subgroup contains linear peptides (e.g., LL-37) without any chemical modifications. In this subclass, some peptides may be rich in certain amino acids (e.g., histatins). An individual sidechain of peptides in the UCLL1b group may be modified (e.g., halogenation, hydroxylation, and glycosylation). This allows a further classification based on the types of modifications. Naturally, any backbone modification of a single amino acid (terminal capping, D-amino acid, dehydration, and heterocycle) generates the third subgroup (UCLL1c). The UCLL2 peptides consist of two independent chains without any interchain bonding. Normally, the two chains need to be combined to be active (UCLL2a). Examples are class 2b bacteriocins such as mutacin IV and carnobacteriocin XY. Under special circumstances, the two peptide chains of gramicidin A can be identical (UCLL2b). As an ionic channel, gramicidin A forms a head-to-head dimer between two single-stranded β-helices (Jadhav et al., 2017).
5. RELATIONSHIP WITH SOURCE-DEPENDENT CLASSIFICATIONS OF ANTIMICROBIAL PEPTIDES
The above-described universal peptide classification serves as a master key to manage the natural antimicrobial peptide information on the same APD platform. It is complementary to the existing classification methods. To illustrate this, Table 1 summarizes the relationships of our universal peptide classification with other kingdom methods. According to this universal classification system, amphibian AMPs fall into two major classes: UCLL and UCSS. The UCLL contains linear peptides that form amphipathic helices or are rich in certain amino acids such as glycine. This class also includes linear AMPs containing D-amino acids (Table 1). The UCSS class includes two major subclasses: those with a Rana box stabilized by intramolecular disulfide bonds and distinctin with an intermolecular disulfide bond that links the two chains. At present, no members from the UCSB or UCBB class have been reported from amphibians.
Table 1.
Relationship between universal classification and other existing methods
| Universal classification | Subfamily | Bacteria (3 classes) | Plants | Insects | amphibians |
|---|---|---|---|---|---|
| UCLL (Class IV) | UCLL1a | cOB1 (class IId) | (1) Not-rich: Skh-AMP1 (2) Pro-rich: Gly-rich |
Gloverin; diptericin | (1) Not-rich XT-2; (2) Rich: Leptoglycin (Gly-rich) |
| UCLL1b (sidechain modified) | Delftibactin-A | ||||
| UCLL1c (backbone modified) | Microcin B17 (Class Id) | Antapin | Bombinin H3 | ||
| UCLL2 | Two-chain peptides (class IIb) | ||||
| UCSS (Class III) | UCSS1a | (1) Pediocin-like peptides (class IIa); (2) Glycocins (Ie) |
(1) Defensins, (2) thionins; (3) Lipid-transfer proteins; (4) Hevein-like; (5) Knottin-type; (6) Snakin. |
Defensins (3S-S); Thanatin (1S-S) Drosomycin (4S-S) |
Brevinin-1Ea (1S-S) |
| UCSS1b (C-S-C) | Lantibiotics (class Ia) | ||||
| UCSS1c (fused Sidechain) | Darobactin | ||||
| UCSS2a (inter) | Delta-Myrtoxin-Mp1a | Distinctin | |||
| UCSS2b (intra) | Lacticin-3147, Smb (class IIb) | ||||
| UCSB (Class II) | UCSB1a (CO-NH, lactam) | Microcin J25, polymyxin B (lasso, class If) | |||
| UCSB1b (CO-O, lactone) | Fusaricidin, daptomycin | ||||
| UCSB1c (Cβ-S-Cα | Thuricin CD (class Ic: sactibiotic) | ||||
| UCBB (Class I) | UCBB1aA (Unmodified) | AS-48 (class Ib, circular) | Cyclosaplin (no S-S) | ||
| UCBB1aB (S-S) | Closticin 574 | Circulin A, Kalata B1 | |||
| UCBB1aC (3S-CA) | Subtilosin A (class Ic: sactibiotic) | ||||
| UCBB1aD (backbone modified) | Gramicidin S (D-amino acids) | ||||
| UCBB1b | Lugdunin |
Like amphibians, insects also make two major classes of AMPs: UCLL and UCSS. The linear UCLL class comprises two subclasses: (1) those that form amphipathic helix in membrane-mimetic environments, and (2) those that are rich in glycines or alanines. Likewise, the UCSS class currently contains two subclasses: (1) those containing intramolecular disulfide bonds (thanatin, drosomycin, and defensin), and (2) those two-chain peptides connected by intermolecular disulfide bonds (delta-myrtoxin-Mp1a) (Table 1).
The plant AMPs consist of three classes: UCLL, UCSS, and UCBB. The linear subclass comprises proline or glycine-rich peptides and “not rich” AMPs. The UCSS class covers a variety of disulfide-bonded defense peptides (Table 1). Numerous circular cyclotides belong to the UCBB class. Finally, bacteria are remarkable organisms that are capable of making all kinds of bacteriocins, covering all the universal classes. The recently proposed bacteriocin families (Alvarez-Sieiro et al., 2016) are well incorporated into this universal classification system (Table 1).
Table 1 also maps the landscape for the currently known antimicrobial peptide scaffolds in various life kingdoms. The linear UCLL class is most widespread and exists in bacteria, plants, and animals. Linear two-chain peptides (UCLL2) are only found in bacteria. However, disulfide bond linked two-chain AMPs (UCSS2a) have been reported in both insects and amphibians, but not in bacteria or plants. Interestingly, the UCSB peptides are only discovered in bacteria. Finally, circular UCBB peptides have examples from bacteria, plants, and non-human primates. Hence, different life kingdoms utilize varying defense chemistries for survival. Because bacteria are able to manufacture a variety of peptide scaffolds (Table 1), they are an important source for identifying future antibiotics. The current interest in microbiota further extends our antimicrobial strategies as well as the search scope.
6. CONCLUDING REMARKS
Despite numerous challenges, over 3000 thousand antimicrobial peptides have been discovered from nature. In order to improve our understanding, it is important to group them into classes. We advanced peptide classification by achieving unified classifications on the same database platform. Our practice facilitates effective information registration, storage, exchange, and use of antimicrobial peptides. This universal classification can be readily expanded to incorporate new peptide classes. Our systematic classification may enable the development of more effective peptide prediction approaches. Finally, a specific peptide with a defined scaffold and parameters lays out a blueprint for synthetic biologists to engineer future medicine.
ACKNOWLEDGMENT
This study is supported by the NIH grants GM138552, AI137161, and the University of Nebraska Collaborative Initiation Award 2021.
REFERENCES
- Alvarez-Sieiro P, Montalbán-López M, Mu D, & Kuipers OP (2016). Bacteriocins of lactic acid bacteria: extending the family. Appl Microbiol Biotechnol. 100, 2939–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiche M, Ladram A, & Nicolas P (2008) A consistent nomenclature of antimicrobial peptides isolated from frogs of the subfamily Phyllomedusinae. Peptides. 29, 2074–82. [DOI] [PubMed] [Google Scholar]
- Andersson M, Boman A, & Boman HG (2003). Ascaris nematodes from pig and human make three antibacterial peptides: isolation of cecropin P1 and two ASABF peptides. Cell Mol Life Sci. 60, 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa K, Kawai Y, Ito Y, Nakamura K, Chujo T, Nishimura J, Kitazawa H, & Saito T (2010). HPLC purification and re-evaluation of chemical identity of two circular bacteriocins, gassericin A and reutericin 6. Lett Appl Microbiol. 50, 406–11. [DOI] [PubMed] [Google Scholar]
- Blond A, Péduzzi J, Goulard C, Chiuchiolo MJ, Barthélémy M, Prigent Y, Salomón RA, Farías RN, Moreno F, & Rebuffat S (1999). The cyclic structure of microcin J25, a 21-residue peptide antibiotic from Escherichia coli. Eur J Biochem. 259, 747–55. [DOI] [PubMed] [Google Scholar]
- Boman HG (2003). Antibacterial peptides: basic facts and emerging concepts. J Intern Med. 254, 197–215. [DOI] [PubMed] [Google Scholar]
- Brewer D, Hunter H, & Lajoie G (1998). NMR studies of the antimicrobial salivary peptides histatin 3 and histatin 5 in aqueous and nonaqueous solutions. Biochem Cell Biol. 76, 247–56. [DOI] [PubMed] [Google Scholar]
- Brogden KA, De Lucca AJ, Bland J, & Elliott S (1996). Isolation of an ovine pulmonary surfactant-associated anionic peptide bactericidal for Pasteurella haemolytica. Proc Natl Acad Sci U S A. 93, 412–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulet P, Hetru C, Dimarcq JL, & Hoffmann D (1999). Antimicrobial peptides in insects: Structure and function. Dev Comp Immunol. 23, 329–344. [DOI] [PubMed] [Google Scholar]
- Conlon JM (2008). Reflections on a systematic nomenclature for antimicrobial peptides from the skins of frogs of the family Ranidae. Peptides. 29, 1815–9. [DOI] [PubMed] [Google Scholar]
- Cotter PD, Hill C, & Ross RP (2005). Bacteriocins: developing innate immunity for food. Nat Rev Microbiol. 3, 777–88. [DOI] [PubMed] [Google Scholar]
- Craik DJ, & Kan MW (2021). How can we improve peptide drug discovery? Learning from the past. Expert Opin Drug Discov. In press. doi: 10.1080/17460441.2021.1961740. [DOI] [PubMed] [Google Scholar]
- Egorov TA, Odintsova TI, Pukhalsky VA, & Grishin EV (2005). Diversity of wheat antimicrobial peptides. Peptides. 26, 2064–73. [DOI] [PubMed] [Google Scholar]
- Faye I, & Lindberg BG (2016). Towards a paradigm shift in innate immunity-seminal work by Hans G. Boman and co-workers. Philos Trans R Soc Lond B Biol Sci. 371, 20150303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flühe L, Burghaus O, Wieckowski BM, Giessen TW, Linne U, & Marahiel MA (2013). Two [4Fe-4S] clusters containing radical SAM enzyme SkfB catalyze thioether bond formation during the maturation of the sporulation killing factor. J Am Chem Soc. 135, 959–62. [DOI] [PubMed] [Google Scholar]
- Gross E, & Morell JL (1971). The structure of nisin. J Am Chem Soc. 93, 4634–5. [DOI] [PubMed] [Google Scholar]
- Heng NCK, & Tagg JR (2006). What’s in a name? Class distinction for bacteriocins. Nat Rev Microbiol. 4, 160. [Google Scholar]
- Imai Y, Meyer KJ, Iinishi A, Favre-Godal Q, Green R, Manuse S, et al. (2019). A new antibiotic selectively kills Gram-negative pathogens. Nature 576, 459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav KB, Stein C, Makarewicz O, Pradel G, Lichtenecker RJ, Sack H, et al. (2017). Bioactivity of topologically confined gramicidin A dimers. Bioorg Med Chem. 25, 261–268. [DOI] [PubMed] [Google Scholar]
- Klaenhammer TR (1993). Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 12, 39–85. [DOI] [PubMed] [Google Scholar]
- Krizsan A, Volke D, Weinert S, Sträter N, Knappe D, & Hoffmann R (2014). Insect-derived proline-rich antimicrobial peptides kill bacteria by inhibiting bacterial protein translation at the 70S ribosome. Angew Chem Int Ed Engl. 53, 12236–9. [DOI] [PubMed] [Google Scholar]
- Lakshmaiah Narayana J, Mishra B, Lushnikova T, Wu Q, Chhonker YS, Zhang Y, et al. (2020). Two distinct amphipathic peptide antibiotics with systemic efficacy. Proc Natl Acad Sci U S A. 117, 19446–19454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min HJ, Yun H, Ji S, Rajasekaran G, Kim JI, Kim JS, et al. (2017). Rattusin structure reveals a novel defensin scaffold formed by intermolecular disulfide exchanges. Sci Rep. 7, 45282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggemann J, Bozko P, Bruns N, Wodtke A, Gieseler MT, Thomas K, et al. (2014). Baceridin, a cyclic hexapeptide from an epiphytic bacillus strain, inhibits the proteasome. Chembiochem. 15, 1021–9. [DOI] [PubMed] [Google Scholar]
- Paik SH, Chakicherla A, & Hansen JN (1998). Identification and characterization of the structural and transporter genes for, and the chemical and biological properties of, sublancin 168, a novel lantibiotic produced by Bacillus subtilis 168. J Biol Chem. 273, 23134–42. [DOI] [PubMed] [Google Scholar]
- Resende JM, Moraes CM, Munhoz VH, Aisenbrey C, Verly RM, Bertani P, et al. (2009). Membrane structure and conformational changes of the antibiotic heterodimeric peptide distinctin by solid-state NMR spectroscopy. Proc Natl Acad Sci U S A. 106, 16639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren KJ, Clark RJ, Daly NL, Göransson U, Jones A, & Craik DJ (2003). Microcin J25 has a threaded sidechain-to-backbone ring structure and not a head-to-tail cyclized backbone. J Am Chem Soc. 125, 12464–74. [DOI] [PubMed] [Google Scholar]
- Rued BE, Covington BC, Bushin LB, Szewczyk G, Laczkovich I, Seyedsayamdost MR, & Federle MJ (2021). Quorum Sensing in Streptococcus mutans Regulates Production of Tryglysin, a Novel RaS-RiPP Antimicrobial Compound. mBio. 12, e02688–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JJ, Unholzer A, Schaller M, Schäfer-Korting M, & Korting HC (2005). Human defensins. J Mol Med (Berl). 83, 587–95. [DOI] [PubMed] [Google Scholar]
- Scocchi M, Wang S, & Zanetti M (1997). Structural organization of the bovine cathelicidin gene family and identification of a novel member. FEBS Lett. 417, 311–5. [DOI] [PubMed] [Google Scholar]
- Selsted ME, Harwig SS, Ganz T, Schilling JW, & Lehrer RI (1985). Primary structures of three human neutrophil defensins. J Clin Invest. 76, 1436–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos D, Andersson M, & Ehrenberg A (1992). The structure of the mammalian antibacterial peptide cecropin P1 in solution, determined by proton-NMR. Eur J Biochem. 209, 163–9. [DOI] [PubMed] [Google Scholar]
- Stepper J, Shastri S, Loo TS, Preston JC, Novak P, Man P, et al. (2011). Cysteine S-glycosylation, a new post-translational modification found in glycopeptide bacteriocins. FEBS Lett. 585, 645–50. [DOI] [PubMed] [Google Scholar]
- van Heusden HE, de Kruijff B, & Breukink E (2002). Lipid II induces a transmembrane orientation of the pore-forming peptide lantibiotic nisin. Biochemistry. 41, 12171–8. [DOI] [PubMed] [Google Scholar]
- Wang G ed. (2010). Antimicrobial Peptides: Discovery, Design, and Novel Therapeutic Strategies, CABI, England: (version 2, 2017). [Google Scholar]
- Wang G (2015) Improved methods for classification, prediction, and design of antimicrobial peptides. Methods Mol Biol. 1268, 43–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G (2020a). The antimicrobial peptide database provides a platform for decoding the design principles of naturally occurring antimicrobial peptides. Protein Science, 29, 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G (2020b). Bioinformatic Analysis of 1000 Amphibian Antimicrobial Peptides Uncovers Multiple Length-Dependent Correlations for Peptide Design and Prediction. Antibiotics (Basel). 9, 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Li X, & Wang Z (2009). APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 37, D933–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Li X, & Wang Z (2016). APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 44, D1087–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Mishra B, Lau K, Lushnikova T, Golla R, & Wang X (2015) Antimicrobial peptides in 2014. Pharmaceuticals (Basel). 8, 123–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Narayana JL, Mishra B, Zhang Y, Wang F, Wang C, Zarena D, Lushnikova T, & Wang X (2019). Design of Antimicrobial Peptides: Progress Made with Human Cathelicidin LL-37. Adv Exp Med Biol. 1117, 215–240. [DOI] [PubMed] [Google Scholar]
- Wang Z, & Wang G (2004). APD: the Antimicrobial Peptide Database. Nucleic Acids Res. 32, D590–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KA, Kalkum M, Ottesen J, Yuzenkova J, Chait BT, Landick R, et al. (2003). Structure of microcin J25, a peptide inhibitor of bacterial RNA polymerase, is a lassoed tail. J Am Chem Soc. 125, 12475–83. [DOI] [PubMed] [Google Scholar]
- Zasloff M (1987). Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 84, 5449–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipperer A, Konnerth MC, Laux C, Berscheid A, Janek D, Weidenmaier C, et al. (2016). Human commensals producing a novel antibiotic impair pathogen colonization. Nature 535, 511–6. [DOI] [PubMed] [Google Scholar]


