Abstract
A structurally diverse library of 93 lipophilic di- and tricyclic diaminopyrimidine derivatives was tested for the ability to inhibit recombinant dihydrofolate reductase (DHFR) cloned from human and bovine isolates of Cryptosporidium parvum (J. R. Vásquez et al., Mol. Biochem. Parasitol. 79:153–165, 1996). In parallel, the library was also tested against human DHFR and, for comparison, the enzyme from Escherichia coli. Fifty percent inhibitory concentrations (IC50s) were determined by means of a standard spectrophotometric assay of DHFR activity with dihydrofolate and NADPH as the cosubstrates. Of the compounds tested, 25 had IC50s in the 1 to 10 μM range against one or both C. parvum enzymes and thus were not substantially different from trimethoprim (IC50s, ca. 4 μM). Another 25 compounds had IC50s of <1.0 μM, and 9 of these had IC50s of <0.1 μM and thus were at least 40 times more potent than trimethoprim. The remaining 42 compounds were weak inhibitors (IC50s, >10 μM) and thus were not considered to be of interest as drugs useful against this organism. A good correlation was generally obtained between the results of the spectrophotometric enzyme inhibition assays and those obtained recently in a yeast complementation assay (V. H. Brophy et al., Antimicrob. Agents Chemother. 44:1019–1028, 2000; H. Lau et al., Antimicrob. Agents Chemother. 45:187–195, 2001). Although many of the compounds in the library were more potent than trimethoprim, none had the degree of selectivity of trimethoprim for C. parvum versus human DHFR. Collectively, the results of these assays comprise the largest available database of lipophilic antifolates as potential anticryptosporidial agents. The compounds in the library were also tested as inhibitors of the proliferation of intracellular C. parvum oocysts in canine kidney epithelial cells cultured in folate-free medium containing thymidine (10 μM) and hypoxanthine (100 μM). After 72 h of drug exposure, the number of parasites inside the cells was quantitated by indirect immunofluorescence microscopy. Sixteen compounds had IC50s of <3 μM, and five of these had IC50s of <0.3 μM and thus were comparable in potency to trimetrexate. The finding that submicromolar concentrations of several of the compounds in the library could inhibit in vitro growth of C. parvum in host cells in the presence of thymidine (dThd) and hypoxanthine (Hx) suggests that lipophilic DHFR inhibitors, in combination with leucovorin, may find use in the treatment of intractable C. parvum infections.
Diaminopyrimidine inhibitors of dihydrofolate reductase (DHFR) such as trimethoprim, pyrimethamine, trimetrexate, and piritrexim (compounds 1 to 4, respectively, in Fig. 1) are used in the prophylaxis and treatment of opportunistic infections in patients whose immune systems are impaired as a result of human immunodeficiency virus infection or immunosuppressive chemotherapy (14, 38). Typically, these agents are coadministered with a sulfonamide or sulfone inhibitor of dihydropteroate synthetase (44, 49), and in the case of trimetrexate or piritrexim, with leucovorin to minimize the toxic side effects of the antifolate (13, 74). Although other microbial parasites are known to cause life-threatening secondary infections in AIDS patients in other parts of the world, the organisms most frequently found to cause opportunistic disease in patients in the United States and other industrialized countries, sometimes at the same time, are Pneumocystis carinii and Toxoplasma gondii. The rationale for combining sulfa drugs with DHFR inhibitors is that P. carinii and T. gondii, but not mammalian cells, can synthesize essential tetrahydrofolate cofactors de novo with the help of dihydropteroate synthetase (39). Because of the lack of this mechanism in humans, sulfa drugs are selective in that they do not affect the folate status of the patient's cells. In the case of DHFR inhibitors that are more potent than trimethoprim, such as trimetrexate or piritrexim, leucovorin can be added to the regimen as a rescue agent to prevent dose-limiting hematological toxicity. In this scenario, the selectivity of the host-protective effect results from the fact that the parasites lack an active transport pathway for reduced folates and thus are impervious to rescue (39). Although combinations of DHFR inhibitors with sulfa drugs have a sound theoretical rationale and, indeed, are effective in a significant number of patients, a subset of patients experience severe cutaneous allergy to the sulfa drug and thus have to discontinue this type of treatment (73).
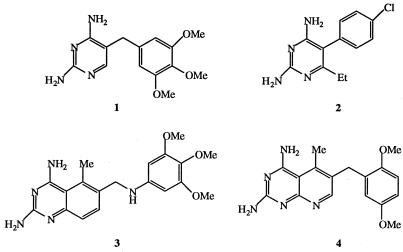
FIG. 1.
Structures of trimethoprim (compound 1), pyrimethamine (compound 2), trimetrexate (compound 3), and piritrexim (compound 4).
A vigorous program of chemical synthesis was launched by several groups in the early 1990s with the goal of discovering new inhibitors of P. carinii and/or T. gondii DHFR that might be potent enough not to require coadministration of a sulfa drug while being selective enough not to require leucovorin rescue. Hundreds of lipophilic condensed diaminopyrimidine antifolates, embodying a rich spectrum of chemical diversity (see references in Table 1), were tested as inhibitors of DHFR in cell-free assays (8, 10) and, in some cases, as inhibitors of the growth of the intact organisms in vitro or in laboratory animals (4, 51). However, with the exception of the older agents trimethoprim and pyrimethamine, the only newer antifolates tested in large, controlled clinical trials in AIDS patients suffering from, or at risk of developing, P. carinii pneumonia and/or toxoplasmosis have been trimetrexate (74) and piritrexim (13).
TABLE 1.
Condensed diaminopyrimidine ring systems tested as inhibitors of P. carinii and T. gondii DHFRs
| Heterocyclic system | Reference(s) |
|---|---|
| 5/6 systems | |
| Thieno[2,3-d]pyrimidines | 64, 66, 69 |
| Furo[2,3-d]pyrimidines | 16, 17, 19, 20, 21 |
| Pyrrolo[2,3-d]pyrimidines | 22, 23 |
| Pyrazolo[2,3-c]pyrimidines (purines) | 26 |
| Cyclopenta[d]pyrimidines | 70 |
| 6/6 systems | |
| Quinazolines | 28, 29, 41, 61, 64, 65, 67, 68 |
| Pyrido[2,3-d]pyrimidines | 15, 18, 24, 27, 60 |
| Pyrido[3,2-d]pyrimidines | 30, 31, 58 |
| Pyrido[4,3-d]pyrimidines | 63 |
| Pteridines | 36, 48, 56, 59, 61 |
| Tricyclic and other ring systems | |
| Indeno[2,3-d]pyrimidines | 72 |
| Benzo[f]quinazolines | 72 |
| Pyrimido[4,5-c]isoquinolines | 61 |
| Benzo[3,4]cycloheptal[1,2-d]pyrimidines | 72 |
| Pyrimido[4,5-c][2,7]naphthyridines | 25 |
| Pyrido[4′,3′:4,5]furo[2,3-d]pyrimidines | 19 |
| Miscellaneous 5,6-(bicycloalkano) tetrahydroquinazolines | 71 |
Among the opportunistic pathogens other than P. carinii and T. gondii that are known to pose a significant risk in the management of AIDS is the intestinal parasite Cryptosporidium parvum, which can be life threatening because of its ability to bring on repetitive episodes of violent diarrhea and, in the most extreme cases, massive exfoliation of the intestinal mucosa (33, 75). Cryptosporidiosis is typically acquired from unsterilized drinking water and is usually self-limiting, except in immunosuppressed individuals. Some success has been reported with pyrimethamine and trimethoprim-sulfamethoxazole in patients infected with Isospora belli, another opportunistic intestinal parasite whose clinical effects include severe and unremitting diarrhea and thus resemble those produced by C. parvum (12, 77). Several other experimental treatments using non-antifolate drugs have also been reported, some of which are likely to act primarily on the host cell rather than the parasite (1, 5, 6, 32, 34, 37, 42, 43, 47, 78). The efficacy of these regimens is, at best, marginal. Thus, until a consistently safe and effective drug against acute cryptosporidiosis in AIDS patients is found, the main treatment of this disease remains palliation with antidiarrheal drugs such as octeotride (35).
With the availability of a large and structurally diverse library of lipophilic DHFR inhibitors generated by our synthetic effort over the years, the opportunity presented itself to test these compounds as inhibitors of the DHFR activity of the difunctional C. parvum DHFR-thymidylate synthase (TS) enzyme, which was recently cloned and sequenced (76). A number of the compounds in our library, along with others from the archives of the National Cancer Institute and the Walter Reed Army Institute of Research, were recently found to be active in a yeast complementation assay using Saccharomyces cerevisiae in which the native yeast gene was replaced with either the entire difunctional DHFR-TS gene or the discrete DHFR domain of C. parvum (7, 40). In the present paper, we report data on 93 compounds from the archival collection of the Dana-Farber Cancer Institute (DFCI) as in vitro inhibitors of recombinant C. parvum and human DHFR activity in a spectrophotometric assay. To our knowledge, this constitutes the largest published database on lipophilic polycyclic diaminopyrimidines as inhibitors of these enzymes. Also reported for comparison are the 50% inhibitory concentrations (IC50s) of the same compounds against Escherichia coli DHFR. Although E. coli infection is not among the more dangerous complications of AIDS, drug-resistant E. coli strains are gradually becoming more prevalent in the food supply and would almost certainly pose a greater risk to an AIDS patient than to an individual whose immune defense system is not impaired.
MATERIALS AND METHODS
Test compounds.
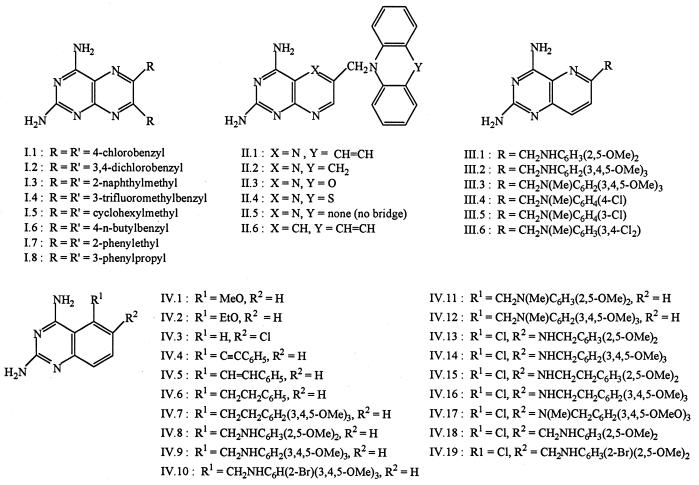
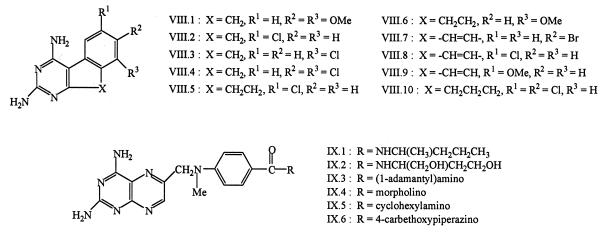
Data were obtained for a total of 93 compounds, comprising the nine structural families shown in Fig. 2 to 4. Symmetrically 5,6-disubstituted pteridines I.1 to I.8 were synthesized from tetraaminopyrimidine and 1,2-diketones (52). Pteridines II.1 to II.5 and pyrido[2,3-d]pyrimidine II.6 were obtained by N-alkylation of secondary amines with 2,4-diamino-6-bromomethylpteridine (56) or 2,4-diamino-6-bromomethylpyrido[2,3-d]pyrimidine (60). Pyrido[3,2-d]pyrimidines III.1 to III.6 were prepared from 2,4-diamino-6-bromomethylpyrido[3,2-d]pyrimidine and anilines or N-methylanilines (58). Quinazolines IV.1 to IV.3 were obtained from the corresponding anthranilonitriles and chloroformamidine hydrochloride (62). Quinazolines IV.4 to IV.7 were obtained from 2,4-diamino-5-iodoquinazoline and an alkene or alkyne via a palladium-catalyzed Heck reaction, followed by catalytic hydrogenation in the case of IV.6 and IV.7 (65). N10-unsubstituted quinazolines IV.8 to IV.12 were obtained from 2,4-diaminoquinazoline-5-carbonitrile and the appropriate substituted anilines by reductive coupling in the presence of Raney nickel (65). 5-Chloro-6-(arylaminoalkyl)quinazolines IV.13 to IV.16, IV.18 and IV.19 were derived from 2,4,6-triamino-5-chloroquinazoline and the appropriate substituted aldehydes by similar reductive coupling (67). Compounds IV.11, IV.12, and IV.17 were obtained from IV.8, IV.9, and IV.16, respectively, by reaction with formaldehyde and sodium cyanoborohydride (65, 67). Tetrahydroquinazolines V.1 to V.16 were synthesized from 4-substituted cyclohexanones by heating with cyanoguanidine (68). Thieno[2,3-d]pyrimidines VI.1 to VI.12 were derived from 5,6-substituted 2-aminothiophene-3-carbonitriles and chloroform-amidine hydrochloride (64, 66), whereas analogs VI.13 to VI.18, with an arylamine side chain, were synthesized in several steps from preformed 2,4-diamino-5-methylthieno[2,3-d]pyrimidine (69). Pyrido[4,3-d]-pyrimidines VII.1 to VII.4 were made by alkylation of the parent amine (63). 9H-Indeno[2,1-d]pyrimidines VIII.1 to VIII.4 were synthesized from 3-cyano-2-alkoxy-1H-indenes and guanidine (57). 5,6-Dihydrobenzo[f]quinazolines VIII.5 and VIII.6 were prepared by heating 2-tetralones with cyanoguanidine (55). Seven-membered ring analog VIII.10 was prepared similarly from 6,7-dichloro-2-benzosuberone (53). Fully aromatic benzo[f]quinazolines VIII.7 to VIII.9 were prepared by thermal cyclization of symmetrical N1,N5-diarylbiguanides, by condensation of 2-aminonaphthalene-1-carbonitriles with guanidine, or by oxidation of 1,3-diamino-5,6-dihydrobenzo[f]quinazolines with selenium dioxide (54). Trimethoprim and trimetrexate were obtained from the National Cancer Institute, Bethesda, Md. All compounds were of recent or archival origin and were judged to be >95% pure at the time of assay.
FIG. 2.
Structures of dicyclic diaminopyrimidines tested as DHFR inhibitors (groups I to IV).
FIG. 4.
Structures of di- and tricyclic diaminopyrimidines tested as DHFR inhibitors (groups VIII and IX).
Enzyme assays.
Recombinant C. parvum and human enzymes were obtained and used as described earlier (7, 76). The C. parvum type 1 DHFR-TS allele was cloned from an isolate obtained from an infected AIDS patient, and the C. parvum type 2 allele was cloned from an isolate obtained from an infected calf. The designations Cp-I and Cp-II will be used here in order to conform to the nomenclature used earlier for the two cloned proteins (76; for a discussion of C. parvum genotypes 1 and 2, see reference 46). The type 1 enzyme is found exclusively in humans, whereas the type 2 enzyme can infect either humans or animals. Molecular characterization of the constructs SFGH-1 (from Cp-I) and NINC-1 (from Cp-I), the latter of which was re-engineered to encode a 22-residue C-terminal TS domain of Cp-I, was documented earlier (76). Briefly, all three proteins were expressed from dhfr mutant E. coli strain PA414 after transfection with the appropriate coding sequence and subcloning into expression plasmid pTrc99A, which contains a promoter induced with IPTG (isopropyl-β-d-thiogalactopyranoside). In the case of the human enzyme, the E. coli mutant was transfected with previously described plasmid pDFR (50). All three enzymes were purified to homogeneity from their respective bacterial lysates by chromatography on a methotrexate-Sepharose affinity column. Assays of enzyme activity were performed at 37°C in a microtiter plate spectrophotometer by monitoring the change in UV absorbance at 340 nm in a solution containing 50 mM N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid (pH 7.0), 1 mM EDTA, 75 μM 2-mercaptoethanol, 0.1% bovine serum albumin, 20 μM dihydrofolate, and 100 μM NADPH. Enzyme concentrations were adjusted to give linear rates over the 5-min duration of the assay. Reactions were initiated by adding 100 μl of 40 μM dihydrofolate in assay buffer to an equal volume of assay buffer lacking dihydrofolate and containing various concentrations of the inhibitor. Each titration was performed twice, and the mean DHFR activity was plotted against the inhibitor concentration to obtain the IC50. Assays using E. coli DHFR were performed under the same conditions as those done with the human enzyme. Stock solutions of all of the test compounds were made up in dimethyl sulfoxide, and appropriate control experiments were done to ensure that the final amount of dimethyl sulfoxide in the assay solution was low enough to have no appreciable effect on the rate of the DHFR-catalyzed reaction or the ability of cells to grow in culture.
RESULTS AND DISCUSSION
Binding of inhibitors to C. parvum DHFR versus their binding to human DHFR.
The availability of the compounds shown in Fig. 2 to 4 offered the opportunity to screen a structurally diverse library of 93 lipophilic antifolates from the DFCI archive as inhibitors and to determine whether any of them are selective for the C. parvum enzyme. Yeast complementation assays had been concurrently used to test a number of these compounds for the ability to inhibit DHFRs from several different species, including C. parvum and humans (7, 40). Fifteen other compounds provided by the National Cancer Institute were also tested by direct spectrophotometric assay (7). One of these, trimethoprim, proved to be >100 times more potent against the C. parvum enzyme than against the human enzyme. Trimetrexate was considerably more potent than trimethoprim but was completely nonselective. Three other compounds were also more potent than trimethoprim against both enzymes, but three of them contained a polar glutamic or aspartic acid side chain and thus were not considered to be of the lipophilic type. Trimethoprim emerged as the most selective member of this initial panel and thus was considered the first promising lead in our search for anticryptosporidial drugs among lipophilic DHFR inhibitors. Although interesting patterns of activity were observed in the DHFR complementation assays (7, 40), this method lacks the quantitative power of a direct measurement of the kinetics of an enzyme reaction. More importantly, compounds can only be assumed to be equipotent as enzyme inhibitors by this method if they achieve the same intracellular concentration over the time course of the assay, which may not necessarily be the case, even for lipophilic antifolates. Thus, the yeast complementation assay should be viewed as only a preliminary screen. In the present study, the spectrophotometric assay was used to assess the potency and selectivity of a larger group of lipophilic antifolates from the DFCI collection with a view to uncovering additional leads. Some of these compounds had already been tested in the yeast assay, but many had not.
As shown in Table 2, the IC50s of trimethoprim against the Cp-I and Cp-II enzymes were 4.0 and 3.8 μM, respectively, whereas that against human DHFR was 890 μM. Thus, as previously noted (7), trimethoprim was equally active against the human and bovine strains of C. parvum DHFR and was remarkably selective for these enzymes relative to human DHFR. Of the 93 additional diaminopyrimidine antifolates that have now been tested against the Cp-I and Cp-II DHFRs, 25 had IC50s in the 1 to 10 μM range against at least one of the C. parvum enzymes and thus were not substantially different from trimethoprim. A second group of 25 compounds had IC50s of <1.0 μM, and of these, 9 had IC50s of <0.1 μM and thus were at least 40 times more potent than trimethoprim. A third group, comprising 42 compounds, afforded <50% inhibition at 10 μM, the concentration arbitrarily chosen as a reasonable upper limit for the screen, and thus are merely footnoted in Table 2. The fact that roughly half of the compounds in our library were at least as potent as trimethoprim raises doubt about a recent comment in the literature that C. parvum DHFR may be intrinsically resistant to 2,4-diaminopyrimidine inhibitors (11).
TABLE 2.
Inhibition of C. parvum and human DHFRs by di- and tricyclic diaminopyrimidines
| Compound | IC50(μM)a
|
Selectivityb
|
|||
|---|---|---|---|---|---|
| Cp-I | Cp-II | Human | Cp-I | Cp-II | |
| Trimethoprim | 4.0 ± 1.7 (1.0) | 3.8 ± 1.8 (1.0) | 890 ± 60 (1) | 220 | 230 |
| I.1 | 1.2 ± 0.32 (3.3) | 1.6 ± 0.38 (2.4) | 5.7 ± 0.63 (160) | 4.8 | 3.6 |
| I.2 | 0.25 ± 0.057 (16) | 0.35 ± 0.052 (11) | 3.5 ± 5.6 (250) | 14 | 10 |
| II.2 | 1.7 ± 0.85 (2.4) | 1.5 ± 0.29 (2.5) | 0.56 ± 0.15 (1,600) | 0.33 | 0.37 |
| II.3 | 0.82 ± 0.48 (4.9) | 0.75 ± 0.29 (5.1) | 0.23 ± 0.088 (3,900) | 0.28 | 0.31 |
| II.4 | 1.8 ± 0.84 (2.2) | 1.6 ± 0.88 (2.4) | 0.81 ± 0.40 (1,100) | 0.45 | 0.51 |
| II.6 | 2.1 ± 1.9 (1.9) | 1.9 ± 0.34 (2.0) | 1.4 ± 0.52 (640) | 0.67 | 0.74 |
| III.1 | 3.2 ± 3.4 (1.3) | 7.2 ± 2.9 (0.53) | 0.83 ± 0.17 (1,100) | 0.26 | 0.12 |
| III.2 | 1.3 ± 0.11 (3.1) | 1.5 ± 0.76 (2.5) | 0.49 ± 0.12 (1,800) | 0.38 | 0.33 |
| III.3 | 0.075 ± 0.039 (53) | 0.14 ± 0.060 (27) | 0.0089 ± 0.0044 (100,000) | 0.12 | 0.037 |
| III.4 | 9.1 ± 1.6 (0.44) | 8.3 ± 2.9 (0.46) | 0.31 ± 0.20 (2,900) | 0.034 | 0.037 |
| III.5 | 0.024 ± 0.012 (170) | 0.049 ± 0.029 (78) | 0.027 ± 0.019 (33,000) | 1.1 | 0.55 |
| III.6 | 0.020 ± 0.015 (200) | 0.029 ± 0.014 (130) | 0.00037 ± 0.00027 (2,400,000) | 0.019 | 0.013 |
| IV.1 | 0.56 ± 0.30 (7.1) | 1.9 ± 0.23 (2.0) | 2.8 ± 2.0 (320) | 5.0 | 1.5 |
| IV.2 | 0.35 ± 0.23 (11) | 0.80 ± 0.095 (4.8) | 0.75 ± 0.50 (1,200) | 2.1 | 0.94 |
| IV.13 | 0.023 ± 0.0060 (170) | 0.040 ± 0.010 (95) | 0.0039 ± 0.0016 (120,000) | 0.17 | 0.098 |
| IV.14 | 0.027 ± 0.0025 (150) | 0.045 ± 0.0064 (84) | 0.0013 ± 00044 (680,000) | 0.048 | 0.029 |
| IV.17 | 0.038 ± 0.011 (105) | 0.022 ± 0.0069 (170) | 0.010 ± 0.0020 (89,000) | 0.26 | 0.45 |
| IV.18 | 0.0065 ± 0.0021 (620) | 0.011 ± 0.0025 (350) | 0.010 ± 0.00028 (89,000) | 1.5 | 0.91 |
| V.1 | 7.6 ± 2.4 (0.53) | 9.0 ± 0.92 (0.42) | 9.4 ± 1.0 (95) | 1.2 | 1.0 |
| V.2 | 0.21 ± 0.025 (19) | 0.13 ± 0.017 (29) | 0.022 ± 0.0080 (40,000) | 0.10 | 0.17 |
| V.4 | 1.0 ± 0.36 (2.9) | 0.65 ± 0.21 (4.2) | 0.59 ± 0.20 (1,500) | 0.59 | 0.91 |
| V.5 | 0.18 ± 0.040 (22) | 0.14 ± 0.026 (27) | 0.094 ± 0.031 (9,500) | 0.52 | 0.67 |
| V.6 | 1.2 ± 0.36 (3.3) | 0.73 ± 0.14 (5.2) | 0.23 ± 0.032 (3,900) | 0.19 | 0.32 |
| V.7 | 1.1 ± 0.48 (3.6) | 1.9 ± 0.26 (2.0) | 0.29 ± 0.068 (3,100) | 0.26 | 0.15 |
| V.8 | 0.33 ± 0.10 (12) | 0.28 ± 0.010 (14) | 0.074 ± 0.018 (12,000) | 0.22 | 0.26 |
| V.9 | 0.40 ± 0.17 (10) | 0.69 ± 0.16 (5.5) | 0.19 ± 0.036 (4,700) | 0.48 | 0.28 |
| V.10 | 0.56 ± 0.56 | 0.22 ± 0.064 | 0.16 ± 0.052 (5,600) | 0.29 | 0.73 |
| V.11 | 0.15 ± 0.010 (27) | 0.16 ± 0.040 (24) | 0.094 ± 0.048 (9,500) | 0.63 | 0.59 |
| V.12 | 0.81 ± 0.34 (3.6) | 0.53 ± 0.068 (6.9) | 0.38 ± 0.048 (3,400) | 0.47 | 0.72 |
| V.13 | 0.45 ± 0.11 (8.9) | 0.34 ± 0.092 (11) | 0.19 ± 0.092 (4,700) | 0.42 | 0.56 |
| V.14 | 0.40 ± 0.10 (10) | 0.26 ± 0.021 (15) | 0.15 ± 0.084 (5,900) | 0.38 | 0.58 |
| V.15 | 0.53 ± 0.044 (7.5) | 0.76 ± 0.14 (5.0) | 0.31 ± 0.12 (2,900) | 0.58 | 0.41 |
| V.16 | 0.065 ± 0.018 (62) | 0.089 ± 0.00487 (43) | 0.094 ± 0.022 (9,500) | 1.4 | 1.1 |
| VI.1 | 2.6 ± 1.1 (1.5) | 1.7 ± 0.40 (2.2) | 0.98 ± 0.30 (910) | 0.38 | 0.58 |
| VI.2 | 8.4 ± 2.8 (0.48) | 8.3 ± 2.9 (0.45) | 0.64 ± 0.24 (1,400) | 0.076 | 0.077 |
| VI.4 | 2.3 ± 1.0 (1.7) | 3.6 ± 0.64 (1.1) | 3.0 ± <0.0001 (300) | 1.3 | 0.83 |
| VI.5 | 0.78 ± 0.28 (5.1) | 0.65 ± 0.11 (5.8) | 1.6 ± 0.31 (560) | 2.1 | 2.5 |
| VI.12 | 5.1 ± 2.2 (0.78) | 8.5 ± 1.6 (0.45) | 7.3 ± 2.3 (120) | 1.4 | 0.86 |
| VII.2 | 2.1 ± 0.38 (1.9) | 2.3 ± 0.60 (1.7) | 2.8 ± 0.356 (320) | 1.3 | 1.2 |
| VIII.6 | 0.72 ± 0.52 (5.6) | 1.4 ± 0.20 (2.7) | 0.12 ± 0.056 (7,400) | 0.17 | 0.086 |
| VIII.8 | 0.023 ± 0.0078 (170) | 0.019 ± 0.0040 (200) | 0.012 ± 0.017 (74,000) | 0.52 | 0.63 |
| VIII.9 | 1.2 ± 0.88 (3.3) | 1.4 ± 0.48 (2.7) | 0.17 ± 0.072 (5,200) | 0.14 | 0.12 |
| IX.1 | 1.3 ± 0.68 (3.1) | 0.66 ± 0.22 (5.8) | 0.60 ± 0.13 (1,500) | 0.46 | 0.91 |
| IX.2 | 2.4 ± 0.26 (1.7) | 2.2 ± 0.44 (1.7) | 1.9 ± 0.96 (470) | 0.79 | 0.86 |
| IX.3 | 1.8 ± 0.44 (2.2) | 1.2 ± 0.056 (3.2) | 0.77 ± 0.37 (1,200) | 0.43 | 0.64 |
| IX.4 | 0.65 ± 0.21 (6.2) | 0.60 ± 0.16 (6.3) | 1.9 ± 1.0 (470) | 2.9 | 3.2 |
| IX.5 | 4.7 ± 4.4 (0.85) | 1.3 ± 0.38 (2.9) | 0.58 ± 0.26 (1,500) | 0.12 | 0.45 |
| IX.6 | 3.2 ± 0.92 (1.3) | 1.3 ± 0.15 (2.9) | 0.81 ± 0.17 (1,100) | 0.25 | 0.62 |
Values reported are means ± standard deviations from a minimum of three, and in some cases as many as 7, separate determinations. The values in parentheses are normalized relative to those for trimethoprim and rounded off to two significant figures. The following compounds had IC50 of >10 μM against one or both C. parvum enzymes: I.3 to I.8, II.1, II.5, IV.3 to IV.12, IV.15, IV.16, and IV.19 (Fig. 2); V.3, VI.3, VI.6 to VI.11, and VI.13 to VI.18 (Fig. 3); VII.1, VII.3, VIII.1 to VIII.5, VIII.7, and VIII.10 (Fig. 4). Of these, however, the following had IC50s of <10 μM against human DHFR: I.5, 5.3; I.7, 3.7; II.1, 7.2; IV.4, 1.9; IV.5, 6.5; IV.10, 1.9; V.3, 5.3; VIII.1, 7.3; VIII.2, 9.9; VIII.5, 7.2; VIII.10, 0.0029. The other compounds with IC50s of >10 μM against Cp-I and/or Cp-II also had IC50 of >10 μM against human DHFR.
Selectivity is defined as the ratio IC50 for human DHFR/IC50 for Cp-I or IC50 for human DHFR/IC50 for Cp-II. Selectivity ratios were calculated only for compounds whose IC50s were <10 μM against both the C. parvum and human DHFRs.
The best compound tested was IV.18, whose IC50 of 0.0065 μM against the Cp-I enzyme approached the value obtained earlier for trimetrexate (7). Like trimetrexate, however, this compound was nonselective. With the exception of I-2, which had 14-fold selectivity for the Cp-I enzyme and 10-fold selectivity for the Cp-II enzyme, almost all of the compounds were more like trimetrexate than trimethoprim, in that they inhibited the C. parvum and human DHFRs with about the same potency or were actually better inhibitors of the human enzyme. For the purpose of this discussion, we have arbitrarily chosen a 10-fold difference in IC50s as the minimum threshold for selectivity. This places I.1, IV.1, IV.2, and IX.4 in the nonselective category even though they are, in fact, slightly selective for the Cp-I enzyme. It is of interest that five of the nine compounds with IC50s of <0.1 μM against at least one of the C. parvum enzymes contained either the 3,4,5-trimethoxyphenyl substitution of trimethoprim and trimetrexate (cf. III.3, IV.14, and IV.17) or the 2,5-dimethoxyphenyl substitution of piritrexim (cf. IV.13 and IV.18), while four others contained 3- and/or 4-chloro substitutions on the phenyl ring (cf. III.5, III.6, V.16, and VIII.8). However, 10 of the other 13 compounds with a 3,4,5-trimethoxyphenyl ring in the side chain and 5 of the other 9 compounds with a 2,5-dimethoxyphenyl ring in the side chain had IC50s of >10 μM, indicating that these substitutions are not necessarily favorable with every diaminopyrimidine ring system.
Structural differences between pairs of analogs with widely divergent potencies as DHFR inhibitors were, in some cases, astonishingly small. Among the pyrido[3,2-d]pyrimidine analogs, for example, methylation of the bridge nitrogen increased potency by approximately 1 order of magnitude (cf. III.2 and III.3) and replacement of a 4-chlorophenyl or 3,4-dichlorophenyl group with a 3-chlorophenyl group in the side chain had a comparable effect (cf. IV.4 to IV.6). However, moving a substituent from the para to the meta position of the phenyl ring was not necessarily favorable and appeared to depend on the nature of the heterocyclic moiety and the bridge (cf. V.6 and V.7 versus III.4 and III.5 and V.14 and V.15 versus III.4 and III.5). Insertion of an extra CH2 group into the bridge of quinazoline analogs IV.13 and IV.14 caused potency to decrease by 2 orders of magnitude (cf. IV.15 and IV.16). In other cases, there was remarkably little variation in binding among compounds with considerably different patterns of aromatic substitution (cf. V.4 to V.15). Even though some of the individual structure-activity correlations in Table 2 were intriguing, the limited data obtained did not yield any clear guidelines as to how selective inhibitors of C. parvum DHFR might be designed. Moreover, none of the compounds approached the dramatic selectivity reported earlier for trimethoprim as an inhibitor of the C. parvum enzyme (7). A better understanding of how an optimal combination of high potency and high selectivity might be designed into an inhibitor of C. parvum DHFR will no doubt be easier to obtain once the first three-dimensional structure of a ternary complex of the enzyme has been solved, e.g., by X-ray crystallography or nuclear magnetic resonance analysis. High-resolution crystallographic structural analysis of several such complexes is in progress (R. G. Nelson, personal communication).
Anticryptosporidial activities of DHFR inhibitors in culture.
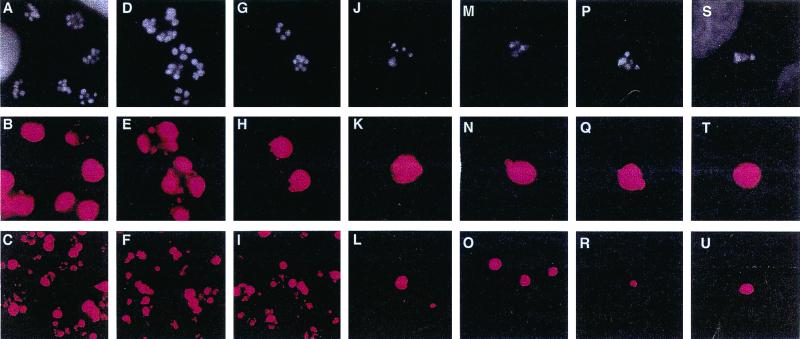
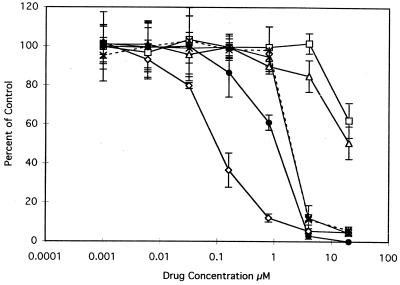
In order to determine whether the potent inhibition of isolated DHFR translates into antiparasitic activity in culture, three arbitrarily chosen examples of the tetrahydroquinazoline type (V.4, V.10, and V.16) were tested for the ability to block proliferation of the intracellular forms of the organism in Madin-Darby canine kidney (MDCK) epithelial cell monolayers grown in microscope chamber slides and infected with C. parvum oocysts. To rule out the possibility that inhibition of parasite growth was due to an antifolate effect on the host cell, the medium was supplemented with 10 μM dThd and 100 μM Hx. In some experiments, the medium could be supplemented effectively with as little as 0.1 μM leucovorin alone, instead of dThd and Hx (R. G. Nelson, results not shown). Parasites were visualized by an indirect immunofluorescence assay in which the cells were fixed and treated sequentially with rat polyclonal anti-C. parvum serum, biotinylated anti-rat serum, and fluoresceinated streptavidin. The fixed cells were also stained for DNA, and the doubly stained cells were enumerated under the microscope. Photomicrographs of control cells and of cells incubated for 48 h with trimethoprim, pyrimethamine, trimetrexate, and tetrahydroquinazolines V.4, V.10, and V.16, all at the same concentration (4 μM), are presented in Fig. 5 and 6. As can be seen in these figures, trimethoprim (panels E and F) and pyrimethamine (panels H and I) had little or no effect on the number of red-stained parasite-containing vacuoles relative to those in untreated controls (panels B and C), presumably because 4 μM is lower than the optimal concentration of these drugs. In contrast, 4 μM trimetrexate led to marked diminution of the number of parasitemic vacuoles (panels K and L) and this was accompanied by aberrant nuclear morphology of the parasites in these vacuoles (panels G and J) compared to that of controls (panel A). Gratifyingly, compounds V.4 (panels M to O), V.10 (panels P to R), and V.16 (panels S to U) appeared to be equipotent with trimetrexate in blocking parasitemia. A dose-response analysis was then performed by repeating this experiment over a range of drug concentrations of 0.001 to 30 μM. As shown in Fig. 6, trimethoprim at 30 μM decreased the number of intracellular parasites by 50% whereas trimetrexate elicited the same result at 0.1 μM, in rough agreement with the 1,000-fold tighter binding of the latter drug to DHFR. The tetrahydroquinazoline analogs had IC50s in an intermediate range (1 to 3 μM).
FIG. 5.
Inhibition of the development and multiplication of C. parvum intracellular life-cycle stages by lipophilic antifolates. MDCK epithelial cells (2.5 × 104/cm2) were plated in eight-well microscope chamber slides and grown to confluence for 48 h in para-aminobenzoic acid and folate-free RPMI 1640 medium (deficient medium) supplemented with 100 μM Hx and 10 μM dThd and containing 5% dialyzed fetal calf serum (dFCS). The cells were transferred to deficient medium–1% dFCS lacking Hx and dThd for 24 h and subsequently infected by incubation with C. parvum oocysts (3.5 × 103/cm2) for 3 h at 37°C. After three washes to remove unexcysted oocysts and extracellular sporozoites, fresh deficient medium–1% dFCS containing 4 μM drug (or no drug) was added. Drugs and media were renewed at 24 h postinfection, and the experiment was terminated at 48 h. After formaldehyde fixation, 1% Triton X-100 extraction, and blockade in 0.5% bovine serum albumin, the infected monolayers were processed for indirect immunofluorescence assay by consecutive 60-min incubations with 1:500 dilutions of rat polyclonal anti-C. parvum serum, biotin-conjugated anti-rat serum, and fluorophore CY3-conjugated streptavidin containing the DNA stain 4′,6′-diamidino-2-phenylindole (DAPI) at 1 μM. Panels in the first and second horizontal rows are photomicrographs (magnification, ca. ×1,000) of the same microscopic fields stained for nuclei with DAPI and for parasite-containing vacuoles with the anti-C. parvum polyclonal antibody, respectively. Panels in the third horizontal row are lower-power photomicrographs (magnification, ca. ×400) demonstrating the overall level of C. parvum infection and the inhibition of parasite multiplication by the antifolates. Results are representative examples of a number of experiments repeated on different days. Panels: A to C, controls (no drug); D to F, trimethoprim; G to I, pyrimethamine; J to L, trimetrexate; M to O, compound V.10; P to R, compound V.15; S to T, compound V.16. All drugs were present at a concentration of 4 μM.
FIG. 6.
Inhibition of the development and multiplication of intracellular C. parvum parasites by lipophilic antifolates. MDCK epithelial cells were plated, grown, and infected as described in the legend to Fig. 5. Following postinfection washes to remove unexcysted oocysts and extracellular sporozoites, deficient medium–1% dFCS was added to each well of the chamber slide and 20 mM stock solutions of the indicated antifolate drugs were serially diluted fivefold across seven wells. The last well received no drug and served as a control to quantify uninhibited growth. Drugs and media were renewed at 24 h postinfection, the experiment was terminated at 48 h, and the monolayers were processed as described in the legend to Fig. 5. The slides were microscopically examined for epifluorescence, and the numbers of parasite-containing fluorescent vacuoles were counted in each of five microscopic fields per well (magnification, ca. ×400). Results are expressed as percentages of the control determined as follows: (average number of parasite-containing vacuoles in drug-treated cells ÷ average number of vacuoles in drug-free controls) × 100. Error bars indicate the standard deviation at each drug concentration. Each drug was tested at least twice with similar results. Control experiments using uninfected MDCK cells showed that the drug concentrations used were not toxic to the cells (data not shown). Symbols: ◊, trimetrexate; □, trimethoprim; ▵, pyrimethamine; ×, compound V.10; ∗, compound V.15; ●, compound V.16.
On the basis of the pilot experiment whose results are depicted in Fig. 5 and 6, we reasoned that most of the compounds in the DFCI library would be effective somewhere in the 0.3 to 30 μM range, with trimethoprim and pyrimethamine at one end of the spectrum and trimetrexate at the other. However, because we felt that a compound would not be worth studying further unless its potency could be shown to be at least 10-fold greater than that of trimethoprim, we selected 3 μM as a reasonable upper drug concentration limit for all subsequent experiments. Of the 93 compounds tested, the majority had IC50s of >3.0 μM and thus did not meet this rather stringent criterion. However, nine compounds with IC50s of ≤0.6 μM, i.e., the pteridine I.2, the pyrido[3,2-d]pyrimidines III.5 and III.6, the 5-chloroquinazolines IV.14, IV.17, and IV.18, the tetrahydroquinazolines V.2 and V.5, and the benzo[f]quinazoline VIII.8 appeared to be somewhat more potent than V.4, V.10, or V.16 and >50 times more potent than trimethoprim. Because our target endpoint was 50% inhibition, which was 0.2 μM for several of the compounds in the library, concentrations of <0.2 μM were not tested. As shown in Fig. 6, the IC50 of trimetrexate was ca. 0.1 μM. The four compounds with the lowest IC50s, the pyrido[3,2-d]pyrimidine III.6, the quinazolines IV.17 and IV.18, and the tetrahydroquinazolines V.2 and V.5 approached trimetrexate in potency. However, because of their lack of enzyme selectivity (Table 2), in vivo use of these compounds would presumably require coadministration of leucovorin.
An interesting structure-activity correlation that becomes evident from the results in Tables 2 and 3 is that a low IC50 in the spectrophotometric assay of DHFR inhibition is not always accompanied by proportionally high potency in the C. parvum growth assay. For example, among the 20 compounds whose IC50s in the spectrophotometric DHFR assay against the Cp-I enzyme were <0.6 μM, there were 8 (40%) whose IC50s in the growth assay were at least 50-fold higher (i.e., >3.0 μM). Conversely, there were no compounds whose IC50s in the growth assay were lower than the IC50s in the enzyme assay. A reasonable explanation for divergence between DHFR inhibition and growth inhibition is that the uptake of some of the lipophilic diaminopyrimidine antifolates into cells, and ultimately into the intracellular parasite, may occur via a mechanism that is more complex than simple diffusion. Evidence has been presented that shows that trimetrexate and piritrexim are both substrates for the P-glycoprotein efflux pump in mammalian cells (2). Because of the very unusual nature of the intracellular localization and mode of nutrient uptake of C. parvum within its host cell (11), very little is known about how drugs actually get into this organism at different stages of its lifecycle. However, it is easily conceivable that C. parvum may express some type of multidrug resistance pump whose affinity for lipophilic substrates extends to nonclassical antifolates. Whatever the exact details of how these compounds actually get into the parasite, it seems clear that in designing and evaluating lipophilic antifolates as candidate drugs against cryptosporidiosis, account must be taken of their ability to reach the target enzyme.
TABLE 3.
Growth inhibition of C. parvum intracellular forms by lipophilic diaminopyrimidine antifolates
| Compound | IC50 (μM)a |
|---|---|
| I.2 | 0.6 |
| III.5 | 0.6 |
| III.6 | 0.3 |
| IV.4 | 2 |
| IV.13 | 2 |
| IV.14 | 0.6 |
| IV.17 | 0.3 |
| IV.18 | 0.2 |
| V.2 | 0.3 |
| V.5 | 0.2 |
| V.14 | 2 |
| V.15 | 2 |
| V.16 | 1 |
| VI.6 | 2 |
| VIII.8 | 0.6 |
| VIII.9 | 2 |
The relatively low activities of V.9 and V.10 (IC50s, >3.0 μM) against C. parvum in culture were of interest because, in a standard biochemical assay based on selective [3H]uracil incorporation into the nuclear DNA of T. gondii tachyzoites cocultured with human embryonic lung cells in folate-containing medium, these compounds and the 3-trifluoromethoxybenzyl analog V.14 had previously been found to have IC50s as low as 0.1 to 0.3 μM (69). Compound V.10 was also active against P. carinii trophozoites cocultured with rat embryonic lung fibroblasts in medium containing 10 μM folic acid, but complete suppression of growth was achieved only at a fairly high concentration of 30 μM (69). Thus, it appears that lipophilic antifolates with potent in vitro activity against T. gondii are not necessarily as potent against either C. parvum or P. carinii. A compound with the same potency against T. gondii, P. carinii, and C. parvum would potentially be of therapeutic interest because AIDS patients are sometimes infected by two or more of these organisms. However, the likelihood of finding a single potent inhibitor that binds equally well to the DHFRs of all three species without also binding tightly to mammalian DHFR seems remote.
The enzyme inhibition data for 20 of the compounds in Table 2 were correlated with the results of the yeast complementation assay reported earlier (7). Seven compounds (IV.3, VI.3, VI.4, VI.10, VI.13, VI.14, and VIII.5) were inactive in the yeast assay, and five of these had IC50s of >10 μM in the spectrophotometric assay against at least one of the C. parvum enzymes. Compound VI.4 was anomalous in that it was ineffective in the yeast assay even though its IC50 against both C. parvum enzymes was in the 1 to 5 μM range. Low activity in the yeast assay could occur if drug diffusion through the plate is slow or if drug penetration through the yeast cell wall is inefficient. Seven compounds (VI.6, VI.7, VII.1, and VII.6) had some activity in the yeast assay but were less effective than trimetrexate, and these compounds also had an IC50s of >10 μM in the enzyme assay. Five compounds (IV.1, IV.2, V.9, V.10, and VIII.8) were almost as effective as trimetrexate in the yeast assay (7), and all of them had IC50s of <1 μM in the enzyme assay. Thus, with the exception of VI.4, which had an IC50 in the 1 to 5 μM range against both C. parvum enzymes by spectrophotometric assay but was inactive in the yeast assay, there was good agreement between the results of the two methods.
While there are, to date, no reported clinical attempts to use trimetrexate and leucovorin to treat severe C. parvum infection, it is conceivable that this approach might be worth trying when the disease fails to respond to other forms of treatment. Our finding that several compounds in the test library inhibited the in vitro growth of C. parvum in mammalian cells at physiologically reasonable low micromolar concentrations comparable to those at which trimetrexate was similarly effective suggests that lipophilic DHFR inhibitors deserve to be more extensively tested in combination with leucovorin for the treatment of the most difficult cases of cryptosporidiosis.
It is important to note that the purpose of this study was simply to explore, at a molecular level (i.e., in a cell-free assay), whether any particular structural class among the diaminopyrimidine DHFR inhibitors we tested might serve as a starting point for systematic structural modifications aimed at the eventual discovery of a drug whose potency and selectivity profile is superior to that of trimethoprim or pyrimethamine. However, it should be remembered that DHFR inhibition data, by themselves, are only an early step in predicting whether an antifolate will be active in an infected cell or a whole animal; indeed, even screening for antifolate activity in culture can provide only a preliminary estimate of whether a drug will be an effective anticryptosporidial agent in patients. Thus, even though trimethoprim was found in this study to be very selective in its binding affinity for C. parvum DHFR versus mammalian DHFR and was also found to suppress parasite growth in a cell assay, as others have previously noted (79), the ability of this agent to control cryptosporidial diarrhea in AIDS patients, even in combination with sulfamethoxazole, has actually been disappointing. It is axiomatic that the activity of a drug in a whole animal is determined by a host of factors, only one of which is binding to the drug's target. Thus, while we consider the database reported in this paper to be of interest from the standpoint of structure-activity correlation at a molecular level, our results are not intended to be seen as anything more than a preliminary indication of therapeutic potential.
Binding of inhibitors to E. coli DHFR.
As shown in Table 4, the IC50 of trimethoprim against E. coli DHFR, determined under the same assay conditions as were used with the C. parvum and human enzymes, was 0.012 μM, in good agreement with previously published results (3, 9). Among the 86 out of 93 compounds tested against the E. coli enzyme, 31 (36%) were found to be better inhibitors than trimethoprim. Moreover, 12 of these (II.2, III.3, III.5, IV.14, IV.18, V.2, V.8, V.10, V.13, V.14, V.16, and VI.5) had IC50s in the 0.1 to 1.0 nM range and 4 others (II.3, III.6, IV.13, and IV.17) had IC50s that could not be accurately determined because they were <0.1 nM. However, because the most active compounds against E. coli DHFR also proved to be very potent against the human enzyme (cf. Table 2), they did not match the remarkable selectivity of trimethoprim.
TABLE 4.
Inhibition of E. coli DHFR by di- and tricyclic diaminopyrimidines
| Compound | IC50 (μM)a |
|---|---|
| Trimethoprim | 0.012 ± 0.003 |
| I.1 | 0.026 ± 0.0076 |
| I.2 | 0.036 ± 0.015 |
| I.3 | 1.2 ± 0.44 |
| I.4 | 3.1 ± 1.5 |
| I.5 | 0.30 ± 0.064 |
| I.6 | 3.1 ± 2.6 |
| I.7 | 0.23 ± 0.27 |
| II.2 | 0.00053 ± 0.0022 |
| II.3 | <0.0001 |
| II.4 | 0.0029 ± 0.0029 |
| II.5 | 0.024 ± 0.017 |
| II.6 | 0.059 ± 0.044 |
| III.1 | 0.0084 ± <0.001 |
| III.2 | 0.0061 ± 0.0025 |
| III.3 | 0.00045 ± <0.0002 |
| III.4 | 0.012 ± 0.0024 |
| III.5 | 0.00011 ± <0.00002 |
| III.6 | <0.0001 |
| IV.1 | 0.0034 ± 0.0028 |
| IV.2 | 0.046 ± 0.015 |
| IV.3 | 0.087 ± 0.010 |
| IV.4 | 8.5 ± 2.5 |
| IV.5 | 0.21 ± 0.088 |
| IV.6 | 0.28 ± <0.0001 |
| IV.7 | 0.068 ± 0.00887 |
| IV.8 | 3.1 ± 0.68 |
| IV.9 | 7.5 ± 2.2 |
| IV.19 | 4.5 ± 2.6 |
| IV.11 | 7.9 ± 1.8 |
| IV.12 | 2.0 ± 1.3 |
| IV.13 | <0.0001 |
| IV.14 | 0.00014 ± <0.0001 |
| IV.15 | 1.5 ± 0.20 |
| IV.16 | 1.1 ± 0.16 |
| IV.17 | <0.0001 |
| IV.18 | 0.00012 ± <0.0001 |
| V.1 | 0.041 ± 0.014 |
| V.2 | 0.00084 ± <0.0004 |
| V.3 | 0.080 ± 0.020 |
| V.4 | 0.011 ± 0.0020 |
| V.5 | 0.0014 ± 0.0011 |
| V.6 | 0.0036 ± 0.0016 |
| V.7 | 0.0066 ± 0.0013 |
| V.8 | 0.00054 ± 0.00025 |
| V.9 | 0.0011 ± 0.0010 |
| V.10 | 0.00016 ± 0.00012 |
| V.11 | 0.0019 ± 0.00026 |
| V.12 | 0.0030 ± 0.00080 |
| V.13 | 0.00052 ± 0.00032 |
| V.14 | 0.00025 ± 0.00026 |
| V.15 | 0.0021 ± 0.0016 |
| V.16 | 0.00024 ± 0.00012 |
| VI.3 | 0.0036 ± <0.001 |
| VI.4 | 0.031 ± 0.016 |
| VI.5 | 0.018 ± 0.0052 |
| VI.6 | 0.075 ± 0.015 |
| VI.7 | 0.031 ± 0.019 |
| VI.12 | 0.0075 ± 0.,0023 |
| VII.1 | 0.097 ± 0.031 |
| VII.4 | 3.3 ± 1.1 |
| VIII.1 | 1.8 ± 0.60 |
| VIII.2 | 0.19 ± 0.025 |
| VIII.3 | 0.18 ± 0.060 |
| VIII.6 | 0.0035 ± 0.0013 |
| VIII.7 | 0.0077 ± 0.012 |
| VIII.8 | 0.032 ± 0.00565 |
| VIII.9 | 0.087 ± 0.052 |
| VIII.10 | 0.62 ± 0.33 |
| IX.1 | 0.012 ± 0.0044 |
| IX.2 | 0.026 ± 0.014 |
| IX.3 | 0.018 ± 0.0064 |
| IX.4 | 0.011 ± 0.0056 |
The values shown are the means ± the standard deviations of several determinations. Compounds VI.9 and VI.12 to VI.17 are not listed because their IC50s were >10 μM. Compounds I.8, II.1, VI.1, VI.2, VI.10, VII.2, and VII.3 were not tested. Structures are shown in Fig. 2.
The preferential affinity of trimethoprim for E. coli DHFR versus human DHFR has been tentatively ascribed to the fact that, in the case of the bacterial enzyme (at least in the binary complex without NADPH present), the trimethoxybenzyl group can interact with two different hydrophobic pockets at the active site, called the upper and lower clefts, whereas in the human enzyme the trimethoxybenzyl group binds only to the upper cleft (45). The decreased ability of our compounds to discriminate between the E. coli and human DHFRs, relative to that of trimethoprim, may be due to the increased separation between the aryl group of the side chain and the diaminopyrimidine moiety, as well as to the fact that the diaminopyrimidine moiety is part of a di- or tricyclic ring system. This combination of features apparently causes the diaminopyrimidine derivatives to lose the ability to interact only with the upper cleft of the human enzyme. It may be noted that trimethoprim displays much more selectivity for the E. coli enzyme than for the C. parvum enzyme under identical assay conditions. Thus, we believe that finding di- or tricyclic DHFR inhibitors with selectivity for the C. parvum enzyme comparable to that of trimethoprim for the E. coli enzyme may prove very difficult and that greater success might be achieved by seeking to increase the potency of trimethoprim rather than to increase the selectivity of trimetrexate or piritrexim.
FIG. 3.
Structures of dicyclic diaminopyrimidines tested as DHFR inhibitors (groups V to VII).
ACKNOWLEDGMENTS
Support of these studies was provided in part by grant RO-AI29904 to A.R., contract UO1-AI40319 to R.G.N., and contract NO1-AI35171 to S.F.Q. from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Armitage K, Flanigan T, Carey J, Frank I, MacGregor R R, Ross P, Goodgame R, Turner J. Treatment of cryptosporidiosis with paromomycin. A report of five cases. Arch Intern Med. 1992;152:2497–2499. [PubMed] [Google Scholar]
- 2.Assaraf Y G, Molina A, Schimke R. Sequential amplification of dihydrofolate reductase and multidrug resistance genes in Chinese hamster ovary cells selected for stepwise resistance to the lipid-soluble antifolate trimetrexate. J Biol Chem. 1989;31:18326–18334. [PubMed] [Google Scholar]
- 3.Baccanari D P, Daluge S, King R W. Inhibition of dihydrofolate reductase: effect of reduced nicotinamide adenine dinucleotide phosphate on the selectivity and affinity of diaminobenzylpyrimidines. Biochemistry. 1982;21:5068–5075. doi: 10.1021/bi00263a034. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett M S, Shaw M, Navaran P, Smith J W, Queener S F. Evaluation of potent inhibitors of dihydrofolate reductase in a culture model for growth of Pneumocystis carinii. Antimicrob Agents Chemother. 1995;39:2436–2441. doi: 10.1128/aac.39.11.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blagburn B, Drain K L, Land T M, Moore P H, Kinard R G, Lindsay D S, Kumar A, Shi J, Boykin D W, Tidwell R R. Dicationic furans inhibit development of Cryptosporidium parvum in HSD/ICR suckling Swiss mice. J Parasitol. 1998;84:851–856. [PubMed] [Google Scholar]
- 6.Blanshard C, Shanson D C, Gazzard B G. Pilot studies of azithromycin, letrazuril and paromomycin in the treatment of cryptosporidiosis. Int J STD AIDS. 1997;8:124–129. doi: 10.1258/0956462971919543. [DOI] [PubMed] [Google Scholar]
- 7.Brophy V H, Vasquez J, Nelson R G, Forney J R, Rosowsky A, Sibley C H. Identification of Cryptosporidium parvum dihydrofolate reductase inhibitors by complementation in Saccharomyces cerevisiae. Antimicrob Agents Chemother. 2000;44:1019–1028. doi: 10.1128/aac.44.4.1019-1028.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broughton M C, Queener S F. Pneumocystis carinii dihydrofolate reductase used to screen potential antipneumocystis drugs. Antimicrob Agents Chemother. 1991;35:1348–1355. doi: 10.1128/aac.35.7.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burchall J J, Hitchings G H. Inhibitor binding analysis of dihydrofolate reductases from various species. Mol Pharmacol. 1965;1:126–136. [PubMed] [Google Scholar]
- 10.Chio L-C, Queener S F. Identification of potent inhibitors of Toxoplasma gondii dihydrofolate reductase. Antimicrob Agents Chemother. 1993;37:1914–1923. doi: 10.1128/aac.37.9.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coombs G H. Biochemical peculiarities and drug targets in Cryptosporidium parvum: lessons from other coccidian parasites. Parasitol Today. 1999;15:333–338. doi: 10.1016/s0169-4758(99)01474-x. [DOI] [PubMed] [Google Scholar]
- 12.DeHovitz J A, Pape J W, Boncy M, Johnson W D., Jr Clinical manifestations and therapy of Isospora belli infection in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1986;315:87–90. doi: 10.1056/NEJM198607103150203. [DOI] [PubMed] [Google Scholar]
- 13.Falloon J, Allegra C J, Kovacs J, O'Neill D, Ogata-Arakaki D, Feuerstein I, Polis M, Davey R, Lane H C, LaFon S, Rogers M, Zunich K, Turlo J, Tuazon C, Parenti D, Simon G, Masur H. Piritrexim with leucovorin for the treatment of pneumocystis pneumonia (PCP) in AIDS patients. Clin Res. 1990;38:361A. [Google Scholar]
- 14.Falloon J, Masur H. Infectious complications of HIV. In: DeVita V T, Hellman S, Rosenberg S A, editors. AIDS etiology, diagnosis, treatment and prevention. 3rd ed. Philadelphia, Pa: Lippincott; 1992. pp. 157–162. [Google Scholar]
- 15.Gangjee A, Adair O, Queener S F. Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase inhibitors and antitumor agents: synthesis and biological activities of 2,4-diamino-5-methyl-6-[(monosubstituted anilino)methyl]pyrido[2,3-d]pyrimidines. J Med Chem. 1999;42:2447–2455. doi: 10.1021/jm990079m. [DOI] [PubMed] [Google Scholar]
- 16.Gangjee A, Devraj R, McGuire J J, Kisliuk R L. Effect of bridge variation on antifolate and antitumor activity of classical 5-substituted 2,4-diaminofuro[2,3-d]pyrimidines. J Med Chem. 1995;38:3798–3805. doi: 10.1021/jm00019a009. [DOI] [PubMed] [Google Scholar]
- 17.Gangjee A, Devraj R, McGuire J J, Kisliuk R L, Queener S F, Barrows L R. Classical and nonclassical furo[2,3-d]pyrimidines as novel antifolates: synthesis and biological activities. J Med Chem. 1994;37:1169–1176. doi: 10.1021/jm00034a015. [DOI] [PubMed] [Google Scholar]
- 18.Gangjee A, Devraj R, Queener S F. Synthesis and dihydrofolate reductase inhibitor activities of 2,4-diamino-5-deaza- and 2,4-diamino-5,10-dideaza lipophilic antifolates. J Med Chem. 1997;40:470–478. doi: 10.1021/jm9606913. [DOI] [PubMed] [Google Scholar]
- 19.Gangjee A, Dubash N P, Queener S F. The synthesis of new 2,4-diaminofuro[2,3-d]pyrimidines with 5-biphenyl, phenoxyphenyl and tricyclic substitutions as dihydrofolate reductase inhibitors. J Heterocycl Chem. 2000;37:935–942. [Google Scholar]
- 20.Gangjee A, Elzein E, Queener S F, McGuire J J. Synthesis and biological activities of tricyclic conformationally restricted tetrahydropyrido annulated furo[2,3-d]pyrimidines as inhibitors of dihydrofolate reductases. J Med Chem. 1998;41:1409–1416. doi: 10.1021/jm9705420. [DOI] [PubMed] [Google Scholar]
- 21.Gangjee A, Guo X, Queener S F, Cody V, Galitsky N, Luft J R, Pangborn W. Selective Pneumocystis carinii dihydrofolate reductase inhibitors: design, synthesis, and biological evaluation of new 2,4-diamino-5-substituted-furo[2,3-d]pyrimidines. J Med Chem. 1998;44:1263–1271. doi: 10.1021/jm970537w. [DOI] [PubMed] [Google Scholar]
- 22.Gangjee A, Mavandadi F, Queener S F. Effect of N9-methylation and bridge atom variation on the activity of 5-substituted 2,4-diaminopyrrolo[2,3-d]pyrimidines against dihydrofolate reductases from Pneumocystis carinii and Toxoplasma gondii. J Med Chem. 1997;40:1173–1177. doi: 10.1021/jm960717q. [DOI] [PubMed] [Google Scholar]
- 23.Gangjee A, Mavandadi F, Queener S F, McGuire J J. Novel 2,4-diamino-5-substituted-pyrrolo[2,3-d]pyrimidines as classical and nonclassical antifolate inhibitors of dihydrofolate reductase. J Med Chem. 1995;38:2158–2165. doi: 10.1021/jm00012a016. [DOI] [PubMed] [Google Scholar]
- 24.Gangjee A, Shi J, Queener S F. Synthesis of 5-methyl-5-deaza non-classical antifolates as inhibitors of dihydrofolate reductases and as potential antipneumocystis, antitoxoplasma, and antitumor agents. J Med Chem. 1993;36:3437–3443. doi: 10.1021/jm00074a026. [DOI] [PubMed] [Google Scholar]
- 25.Gangjee A, Shi J, Queener S F. Synthesis and biological activities of conformationally restricted, tricyclic nonclassical antifolates as inhibitors of dihydrofolate reductase. J Med Chem. 1997;40:1930–1936. doi: 10.1021/jm960693n. [DOI] [PubMed] [Google Scholar]
- 26.Gangjee A, Vasudevan A, Queener S F. Conformationally restricted analogues of trimethoprim: 2,6-diamino-8-substituted purines as potential dihydrofolate reductase inhibitors from Pneumocystis carinii and Toxoplasma gondii. J Med Chem. 1997;40:3032–3039. doi: 10.1021/jm970271t. [DOI] [PubMed] [Google Scholar]
- 27.Gangjee A, Vasudevan A, Queener S F, Kisliuk R L. 6-Substituted 2,4-diamino-5-methyl-pyrido[2,3-d]pyrimidines as inhibitors of dihydrofolate reductases from Pneumocystis carinii and Toxoplasma gondii and as antitumor agents. J Med Chem. 1995;38:1778–1785. doi: 10.1021/jm00010a022. [DOI] [PubMed] [Google Scholar]
- 28.Gangjee A, Vidwans A P, Vasudevan A, Queener S F, Kisliuk R L, Cody V, Li R, Galitsky N, Luft J E, Pangborn W. Structure-based design and synthesis of lipophilic 2,4-diamino-6-substituted quinazolines and their evaluation as inhibitors of dihydrofolate reductases and potential antitumor agents. J Med Chem. 1998;41:3426–3434. doi: 10.1021/jm980081y. [DOI] [PubMed] [Google Scholar]
- 29.Gangjee A, Zaveri N, Kothare M, Queener S F. Non-classical 2,4-diamino-6-(amino-methyl)-5,6,7,8-tetrahydroquinazoline antifolates: synthesis and biological activities. J Med Chem. 1995;38:1959–1966. doi: 10.1021/jm00018a027. [DOI] [PubMed] [Google Scholar]
- 30.Gangjee A, Zhu Y, Queener S F. 6-Substituted 2,4-diaminopyrido[3,2-d]pyrimidine analogues of piritrexim as inhibitors of dihydrofolate reductase from rat liver, Pneumocystis carinii, and Toxoplasma gondii and as antitumor agents. J Med Chem. 1998;41:4533–4541. doi: 10.1021/jm980206z. [DOI] [PubMed] [Google Scholar]
- 31.Gangjee A, Zhu Y, Queener S F, Francom P, Broom A D. Nonclassical 2,4-diamino-8-deazafolate analogues as inhibitors of dihydrofolate reductases from rat liver. Pneumocystis carinii, and Toxoplasma gondii. J Med Chem. 1996;39:1836–1845. doi: 10.1021/jm950918e. [DOI] [PubMed] [Google Scholar]
- 32.Gargala G, Delaunay A, Li X, Brasseur P, Favennec L, Ballet J J. Efficacy of nitazoxanide, tizoxanide and tizoxanide glucuronide against Cryptosporidium parvum development in sporozoite-infected HCT-8 enterocytic cells. J Antimicrob Chemother. 2000;46:57–60. doi: 10.1093/jac/46.1.57. [DOI] [PubMed] [Google Scholar]
- 33.Griffiths J K. Human cryptosporidiosis: epidemiology, transmission, clinical disease, treatment, and diagnosis. Adv Parasitol. 1998;40:37–85. doi: 10.1016/s0065-308x(08)60117-7. [DOI] [PubMed] [Google Scholar]
- 34.Griffith J K, Balankrishnan R, Widmer G, Tzipori S S. Paromomycin and Geneticin inhibit intracellular Cryptosporidium parvum without trafficking through the host cell cytoplasm: implications for drug delivery. Infect Immun. 1998;66:3874–3883. doi: 10.1128/iai.66.8.3874-3883.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guarino A, Berni Canani R, Spagnuolo M I, Bisceglia M, Boccia M C, Rubino A. In vivo and in vitro efficacy of octreotide for treatment of enteric cryptosporidiosis. Dig Dis Sci. 1998;43:436–441. doi: 10.1023/a:1018839329759. [DOI] [PubMed] [Google Scholar]
- 36.Jackson H C, Biggadike K, McKilligin E, Kinsman O S, Queener S F, Lane A, Smith J E. 6,7-Disubstituted 2,4-diaminopteridines: novel inhibitors of Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase. Antimicrob Agents Chemother. 1996;40:1371–1374. doi: 10.1128/aac.40.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khalifa L, Rosales M J, Mascaro C, Karolak-Wojciechowska J, Bsiri N, Brouant P, Barbe J. Inhibition of Cryptosporidium parvum in vitro by 9-(alkylthio)acridine derivatives. Arzneim-Forsch. 2000;50:163–166. doi: 10.1055/s-0031-1300183. [DOI] [PubMed] [Google Scholar]
- 38.Klepser M E, Klepser T B. Drug treatment of HIV-related opportunistic infections. Drugs. 1997;53:40–73. doi: 10.2165/00003495-199753010-00004. [DOI] [PubMed] [Google Scholar]
- 39.Kovacs J A, Allegra C J, Beaver J J, Boarman D, Lewis M, Parrillo J E, Chabner B, Masur H. Characterization of de novo folate synthesis in Pneumocystis carinii and Toxoplasma gondii: potential for screening therapeutic agents. J Infect Dis. 1989;160:312–320. doi: 10.1093/infdis/160.2.312. [DOI] [PubMed] [Google Scholar]
- 40.Lau H, Ferlan J T, Brophy V T, Rosowsky A, Sibley C H. Efficacies of lipophilic inhibitors of dihydrofolate reductase against parasitic protozoa. Antimicrob Agents Chemother. 2001;45:187–195. doi: 10.1128/AAC.45.1.187-195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L, Queener S F, Abraham A, Desai A, Nair M G. Nonclassical analogues of 10-deazaminopterin: synthesis and inhibition of rat liver, P. carinii, and T. gondii dihydrofolate reductase. Med Chem Res. 1993;4:211–221. [Google Scholar]
- 42.Mead J R, Benbow J W, Garmon D, Stewart J. Improved efficacy of dinitroaniline analogs for use as anti-cryptosporidial drugs. J Eukaryot Microbiol. 1999;46:62S–63S. [PubMed] [Google Scholar]
- 43.Mead J R, You X, Pharr J E, Belenkaya Y, Arrowood M J, Fallon M T, Schinazi R F. Evaluation of maduramicin and alborixin in a SCID mouse model of chronic cryptosporidiosis. Antimicrob Agents Chemother. 1995;39:854–858. doi: 10.1128/aac.39.4.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medina I, Mills J, Leoung G, Hopewell P C, Modin G, Benowitz N, Wofsy C B. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med. 1990;323:773–782. doi: 10.1056/NEJM199009203231202. [DOI] [PubMed] [Google Scholar]
- 45.Oefner C, D'Arcy A, Winkler F K. Crystal structure of human dihydrofolate reductase complexed with folate. Eur J Biochem. 1988;174:377–385. doi: 10.1111/j.1432-1033.1988.tb14108.x. [DOI] [PubMed] [Google Scholar]
- 46.Peng M M, Xiao L, Freemam A R, Arrowood M J, Escalante A A, Weltman A C, Ong C S, MacKenzie W R, Lal A A, Beard C B. Genetic polymorphism among Cryptosporidium parvum isolates: evidence of two distinct human transmission cycles. Emerg Infect Dis. 1997;3:567–573. doi: 10.3201/eid0304.970423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perkins M E, Wu T W, Le Blancq S E. Cyclosporin analogs inhibit in vitro growth of Cryptosporidium parvum. Antimicrob Agents Chemother. 1998;42:843–848. doi: 10.1128/aac.42.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piper J R, Johnson C A, Krauth C A, Carter R L, Hosmer C A, Queener S F, Borotz S E, Pfefferkorn E R. Lipophilic antifolates as agents against opportunistic infections. 1. Agents superior to trimetrexate and piritrexim against Toxoplasma gondii and Pneumocystis carinii in in vitro evaluations. J Med Chem. 1996;39:1271–1280. doi: 10.1021/jm950760y. [DOI] [PubMed] [Google Scholar]
- 49.Podzamczer D, Salazar A, Jimenes J, Santin M, Consiglio E, Casanova A, Rufi G, Gudiol F. Intermittent trimethoprim-sulfamethoxazole compared with dapsone-pyrimethamine for the simultaneous prophylaxis of pneumocystis pneumonia and toxoplasmosis in patients infected with HIV. Ann Intern Med. 1995;122:755–761. doi: 10.7326/0003-4819-122-10-199505150-00004. [DOI] [PubMed] [Google Scholar]
- 50.Prendergast N J, Delcamp T J, Smith P L, Freisheim J H. Expression and site-directed mutagenesis of human dihydrofolate reductase. Biochemistry. 1988;27:3664–3671. doi: 10.1021/bi00410a022. [DOI] [PubMed] [Google Scholar]
- 51.Queener S F, Bartlett M S, Jay M A, Durkin M M, Smith J W. Activity of lipid-soluble inhibitors of dihydrofolate reductase against Pneumocystis carinii in culture and in a rat model of infection. Antimicrob Agents Chemother. 1987;31:1323–1327. doi: 10.1128/aac.31.9.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosowsky A, Chaykovsky M, Lin M, Modest E J. Pteridines. 2. New 6,7-disubstituted pteridines as potential antimalarial and antitumor agents. J Med Chem. 1973;16:869–875. doi: 10.1021/jm00266a001. [DOI] [PubMed] [Google Scholar]
- 53.Rosowsky A, Chen K K N, Lin M, Nadel M E, St. Amand R A, Yeager S A. Synthesis of two new heterocyclic ring systems: benzo[3,4]cyclohepta[1,2-d]pyrimidines and benzo[3,4]cyclohepta[2,1-d]pyrimidines. J Heterocycl Chem. 1971;8:789–795. [Google Scholar]
- 54.Rosowsky A, Chen K K N, Nadel M E, Papathanasopoulos N, Modest E J. Quinazolines. VIII. Synthesis of 1,3-diaminobenzo[f]quinazolines. J Heterocycl Chem. 1972;9:275–283. [Google Scholar]
- 55.Rosowsky A, Chen K K N, Papathanasopoulos N, Modest E J. Quinazolines. VII. Synthesis of 1,3-diamino-5,6-dihydrobenzo[f]quinazolines. J Heterocycl Chem. 1972;9:263–273. [Google Scholar]
- 56.Rosowsky A, Cody V, Galitsky N, Fu H, Papoulis A T, Queener S F. Structure-based design of selective inhibitors of dihydrofolate reductase: synthesis and antiparasitic activity of 2,4-diaminopteridine analogues with a bridged diarylamine side chain. J Med Chem. 1999;42:4853–4860. doi: 10.1021/jm990331q. [DOI] [PubMed] [Google Scholar]
- 57.Rosowsky A, Dey A S, Battaglia J, Modest E J. Synthesis of 2,4-diamino-9H-indeno[2,1-d]pyrimidines. J Heterocycl Chem. 1969;6:613–622. [Google Scholar]
- 58.Rosowsky A, Forsch R A, Queener S F. 2,4-Diaminopyrido[3,2-d]pyrimidine inhibitors of dihydrofolate reductase form Pneumocystis carinii and Toxoplasma gondii. J Med Chem. 1995;38:2615–2620. doi: 10.1021/jm00014a014. [DOI] [PubMed] [Google Scholar]
- 59.Rosowsky A, Forsch R A, Queener S F. Synthesis of 2,4-diaminopteridines with bulky lipophilic groups at the 6 position as inhibitors of Pneumocystis carinii, Toxoplasma gondii, and mammalian dihydrofolate reductase. Pteridines. 1997;8:173–187. [Google Scholar]
- 60.Rosowsky A, Fu H, Queener S F. Synthesis of 2,4-diaminopyrido[2,3-d]pyrimidines and 2,4-diaminoquinazolines with bulky dibenz[b,f]azepine and dibenzo[a,d]cycloheptene substituents at the 6 position as inhibitors of dihydrofolate reductases from Pneumocystis carinii, Toxoplasma gondii, and Mycobacterium avium. J Heterocycl Chem. 2000;37:921–926. [Google Scholar]
- 61.Rosowsky A, Hynes J B, Queener S F. Structure-activity and structure-selectivity studies on diaminoquinazolines and other inhibitors of Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase. Antimicrob Agents Chemother. 1995;39:79–86. doi: 10.1128/aac.39.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosowsky A, Marini J L, Nadel M E, Modest E J. Quinazolines. VI. Synthesis of 2,4-diaminoquinazolines from anthranilonitriles. J Med Chem. 1970;13:882–886. doi: 10.1021/jm00299a021. [DOI] [PubMed] [Google Scholar]
- 63.Rosowsky A, Mota C E, Queener S F. Synthesis and antifolate activity of 2,4-diamino-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine analogues of trimetrexate and piritrexim. J Heterocycl Chem. 1995;32:335–340. [Google Scholar]
- 64.Rosowsky A, Mota C E, Queener S F. Brominated trimetrexate analogues as inhibitors of Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase. J Heterocycl Chem. 1996;33:1959–1966. [Google Scholar]
- 65.Rosowsky A, Mota C E, Queener S F, Waltham M, Ercikan-Abali E, Bertino J R. 2,4-Diamino-5-substituted-quinazolines as inhibitors of a human dihydrofolate reductase with a site-directed mutation at position 22 and of the dihydrofolate reductases from Pneumocystis carinii and Toxoplasma gondii. J Med Chem. 1995;38:745–752. doi: 10.1021/jm00005a002. [DOI] [PubMed] [Google Scholar]
- 66.Rosowsky A, Mota C E, Wright J E, Freisheim J E, Heusner J J, McCormack J J, Queener S F. 2,4-Diaminothieno[2,3-d]pyrimidine analogues of trimetrexate and piritrexim as potential inhibitors of Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase. J Med Chem. 1993;36:3103–3112. doi: 10.1021/jm00073a009. [DOI] [PubMed] [Google Scholar]
- 67.Rosowsky A, Mota C E, Wright J E, Queener S F. 2,4-Diamino-5-chloroquinazoline analogues of trimetrexate and piritrexim as potential inhibitors of Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase. J Med Chem. 1994;37:4522–4528. doi: 10.1021/jm00052a011. [DOI] [PubMed] [Google Scholar]
- 68.Rosowsky A, Papoulis A T, Forsch R A, Queener S F. Synthesis and antiparasitic and antitumor activity of 2,4-diamino-6-arylmethyl-5,6,7,8-tetrahydroquinazoline analogues of piritrexim. J Med Chem. 1999;42:1007–1017. doi: 10.1021/jm980572i. [DOI] [PubMed] [Google Scholar]
- 69.Rosowsky A, Papoulis A T, Queener S F. 2,4-Diaminothieno[2,3-d]pyrimidine lipophilic antifolates as inhibitors of Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase. J Med Chem. 1997;40:3694–3699. doi: 10.1021/jm970399a. [DOI] [PubMed] [Google Scholar]
- 70.Rosowsky A, Papoulis A T, Queener S F. 2,4-Diamino-6,7-dihydro-5H-cyclopenta[d]pyrimidine analogues of trimethoprim as inhibitors of Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase. J Med Chem. 1998;41:913–918. doi: 10.1021/jm970614n. [DOI] [PubMed] [Google Scholar]
- 71.Rosowsky A, Papoulis A T, Queener S F. One-step synthesis of novel 2,4-diaminopyrimidine antifolates from bridged alicyclic ketones and cyanoguanidine. J Heterocycl Chem. 1999;36:723–728. [Google Scholar]
- 72.Rosowsky A, Queener S F, Cody V. Inhibition of dihydrofolate reductases from Toxoplasma gondii, Pneumocystis carinii, and rat liver by rotationally restricted analogues of pyrimethamine and metoprine. Drug Design Discovery. 1999;16:25–40. [PubMed] [Google Scholar]
- 73.Roudier C, Caumes E, Rogeauz O, Bricaire F, Gentilini M. Adverse cutaneous reactions to trimethoprim-sulfamethoxazole in patients with the acquired immunodeficiency syndrome and Pneumocystis carinii pneumonia. Arch Dermatol. 1994;30:1383–1386. [PubMed] [Google Scholar]
- 74.Sattler F R, Frame P, Davis R, Nichols L, Shelton N, Akil B, Baughman R, Hughlett C, Weiss W, Boylen C T, van der Horst C, Black J, Powderly W, Steigbigel R T, Leedom J M, Masur H, Feinberg J, Benoit S, Eyste E, Gocke D, Beck K, Aldermen M, Phari J, Reichman R, Sacks H S, Soiero R. Trimetrexate with leucovorin versus trimethoprim-sulfamethoxazole for moderate to severe episodes of Pneumocystis carinii pneumonia in patients with AIDS—a prospective controlled multicenter investigation of the AIDS Clinical Trials Group Protocol 029/031. J Infect Dis. 1994;170:165–172. doi: 10.1093/infdis/170.1.165. [DOI] [PubMed] [Google Scholar]
- 75.Tzipori S. Cryptosporidiosis: laboratory investigations and chemotherapy. Adv Parasitol. 1998;40:187–221. doi: 10.1016/s0065-308x(08)60121-9. [DOI] [PubMed] [Google Scholar]
- 76.Vásquez J R, Goozé L, Kim K, Gut J, Petersen C, Nelson R G. Potential antifolate resistance determinants and genotypic variation in the bifunctional dihydrofolate reductase-thymidylate synthase gene from human bovine isolates of Cryptosporidium parvum. Mol Biochem Parasitol. 1996;79:153–165. doi: 10.1016/0166-6851(96)02647-3. [DOI] [PubMed] [Google Scholar]
- 77.Weiss L M, Perlman D C, Sherman J, Tanowitz H, Wittner M. Isospora belli infection: treatment with pyrimethamine. Ann Intern Med. 1988;109:474–475. doi: 10.7326/0003-4819-109-6-474. [DOI] [PubMed] [Google Scholar]
- 78.Wiest P M, Johnson J H, Flanigan T P. Microtubule inhibitors block Cryptosporidium parvum infection of a human enterocyte cell line. Infect Immun. 1993;61:4888–4890. doi: 10.1128/iai.61.11.4888-4890.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woods K M, Nesterenko M V, Upton S J. Efficacy of 101 antimicrobials and other agents on the development of Cryptosporidium parvum in vitro. Ann Trop Med Parasitol. 1996;90:603–615. doi: 10.1080/00034983.1996.11813090. [DOI] [PubMed] [Google Scholar]