Abstract
Background:
Asthma is the most common chronic disease affecting pregnancy and poor asthma control has been associated with adverse pregnancy outcomes. However, the trajectory of asthma control during pregnancy is not well understood or characterized.
Objective:
We aimed to identify and characterize trajectories of gestational asthma control in a US-based prospective pregnancy cohort.
Methods:
A k-means algorithm for joint longitudinal data was used to cluster pregnant women with and without asthma into gestational asthma control trajectories based on daily activity limitation, nighttime symptoms, inhaler use, and respiratory symptoms.
Results:
Among 308 women with asthma, two trajectories of gestational asthma control were identified and labeled “same” (n=184; 59.5%) or “worse” (n=124; 40.5%). Contrary to prior studies, we did not observe women with better asthma control in pregnancy. Women belonging to the “worse” trajectory experienced frequent and stable activity limitation and inhaler use, as well as frequent and increasing nighttime symptoms (~3 days/gestational week) and respiratory symptoms (~5 times/week). Women belonging to the “same” trajectory experienced infrequent and stable activity limitation, inhaler use, and respiratory symptoms, as well as infrequent and slightly increasing (~1 day/gestational week) nighttime symptoms. Results from pregnant women without asthma (n=107) suggest pregnancy alone was not responsible for changes in symptoms over time.
Conclusion:
In this US-based obstetric cohort receiving care according to standard clinical practice, gestational asthma control worsened for about 40% of women.
Keywords: Asthma, pregnancy, machine learning, prognosis, exacerbations, algorithms, cohort studies
INTRODUCTION
Affecting approximately 10% of women, asthma is the most common chronic disease among pregnant women(1). Compared to women without asthma, women with asthma are more likely to experience adverse pregnancy outcomes including preterm delivery, gestational diabetes, preeclampsia, and neonatal death(2–6). Prior studies suggest that better asthma control during pregnancy may help to ameliorate some of these outcomes(2, 7).
Convention holds that a third of women with asthma will experience increased control (i.e., get better), a third will experience no change in control (i.e., stay the same), and a third will experience decreased control (i.e., get worse) during pregnancy(8–10). However, few studies have been adequately designed to examine longitudinal changes in asthma control during pregnancy, and more recent studies have begun challenging this convention(2, 8–14). Notably, most prior studies rely on assessments of asthma control based on a self-reported predetermined “better, same, or worse” framework(2, 11–14). No prior studies have employed agnostic, data-driven approaches to examine changes in asthma control during pregnancy. Such a method may be particularly relevant, as there are multiple factors that contribute to this determination; it is possible that some aspects of asthma control may be modified differentially across pregnancy. Further, there is substantial overlap between symptoms of asthma and those of pregnancy (e.g., shortness of breath), and prior studies have not adequately accounted for this potential overlapping symptomatology(15, 16). Understanding changes in specific asthma symptoms – including whether pregnant women without asthma experience similar symptoms during this timeframe – is key to understanding the course of asthma control during pregnancy.
Thus, the primary aim of the current study was to identify and describe trajectories of gestational asthma control among a prospective cohort of women with and without asthma followed from early pregnancy. Our secondary aim was to determine sociodemographic and clinical characteristics related to gestational asthma control.
METHODS
Study design
Analyses were conducted using data from the Breathe - Wellbeing, Environment, Lifestyle, and Lung function (B-WELL-Mom) Cohort, a prospective pregnancy cohort of women with active asthma (n=311) or no history of asthma (n=107). Women were recruited from obstetric facilities at Northwestern University and University of Alabama-Birmingham. Potentially eligible participants were identified from medical record review and then screened for eligibility and consent. Women were categorized as having asthma if they reported an asthma diagnosis and either used prescription medication for asthma or had asthma symptoms in the year prior to the pregnancy. Women were categorized as having no asthma if they had no history of asthma and no current asthma or asthma medication use. Table E1 presents participant characteristics by asthma status.
Women were followed during pregnancy with three in-person study visits that included assessments and questionnaires at <15 weeks (baseline visit 1), 20–22 weeks (visit 2), and at 30–32 weeks (visit 3). Medical record abstraction provided additional information on maternal and perinatal health including delivery information. Women were also asked to complete daily diaries. Data collection was for research purposes only and study data were not provided to clinical care providers or used to direct clinical management of asthma. Human subjects’ approval was obtained from all participating sites and all subjects provided informed consent. Eligibility criteria is summarized in Table E2, and data collection materials are available on the study website (https://b-well-mom.org).
Sociodemographic and clinical characteristics
At baseline, study visit questionnaires collected data on participant sociodemographics including age, self-identified race/ethnicity, parity, household income, education, pre-pregnancy cigarette smoke exposure, and pre-pregnancy health conditions. We additionally collected data on body mass index (kg/m2; BMI) measured by study staff, season of conception, and study site.
Study visit questionnaires also collected data on clinical characteristics, including baseline asthma control from the Asthma Control Test(17), asthma course in prior pregnancy, age at asthma onset, exercise-induced asthma, atopy, medication use, and asthma exacerbations. Pre-pregnancy medication use was determined based on asthma medications participants reported taking in the 6 months prior to the baseline study visit. Baseline medication use was determined based on asthma medications participants reported taking on a regular basis on the baseline questionnaire. We then calculated pre-pregnancy and baseline asthma severity from medication use based on the American College of Obstetricians and Gynecologists guidelines for asthma management during pregnancy (Table E3)(18). Changes in asthma medication from pre-pregnancy course to baseline (step up, step down, no change) were determined by comparing asthma severity pre-pregnancy to baseline. A step up or step down refers to any change in medications that would cause someone to be classified at a higher or lower asthma severity level, respectively. No change refers to maintaining the same asthma severity level between pre-pregnancy and baseline. Pre/early pregnancy asthma exacerbations included oral corticosteroid use in the past 6 months, and any asthma attacks or asthma-related medical encounters (i.e., unscheduled doctor’s visits, emergency room/urgent care visits, or hospitalizations) occurring in the 12 months prior to baseline. Asthma exacerbations during pregnancy included oral corticosteroid use, asthma attacks, or asthma-related medical encounters reported during pregnancy.
During study visit assessments, trained study staff collected data on pulmonary function and inflammation. Pulmonary function tests (forced expiratory volume in 6 seconds (FEV6) and 1 second (FEV1), forced vital capacity (FVC), FEV1/FVC, and peak flow) were collected in triplicate and – excepting FEV1/FVC – were converted to a percent predicted measure. The maximum value recorded at each visit was used in analyses. Pulmonary inflammation, assessed by FeNO (parts per billion (ppb)), was collected using a NIOX® device (Circassia Limited, Morrisville, NC).
Asthma control measures
Daily diaries collected information on nighttime symptoms (“Did you wake due to difficulty breathing or coughing in the middle of the night?”), activity limitation (“Did you miss school, work, or normal daily activities because of your illness or symptoms?”), inhaler use (“What prescription medication(s) did you use?”), and respiratory symptoms (wheezing, coughing, chest tightness, chest pain, shortness of breath). Respiratory symptoms were aggregated (i.e., to a total of 5 possible symptoms per day) in analyses. Daily measures of gestational asthma control were aggregated to obtain the total number of days or times that women reported each of the asthma control variables for each week of gestation they were in the study (i.e., between baseline and delivery or loss to follow-up). If a woman reported having a cold, the flu, or a sore throat on a given day, we did not consider their respiratory symptoms for that day in analyses.
Statistical Analysis
Analyses proceeded in two stages: in the first, we identified and described gestational asthma control trajectories and, in the second, we associated sociodemographic and clinical characteristics with gestational asthma control trajectories. Prior to running analyses, a two-step multiple imputation procedure was used to address missing data in daily diaries (see E1). Trajectories of gestational asthma control were then identified using the k-means algorithm, an unsupervised machine learning approach. This was run using the kml3d package in R with default settings (2–6 clusters run 20 times)(19). Women were partitioned into asthma control groups based on weekly activity limitation (days/gestational week), nighttime symptoms (days/gestational week), inhaler use (days/gestational week), and respiratory symptoms (number of times/gestational week) based on the Euclidean distance (pairwise distance between data points). The optimal number of groups was selected based on the Calinski-Harabatz criteria (the ratio of the sum of between-group dispersion and of inter-group dispersion for all groups), with restriction to a group size of at least 10% of our population. There was high agreement in gestational asthma control trajectory assignment across imputations (Table E4). Thus, results from across imputations were combined to assign women to one gestational asthma control trajectory. Women were assigned to a trajectory based on the group to which they were more likely to belong; one woman with a 50% chance of being placed in either group was assigned to the “worse” asthma control trajectory.
Chi-squared, Fisher’s exact, and t-tests were used to examine associations between sociodemographic and clinical characteristics and gestational asthma control trajectory. Generalized linear mixed models were used to examine differences in mean pulmonary function and inflammation across pregnancy by gestational asthma control trajectory.
Robustness of results from our primary analysis was assessed through several sensitivity analyses. First, to determine whether overlapping symptomology between pregnancy and asthma may explain our findings, we performed the following analyses: 1) re-ran the primary analysis among the full cohort (i.e., including women with and without asthma), 2) re-ran the primary analysis among the full cohort but excluded inhaler use from the algorithm, and 3) re-ran the primary analysis only among women without asthma. Second, to determine whether changes in medication use influenced our findings, we re-ran the primary analysis by changes in medication use (no change, step up, step down) from pre-pregnancy to baseline. Third, we re-ran the primary analysis for a complete case, last-observation-carried-forward, and a “worst” and “best” case scenario. The “worst” case scenario assumed women who missed a daily diary entry experienced their personal worst asthma control. The “best” case scenario assumed women who missed a daily diary entry experienced no asthma symptoms. Next, we re-ran the primary analysis including individual respiratory symptoms (i.e., wheezing, coughing, chest tightness, chest pain, and shortness of breath) alone as well as in conjunction with asthma control variables used in the primary analysis. Finally, group-based multi-trajectory models in the full sample and asthma-only subgroup were fit with 2–6 groups as per Nagin et al(20) to assess whether a model-based algorithm would corroborate our findings.
Analyses were conducted using SAS version 9.4 (Cary, NC) and R version 3.6.1. A p-value of 0.05 was used to determine statistical significance.
RESULTS
Of the 418 women with and without asthma enrolled, three failed to complete any study procedures and were therefore excluded from analyses (Figure 1). Of the remaining 415 women, 83% had at least one daily diary entry completed per gestational week between baseline and delivery or loss to follow-up.
Figure 1.
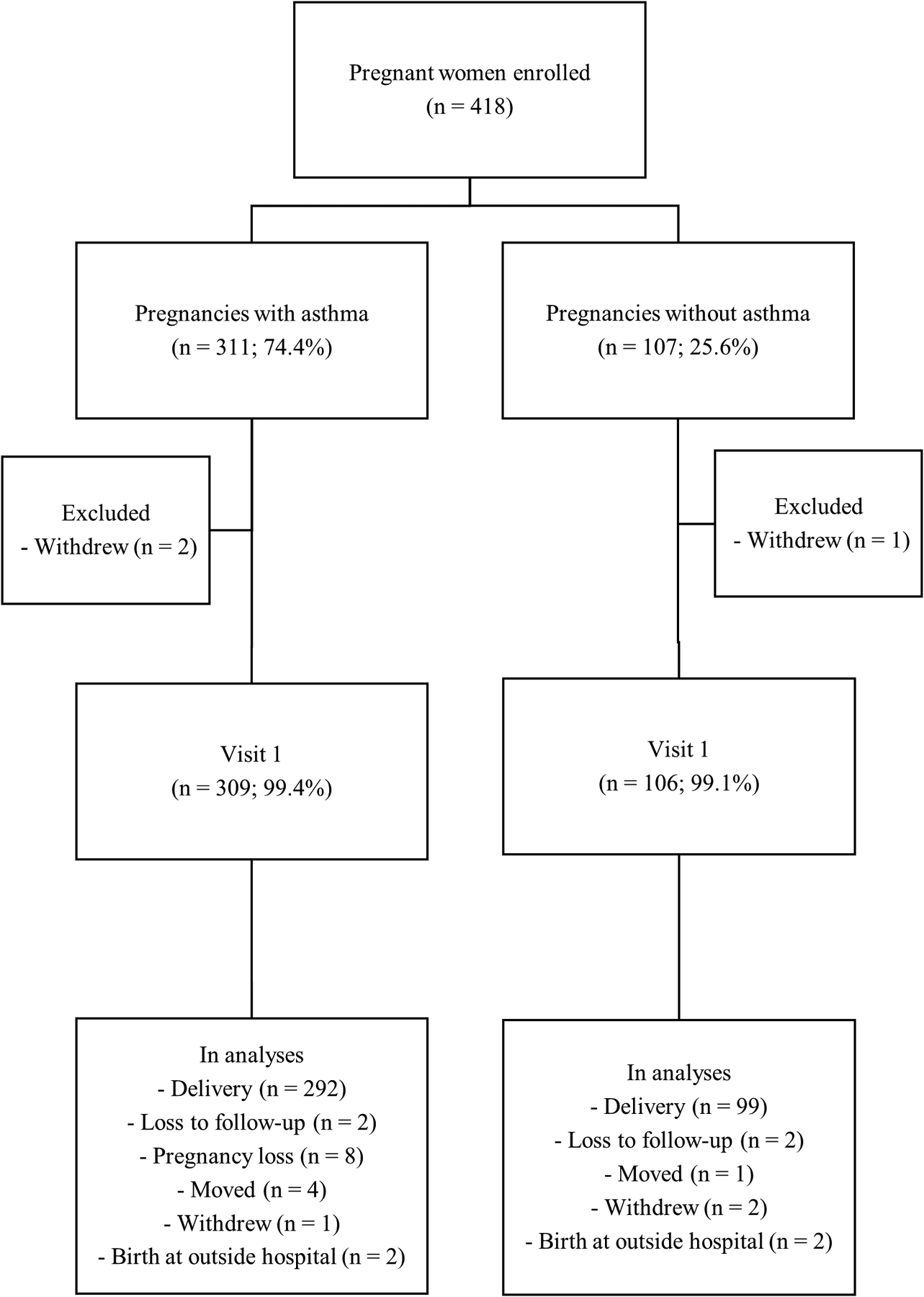
Study population flow chart for participants in the Breathe-Wellbeing, Environment, Lifestyle, and Lung Function Study, USA (2015–2019)
Among the 309 women with asthma, two gestational asthma control trajectories were identified: “same” (n=184; 59.5%) or “worse” (n=124; 40.5%) (Figure 2). One woman was not assigned to a trajectory due to insufficient time in the study (i.e., only one week of data). Women belonging to the “worse” trajectory experienced frequent and stable activity limitation and inhaler use, as well as frequent and increasing nighttime symptoms and respiratory symptoms. Women belonging to the “same” trajectory experienced infrequent and stable activity limitation, inhaler use, and respiratory symptoms, as well as infrequent and increasing (~1 day/gestational week) nighttime symptoms.
Figure 2.
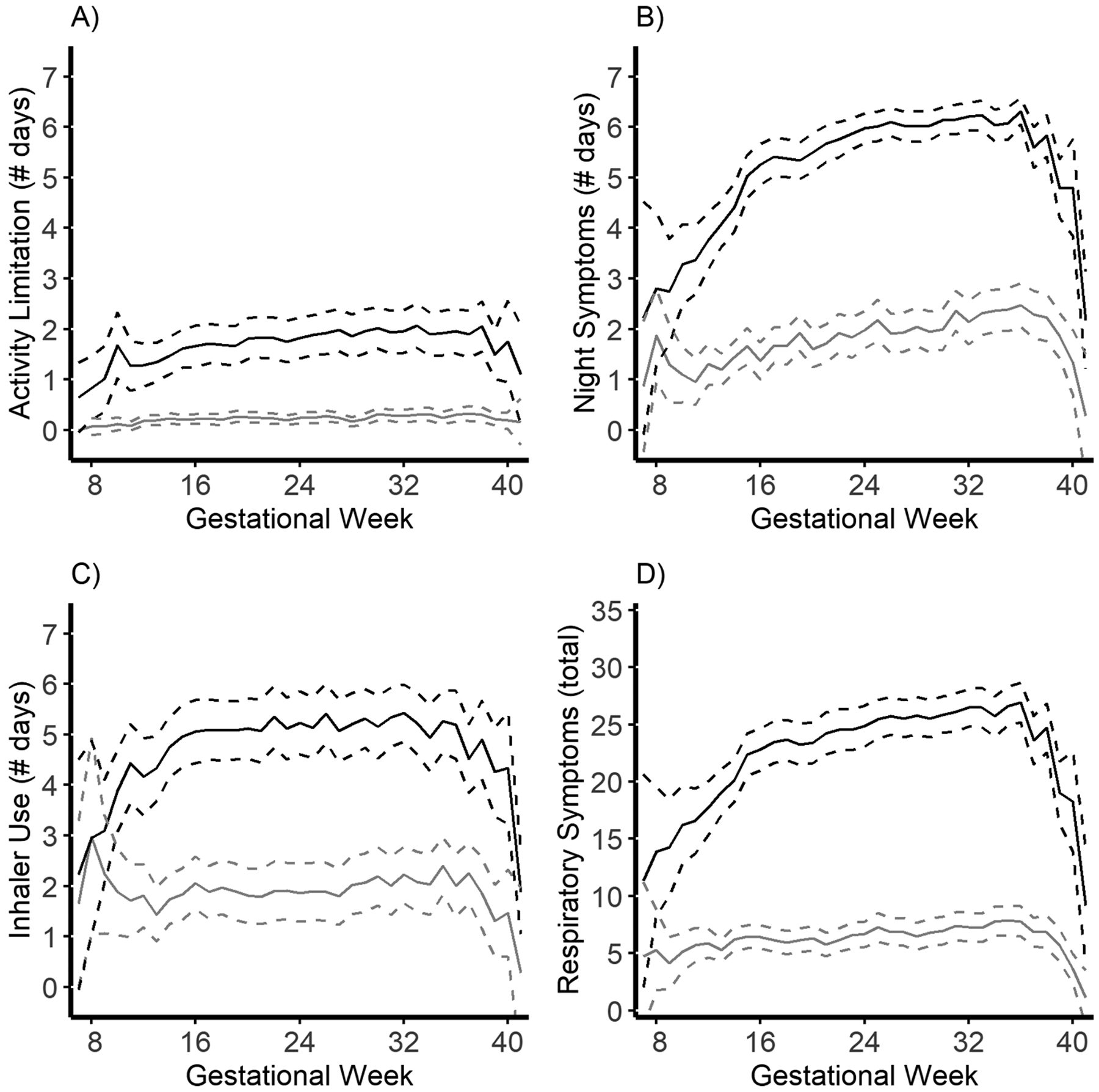
Mean multivariate trajectories of algorithm-assigned asthma control during pregnancy restricted to women with asthma in the Breathe-Wellbeing, Environment, Lifestyle, and Lung Function Study, USA (2015–2019). Panel A) Activity limitation (days/gestational week). Panel B) Night wakening (days/gestational week). Panel C) Inhaler use (days/gestational week). Panel D) Respiratory symptoms (total instances of wheeze, cough, chest tightness, chest pain, and shortness of breath/gestational week). Black lines indicate mean trajectory (dashed lines are 95% confidence intervals (CI)) for women assigned to the “worse” gestational asthma control category. Grey lines indicate mean trajectory (95% CI) for women assigned to the “same” gestational asthma control category.
Table 1 presents sociodemographics by these gestational asthma control trajectories. Compared to women belonging to the “same” gestational asthma control trajectory, women with a “worse” gestational asthma control trajectory were more likely to be younger, non-Hispanic Black, multiparous, to have lower income and educational attainment, higher BMI at baseline, pre-pregnancy hypertension, and to be recruited from the University of Alabama-Birmingham. Table 2 and Table E5 present clinical characteristics and medication use by gestational asthma control trajectories during pregnancy, respectively. Compared to women belonging to the “same” gestational asthma control trajectory, women with a “worse” gestational asthma control trajectory were more likely to have poorly-controlled asthma at baseline, experience poor asthma control in a prior pregnancy, to have exercise-induced asthma and increased pre-pregnancy asthma severity, and were more likely to have had asthma exacerbations (medical encounters and attacks) both pre/early -pregnancy and during pregnancy. There was no statistically significant difference in the trajectory of pulmonary function by gestational asthma control during pregnancy for any of the spirometry measures, but women with worse gestational asthma control had poorer pulmonary function at baseline and across all study visits for FEV1, peak flow, and FEV1/FVC, and across visits 2 and 3 for FVC (Figure 3, Table E6).
Table 1.
Participant sociodemographics associated with gestational asthma control in the Breathe-Wellbeing, Environment, Lifestyle, and Lung Function Study, USA (2015–2019)
| Sociodemographics | Same (N=184) |
Worse (N=124) |
p-valueb | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age (years)a | 30.5 | 5.9 | 28.5 | 5.9 | 0.004 |
| Race/ethnicity | 0.044 | ||||
| Black | 88 | 47.8 | 75 | 60.5 | |
| Hispanic | 16 | 8.7 | 13 | 10.5 | |
| Mixed race/other | 12 | 6.5 | 9 | 7.3 | |
| White | 68 | 37.0 | 27 | 21.8 | |
| Parity | 0.020 | ||||
| Nulliparous | 93 | 50.5 | 46 | 37.1 | |
| Multiparous | 91 | 49.5 | 78 | 62.9 | |
| Household income | |||||
| < $15,000 | 44 | 23.9 | 44 | 35.5 | 0.006 |
| $15,000-$40,000 | 34 | 18.5 | 33 | 26.6 | |
| $40,000-$120,000 | 52 | 28.3 | 27 | 21.8 | |
| >$120,000 | 54 | 29.3 | 20 | 16.1 | |
| Education | |||||
| High school or less | 50 | 27.2 | 49 | 39.5 | <0.001 |
| Associate’s/Some college | 49 | 26.6 | 44 | 35.5 | |
| Bachelor’s degree | 41 | 22.3 | 7 | 5.6 | |
| Master’s or advanced degree | 44 | 23.9 | 24 | 19.4 | |
| Enrollment BMI (kg/m2) | 0.024 | ||||
| BMI < 25 | 70 | 38.0 | 31 | 25.0 | |
| BMI 25–30 | 44 | 23.9 | 28 | 22.6 | |
| BMI ≥ 30 | 70 | 38.0 | 65 | 52.4 | |
| Cigarette smoking pre-pregnancy | 0.23 | ||||
| No | 149 | 81.4 | 94 | 75.8 | |
| Yes | 34 | 18.6 | 30 | 24.2 | |
| Pre-pregnancy diabetes | 0.11 | ||||
| No | 177 | 96.2 | 114 | 91.9 | |
| Yes | 7 | 3.8 | 10 | 8.1 | |
| Pre-pregnancy hypertension | 0.012 | ||||
| No | 172 | 93.5 | 105 | 84.7 | |
| Yes | 12 | 6.5 | 19 | 15.3 | |
| Pre-pregnancy depression | 0.43 | ||||
| No | 118 | 64.1 | 74 | 59.7 | |
| Yes | 66 | 35.9 | 50 | 40.3 | |
| Season of conception | 0.19 | ||||
| Spring | 39 | 21.2 | 37 | 29.8 | |
| Summer | 38 | 20.7 | 30 | 24.2 | |
| Fall | 53 | 28.8 | 28 | 22.6 | |
| Winter | 54 | 29.3 | 29 | 23.4 | |
| Study site | 0.004 | ||||
| Northwestern University | 112 | 60.9 | 55 | 44.4 | |
| University of Alabama-Birmingham | 72 | 39.1 | 69 | 55.6 | |
Mean and standard deviation
p-value from Chi-squared or Fisher’s exact tests, and t-tests
Table 2.
Participant clinical characteristics associated with gestational asthma control in the Breathe-Wellbeing, Environment, Lifestyle, and Lung Function Study, USA (2015–2019)
| Characteristics | Same (N=184) |
Worse (N=124) |
p-valuea | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Baseline asthma control | <0.001 | ||||
| Well-controlled asthma | 112 | 60.9 | 34 | 27.4 | |
| Poorly-controlled asthma | 72 | 39.1 | 90 | 72.6 | |
| Asthma control in prior pregnancy | 0.004 | ||||
| Better | 12 | 6.6 | 4 | 3.2 | |
| Worse | 36 | 19.7 | 44 | 35.5 | |
| Same | 72 | 39.3 | 50 | 40.3 | |
| No prior pregnancies | 63 | 34.4 | 26 | 21.0 | |
| Asthma onset | 0.99 | ||||
| Adult-onset asthma | 41 | 22.5 | 28 | 22.6 | |
| Child-onset asthma | 141 | 77.5 | 96 | 77.4 | |
| Exercise-induced asthma | 0.006 | ||||
| No | 82 | 47.1 | 33 | 26.8 | |
| Yes | 76 | 43.7 | 66 | 53.7 | |
| Don’t exercise | 16 | 9.2 | 24 | 19.5 | |
| Asthma atopy | 0.16 | ||||
| Atopic asthma | 145 | 79.2 | 106 | 85.5 | |
| Non-atopic asthma | 38 | 20.8 | 18 | 14.5 | |
| Pre-pregnancy asthma severityb | 0.012 | ||||
| Mild intermittent | 110 | 59.8 | 60 | 48.4 | |
| Mild persistent | 35 | 19.0 | 27 | 21.8 | |
| Moderate persistent | 30 | 16.3 | 18 | 14.5 | |
| Severe persistent | 9 | 4.9 | 19 | 15.3 | |
| Baseline asthma severityb | 0.15 | ||||
| Mild intermittent | 143 | 77.7 | 86 | 69.4 | |
| Mild persistent | 25 | 13.6 | 17 | 13.7 | |
| Moderate persistent | 13 | 7.1 | 15 | 12.1 | |
| Severe persistent | 3 | 1.6 | 6 | 4.8 | |
| Change in medication usec | 0.20 | ||||
| No change | 127 | 69.0 | 75 | 60.5 | |
| Step down | 50 | 27.2 | 40 | 32.3 | |
| Step up | 7 | 3.8 | 9 | 7.3 | |
| Asthma exacerbations | |||||
| Asthma attacks pre/early pregnancy | 98 | 53.8 | 49 | 39.5 | 0.014 |
| Asthma attacks during pregnancy | 114 | 69.1 | 68 | 58.6 | 0.07 |
| Medical encounters pre/early pregnancy | 126 | 69.2 | 64 | 51.6 | 0.002 |
| Medical encounters during pregnancy | 148 | 89.7 | 84 | 72.4 | <0.001 |
| Oral corticosteroids pre-pregnancy | 7 | 3.8 | 18 | 14.5 | <0.001 |
| Oral corticosteroids during pregnancy | 3 | 1.7 | 3 | 2.4 | 0.69 |
p-value from Chi-squared or Fisher’s Exact tests
Asthma severity defined according to guidelines set out in Table E1 using medication regimens from pre-pregnancy (past 6 months) and baseline (first trimester)
Changes in medication use occurred between pre-pregnancy (past 6 months) and baseline (first trimester). A step up is referred to as any increase in medications that would cause someone to be classified at a higher asthma severity level. A step down is referred to as any decrease in medications that would cause someone to be classified at a lower asthma severity level. No change refers to maintaining the same asthma severity level between pre-pregnancy and baseline. Women in this group could have changed medications, but not to the degree that they would be re-classified
Figure 3.
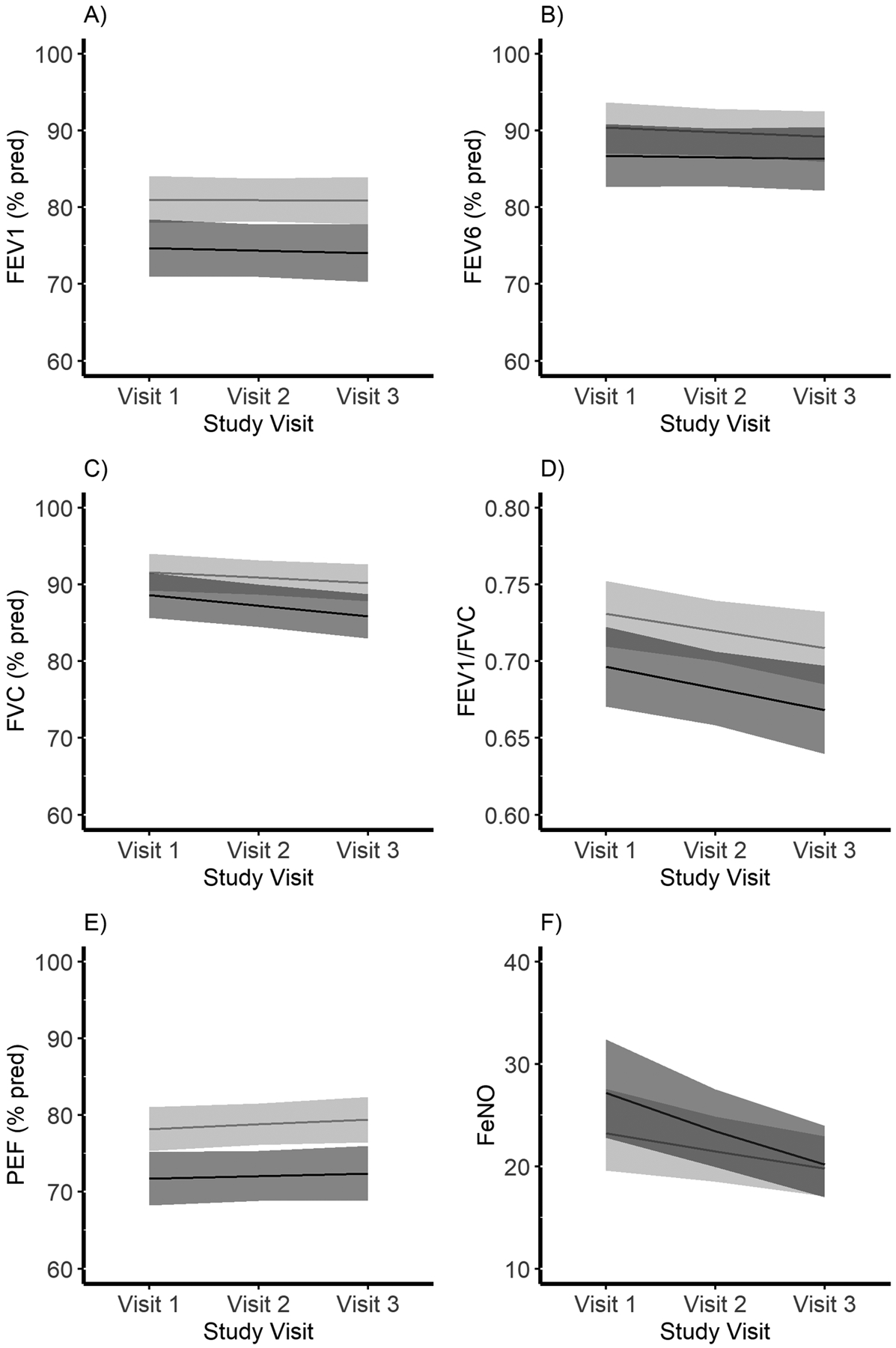
Pulmonary function by gestational asthma control during pregnancy among women with asthma in the Breathe-Wellbeing, Environment, Lifestyle, and Lung Function Study, USA (2015–2019). Panel A) FEV1 (% predicted). Panel B) FEV6 (% predicted). Panel C) FVC (% predicted). Panel D) FEV1/FVC. Panel E) peak flow (% predicted). Panel F) FeNO (ppb). Black lines indicate mean trajectory (95% confidence intervals (CI) shaded) for women assigned to the “worse” gestational asthma control category. Light grey lines indicate mean trajectory (95% CI shaded) for women assigned to the “same” gestational asthma control category
Figure E1 presents results from sensitivity analyses in the full cohort (i.e., including women with and without asthma; n=415). Similar to the primary analyses, two gestational “asthma control” trajectories were identified: “same” (n=272; 66.2%) and “worse” (n=139; 33.8%). Four women were not assigned to a trajectory due to insufficient time in the study (i.e., only one week of data). Almost all women without asthma belonged to the “same” trajectory (n=102; 99% of women without asthma); one woman without asthma was assigned to the “worse” trajectory due to frequent and stable respiratory symptom burden. These results were the same even after excluding inhaler use from the algorithm, suggesting that differences in group assignment by asthma status were not solely driven by inhaler use. Next, we re-ran primary analyses among women without asthma (n=106). Though two groups (“same” and “worse”) were identified, only one woman was assigned to the “worse” trajectory (data not presented).
A summary of findings from additional sensitivity analyses, which confirmed results from our primary analyses, can be found in Table E7 and E2.
DISCUSSION
In this prospective pregnancy cohort, we observe that asthma worsens for 40% of women and stays the same for 60% of women during pregnancy. Contrary to prior research, asthma does not appear to improve (i.e., get better) during pregnancy. Specific symptoms that worsened during pregnancy were respiratory symptoms (i.e., wheeze, cough, shortness of breath, chest pain, chest tightness) and nighttime symptoms, with increases in frequency typically occurring around the second trimester and continuing until around delivery. We identify several sociodemographics and clinical characteristics associated with worse gestational asthma control, including a more severe asthma phenotype pre-pregnancy and poor asthma control in the first trimester. Changes in medication use did not appear to impact gestational asthma control, though further study is needed due to small sample sizes in certain groups (e.g., only 16 women had a step up in medications between pre-pregnancy and baseline). Finally, we note that increases in symptomology were restricted to women with asthma, suggesting that pregnancy itself does not substantially contribute to asthma-like symptom burden. This is the first study to use a data-driven approach to identify and characterize trajectories of gestational asthma control. Given that multiple factors contribute to the determination of asthma control, our multivariate data-driven approach was especially suited to address this aim.
Gestational asthma control has previously been defined and assessed in many ways(2, 7). Older studies rely on self-reported assessments of asthma course that fit within a “better, same, or worse” framework and which compare the non-pregnant to the pregnant state(11, 12). Only a few prior studies have included asthma symptomology during pregnancy(8, 13, 21, 22). Of those that do, symptoms are often grouped according to existing asthma guidelines(23, 24). While clinically relevant, grouping obscures potentially meaningful information regarding symptomology over the course of pregnancy and precludes the possibility of more nuanced changes in asthma being experienced during this time. For example, we observed clinically meaningful increases in night symptoms (~3 days/week) and respiratory symptoms (~5 times/week) for women with worsening asthma during pregnancy, but little change in activity limitation and inhaler use. Two prior studies have reported that the first and last month of pregnancy are characterized by lack of exacerbations and fewer asthma symptoms(10, 25). Due to differences in timing of study entry and exit, as well as increased risk of preterm birth among women with asthma and poor asthma control, the decrease in asthma symptoms observed towards the end of gestation should be interpreted with caution(26–30). Thus, we interpret our trajectories to suggest that respiratory and nighttime symptoms occur with increasing frequency across gestation, with the largest increases occurring around the start of the second trimester.
Asthma control is assessed based on symptomology and exacerbation risk(24). Our assessment of gestational asthma course relied on four domains common across existing guidelines and assessments of asthma control: activity limitation, nighttime symptoms, inhaler use, and respiratory symptoms(17, 18, 23, 24). Asthma exacerbations were not able to be included in the original algorithm but were strongly associated with trajectory assignment, providing further evidence that our algorithm identified women with differing gestational asthma control. Pulmonary function and inflammation are not consistently employed in asthma control guidelines but are a key part of asthma assessment in clinical practice(18, 23, 24). Though we observed overall poorer pulmonary function and increased pulmonary inflammation among women assigned to the “worse” compared to the “same” gestational asthma control trajectory, we did not observe expected changes in pulmonary function and inflammation across pregnancy by trajectory assignment. For example, the mean percent predicted FEV1 for women assigned to the “worse” group was about 6 units lower than the mean percent predicted FEV1 for women assigned to the “same group across all trimesters (study visits). However, given the strong association between our algorithm, exacerbations, and other clinical characteristics (e.g., baseline asthma control from the Asthma Control Test), we are confident that we accurately assessed and captured changes in asthma control during pregnancy.
Changes in asthma control during pregnancy may be due to changes in underlying disease severity, poor medication adherence, medication clearance rates, and/or other exacerbating factors during this time(8, 13, 21, 22). Similar to prior studies, we report that a more severe asthma phenotype is associated with worse gestational asthma control; 68% of women with severe persistent asthma pre-pregnancy experienced worsening asthma control during pregnancy(13, 25, 31). Appropriate treatment and adherence to prescribed treatment is key to preventing poor asthma control during pregnancy, though higher asthma severity may still confer greater risk even with appropriate treatment and adherence(2, 13). During pregnancy, medication cessation is common among women with asthma, with most changes in medication use occurring in the first trimester(32). We observed no differences in gestational asthma control with a step up or step down in medication use between pre-pregnancy and the first trimester, though very few women (5.2%) stepped up during this time. We also observe the same two-trajectory schema in sensitivity analyses examining trajectories for women who did and did not change their medications. Importantly, more active management of asthma may have resulted in improved gestational asthma control. We are unable to address this key literature gap in this observational study. However, this study represents a current obstetric population receiving asthma treatment according to standard clinical practice; as such, findings reflect changes in gestational asthma control experienced in a real-world setting.
Despite prior reports suggesting overlap between pregnancy and asthma symptoms (e.g., shortness of breath, nighttime symptoms), our study finds that symptoms related to pregnancy per se appear not contribute to the trajectory of asthma symptomatology in pregnancy(15, 16). This is an important insight, as some prior studies of the course of asthma during pregnancy have accounted for assumed pregnancy-specific effects on asthma by excluding symptoms thought to be due to pregnancy(13). Our study suggests this should not be necessary. Instead, changes in asthma symptoms during pregnancy were confined to women with asthma. Potential mechanisms influencing the course of asthma during pregnancy include increased pulmonary capillary permeability and upper airway resistance, decreased functional residual capacity, prostaglandin F2 α-mediated bronchoconstriction, placental major basic protein interacting with the pulmonary system, gastroesophageal reflux-induced asthma, stress, dynamic changes in maternal immune status, and elevation of the diaphragm from the expanding uterus in the second and third trimesters(10).
Limitations
This study possesses several notable limitations. First, daily diary data was prone to missingness, the exclusion of which can result in substantial bias(33). Thus, our primary analysis was conducted on an imputed dataset. Moreover, the two-group trajectory assignment was maintained across various sensitivity analyses for missing data. Second, k-means clustering algorithms are prone to limitations inherent to all clustering algorithms as summarized previously(34), as well as limitations inherent to non-model-based algorithms(19). However, despite lacking methods for goodness-of-fit testing, non-model-based algorithms possess a number of strengths (flexible, non-parametric) and sensitivity analyses using group-based multi-trajectory models corroborated our main findings(19). Though k-means clustering can accommodate variables of differing structure over time as well as missingness, we were not able to accommodate data collected outside of the daily diary context (i.e., spirometry or exacerbations collected at study visits) due to sparseness of data collection for those variables. However, we note strong associations between trajectory assignment and pulmonary function and exacerbations. Finally, we could not assess how closely patients’ medication use adhered to that prescribed by their providers, and thus could not assess whether medication was appropriately administered and taken across gestation. However, we do present results by pre-pregnancy (last 6 months) and baseline (first trimester) medications.
Strengths
Among the strengths of this study are the prospectively collected data and multivariate trajectory modelling, which allowed us to provide detailed, clinically relevant information on the course of asthma during pregnancy without relying upon assumed patterns or groupings of asthma control. In particular, we note the use of daily diaries as our primary assessment of asthma control, which has been shown to provide more sensitive estimates of asthma control than retrospective questionnaires like the Asthma Control Test(35). Second, we are one of the only studies of asthma status during pregnancy to include a comparison group of women without asthma. Prior studies with a comparison group of women without asthma have had small sample sizes and variable results; most have focused on only one aspect of asthma course: pulmonary function(10). Finally, we performed a large number of sensitivity analyses which confirm our main findings.
Conclusion
In this prospective pregnancy cohort of women with and without asthma, 40% of women with asthma experienced worsening asthma control during pregnancy. Given the association between worsening gestational asthma control and adverse pregnancy outcomes(2, 7), it is imperative that further studies be performed to identify mechanisms underlying worsening control and to elucidate interventions to enhance stability or improvement in asthma control during pregnancy. Several sociodemographic and clinical characteristics – including poorer pre-pregnancy asthma control – were associated with worsening gestational asthma control. These at-risk patients may require closer symptom monitoring as well as additional counselling in trigger identification and avoidance during pregnancy.
Supplementary Material
HIGHLIGHTS.
What is already known about the topic? Convention holds that asthma control follows a rule of thirds during pregnancy: a third get better, a third get worse, and the rest stay the same.
What does this article add to our knowledge? Using an unsupervised machine learning approach, we show that asthma control does not improve during pregnancy. Rather, ~60% of women experience no change in asthma control during pregnancy.
How does this study impact current management guidelines? In this contemporary pregnancy cohort of women with asthma managed according to standard practice, ~40% of women may have benefitted from more active asthma management.
Funding Acknowledgements:
This work was supported by the National Institutes of Health’s Intramural Research Program at the Eunice Kennedy Shriver National Institute of Child Health and Human Development (clinical site contracts HHSN275201300013C to Northwestern University, HHSN275201300014C to the University of Alabama at Birmingham; and the Emmes Company for the Data Coordinating Center HHSN275201300026I, HHSN27500001, HHSN275000017). Funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Abbreviations:
- BMI
body mass index
- B-WELL-Mom
Breathe - Wellbeing, Environment, Lifestyle, and Lung function
- FeNO
fractional exhaled Nitric Oxide
- FEV1
forced expiratory volume in 1 second
- FEV6
forced expiratory volume in 6 seconds
- FVC
forced vital capacity
- kg
kilograms
- m
meter
Footnotes
Conflict of Interest Data: The authors have no conflicts of interest relevant to disclose.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2018 National Health Interview Survey (NHIS) Data 2018 [updated December 17, 2019. Available from: https://www.cdc.gov/asthma/nhis/2018/table4-1.htm.
- 2.Murphy VE, Jensen ME, Gibson PG. Asthma during Pregnancy: Exacerbations, Management, and Health Outcomes for Mother and Infant. Semin Respir Crit Care Med. 2017;38(2):160–73. [DOI] [PubMed] [Google Scholar]
- 3.Murphy VE, Jensen ME, Powell H, Gibson PG. Influence of Maternal Body Mass Index and Macrophage Activation on Asthma Exacerbations in Pregnancy. J Allergy Clin Immunol Pract. 2017;5(4):981–7 e1. [DOI] [PubMed] [Google Scholar]
- 4.Flores KF, Robledo CA, Hwang BS, Leishear K, Laughon Grantz K, Mendola P. Does maternal asthma contribute to racial/ethnic disparities in obstetrical and neonatal complications? Ann Epidemiol. 2015;25(6):392–7 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandoli G, Palmsten K, Forbess Smith CJ, Chambers CD. A Review of Systemic Corticosteroid Use in Pregnancy and the Risk of Select Pregnancy and Birth Outcomes. Rheum Dis Clin North Am. 2017;43(3):489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemppainen M, Lahesmaa-Korpinen AM, Kauppi P, Virtanen M, Virtanen SM, Karikoski R, et al. Maternal asthma is associated with increased risk of perinatal mortality. PLoS One. 2018;13(5):e0197593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labor S, Dalbello Tir AM, Plavec D, Juric I, Roglic M, Pavkov Vukelic J, et al. What is safe enough - asthma in pregnancy - a review of current literature and recommendations. Asthma Res Pract. 2018;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schatz M, Harden K, Forsythe A, Chilingar L, Hoffman C, Sperling W, et al. The course of asthma during pregnancy, post partum, and with successive pregnancies: a prospective analysis. J Allergy Clin Immunol. 1988;81(3):509–17. [PubMed] [Google Scholar]
- 9.Juniper E, Newhouse M. Effect of pregnancy on asthma: systematic review and meta-analysis. Schatz M, Zeiger R, Claman H, editors. New York: Marcel Dekker; 1993. 1993. [Google Scholar]
- 10.Gluck JC. The change of asthma course during pregnancy. Clin Rev Allergy Immunol. 2004;26(3):171–80. [DOI] [PubMed] [Google Scholar]
- 11.Juniper EF, Daniel EE, Roberts RS, Kline PA, Hargreave FE, Newhouse MT. Improvement in airway responsiveness and asthma severity during pregnancy. A prospective study. The American review of respiratory disease. 1989;140(4):924–31. [DOI] [PubMed] [Google Scholar]
- 12.Grosso A, Locatelli F, Gini E, Albicini F, Tirelli C, Cerveri I, et al. The course of asthma during pregnancy in a recent, multicase-control study on respiratory health. Allergy Asthma Clin Immunol. 2018;14:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belanger K, Hellenbrand ME, Holford TR, Bracken M. Effect of pregnancy on maternal asthma symptoms and medication use. Obstet Gynecol. 2010;115(3):559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kircher S, Schatz M, Long L. Variables affecting asthma course during pregnancy. Ann Allergy Asthma Immunol. 2002;89(5):463–6. [DOI] [PubMed] [Google Scholar]
- 15.Lee SY, Chien DK, Huang CH, Shih SC, Lee WC, Chang WH. Dyspnea in pregnancy. (1875–6263 (Electronic)). [DOI] [PubMed]
- 16.Varnier NA, Chwah S, Miller T, Pettit F, Brown M, Rees D, et al. All that wheezes is not asthma: A cautionary case study of shortness of breath in pregnancy. (1753–495X (Print)). [DOI] [PMC free article] [PubMed]
- 17.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. [DOI] [PubMed] [Google Scholar]
- 18.ACOG Committee on Practice Bulletins. ACOG Practice Bulletin: Clinical Management Guidelines for Obstetrician-Gynecolosits Number 90. Obstet Gynecol. February 2008;111:457–64. [DOI] [PubMed] [Google Scholar]
- 19.Genolini C, Pingault JB, Driss T, Cote S, Tremblay RE, Vitaro F, et al. KmL3D: a non-parametric algorithm for clustering joint trajectories. Comput Methods Programs Biomed. 2013;109(1):104–11. [DOI] [PubMed] [Google Scholar]
- 20.Nagin DS, Jones BL, Passos VL, Tremblay RE. Group-based multi-trajectory modeling. Stat Methods Med Res. 2018;27(7):2015–23. [DOI] [PubMed] [Google Scholar]
- 21.Schatz M, Dombrowski MP, Wise R, Thom EA, Landon M, Mabie W, et al. Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol. 2003;112(2):283–8. [DOI] [PubMed] [Google Scholar]
- 22.Sawicki E, Stewart K, Wong S, Paul E, Leung L, George J. Management of asthma by pregnant women attending an Australian maternity hospital. The Australian & New Zealand journal of obstetrics & gynaecology. 2012;52(2):183–8. [DOI] [PubMed] [Google Scholar]
- 23.National Asthma Educatio and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Heart, Lung, and Blood Institute; 2007. [Google Scholar]
- 24.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2020.
- 25.Schatz M Interrelationships between asthma and pregnancy: a literature review. J Allergy Clin Immunol. 1999;103(2 Pt 2):S330–6. [DOI] [PubMed] [Google Scholar]
- 26.Baghlaf H, Spence AR, Czuzoj-Shulman N, Abenhaim HA. Pregnancy outcomes among women with asthma. J Matern Fetal Neonatal Med. 2019;32(8):1325–31. [DOI] [PubMed] [Google Scholar]
- 27.Enriquez R, Griffin MR, Carroll KN, Wu P, Cooper WO, Gebretsadik T, et al. Effect of maternal asthma and asthma control on pregnancy and perinatal outcomes. J Allergy Clin Immunol. 2007;120(3):625–30. [DOI] [PubMed] [Google Scholar]
- 28.Mendola P, Laughon SK, Mannisto TI, Leishear K, Reddy UM, Chen Z, et al. Obstetric complications among US women with asthma. Am J Obstet Gynecol. 2013;208(2):127. e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendola P, Mannisto TI, Leishear K, Reddy UM, Chen Z, Laughon SK. Neonatal health of infants born to mothers with asthma. J Allergy Clin Immunol. 2014;133(1):85–90. e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy VE, Schatz M. Asthma in pregnancy: A hit for two. European Respiratory Review. 2014;23(131):64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy VE, Gibson P, Talbot PI, Clifton VL. Severe asthma exacerbations during pregnancy. Obstet Gynecol. 2005;106(5 Pt 1):1046–54. [DOI] [PubMed] [Google Scholar]
- 32.Enriquez R, Wu P, Griffin MR, Gebretsadik T, Shintani A, Mitchel E, et al. Cessation of asthma medication in early pregnancy. Am J Obstet Gynecol. 2006;195(1):149–53. [DOI] [PubMed] [Google Scholar]
- 33.Sundermann AC, Hartmann KE, Jones SH, Torstenson ES, Velez Edwards DR. Validation of maternal recall of early pregnancy medication exposure using prospective diary data. Ann Epidemiol. 2017;27(2):135–9. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aghabozorgi S, Seyed Shirkhorshidi A, Ying Wah T. Time-series clustering – A decade review. Information Systems. 2015;53:16–38. [Google Scholar]
- 35.Okupa AY, Sorkness CA, Mauger DT, Jackson DJ, Lemanske RF Jr. Daily diaries vs retrospective questionnaires to assess asthma control and therapeutic responses in asthma clinical trials: is participant burden worth the effort? Chest. 2013;143(4):993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


