Abstract
Sordarins constitute a new class of antifungal agents with a novel mechanism of action involving the selective inhibition of fungal protein synthesis. A further evolution of this class of antifungals has led to a new family of sordarin derivatives called azasordarins. The therapeutic efficacies of two new azasordarins, GW471552 and GW471558, were studied in experimental models of oral and vulvovaginal candidiasis in immunosuppressed rats. In all cases rats were immunosuppressed with dexamethasone in the drinking water. Oral candidiasis was established by inoculating 0.1 ml of a yeast suspension containing 5 × 108 cells of Candida albicans 4711E with a cotton swab on three alternate days. Vulvovaginal candidiasis was established in ovariectomized and estrus-induced rats by intravaginal inoculation of 107 CFU of C. albicans 4711E in 0.1 ml of saline. GW471552 and GW471558 were administered at 1, 5, and 10 mg/kg of body weight via the subcutaneous route. In oral candidiasis, azasordarins were administered each 8 h for 7 consecutive days, while in vaginal candidiasis the compounds were given each 4 h for 3 consecutive days. Antifungal activity of azasordarins was assessed by colony counts and by histological examination 1 day after treatment. In the oral infection model, GW471552 and GW471558 administered at 5 mg/kg significantly reduced (P < 0.05) the number of CFU of C. albicans compared with untreated controls. In addition, GW471552 and GW471558 given at 10 mg/kg eradicated C. albicans from the oral cavities of 100% of infected animals. Against vulvovaginal infection, both compounds showed significant therapeutic efficacy. GW471552 was able to eradicate the vaginal fungal burden at a dose of 10 mg/kg, and it significantly reduced the number of CFU of C. albicans in vaginas of rats treated with a dose of 5 mg/kg (P < 0.05). GW471558 showed greater efficacy, eradicating the fungal burden of 100% of infected rats at a dose of 5 mg/kg and significantly reducing (P < 0.05) the C. albicans vaginal counts even at a dose of 1 mg/kg. In both therapeutic efficacy studies, the histological findings confirmed the microbiological results. The experimental results presented show that the tested azasordarins are effective against oral and vulvovaginal candidiasis in immunosuppressed rats and could be promising antifungal agents for use in humans.
Infections caused by Candida albicans have increased in prevalence worldwide (3, 19). These infections range from mucosal candidiasis, including oropharyngeal and esophageal candidiasis, which is frequently observed in immunocompromised patients, to vulvovaginal candidiasis (VVC), which affects a large number of otherwise healthy women (19).
Oropharyngeal candidiasis is the most common opportunistic infection in immunosuppressed individuals and is strongly correlated with impairment of the immune system. In addition, several conditions, such as hyposalivation, diabetes mellitus, and prolonged antibiotic use can predispose an individual to oral infection.
C. albicans vaginitis has been increasing in medical importance, since a significant proportion of women suffer from acute episodes and recurrent infections may often occur after therapy. It has been estimated that 75% of all women will experience an episode of Candida vaginitis once in their lifetime, with up to 5% showing recurrence (8). VVC is often associated with conditions such as diabetes mellitus, antibiotherapy, and pregnancy, although in many cases there are no clear predisposing factors (18).
In recent years azole agents have become the drugs of choice for treating both oropharyngeal candidiasis and VVC (16, 18). However, recent studies have indicated the possibility of treatment failures associated with C. albicans resistance to azoles (4, 12). New and effective drugs are therefore needed to treat these fungal infections.
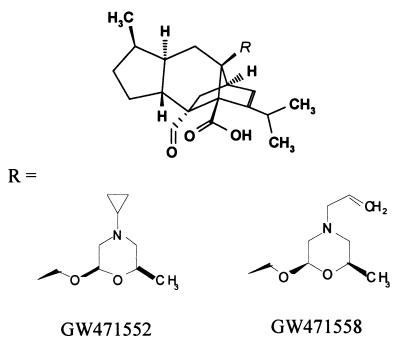
Sordarins are a new class of antifungal agents that act by inhibiting the protein synthesis elongation cycle (5, 6). Sordarin derivatives have demonstrated potent and relatively broad-spectrum antifungal activity in several in vitro (10) and in vivo (1, 13, 14) studies. A further evolution of this class of compounds has led to a new family of substances called azasordarins. Azasordarins are chemically characterized by the presence of a 6-methylmorpholin-2-yl group with different N-4′ substituents at position 8a of the sordaricin indacene ring system (Fig. 1). They have demonstrated excellent activity against key fungal pathogens related to mucosal infections, such as C. albicans and other, non-C. albicans species (9a).
FIG. 1.
Chemical structures of new azasordarins.
In order to understand the potential use of azasordarins for the treatment of mucosal candidiasis, the therapeutic efficacies of GW471552 and GW471558, as representatives of these new family of antifungal agents, have been evaluated in two experimental infection models of oral candidiasis and VVC in immunosuppressed rats. Taking into account that C. albicans infective forms are pseudohyphae, which are able to penetrate epithelial layers, the therapeutic efficacy of azasordarins against C. albicans infections has been evaluated by microbiological but also by histopathological studies.
(This work was presented in part at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, 17–20 September 2000 [A. Martinez, S. Ferrer, E. Jimenez, J. Sparrowe, J. Caballero, and D. Gargallo-Viola, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1689, 2000].)
MATERIALS AND METHODS
Antifungal agents.
The azasordarin derivatives GW471552 and GW471558 (Fig. 1) were synthesized and supplied by the Organic Chemistry Department of GlaxoSmithKline, Tres Cantos, Spain. The compounds, as sodium salts, were individually dissolved and diluted to the appropriate concentration in sterile physiological saline solution (SPSS) immediately prior to administration. Doses of antifungal agents are given as milligrams of base per kilogram of body weight.
Organisms and inoculum preparation.
Therapeutic efficacy studies were performed against C. albicans 4711E, a clinical isolate used in previous in vivo studies (1, 13, 14). C. albicans 4711E was stored at −80°C in Sabouraud dextrose broth containing 15% glycerol in our laboratory until used. For inoculum preparation, C. albicans 4711E was grown on Sabouraud dextrose agar (Difco Laboratories, Madrid, Spain) plates at 30°C for 24 h. Fungal colonies were then scraped off the agar, washed three times in SPSS, and adjusted in this solution to the appropriate concentration using a hemacytometer chamber. In addition, the viable inoculum was confirmed by quantitative cultures of serial 10-fold dilutions on Sabouraud dextrose agar plates at the time of infection. All counts are expressed as CFU of viable organisms.
MIC determination.
The MICs of GW471552 and GW471558 against C. albicans 4711E were determined by the broth microdilution method according to National Committee for Clinical Laboratory Standards methods (15).
Animals.
Six-week-old specific-pathogen-free female Sprague-Dawley rats (Iffa-Credo Laboratories, Lyon, France) weighing approximately 200 g were used. The rats were housed in 480- by 270- by 200-mm Apec cages (Techniplast; Letica Scientific Instruments, Madrid, Spain) on corncob granules (Panlab, Barcelona, Spain). Photoperiods were adjusted to 12 h of light and 12 h of darkness daily, and the environmental temperature was constantly maintained at 21 ± 1°C. Rats were given ad libitum access to food and water. The research complied with European legislation and company policy on the care and use of animals and with related codes of practice.
Oral candidiasis model.
Oral infection in rats was induced basically as reported by Jones and Adams (11) with some modifications (14). Rats were immunosuppressed, starting 1 week before initiation of experimental infection and continued throughout the experiment, by administering dexamethasone (Fortecortin; Merck Laboratories, Madrid, Spain) in drinking water at a dose of 0.5 mg per liter. Also, a 0.1% aqueous solution of tetracycline hydrochloride (Terramycin; Pfizer Laboratories, Madrid, Spain) was given to the animals beginning 7 days before infection. The concentration of tetracycline hydrochloride was reduced to 0.01% when the infection process was started and was maintained throughout the experiment. Experimental infection was established by inoculation of the oral cavities of the rats three times at 48-h intervals with 0.1 ml of a yeast suspension containing 5 × 108 cells of C. albicans 4711E. Oral inoculation was performed by means of a cotton swab rolled twice over all parts of the mouth. Rats were then randomly distributed into groups of 10 animals each. Three days after the last Candida inoculation, infected animals were sampled to quantify the number of CFU in the oral cavity (see below). The mean log10 CFU and standard deviation per sample were calculated and accepted as the basal level of infection before treatment.
VVC model.
The animal model of VVC was established based on previously described models (19, 21) with modifications to obtain a more chronic and homogeneous infection. Briefly, animals were ovariectomized, and estrus was induced with subcutaneous administration of estradiol (Estrogeno Neosan; SmithKline Beecham, Madrid, Spain) at a dose of 10 mg/kg 3 days before infection and subsequently maintained by subcutaneous estradiol at a dose of 4 mg/kg weekly throughout the experiment. Furthermore, animals were immunosuppressed with dexamethasone (Fortecortin; Merck Laboratories) added to drinking water at a final concentration of 2 mg per liter. Immunosuppressive treatment was also started 3 days before infection and maintained throughout the experiment. For establishment of vaginal C. albicans infection, female rats were anesthetized with isoflurane (Forane; Abbott Laboratories, Kent, United Kingdom), using a customized face mask device with a scavenging system, and inoculated intravaginally with 107 yeast cells per 0.1 ml of sterile saline. Inoculation was performed using a micropipette with disposable tips. Infected animals were then randomly distributed into groups of 10 rats each. The vaginal C. albicans burden was evaluated (see below) at day 3 postinfection, 24 h before the start of treatment. The log10 CFU and standard deviation per vaginal sample were calculated to yield the basal level of infection and to ensure that all animals involved in the study were homogeneously infected.
Antifungal treatment.
Antifungal therapy was started 4 days postinfection. Groups of 10 rats each were treated subcutaneously with GW471552 or GW471558 at doses of 1, 5, and 10 mg/kg of body weight in 0.5 ml of SPSS. In the oral candidiasis model, treatment was given every 8 h for 7 consecutive days. In the VVC model, treatment was administered every 4 h for 3 consecutive days. Dosage patterns were established according to pharmacokinetic data for both compounds in rat (P. Aviles, A. Pateman, R. San Roman, M. J. Guillen, and D. Gargallo-Viola, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1690, 2000). In control groups, animals received SPSS by the subcutaneous route.
Quantification of infection level and determination of therapeutic efficacy.
Therapeutic efficacy was assessed by microbiological and histopathological evaluation. After treatment and at least 24 h after the administration of the last dose of antifungal agents or saline, animals were sacrificed by an overdose of pentobarbital (Eutalender, Normon, Spain). From each group of 10 animals, 6 were used for microbiological assessment and 4 were subjected to histopathological examination.
(i) Microbiology.
Drug efficacy was assessed by measuring the numbers of C. albicans organisms (CFU) in the antifungal treatment groups and comparing them with those of the untreated controls at the end of the experiment. Oral and vaginal samples were collected by rolling a sterile cotton swab over the oral cavity or within the vagina by a standard procedure. Samples were then suspended in 1 ml of SPSS and cultured in duplicate on Bengal Rose chloramphenicol agar (Microkit Iberica, S.A., Madrid, Spain) using a Spiral Biotech autoplate (Aplicaciones Analíticas, Barcelona, Spain). Plates were incubated at 37°C for 48 h. The yeast count was expressed as log10 CFU per milliliter, i.e., log CFU per sample. The lowest number of organisms detected by this method was 40 CFU per sample.
(ii) Histopathology.
Twenty-four hours after the end of the treatments, the animals were sacrificed and tongues or vaginas were aseptically removed. Gross observations and photographs were made following gentle rinsing of the organs. After that, tongues and longitudinally opened vaginas were fixed in toto by immersion in neutral buffered 10% formalin solution for 48 h. Serial cross sections of the organs were then obtained and fixed again in formalin for 12 h. Finally, tongue sections and vaginal tissue were embedded in paraffin. Five-micrometer sections were obtained from the paraffin blocks and stained with both hematoxylin and eosin stain and periodic acid-Schiff (PAS) stain for fungal visualization.
Statistical analysis.
An analysis of variance on ranks was used to statistically compare the numbers of CFU of C. albicans isolated from the mouths or vaginas of the experimental groups. Multiple comparisons of treated groups versus the control group were performed by Dunn's method. All statistical evaluations were performed using the analysis of variance program of the SigmaStat statistical package (Jandel Scientific, Erkrath, Germany). P values of ≤0.05 were considered statistically significant. All mean values reported here include the standard deviations of the means.
RESULTS
Two experimental models of mucosal candidiasis, oral and vulvovaginal, were used to evaluate the therapeutic efficacies of GW471552 and GW471558. The MICs of GW471552 and GW471558 against the strain C. albicans 4711E used for experimental infections were 0.001 μg/ml for both compounds. The therapeutic efficacies of the new azasordarins were assessed by microbiological and pathological studies. Pathological examination was needed due to the ability of Candida to penetrate epithelial layers by its pseudohyphal form. From each group of 10 animals, 6 were used for microbiological assessment and 4 were used for histopathological examination.
Therapeutic efficacies of GW471552 and GW471558 against experimental oral C. albicans infections. (i) Microbiology.
Prior to initiation of the study, samples from the oral cavity were obtained from all animals and cultured in duplicate to ensure the absence of a preexisting candidiasis. None of the rats used in the study showed any C. albicans organisms in the oral cavity. One day before antifungal treatment was started, oral swabs were taken from infected animals and cultured quantitatively as described in Materials and Methods. The results indicate that all animals developed oral infection, with a log CFU per sample (mean ± standard deviation) of 4.9 ± 0.5. This infection remained in the untreated rats, although with a certain decrease in the number of organisms when evaluated 24 h after the end of the treatment (3.2 ± 0.4 log CFU/sample). Therapeutic efficacy was assessed by comparing the numbers of C. albicans organisms recovered from mouths of antifungal-treated groups with those from the untreated control at the end of treatment. The experimental results obtained with GW471552 and GW471558 against oral candidiasis in rats are summarized in Table 1. A significant decrease in the percentage of positive cultures was observed in rats treated with GW471552 and GW471558. After antifungal chemotherapy, the challenging organism was recovered from only one-third of the animals treated with 5 mg of GW471552 or GW471558 per kg, and C. albicans was not detected in any of the animals when azasordarins were dosed at 10 mg/kg. In addition, GW471552 and GW471558 administered at 5 mg/kg significantly reduced the log number of CFU per sample in those animals that still showed positive cultures after treatment, compared to the number of CFU per sample in the controls. In this case, the fungal burden reductions were 96.84 and 94.37% for GW471552 and GW471558 treatments, respectively.
TABLE 1.
| Dose (mg/kg) | No. of infected animals/ total (%) infected after treatment | Log CFU/sample (mean ± SD)b | % Reduction of CFU compared to control |
|---|---|---|---|
| Control | 6/6 (100) | 3.2 ± 0.4 | |
| GW471552 | |||
| 1 | 6/6 (100) | 2.9 ± 0.3 | 49.88 |
| 5 | 2/6 (33) | 1.7 ± 0.6* | 96.84 |
| 10 | 0/6 (0) | NOD* | 100 |
| GW471558 | |||
| 1 | 5/6 (83) | 2.6 ± 0.3 | 74.88 |
| 5 | 2/6 (33) | 2.0 ± 0.3* | 94.37 |
| 10 | 0/6 (0) | NOD* | 100 |
Rats were orally infected with 5 × 108 C. albicans 4711E cells/ml. Compounds were administered every 8 h for 7 consecutive days.
NOD, no organisms detected. *, P < 0.05 (versus the control treatment). The limit of detection was 40 CFU/sample.
(ii) Pathology.
Macroscopically, all infected and untreated animals had clinically manifest lesions of the lingual mucosa, consisting of patchy areas of smooth mucosa and well-delimited atrophic areas on the dorsum of the tongue. These lesions were mainly distributed surrounding the giant conical papillae. However, animals treated with GW471552 and GW471558 at 10 mg/kg showed grossly normal dorsal tongue surfaces. Rats treated at doses of 1 and 5 mg of azasordarins per kg showed moderate macroscopic lesions of the tongue. Evident agreement between antifungal chemotherapy and gross observations was obtained.
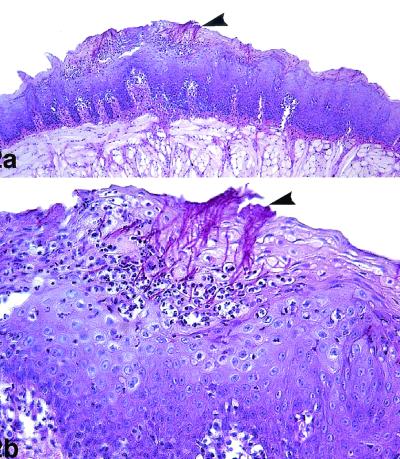
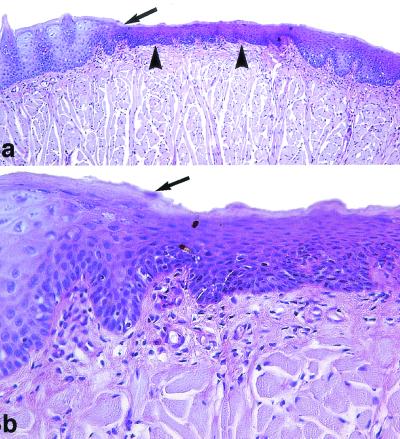
The histological findings for infected and untreated animals showed extensive colonization by numerous hyphae of the epithelium of the dorsal surface of the tongue, and in many areas colonization extended deeply through the superficial layers of the horny and squamous layers. In relation to C. albicans infection, abundant inflammatory cells in the lamina propria were observed. Multifocal leukodiapedesis into the epithelium was observed in association with these inflammatory infiltrates in the lamina propria. In multiple areas, the intraepithelial hyphae caused keratinocyte destruction and the formation of intraepithelial microabscesses (Fig. 2). Similar results were observed in animals treated with 1 mg of antifungal compounds per kg. However, all animals treated with azasordarins at 5 mg/kg showed minimal zones of the tongue occupied by hyphae, with focal hyphal penetration that produced intraepithelial microabscesses. Animals treated with azasordarins at 10 mg/kg showed multiple regenerative areas of the covering epithelium, and no histological evidence of C. albicans within the epithelium of the tongue was seen in these animals (Fig. 3).
FIG. 2.
Oral candidiasis in control immunosuppressed rats. PAS staining was used. (a) The lesional mucosa showed extensive hyperplasia of the epithelium of the dorsum of the tongue, with numerous C. albicans organisms (arrowhead) in the epithelium. Magnification, ×100. (b) Detail of panel a, showing necrosis of the superficial epithelium with intraepithelial microabscesses and numerous candidal hyphae (arrowhead). Magnification, ×400.
FIG. 3.
Oral candidiasis in immunosuppressed rats treated with GW471558 at 10 mg/kg. PAS staining was used. (a) Extensive lingual epithelium atrophy (arrowheads) compared to the normal lingual epithelium of the tongue (arrow) is shown. Magnification, ×100. (b) The atrophic epithelium shows an increase of basal and suprabasal keratinocyte proliferation with incomplete differentiation of the scamous and corneum stratum. The limit between normal and lesional epithelium is very pronounced (arrow). C. albicans was not observed in all lingual mucosa. Magnification, ×400.
Therapeutic efficacies of GW471552 and GW471558 against experimental vaginal C. albicans infections.
The therapeutic efficacies of the new azasordarins for the treatment of experimental VVC induced by C. albicans 4711E were also determined by microbiological and pathological studies.
(i) Microbiology.
Ovariectomized animals were immunosuppressed with dexamethasone, and estrus was induced and maintained with estradiol. Under these conditions, vaginal colonization by C. albicans was homogeneous, with 100% of animals infected at day 3 postinfection. Before the start of antifungal treatment, the mean log CFU per sample recovered from vaginas for the global experimental population was 5.03 ± 0.3. Saline-treated controls maintained the level of infection, with vaginal counts of 4.9 ± 0.3 at 1 day after the end of treatment. The therapeutic effects of GW471552 and GW471558 treatment on the C. albicans vaginal burden in infected rats are summarized in Table 2. A significant decrease in the percentage of animals showing positive vaginal cultures was observed in rats treated with both azasordarins. GW471552 and GW471558 were both able to reduce the vaginal C. albicans burden to below the detection threshold in 100% of animals treated at 10 or 5 mg/kg, respectively. Only one-third of animals treated with GW471552 at 5 mg/kg showed positive Candida cultures. In this case, animals still positive for vaginal Candida presence showed a statistically significant (P < 0.05) reduction in fungal burden compared to controls, with a log CFU per sample of 1.7 ± 0.2, which represents a 99.94% decrease in fungal burden. In the present study, GW471558 showed the greatest antifungal activity. In animals treated with GW471558 at a dose of 1 mg/kg, the challenging microorganism was recovered in only 50% of the animals at the end of antifungal treatment. In addition, animals with positive cultures in this case showed C. albicans vaginal counts significantly (P < 0.05) lower than those obtained from control animals, with a log CFU per sample of 2.6 ± 0.1, which represents a 99.50% decrease in yeast burden compared to the saline-treated controls (Table 2).
TABLE 2.
| Dose (mg/kg) | No. of infected animals/ total (% infected) after treatment | Log CFU/sample (mean ± SD)b | % Reduction of CFU compared to control |
|---|---|---|---|
| Control | 6/6 (100) | 4.9 ± 0.3 | |
| GW471552 | |||
| 1 | 6/6 (100) | 4.6 ± 0.2 | 49.88 |
| 5 | 2/6 (33) | 1.7 ± 0.2* | 99.94 |
| 10 | 0/6 (0) | NOD* | 100 |
| GW471558 | |||
| 1 | 3/6 (50) | 2.6 ± 0.1* | 99.50 |
| 5 | 0/6 (0) | NOD* | 100 |
| 10 | 0/6 (0) | NOD* | 100 |
Rats were intravaginally infected with 108 C. albicans 4711E cells/ml. Compounds were administered every 4 h for 3 consecutive days.
NOD, no organisms detected. *, P < 0.05 (versus the control treatment). The limit of detection was 40 CFU/sample.
(ii) Pathology.
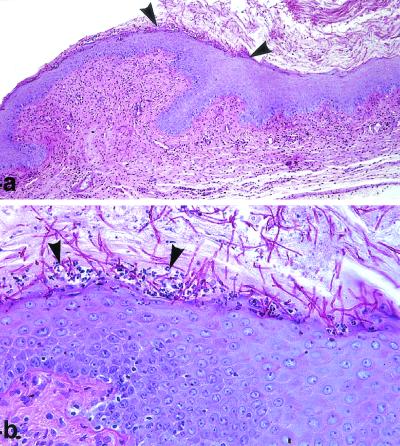
In each antifungal-treated group and among the controls, four animals were subjected to histopathological examination of the vaginal mucosa after the end of treatment. Untreated control rats showed abundant growth of Candida organisms, both as budding yeasts and in the pseudohyphal form, involving the stratum corneum and the luminal keratin debris and accompanied by a minimal inflammatory cell response with characteristic leukocyte microabscesses (Fig. 4). In those experimental groups where C. albicans was still detectable by microbiological means, histopathological findings remained similar to those found in untreated control animals. Some Candida pseudohyphae could be observed penetrating the vaginal epithelium, with a qualitative reduction of fungal luminal presence (data not shown). On the other hand, animals treated with GW471552 and GW471558 at 10 and 5 mg/kg, respectively, that did not show positive cultures showed an absence of Candida (either as budding yeast or in the form of pseudohyphae) in the vaginas. Histopathological findings in animals treated with GW471558 illustrated this result (Fig. 5). Only residual inflammatory infiltrates and ulcerations indicated that a previous infectious process was affecting the vaginal mucosa. Regenerative changes associated with the restoration of the vaginal mucosa to its normal status following antifungal treatment could also be seen.
FIG. 4.
VVC in control immunosuppressed rats. PAS staining was used. (a) C. albicans organisms are plentiful inside the vaginal cavity, and hyphae are adhering to the surface of the epithelium (arrowheads). Magnification, ×100. (b) C. albicans penetrating into the epithelium and causing necrosis on the superficial keratinocyte layer associated with intraepithelial leukocyte microabscesses (arrowheads). Magnification, ×400.
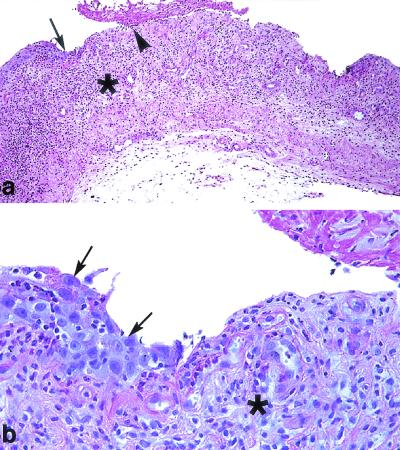
FIG. 5.
VVC in immunosuppressed rats treated with GW471558 at 10 mg/kg. PAS staining was used. (a) C. albicans was not observed in treated rats. Ulceration of the covering vaginal epithelium with fibrin deposits in the lumen (arrowhead) and an important inflammatory response of the lamina propria (asterisk) were observed. The transition between mucosal ulcera and adjacent vaginal epithelium is shown by the arrow. Magnification, ×100. (b) Regenerative changes of the vaginal epithelium were observed in this area (arrows). Also observed was the development of a granulation tissue (asterisk) in the ulcerate lamina propria. Magnification, ×400.
DISCUSSION
Infections caused by C. albicans and a few other related species have increased in prevalence worldwide. They range from mucosal diseases observed in immunosuppressed patients to fatal systemic episodes in severely neutropenic subjects. Although oral candidiasis is not a life-threatening disease, the sustained immunosuppression in these patients facilitates recurrence of the infection. On the other hand, VVC also affects a large number of otherwise healthy women. It has been estimated that 75% of all women of childbearing age will experience an episode of VVC in their lifetime (8).
Sordarins constitute a new class of antifungal agents that are distinguished from other antifungal compounds, such as polyenes, azoles, or allylamines, in that they possess a novel mechanism of action. Sordarin derivatives are highly selective fungal protein synthesis inhibitors that interact with translation elongation factor 2 (5, 6) and the large ribosomal subunit stalk rpP0 (7), thus inhibiting translation elongation in fungal cells. This multiple interaction with elements of the translation machinery may explain the high selectivity of this novel class of antifungal agents (7). Sordarins exhibit broad-spectrum and potent in vitro (10) and in vivo (2, 9, 13) antifungal activity. The presence at position 8a of the sordaricin indacene ring system of a 6-methylmorpholin-2-yl group with different N-4′ substituents instead of the sugar moiety has lead to the development of a new class of molecules called azasordarins. With the aim of assessing the in vivo efficacies of azasordarins, two new compounds, GW471552 and GW471558, have been selected and evaluated against oral candidiasis and VVC in rats. Both animal models were selected because they appear to closely mimic the situation seen in clinical settings; therefore rats were rendered neutropenic by the addition of dexamethasone in the drinking water. These animal models were also of interest because they provide preliminary information about the tissue distribution of the compounds. Furthermore, oral and vaginal candidiasis models have been shown to afford a simple, reliable, and highly reproducible method for studying the efficacies of new antifungal agents (17, 20). On the other hand, strain-related differences in C. albicans pathogenicity in the rat mucosa have been described; consequently, we have used a well-characterized C. albicans strain that has been widely used in previous therapeutic and pharmacodynamic studies in rodents, where its pathogenic properties have been well demonstrated (1, 13, 14). In the present study, immunosuppression in the VVC model ensured homogeneous and chronic infection in the rats.
The data presented indicate that azasordarins GW471552 and GW471558 administered to immunosuppressed rats with oral candidiasis or VVC exerted a clear therapeutic effect. In the oral candidiasis model, azasordarins reduced fungally mediated oral cavity injury, as measured by colony counts, gross pathology, and histological examination. Animals given lower doses of GW471552 and GW471558 (1 mg/kg) showed persistent C. albicans culture positivity, as well as abundant mycelial penetration into the epithelium of the tongue. However, GW471558 at a dose of 5 mg/kg showed a significant decrease in C. albicans CFU obtained from the mouths of infected rats compared with the controls, and in fact there was a reduction of the number of infected animals after treatment (only one-third of treated animals had positive cultures). In these animals, the histological study demonstrated that Candida organisms had disappeared from the surface of the tongue, although some hyphae persisted within the most superficial keratinocyte layers. Agreement between cultures from the oral cavity and the clinical and histological evidence of infection was observed. Rats treated with 10 mg of GW471552 or GW471558 per kg showed negative mouth cultures for all animals inspected and a complete absence of C. albicans on the dorsal surface of the tongue, suggesting that azasordarins at this dose were extremely effective.
Regarding activity against VVC, GW471552 and GW471558 showed efficacies similar to those against oral candidiasis. Both compounds diminished the vaginal Candida burden to below the level of detection in all treated animals when administered at a dose of 10 mg/kg. GW471558 was the most effective compound, being able to eradicate C. albicans from vaginas of infected animals at a dose of 5 mg/kg and significantly reducing the fungal burden when administered at a dose of 1 mg/kg (99.5% reduction compared with untreated infected controls). In this case, the challenging organism was recovered from only 50% of treated rats. Significant efficacy was also achieved with GW471552 administered at 5 mg/kg, with a fungal burden reduction of 100% in two-thirds of treated animals and of 99.94% in the remainder. In all cases evaluated, histopathological examination agreed with the microbiological results, showing the absence of Candida (no budding yeast forms or pseudohyphae) in those experimental groups where the microorganism was not detected by culture following the treatment period. Animals given GW471552 and GW471558 at doses leading to Candida eradication (10 and 5 mg/kg, respectively) showed histopathological findings (such as residual inflammatory infiltrates) that were clearly associated with normal mucosal recovery from the induced infectious process.
In addition to the above-described considerations, the preliminary in vitro and in vivo toxicity profiles of the azasordarins reflected the low toxicity of this new family of compounds (E. Herreros, A. Martinez, M. J. Almela, E. Jimenez, S. Lozano, M. J. Perez, and D. Gargallo-Viola, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1691, 2000).
In conclusion, these results together with the good in vitro antifungal activities of GW471552 and GW471558 (9a) strongly suggest that azasordarins could be promising antifungal agents for the treatment of human oral candida and VVC infections.
ACKNOWLEDGMENTS
A. Martinez and S. Ferrer contributed equally to the work presented in this publication.
We thank Jesus Caballero and members of the Pharmacological Research Center for their excellent technical assistance, Esperanza Herreros for providing all in vitro data, and members of the Organic Chemistry Group for compound synthesis.
REFERENCES
- 1.Aviles P, Falcoz C, San Roman R, Gargallo-Viola D. Pharmacokinetics-pharmacodynamics of a sordarin derivative ( GM237354) in a murine model of lethal candidiasis. Antimicrob Agents Chemother. 2000;44:2333–2340. doi: 10.1128/aac.44.9.2333-2340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemons K V, Stevens D A. Efficacies of sordarin derivatives GM193663, GM211676, or GM237354 in a murine model of systemic coccidioidomycosis. Antimicrob Agents Chemother. 2000;44:1874–1877. doi: 10.1128/aac.44.7.1874-1877.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Bernardis F, Arancia S, Morelli L, Hube B, Sanglard D, Schäfer W, Cassone A. Evidence that members of the secretory aspartyl proteinase gene family, in particular SAP2, are virulence factors for candida vaginitis. J Infect Dis. 1999;179:201–208. doi: 10.1086/314546. [DOI] [PubMed] [Google Scholar]
- 4.Diaz-Guerra T, Martinez-Suarez J, Laguna F, Rodriguez-Tudela J. Comparison of four molecular typing methods for evaluating genetic diversity among Candida albicans isolates from human immunodeficiency virus-positive patients with oral candidiasis. Antimicrob Agents Chemother. 1997;35:856–861. doi: 10.1128/jcm.35.4.856-861.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domínguez J M, Kelly V A, Kinsman O S, Marriott M S, Gómez de las Heras F, Martin J J. Sordarins: a new class of antifungal with selective inhibition of the protein synthesis elongation cycle in yeast. Antimicrob Agents Chemother. 1998;42:2274–2278. doi: 10.1128/aac.42.9.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domínguez J M, Martín J J. Identification of elongation factor 2 as the essential protein targeted by sordarins in Candida albicans. Antimicrob Agents Chemother. 1998;42:2279–2283. doi: 10.1128/aac.42.9.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gargallo-Viola D. Sordarins as antifungical compounds. Curr Opin Anti-Infect Invest Drugs. 1999;1:297–305. [Google Scholar]
- 8.Ghelardi E, Tavanti A, Lupetti A, Celandroni F, Boldrini E, Campa M, Senesi S. Control of Candida albicans murine vaginitis by topical administration of polycarbophil-econazole complex. Antimicrob Agents Chemother. 1998;42:2436. doi: 10.1128/aac.42.9.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graybill J R, Najvar L, Fothergill A, Bocanegra R, Gomez de las Heras F. Activities of sordarins in murine histoplasmosis. Antimicrob Agents Chemother. 1999;43:1716–1718. doi: 10.1128/aac.43.7.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Herreros E, Almela M J, Lozano S, Gomez de las Heras F, Gargallo-Viola D. Antifungal activities and cytotoxicity studies of six new azasordarins. Antimicrob Agents Chemother. 2001;45:3132–3139. doi: 10.1128/AAC.45.11.3132-3139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herreros E, Martinez C M, Almela M J, Marriot M S, Gomez de las Heras F, Gargallo-Viola D. Sordarins: in vitro activities of new antifungal derivatives against pathogenic yeast, Pneumocystis carinii, and filamentous fungi. Antimicrob Agents Chemother. 1998;42:2863–2869. doi: 10.1128/aac.42.11.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones J, Adams D. Experimentally induced acute oral candidosis in the rat. Brit J Dermatol. 1970;83:670–673. doi: 10.1111/j.1365-2133.1970.tb15762.x. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Ribot J, McAtee R, Perea S, Kirkpatrick W, Rinaldi M G, Patterson T. Multiple resistant phenotypes of Candida albicans coexist during episodes of oropharyngeal candidiasis in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 1999;43:1621–1630. doi: 10.1128/aac.43.7.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez A, Aviles P, Jimenez E, Caballero J, Gargallo-Viola D. Activities of sordarins in experimental models of candidiasis, aspergillosis, and pneumocystosis. Antimicrob Agents Chemother. 2000;44:3389–3394. doi: 10.1128/aac.44.12.3389-3394.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez A, Jimenez E, Santos I, Gargallo-Viola D. Antifungal efficacy of GM237354, a sordarin derivative, in experimental oral candidiasis in immunosuppressed rats. Antimicrob Agents Chemother. 2001;45:1008–1013. doi: 10.1128/AAC.45.4.1008-1013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard. Document M-27A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 16.Quereda C, Polanco A, Giner C, Sánchez-Sousa A, Pereira E, Navas E, Fortún J, Guerrero A, Baquero F. Correlation between in vitro resistance to fluconazole and clinical outcome of oropharyngeal candidiasis in human immunodeficiency virus-infected patients. Infect Immun. 1996;58:1514–1517. doi: 10.1007/BF01586182. [DOI] [PubMed] [Google Scholar]
- 17.Samaranayake Y H, Samaranayake L. Experimental oral candidiasis in animal models. Clin Microbiol Rev. 2001;14:398–429. doi: 10.1128/CMR.14.2.398-429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheehan D, Hitchcock C, Sibley C. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12:40–79. doi: 10.1128/cmr.12.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobel J D, Hasegawa A, Debernardis F, Adriani D, Pellegrini G, Cassone A, Fidel P L, Haidaris C, Gigliotti A G, Harmsen D, Fujita S, Yamamoto K, Makimura K, Shibuya K, Uchida K, Yamaguchi H. Selected animal models: vaginal candidosis, pneumocystosis pneumonia, dermatophytosis and trichosporonosis. Med Mycol. 1998;36:129–136. [PubMed] [Google Scholar]
- 20.Sobel J D, Muller G. Comparison of ketoconazole (BAY L9139) in the treatment of experimental vaginal candidiasis. Antimicrob Agents Chemother. 1983;24:434–436. doi: 10.1128/aac.24.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobel J D, Muller G, McCormick J F. Experimental chronic vaginal candidosis in rats. J Med Vet Mycol. 1985;23:199–206. doi: 10.1080/00362178585380301. [DOI] [PubMed] [Google Scholar]