Abstract
A novel CuBr-catalyzed hydroxytrifluoromethylation reaction was investigated. Substituted 3-benzylidene-2-arylisoindolin-1-ones was reacted with sodium trifluoromethanesulfinate to afford substituted-3-hydroxy-2-aryl-3-(2,2,2-trifluoro-1-arylethyl)isoindolin-1-one. The reaction proceeded at 25 °C in air atmosphere in the absence of base and ligands. Our results indicate that trifluoromethyl free radical tends to attack a double bond rather than aryl in this reaction.
A novel CuBr-catalyzed hydroxytrifluoromethylation reaction was investigated. Substituted 3-benzylidene-2-arylisoindolin-1-ones were reacted with sodium trifluoromethanesulfinate to afford substituted-3-hydroxy-2-aryl-3-(2,2,2-trifluoro-1-arylethyl)isoindolin-1-one.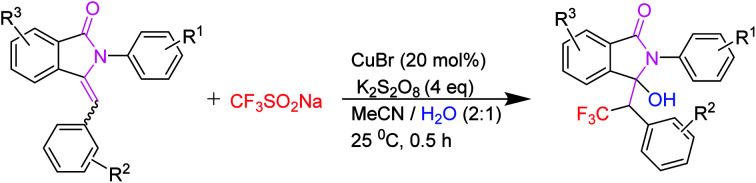
Introduction
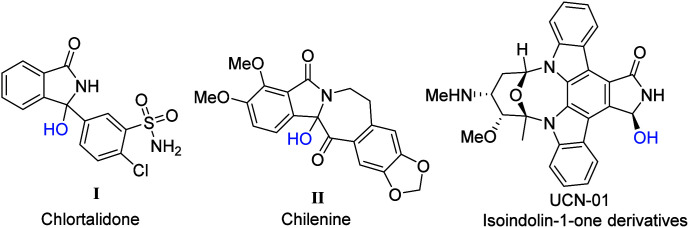
Isoindoles are a series of notable nitrogen-containing compounds known for their bioactivity in nature.1 In particular, 3-hydroxyisoindolin-1-ones such as I and II are the core structural motifs of several compounds of medicinal value (Fig. 1). 3-Hydroxy isoindolin-1-ones are known for their use as diuretic and anticancer drugs.2 As substituted 3-benzylidene-2-arylisoindolin-1-ones have double bonds, we try to find a catalytic system for direct hydroxytrifluoromethylation of substituted 3-benzylidene-2-arylisoindolin-1-one.
Fig. 1. Bioactive and drug value compounds containing 3-hydroxyisoindolin-1-one motifs.
Hydroxytrifluoromethylation of organic molecules has become a research focus in the field of organic synthesis for its unique biological activities.3 In 1991, Langlois and co-workers reported the first use of CF3SO2Na as the trifluoromethyl radical source.4 Since then, a series of trifluoromethylation of olefins by using Langlois reagent has been published in the last twenty years.5 In these reactions, CF3SO2Na was excited by single electron oxidations to generate CF3 free radicals. The oxidative partners included TBHP,6 K2S2O8,7 PhI(OAc)2,8 DTBP,9 I2O5,10 metal (Cu, Mn),11 photoinducers12 and so on. There have only been several examples involving hydroxytrifluoromethylation of olefins to afford useful β-trifluoromethyl alcohols.13 Moreover, there were also studies of the metal-free-catalyzed hydroxytrifluoromethylation reactions of styrenes.14 Recently, manganese-catalyzed direct hydroxytrifluoromethylation reaction of styrene derivatives has been established.15 On the other hand, the CuCF3 system has been used for the synthesis of direct hydroxytrifluoromethylation reaction.16 Visible light promoted C–F functionalization has been developed under mild reaction condition.17 Until now, hydroxytrifluoromethylation of enamides has not been reported. As part of our research on the transition metal-catalyzed free radical reaction of substituted 3-benzylidene-2-arylisoindolin-1-one,18 this communication reports the first example of hydroxytrifluoromethylation reaction of 3-benzylidene-2-arylisoindolin-1-one (the special structure of enamide) with sodium trifluoromethanesulfinate catalyzed by CuBr in the presence of K2S2O8 (Scheme 1).
Scheme 1.
Results and discussion
When the model reaction of 3-benzylidene-2-phenylisoindolin-1-one (1a) with sodium trifluoromethanesulfinate (2) was performed in CH3CN/H2O in the presence of oxidants such as TBHP, DTBP, Mn(OAc)3 and PhI(OAc)2, no desired products were obtained (Table 1, entries 1, 2, 3 and 4). After the addition of K2S2O8 (4 eq.), the reaction proceeded smoothly to afford the desired product, 3-hydroxy-2-phenyl-3-(2,2,2-trifluoro-1-phenylethyl)isoindolin-1-one (3a) in 48% yield (Table 1, entry 5). Further investigation of copper catalysts, the yield of 3a was improved to 72% when we used CuBr as the catalyst (Table 1, entries 9). On the other hand, when we use FeCl3 in place of CuBr, the reaction afforded the desired product in a lower yield (Table 1, entry 12). By screening polar mixed solvents such as DMSO/H2O DMF/H2O, THF/H2O, acetone/H2O, and a representative nonpolar solvent, toluene (Table 1, entries 15–19), we found that CH3CN/H2O (2 : 1) works best for the reaction. Apart from the above-mentioned factors, the effects of catalyst loading, reaction temperature and time were also investigated, and the optimal reaction conditions were determined to be room temperature reaction for 0.5 h in air atmosphere, with the addition of 20 mol% CuBr as catalyst, K2S2O8 as single electron oxidation regent and CH3CN/H2O as solvent (Table 1, entries 20–27).
Optimization of the reaction conditionsa.

| |||||
|---|---|---|---|---|---|
| Entry | Catalyst (%) | Oxidant | Temperature | Solvent (66.7%) | Yieldb |
| 1 | TBHP | 25 °C | CH3CN | N.D | |
| 2 | DTBP | 25 °C | CH3CN | N.D | |
| 3 | Mn(OAc)3 | 25 °C | CH3CN | N.D | |
| 4 | PhI(OAc)2 | 25 °C | CH3CN | N.D | |
| 5 | K2S2O8 | 25 °C | CH3CN | 48% | |
| 6 | CuO (20) | K2S2O8 | 25 °C | CH3CN | 30% |
| 7 | Cu(OAc)2 (20) | K2S2O8 | 25 °C | CH3CN | 55% |
| 8 | CuCl (20) | K2S2O8 | 25 °C | CH3CN | 60% |
| 9 | CuBr (20) | K2S2O8 | 25 °C | CH3CN | 72% |
| 10 | CuI (20) | K2S2O8 | 25 °C | CH3CN | 28% |
| 11 | CuBr2 (20) | K2S2O8 | 25 °C | CH3CN | 50% |
| 12 | FeCl3 (20) | K2S2O8 | 25 °C | CH3CN | 45% |
| 13 | Ag2CO3 (20) | K2S2O8 | 25 °C | CH3CN | 48% |
| 14 | CuBr (20) | K2S2O8 | 25 °C | CH3CN | 48% |
| 15 | CuBr (20) | K2S2O8 | 25 °C | DMSO | 6% |
| 16 | CuBr (20) | K2S2O8 | 25 °C | DMF | 10% |
| 17 | CuBr (20) | K2S2O8 | 25 °C | THF | 37% |
| 18 | CuBr (20) | K2S2O8 | 25 °C | Acetone | 55% |
| 19 | CuBr (20) | K2S2O8 | 25 °C | Toluene | 0% |
| 20 | CuBr (20) | K2S2O8 | 50 °C | CH3CN | 65% |
| 21 | CuBr (20) | K2S2O8 | 80 °C | CH3CN | 60% |
| 22 | CuBr (20) | K2S2O8 | 10 °C | CH3CN | 66% |
| 23c | CuBr (20) | K2S2O8 | 25 °C | CH3CN | 35% |
| 23d | CuBr (20) | K2S2O8 | 25 °C | CH3CN | 28% |
| 23e | CuBr (20) | K2S2O8 | 25 °C | CH3CN | 70% |
| 24f | CuBr (20) | K2S2O8 | 25 °C | CH3CN | 46% |
| 25g | CuBr (20) | K2S2O8 | 25 °C | CH3CN | 70% |
| 26h | CuBr (20) | K2S2O8 | 25 °C | CH3CN | 70% |
| 27 | CuBr (10) | K2S2O8 | 25 °C | CH3CN | 60% |
Reaction conditions: 1a (1 mmol), 2, (3 mmol), CuBr, (0.2 mmol), K2S2O8 (4 mmol), solvent (10 ml), at 25 °C in air atmosphere, 30 min.
Yields are given for isolated products.
CH3CN/H2O = 5/1.
CH3CN/H2O = 1/1.
K2S2O8 (5 mmol) was added.
K2S2O8 (3 mmol) was added.
1 h.
In argon atmosphere.
With the promising results obtained in the model reaction, we subsequently examined the substrate scope of 3-benzylidene-2-arylisoindolin-1-one under the optimized reaction conditions (20 mol% CuBr as catalyst, and K2S2O8 as oxidant in CH3CN/H2O (2 : 1) at 25 °C, for 0.5 h in air atmosphere).
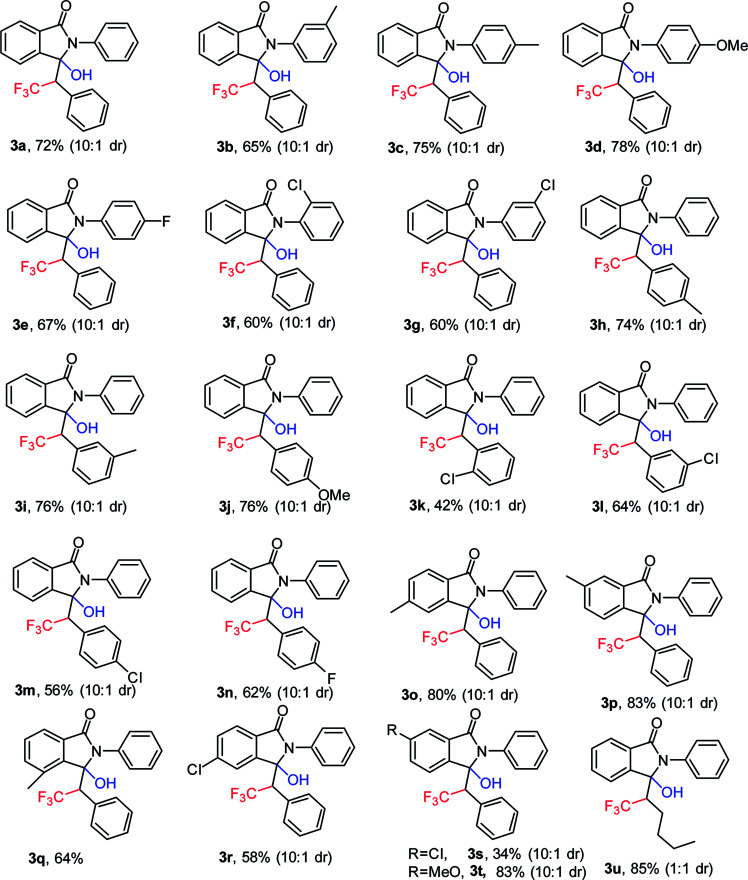
As shown in Table 2, electron-donating substituents such as methyl and methoxy groups on the aryl ring of substituted 3-benzylidene-2-arylisoindolin-1-one (1) facilitated the reaction to afford the hydroxytrifluoromethylation products (3) in moderate to good yields (Table 2, 65–83%, 3a–3d, 3h–3j, 3o–3p, and 3t). On the contrary, election-withdrawing groups such as F and Cl were unfavorable for the reaction and led to lower yields (Table 2, 34–67%, 3e–3g, 3k–3n and 3r–3s). We also found that when the substrate was 3-pentylidene-2-phenylisoindolin-1-one, the target product (3u) was in 85% yield but diastereomeric ratio is 1 : 1.
Scope studies of 3-hydroxy-2-phenyl-3-(2,2,2-trifluoro-1-phenylethyl)isoindolin-1-onea.

|
|---|
|
Reaction conditions: 1 (1 mmol), 2 (3 mmol), CuBr, (0.2 mmol), K2S2O8 (4 mmol), CH3CN/H2O 2 : 1 (10 ml), at 25 °C in air atmosphere, 30 min. Yield of isolated products are given.
In order to understand the reaction mechanism, following control experiments were carried out. We repeated the reaction in the presence of radical quencher 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO) and none of 3a was obtained (Scheme 2a). The result suggested that free radical were probably generated during the reaction. Furthermore, 3a was also not detected when the reaction was performed with the addition of butylated hydroxytoluene (BHT, 3.0 equiv.) under the standard conditions (Scheme 2b). Trifluoromethylation products was obtained when 1,1-diyldibenzene and Langlois reagent were carried out in standard condition (Scheme 2c). On the other hand, neither aryl amine nor benzylamine substrate produced the ortho-position C–H activated products (Scheme 2d).18 These results indicated that the reaction is only suitable for enamine substrates which have enough electron cloud density.
Scheme 2. Control experiments.
As the hydroxytrifluoromethylation always took place under argon atmosphere in the above experiments, we wondered whether the reaction would proceed if isotopically labelled molecular H218O was used. Hence, we did further reactions (Scheme 2e). Surprisingly, the corresponding 18O-containing product 4a was obtained in 70%. These results further indicated that the oxygen source of this reaction is derived from H2O rather than oxygen gas.
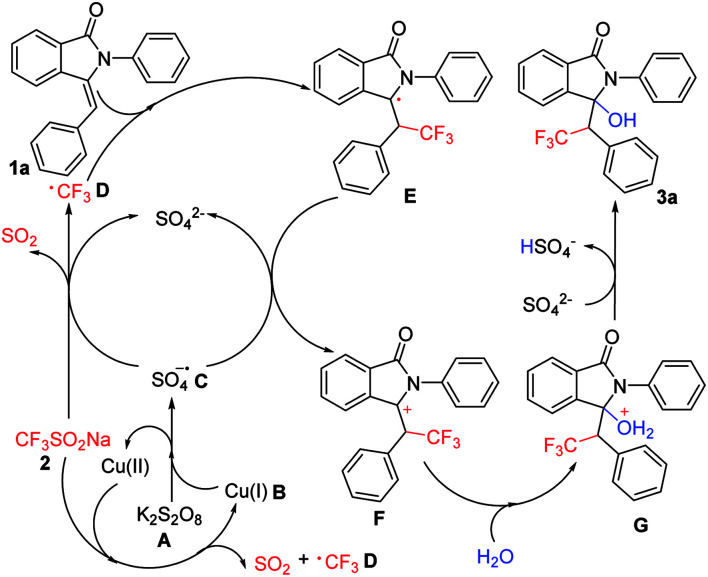
On the basis of the mechanistic studies and experimental results, a plausible mechanism is proposed in Scheme 3.
Scheme 3. Proposed reaction mechanism.
Initially, the K2S2O8 (A) was excited by Cu(i) (B) to generate the intermediate SO4 radical anions (C), which then reacted with CF3SO2Na (2) to form trifluoromethyl free radical (D). D underwent addition with substrate (1a) to form key radical intermediate E. Thereafter, the radical intermediate E was oxidized by SO4 radical anions (C) which can regenerate SO42− to produce the cation intermediate F. Due to the presence of H2O, the cation intermediate F underwent nucleophilic addition to generated the corresponding intermediate G. The cation intermediate G underwent removing protons to generated the corresponding product 3a and HSO4−. Finally, Cu(ii) was reduced to Cu(i) by CF3SO2Na (2) to complete the catalytic cycle.
Conclusions
In summary, we have developed a novel catalytic system for direct hydroxytrifluoromethylation of substituted 3-benzylidene-2-arylisoindolin-1-ones via a radical pathway. The reaction has a high regioselectivity as the CF3 free radical is prone to attacking a double bond rather than the aryl. The method has a broad scope and offers a good yield. The corresponding products are potentially useful in drug discovery.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We gratefully acknowledge financial support from the Prospective Study Program of Jiangsu (BY2015039-08), the Project of Scientific and Technologic Infrastructure of Suzhou (SZS201708), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra04088e
Notes and references
- Mailyan A. K. Eickhoff J. A. Minakova A. S. Gu Z. Lu P. Zakarian A. Chem. Rev. 2016;116:4441. doi: 10.1021/acs.chemrev.5b00712. [DOI] [PubMed] [Google Scholar]
- (a) Fang F.-G. Danishefsky S. J. Tetrahedron Lett. 1989;30:2747. doi: 10.1016/S0040-4039(00)99115-9. [DOI] [Google Scholar]; (b) Fajardo V. Elango V. Cassels B. K. Shamma M. Tetrahedron Lett. 1982;23:39. doi: 10.1016/S0040-4039(00)97526-9. [DOI] [Google Scholar]
- (a) Ojima I., Fluorine in Medicinal Chemistry and Chemical Biology, Wiley-Blackwell, Chichester, 2009 [Google Scholar]; (b) Shimizu M. Hiyama T. Angew. Chem., Int. Ed. 2005;44:214. doi: 10.1002/anie.200460441. [DOI] [PubMed] [Google Scholar]; (c) Schlosser M. Angew. Chem., Int. Ed. 2006;45:5432. doi: 10.1002/anie.200600449. [DOI] [PubMed] [Google Scholar]; (d) Müller K. Faeh C. Diederich F. Science. 2007;317:1881. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]; (e) Purser S. Moore P. R. Swallow S. Gouverneur V. Chem. Soc. Rev. 2008;37:320. doi: 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]; (f) Wang J. Sánchez-Roselló M. Aceña J. L. del Pozo C. Sorochinsky A. E. Fustero S. Soloshonok V. A. Liu H. Chem. Rev. 2014;114:2432. doi: 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]
- (a) Langlois B. R. Laurent E. Roidot N. Tetrahedron Lett. 1992;33:1291. doi: 10.1016/S0040-4039(00)91604-6. [DOI] [Google Scholar]; (b) Clavel J.-L. Langlois B. Laurent E. Roidot N. Phosphorus, Sulfur Silicon Relat. Elem. 1991;59:169. doi: 10.1080/10426509108045716. [DOI] [Google Scholar]; (c) Langlois B. R. Laurent E. Roidot N. Tetrahedron Lett. 1991;32:7525. doi: 10.1016/0040-4039(91)80524-A. [DOI] [Google Scholar]
- For recent examples of difunctionalizing trifluoromethylation of olefins by using Langlois reagent, see:; (a) Zhang H.-Y. Huo W. Ge C. Zhao J. Zhang Y. Synlett. 2017;28:962. doi: 10.1055/s-0036-1588400. [DOI] [Google Scholar]; (b) Yatham V. R. Shen Y. Martin R. Angew. Chem., Int. Ed. 2017;56:10915. doi: 10.1002/anie.201706263. [DOI] [PubMed] [Google Scholar]; (c) Wu Z. Wang D. Liu Y. Huan L. Zhu C. J. Am. Chem. Soc. 2017;139:1388. doi: 10.1021/jacs.6b11234. [DOI] [PubMed] [Google Scholar]; (d) Liu Z.-Q. Liu D. J. Org. Chem. 2017;82:1649. doi: 10.1021/acs.joc.6b02812. [DOI] [PubMed] [Google Scholar]; (e) Kong W. An H. Song Q.-L. Chem. Commun. 2017;53:8968. doi: 10.1039/C7CC03520A. [DOI] [PubMed] [Google Scholar]; (f) Jana S. Verma A. Kadu R. Kumar S. Chem. Sci. 2017;8:6633. doi: 10.1039/C7SC02556D. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Fang J. Wang Z.-K. Wu S.-W. Shen W.-G. Ao G.-Z. Liu F. Chem. Commun. 2017;53:7638. doi: 10.1039/C7CC01903C. [DOI] [PubMed] [Google Scholar]; (h) Zhu L. Wang L.-S. Li B. Fu B. Zhang C.-P. Li W. Chem. Commun. 2016;52:6371. doi: 10.1039/C6CC01944G. [DOI] [PubMed] [Google Scholar]; (i) Yu X.-L. Chen J.-R. Chen D.-Z. Xiao W.-J. Chem. Commun. 2016;52:8275. doi: 10.1039/C6CC03335K. [DOI] [PubMed] [Google Scholar]; (j) Yang B. Xu X.-H. Qing F.-L. Chin. J. Chem. 2016;34:465. doi: 10.1002/cjoc.201500641. [DOI] [Google Scholar]; (k) Li B. Fan D. Yang C. Xia W.-J. Org. Biomol. Chem. 2016;14:5293. doi: 10.1039/C6OB00912C. [DOI] [PubMed] [Google Scholar]; (l) Fu H. Wang S.-S. Li Y.-M. Adv. Synth. Catal. 2016;358:3616. doi: 10.1002/adsc.201600693. [DOI] [Google Scholar]; (m) Yang B. Xu X.-H. Qing F.-L. Qing F.-L. Org. Lett. 2015;17:1906. doi: 10.1021/acs.orglett.5b00601. [DOI] [PubMed] [Google Scholar]; (n) Zhang L. Li Z. Liu Z.-Q. Org. Lett. 2014;16:3688. doi: 10.1021/ol5014747. [DOI] [PubMed] [Google Scholar]; (o) Yang F. Klumphu P. Liang Y.-M. Lipshutz B. H. Chem. Commun. 2014;50:936. doi: 10.1039/C3CC48131J. [DOI] [PubMed] [Google Scholar]; (p) Wei W. Wen J. Yang D. Liu X. Guo M. Dong R. Wang H. J. Org. Chem. 2014;79:4225. doi: 10.1021/jo500515x. [DOI] [PubMed] [Google Scholar]; (q) Lu Y. Li Y. Zhang R. Jin K. Duan C.-Y. J. Fluorine Chem. 2014;161:128. doi: 10.1016/j.jfluchem.2014.01.020. [DOI] [Google Scholar]; (r) Wilger D. J. Gesmundo N. J. Nicewicz D. A. Chem. Sci. 2013;4:3160. doi: 10.1039/C3SC51209F. [DOI] [Google Scholar]; (s) Jiang X.-Y. Qing F.-L. Angew. Chem., Int. Ed. 2013;52:14177. doi: 10.1002/anie.201307595. [DOI] [PubMed] [Google Scholar]; (t) Hang Z. Li Z. Liu Z. Q. Org. Lett. 2014;16:3648. doi: 10.1021/ol501380e. [DOI] [PubMed] [Google Scholar]; (u) Lefebvre Q. Synlett. 2017;28:19. doi: 10.1055/s-0036-1588643. [DOI] [Google Scholar]; (v) Zhang C. Adv. Synth. Catal. 2014;356:2895. doi: 10.1002/adsc.201400370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For recent examples of TBHP activate Langlois reagent to liberate CF3 radicals, see:; (a) van d. W. A. Hribersek M. Selander N. Org. Lett. 2017;19:2374. doi: 10.1021/acs.orglett.7b00908. [DOI] [PubMed] [Google Scholar]; (b) Konik Y. A. Kudrjashova M. Konrad N. Kaabel S. Jarving I. Lopp M. Kananovich D. G. Org. Biomol. Chem. 2017;15:4635. doi: 10.1039/C7OB00680B. [DOI] [PubMed] [Google Scholar]; (c) Jiang H. Huang W. Yu Y. Yi S. Li J. Wu W.-Q. Chem. Commun. 2017;53:7473. doi: 10.1039/C7CC03125D. [DOI] [PubMed] [Google Scholar]; (d) Simon R. C. Busto E. Resch V. Kroutil W. Richter N. Houk K. N. Nat. Commun. 2016;7:13323. doi: 10.1038/ncomms13323. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zhang X. Huang P. Li Y. Duan C. Org. Biomol. Chem. 2015;13:10917. doi: 10.1039/C5OB01516B. [DOI] [PubMed] [Google Scholar]; (f) Zhang K. Xu X.-H. Qing F.-L. J. Org. Chem. 2015;80:7658. doi: 10.1021/acs.joc.5b01295. [DOI] [PubMed] [Google Scholar]; (g) Monir K. Bagdi A. K. Ghosh M. Hajra A. J. Org. Chem. 2015;80:1332. doi: 10.1021/jo502928e. [DOI] [PubMed] [Google Scholar]; (h) Liu Y.-R. Tu H.-Y. Zhang X.-G. Synthesis. 2015;47:3460. doi: 10.1055/s-0034-1378810. [DOI] [Google Scholar]; (i) Hua H.-L. He Y.-T. Qiu Y.-F. Li Y.-X. Song B. Gao P. Song X.-R. Guo D.-H. Liu X.-Y. Liang Y.-M. Chem.–Eur. J. 2015;21:1468. doi: 10.1002/chem.201405672. [DOI] [PubMed] [Google Scholar]; (j) Yin J. Li Y. Zhang R. Jin K. Duan C. Synthesis. 2014;46:607. doi: 10.1055/s-0034-1378358. [DOI] [Google Scholar]; (k) Wu M. Ji X. Dai W. Cao S. J. Org. Chem. 2014;79:8984. doi: 10.1021/jo501221h. [DOI] [PubMed] [Google Scholar]; (l) Fennewald J. C. Lipshutz B. H. Green Chem. 2014;16:1097. doi: 10.1039/C3GC42119H. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Dubbaka S. R. Nizalapur S. Atthunuri A. R. Salla M. Mathew T. Tetrahedron. 2014;70:2118. doi: 10.1016/j.tet.2014.02.005. [DOI] [Google Scholar]; (n) Presset M. Oehlrich D. Rombouts F. Molander G. A. J. Org. Chem. 2013;78:12837. doi: 10.1021/jo4023233. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Musumeci D. Irace C. Santamaria R. Montesarchio D. Med. Chem. Commun. 2013;4:1405. doi: 10.1039/C3MD00159H. [DOI] [Google Scholar]; (p) Li Z. Cui Z. Liu Z.-Q. Org. Lett. 2013;15:406. doi: 10.1021/ol3034059. [DOI] [PubMed] [Google Scholar]; (q) Li Y. Wu L. Neumann H. Beller M. Chem. Commun. 2013;49:2628. doi: 10.1039/C2CC36554E. [DOI] [PubMed] [Google Scholar]; (r) Ye Y. Kunzi S. A. Sanford M. S. Org. Lett. 2012;14:4979. doi: 10.1021/ol3022726. [DOI] [PMC free article] [PubMed] [Google Scholar]; (s) Ji Y. Brueckl T. Baxter R. D. Fujiwara Y. Seiple I. B. Su S. Blackmond D. G. Baran P. S. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14411. doi: 10.1073/pnas.1109059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For recent examples of K2S2O8 activate Langlois reagent to liberate CF3 radicals, see:; (a) Wang D. Fang J. Deng G.-J. Gong H. ACS Sustainable Chem. Eng. 2017;5:6398. doi: 10.1021/acssuschemeng.7b01705. [DOI] [Google Scholar]; (b) Lu Q. Liu C. Huang Z. Ma Y. Zhang J. Lei A.-W. Chem. Commun. 2014;50:14101. doi: 10.1039/C4CC06328G. [DOI] [PubMed] [Google Scholar]; (c) Wang D. Deng G.-J. Chen S. Gong H. Green Chem. 2016;18:5967. doi: 10.1039/C6GC02000C. [DOI] [Google Scholar]; (d) Shen C. Xu J. Ying B. Zhang P.-F. ChemCatChem. 2016;8:3560. doi: 10.1002/cctc.201601068. [DOI] [Google Scholar]; (e) Huang H.-L. Yan H. Gao G.-L. Yang C. Xia W. Asian J. Org. Chem. 2015;4:674. doi: 10.1002/ajoc.201500096. [DOI] [Google Scholar]; (f) Liu J. Zhuang S. Gui Q. Chen X. Yang Z. Tan Z. Eur. J. Org. Chem. 2014:3196. doi: 10.1002/ejoc.201400087. [DOI] [Google Scholar]; (g) Patra T. Deb A. Manna S. Sharma U. Maiti D. Eur. J. Org. Chem. 2013:5247. doi: 10.1002/ejoc.201300473. [DOI] [Google Scholar]; (h) Deb A. Manna S. Modak A. Patra T. Maity S. Maiti D. Angew. Chem., Int. Ed. 2013;52:9747. doi: 10.1002/anie.201303576. [DOI] [PubMed] [Google Scholar]
- For recent examples of PhI(OAc)2 activate Langlois reagent to liberate CF3 radicals, see:; (a) Tan Z. Zhang S. Zhang Y. Li Y. Ni M. Feng B.-N. J. Org. Chem. 2017;82:9384. doi: 10.1021/acs.joc.7b01359. [DOI] [PubMed] [Google Scholar]; (b) Wu Z. He Y. Ma C. Zhou X. Liu X. Li Y. Hu T. Wen P. Huang G. Asian J. Org. Chem. 2016;5:724. doi: 10.1002/ajoc.201600128. [DOI] [Google Scholar]; (c) Shi L. Yang X. Wang Y. Yang H. Fu H. Adv. Synth. Catal. 2014;356:1021. doi: 10.1002/adsc.201300995. [DOI] [Google Scholar]; (d) Xu X. Liu F. Org. Chem. Front. 2017;4:2306. doi: 10.1039/C7QO00635G. [DOI] [Google Scholar]
- (a) Wu L.-H. Zhao K. Shen Z.-L. Loh T.-P. Org. Chem. Front. 2017;4:1872. doi: 10.1039/C7QO00416H. [DOI] [Google Scholar]; (b) Huang P. Li Y. Fu X. Zhang R. Jin K. Wang W. Duan C. Tetrahedron Lett. 2016;57:4705. doi: 10.1016/j.tetlet.2016.09.016. [DOI] [Google Scholar]
- Shang X.-J. Li Z. Liu Z.-Q. Tetrahedron Lett. 2015;56:233. doi: 10.1016/j.tetlet.2014.11.076. [DOI] [Google Scholar]
- (a) Zhang P.-Z. Li C.-K. Zhang G.-Y. Zhang L. Jiang Y.-J. Zou J.-P. Tetrahedron. 2016;72:3250. doi: 10.1016/j.tet.2016.04.048. [DOI] [Google Scholar]; (b) Cao X.-H. Pan X. Zhou P.-J. Zou J.-P. Asekun O. T. Chem. Commun. 2014;50:3359. doi: 10.1039/C3CC49689A. [DOI] [PubMed] [Google Scholar]; (c) Yang H.-B. Selander N. Org. Biomol. Chem. 2017;15:1771. doi: 10.1039/C7OB00203C. [DOI] [PubMed] [Google Scholar]
- For selected examples of photoinduced Langlois reagent liberate CF3 radicals, see:; (a) Corsico S. Fagnoni M. Ravelli D. Photochem. Photobiol. Sci. 2017;16:1375. doi: 10.1039/C7PP00179G. [DOI] [PubMed] [Google Scholar]; (b) Chang B. Shao H. Yan P. Qiu W. Weng Z. Yuan R. ACS Sustainable Chem. Eng. 2017;5:334. doi: 10.1021/acssuschemeng.6b01682. [DOI] [Google Scholar]; (c) Li L. Mu X. Liu W. Wang Y. Mi Z. Li C.-J. J. Am. Chem. Soc. 2016;138:5809. doi: 10.1021/jacs.6b02782. [DOI] [PubMed] [Google Scholar]
- For recent examples of hydroxyltrifluoromethylation of olefins, see:; (a) Yasu Y. Koike T. Akita M. Angew. Chem., Int. Ed. 2012;51:9567. doi: 10.1002/anie.201205071. [DOI] [PubMed] [Google Scholar]; (b) Luo X.-Z. Luo H.-Q. Zhang Z.-P. Dong W. Synlett. 2014;25:1307. doi: 10.1055/s-0033-1341057. [DOI] [Google Scholar]; (c) Yang Y. Liu Y. Jiang Y. Zhang Y. Vicic D. A. J. Org. Chem. 2015;80:6639. doi: 10.1021/acs.joc.5b00781. [DOI] [PubMed] [Google Scholar]; (d) Liu C. Lu Q. Huang Z. Zhang J. Liao F. Peng P. Lei A.-W. Org. Lett. 2015;17:6034. doi: 10.1021/acs.orglett.5b03035. [DOI] [PubMed] [Google Scholar]; (e) Yang X. He L. Tsui G. C. Org. Lett. 2017;19:2446. doi: 10.1021/acs.orglett.7b01085. [DOI] [PubMed] [Google Scholar]
- Luo X.-Z. Luo H.-Q. Zhang Z.-P. Dong W. Synlett. 2014;25:1307. doi: 10.1055/s-0033-1341057. [DOI] [Google Scholar]
- Yang Y. Liu Y. Jiang Y. Zhang Y. Vicic D. A. J. Org. Chem. 2015;80:6639. doi: 10.1021/acs.joc.5b00781. [DOI] [PubMed] [Google Scholar]
- Yang X. He L. Tsui G. C. Org. Lett. 2017;19:2446. doi: 10.1021/acs.orglett.7b01085. [DOI] [PubMed] [Google Scholar]
- Yasu Y. Koike T. Akita M. Angew. Chem., Int. Ed. 2012;51:9567. doi: 10.1002/anie.201205071. [DOI] [PubMed] [Google Scholar]
- Shi P. Wang Q. Zeng X. Zhao Y.-S. Zeng R.-S. RSC Adv. 2017;7:54277. doi: 10.1039/C7RA10902D. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.