Abstract
Immune checkpoint blockade using immune checkpoint inhibitors, including cytotoxic T-lymphocyte-associated antigen–4 and programmed cell death protein-1/programmed cell death ligand–1 inhibitors, has revolutionized systematic treatment for advanced solid tumors, with unprecedented survival benefit and tolerable toxicity. Nivolumab, pembrolizumab, cemiplimab, avelumab, durvalumab, atezolizumab, and ipilimumab are currently approved standard treatment options for various human cancer types. The response rate to immune checkpoint inhibitors, however, is unsatisfactory, and unexpectedly, atypical radiological responses, including delayed responses, pseudoprogression, hyperprogression, and dissociated responses (DRs), are observed in a small subgroup of patients. The benefit of immunotherapy for advanced patients who exhibit atypical responses is underestimated according to the conventional response evaluation criteria in solid tumors (RECIST). In particular, DR is considered a mixed radiological or heterogeneous response pattern when responding and nonresponding lesions or new lesions coexist simultaneously. The rate of DR reported in different studies encompass a wide range of 3.3–47.8% based on diverse definition of DR. Although DR is also associated with treatment efficacy and a favorable prognosis, it is different from pseudoprogression, which has concordant progressive lesions and can be regularly captured by immune RECIST. This review article aims to comprehensively determine the frequency, definition, radiological evaluation, probable molecular mechanisms, prognosis, and clinical management of immune-related DR and help clinicians and radiologists objectively and correctly interpret this specific atypical response and better understand and manage cancer patients with immunotherapy and guarantee their best clinical benefit.
Keywords: atypical response pattern, dissociated response, immune checkpoint inhibitor, programmed cell death ligand–1, programmed cell death protein–1
Introduction
Immune checkpoint blockade using immune checkpoint inhibitors (ICIs), including cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and programmed cell death protein-1 (PD-1)/programmed cell death ligand-1 (PD-L1) inhibitors, has revolutionized the systematic treatment for various human solid tumors at the metastatic stage, with unprecedented survival benefit and tolerable toxicity. ICIs selectively restore and normalize the body’s antitumor immune responses by disrupting the immunoinhibitory signals mediated by the PD-1/PD-L1 and CTLA-4 axes in the tumor microenvironment. 1 Nivolumab, pembrolizumab, cemiplimab, avelumab, durvalumab, atezolizumab, and ipilimumab are currently approved standard treatment options and have shifted the treatment paradigm for various cancer types, including previously treated or untreated non-small cell lung cancer (NSCLC), melanoma, and other human solid tumors. 2
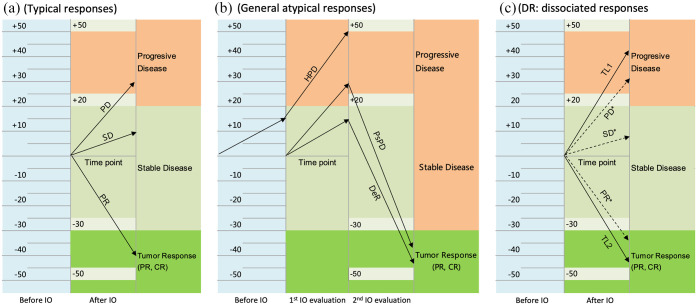
Unlike conventional cytotoxic agents or targeted treatments, immunotherapy with ICIs can result in different response patterns because of their unique mechanisms of pharmacological action. 3 Some patients receiving immunotherapy present with typical responses, including a complete response (CR), a partial response (PR), stable disease (SD), or progressive disease (PD), but atypical patterns of response may occur in a subgroup of patients, including a delayed response (DeR), pseudoprogressive disease (PsPD), hyperprogressive disease (HPD), and a dissociated response (DR). DeR is observed following initial SD and subsequent therapeutic responses. PsPD represents an uncommon response pattern in which objective responses occur after temporary tumor growth.3,4 As an aggressive pattern of cancer progression, HPD causes tumor progression at an accelerated and unexpected rate and an increase in volume within a short period of time. 5 By contrast, DR is considered a type of mixed or heterogeneous radiological response when responding and nonresponding lesions and new lesions coexist within the same patient simultaneously (Figure 1). 6 In particular, PsPD, HPD, and DR are considered a category of atypical tumor responses that are different from conventional tumor responses. PsPD, HPD, and DeR, however, have concordant progressive lesions and can be captured when two consecutive assessments conducted before or after immunotherapy are completed, whereas DR is captured at a single time point for different target lesions that present inverse responses to immunotherapy (Figure 2).
Figure 1.
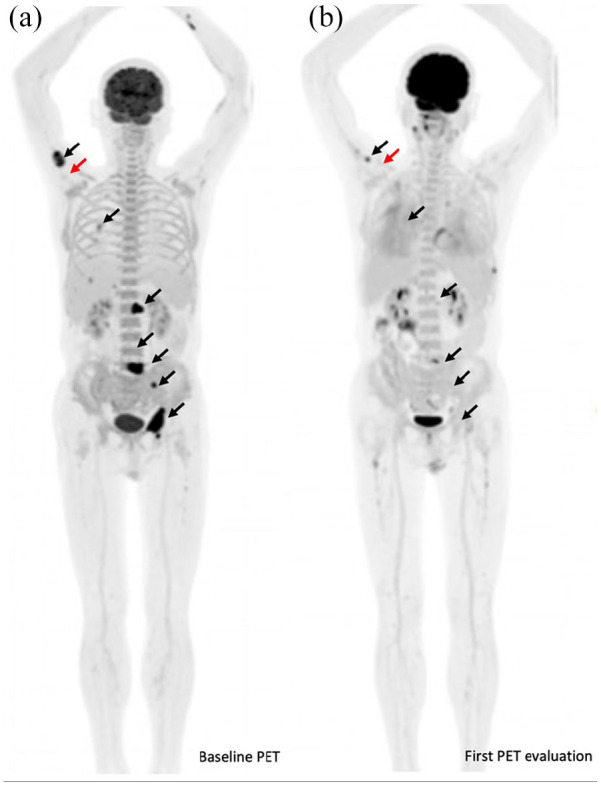
Radiological changes based on PET evaluation for a representative metastatic lung cancer patient who exhibited DR during immunotherapy. The patient was a 68-year-old man with metastatic adenocarcinoma and positive tumor cell PD-L1 expression through immunohistochemistry using the PD-L1 22 C3 pharmDx assay (Dako, Inc.). (a) He initially was treated with a first-line regimen of camrelizumab in combination with chemotherapy. Initial baseline PET showed multiple metabolic lesions in the lower lobe of the right lung, right lobe of the liver, and right adrenal gland. Multiple metabolic bone lesions of the L1 vertebra, left iliac crest, left acetabulum, and left ischium were also visible (black arrows). (b) After two cycles of treatment, the first PET evaluation was performed and showed significant metabolic regression or disappearance of previous primary pulmonary lesions and all metastatic lesions (black arrows) and metabolic progression of the proximal right humerus (red arrows). At that time, CT did not show PD, but PET/CT confirmed PD. He was also classified as having metabolic PD by PERCIST because of the appearance of a new metabolic lesion in the proximal right humerus but a partial metabolic response by other imPERCIST criteria. He had a stable clinical performance status and continued to receive immunotherapy with a durable clinical benefit for 6 months.
Figure 2.
Three types of response patterns based on the radiological evaluation of existing target lesions per RECIST 1.1 criteria. (a) Typical response patterns include complete response, partial response, stable disease, and progressive disease by measuring the variation in the sum of the longest diameters of the target lesions. (b) General atypical response patterns include DeR, PsPD, and HPD that have concordant progressive lesions and can be captured when two consecutive assessments are completed. HPD is often defined as a greater than 50% increase in the tumor burden, twofold or more increase in the tumor growth ratio or a tumor growth kinetics ratio during treatment with immunotherapy compared with that observed before immunotherapy. (c) DR is a specific mixed or heterogeneous response pattern. Unlike general atypical responses, DR is captured at a single time point for different target lesions with inverse responses to immunotherapy.
IO, immunotherapy; SLD, sum of the longest diameters; TL1, target lesion 1; TL2, target lesion 2.
Immunotherapy-associated DR is rarely reported and patients with DR have different biological specifications, clinical benefits, and prognostic significance compared to those with real disease progression. This review article aims to comprehensively determine the frequency, definition, radiological evaluation, probable molecular mechanisms, prognosis, and clinical management of immune-related DR and help clinicians and radiologists objectively and correctly interpret this specific atypical response and better understand and manage cancer patients with immunotherapy and guarantee their best clinical benefit.
Frequency of immune-related DR
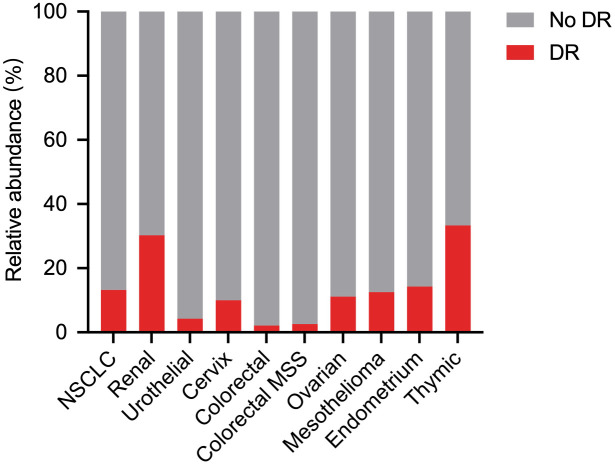
DR has been previously reported in cancer patients treated with systematic chemotherapy and anti-epidermal growth factor receptor (EGFR)-targeted therapy. 6 Almost 13.9–39.0% of NSCLC patients have DR following systematic targeted therapy or chemotherapy.7 –9 In 2015, the first patient with metastatic renal cell carcinoma (RCC) was reported to develop DR when he developed a new lesion following single-agent anti-PD-L1 treatment as part of a clinical trial. 10 Most DR cases have been reported in individual case reports, case series reports, and retrospective studies. 11 In an open, nonrandomized, current care study, 62 NSCLC patients were scheduled to initiate immunotherapy as their first or later systemic treatment and were prospectively evaluated, and five patients developed DR. 12 Different ICI monotherapies, including nivolumab, pembrolizumab, and atezolizumab, contribute to the occurrence of DR. DR has also been found in patients receiving combination immunotherapy, such as nivolumab in combination with ipilimumab and PD-1/PD-L1 inhibitors in combination with chemotherapy, targeted therapy, or radiotherapy.13,14 In a retrospective study including 360 cancer patients who participated in clinical trials for combination immunotherapy, DR was observed in 12 (3.3%) patients and PsPD in 10 (2.8%) patients. 14 Several metastatic solid tumors, including NSCLC and RCC, can develop as DR after immunotherapy.12 –19 Like general atypical response patterns such as PsPD and HPD,20,21 different rates of DR have been described depending on the tumor type. A combination analysis of published documents showed that there seemed to be an association between the frequency of DR and type of solid cancer, with a DR rate of 30.3% in RCC, 14.3% in endometrial carcinoma, 13.2% in NSCLC, and 12.5% in mesothelioma (Figure 3). DR occurred between 4.7% and 22.1% in four retrospective and one prospective trial involving 672 NSCLC patients from 2018 to 2021. Recently, Wong et al. 13 reported that DR occurred in nearly half of patients with advanced RCC via a detailed lesion-by-lesion analysis of serial imaging. Thus, the rate of DR reported in these studies encompasses a wide range of 3.3–47.8%. Conventional medical imaging modalities such as computed tomography (CT) or magnetic resonance imaging (MRI) and nuclear medicine imaging such as positron emission tomography (PET) are clinically performed to explore cancer, notably for the extension evaluation following systematic immunotherapy. The DR rate identified by PET and computerized tomography (CT) was 10.0–47.8% and 3.3–22.1%, respectively (Table 1).
Figure 3.
Association between the frequency of DR and type of solid cancer.
Like general atypical response patterns such as PsPD and HPD, different rates of DR have been described depending on the tumor type. A combination analysis showed that there seemed to be significant difference regarding the frequency of DR in different type of solid cancer (p = 0.0001).
Table 1.
Summary of studies reporting solid tumors with DR associated with immunotherapy.
| Authors | Year | Tumor type | Design of study | Treatment of ICIs | Radiological evaluation | Definition of DR | Frequency of DR (%; n/N) | Prognosis of patients with DR | Treatment of patients with DR |
|---|---|---|---|---|---|---|---|---|---|
| Sato et al. 15 | 2021 | NSCLC | Retro | Nivolumab | CT and MRI | (1) Having both CR/PR in organs and PD (2) having all CR/PR but with the appearance of new lesions or apparent deterioration of unmeasurable lesions |
4.7 (5/107) | OS for DR and concordant PD: 46.9 versus 8.2 months; p = 0.038 | All patients continued nivolumab |
| Wong et al. 13 | 2021 | RCC | Retro | Nivolumab, pembrolizumab, ipilimumab-nivolumab | CT, MRI, and PET/CT | (1) Mixed response with new lesions (2) Mixed stable and progressing or regressing lesions |
47.8 (22/46) | OS for RECIST PD with DR and RECIST PD without DR: HR: 0.40; p = 0.186 | Concurrent surgery, SBRT or GKS for nonresponding lesions without changing their ICI treatment regime |
| Tozuka et al. 12 | 2020 | NSCLC | Retro | Nivolumab, pembrolizumab, atezolizumab | CT | A disease with some shrinking lesions as well as growing or emerging new lesions | 9.2 (11/120) | OS for DR and true PD: 14.0 versus 6.5 months; p = 0.022 | Five patients with continuation of ICIs, and others with chemotherapy or local radiotherapy |
| Bernard‑Tessier et al. 14 | 2020 | Solid tumors | Retro | CTLA-4 and PD-1 inhibitors | CT | A concomitant relative decrease greater than 30% in some tumor lesions and relative increase greater than 20% in others (significant increase ⩾5 mm in the sum of measures) | 3.3 (12/360) | PFS for atypical response and PD: 23.8 versus 1.8 months OS for atypical response and PD: not reached versus 5.1 months |
Among the 203 patients who experienced an initial progression in the 12 weeks of drug exposure, 81 (39.4%) patients were treated beyond progression |
| Zhou et al. 19 | 2020 | NSCLC | Retro | PD-1/PD-L1 inhibitors | CT | DR was defined as a reduction at baseline or increase <20% in target lesions compared with the nadir in the presence of new lesions | 22.1 (52/235) | OS for DR and RECIST 1.1 defined PD without new lesions: 11.70 versus 5.25 months; p = 0.019 | Among the 52 patients who had DR, 32 (61.54%) continued ICIs, 20 (38.46%) instead received subsequent antitumor therapy |
| Humbert et al. 17 | 2019 | NSCLC | Pro | Nivolumab, pembrolizumab | PET/CT | Concomitant decrease in certain hypermetabolic lesions associated with an increase in other lesions. | 10.0 (5/50) | Increased clinical benefit | In all patients showing DR, the treatment was continued (due to an improved, or at least stable, clinical status according to the tumor board) |
| Vaflard et al. 18 | 2019 | Solid tumors | Retro | PD-1/PD-L1 inhibitors | CT | (1) One TL in CR/PR and one progressive TL (DR1) (2) One stable TL and one progressive TL (DR2) 3) One TL in CR/PR and one stable TL (DR3) |
DR1: 8.0 (8/100) DR2: 44.0 (44/100) DR3: 10.0 (10/100) |
NR | NR |
| Tazdait et al. 16 | 2018 | NSCLC | Retro | PD-1/PD-L1 inhibitors | CT | Concomitant decrease in certain tumoral elements and increase in other elements | 7.5 (20/160) | OS for atypical responses (PsPD and DR) and PD: 9.8 versus 6.1 months; p < 0.0001 | The proportion of patients treated beyond RECIST PD was around 90% |
CR, complete response; CT, computed tomography; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; DR, dissociated response; GKS, gamma knife surgery; ICIs, immune checkpoint inhibitors; MRI, magnetic resonance imaging; NR, not reported; NSCLC, non-small cell lung cancer; OS, overall survival; PD-1, programmed cell death protein-1; PD-L1, programmed death ligand-1; PET/CT, positron emission tomography/computed tomography; PFS, progression-free survival; PR, partial response; Pro, prospective; PsPD, pseudoprogressive disease; RCC, renal cell carcinoma; RECIST, response evaluation criteria in solid tumors; Retro, retrospective; SBRT, stereotactic body radiation therapy; TL, target lesion.
Definition of immune-related DR by radiological evaluation criteria
RECIST 1.1 and iRECIST
How are patients with immune-related DR defined? Mixed tumor response phenomena in some patients have been reported, where some target lesions are decreased in size, whereas others have grown. RECIST 1.1, the most commonly used conventional treatment response evaluation criterion, is widely used to determine the occurrence of DR, although it is not clearly defined in radiological criteria (Table 2). As an unconventional immune-related pattern of response, DR occurs across organs and tissues. Like other atypical response patterns, the definition of DR varied across studies (Table 1). DR is mostly defined as the concomitant decrease in certain tumoral elements and increase in other elements.12,14 –16,19 For example, Bernard-Tessier et al. 14 defined DR as a concomitant relative decrease greater than 30% in some tumor lesions and a relative increase greater than 20% in others (significant increase ⩾ 5 mm in the sum of measures). In contrast, three types of DR were recently constructed by Vaflard et al. 18 : (1) one target lesion with CR/PR and one with PD (DR1); (2) one target lesion with SD and one with PD (DR2); and (3) one target lesion with CR/PR and one with SD (DR3). DR2 and DR3 were observed in 44% and 10% of patients, respectively, and the rate of DR1 was 8%, which was consistent with previous evaluation criteria for DR. Similarly, Wong et al. 13 reported a relatively high frequency of DR (47.8%), where DR was defined as a mixed response with new lesions, as well as mixed stable and progressing or regressing lesions. Thus, the rate of DR occurrence could be overestimated because of the incorporation of stable target lesions for DR evaluation. If patients have one target lesion with SD and one with PD, they are evaluated as real PD, but not DR, with unfavorable outcomes. The following criteria should be recommended to define DR on CT: (1) patients who have both CR/PR (at least a 30% decrease in some lesions) and progressive lesions (at least a 20% increase in other lesions) simultaneously and (2) patients who have CR/PR lesions but with the appearance of one or more new lesions or apparent deterioration of unmeasurable lesions. These criteria are consistent with previous suggestions made by other investigators.6,22 The lesions to evaluate DR may be primary lesions or metastatic lesions located in various organs. Although CR/PR and PD can be identified for different target lesions, the overall response evaluation for an individual patient could be PR, SD, or PD, which is dependent on the extent of the change for responding and nonresponding lesions (Figure 2).
Table 2.
Summary of comparison of RECIST 1.1, irRC, irRECIST, iRECIST, PERCIST, and imPERCIST criteria for immunotherapy.
| RECIST 1.1 (2009) | CR | PR | SD | PD | |
| Complete disappearance of target and nontarget lesions | ⩾30% decrease from baseline | Neither PD, PR nor CR | ⩾ 20% increase in the nadir of the sum of target lesions, with a minimum of 5 mm, or new lesions | ||
| irRC (2009) | irCR | irPR | irSD | irPD | |
| Complete disappearance of target lesions and no new nonmeasurable lesions | Disappearance of target lesions and stable or unequivocal progression of nontarget lesions or ⩾50% decrease in target lesion and absent, stable, or unequivocal progression of nontarget lesions | <50% decrease or <25% increase of target lesions and absent, stable, or unequivocal progression of nontarget lesions | >25% increase of target lesions and any change of nontarget lesions | ||
| irRECIST (2013) | Complete disappearance of target and nontarget lesions | ⩾30% decrease from baseline | Neither irPD, irPR nor irCR | ⩾20% increase in total measured tumor burden compared with nadir of progression of nontarget lesions or new lesions Confirmation of progression recommended minimum 4 weeks after the first irPD assessment |
|
| iRECIST (2020) | iCR | iPR | iSD | iUPD | iCPD |
| Complete disappearance of target and nontarget lesions. All lymph nodes must be nonpathological in size (<10 mm in SAD) |
⩾30% decrease of target lesions compared with the baseline, or in the case of complete remission of the TL, when one or more non-TL can still be distinguished | The criteria of iCR or iPR are not met and no tumor progression is present | Further progress of the target sum (⩾5 mm), or any further progress of the non-TL, and/or progress of the new measurable and not measurable lesions either in number or in size (sum ⩾ 5 mm) | An increase in the sum of all TL by at least ⩾20% (but at least ⩾ 5 mm) compared with the time point with the lowest TL sum (Nadir), or an unequivocal progression of non-TL, or by the occurrence of new measurable and/or nonmeasurable tumor lesions Need to be confirmed 4–8 weeks later; if progression is followed by tumor shrinkage, the bar is reset |
|
| PERCIST (2009) | CMR | PMR | SMD | PMD | |
| Complete disappearance of all metabolically active tumors | ⩾30% decrease of SULpeak in target lesions, and absolute drop in SUL by at least 0.8 SUL units | Neither PMD, PMR nor CMR | ⩾30% increase of SULpeak or the appearance of any new metabolically active lesions | ||
| ImPERCIST (2019) | CMR | PMR | SMD | UPMD | CPMD |
| Disappearance of all metabolically active tumors | ⩾30% decrease of SULpeak in target lesions, and absolute drop in SUL by at least 0.8 SUL units | Neither PMD, PMR nor CMR | ⩾30% increase of SULpeak or the appearance of any new metabolically active lesions | ⩾30% increase of SULpeak or the appearance of any new metabolically active lesions Need to be confirmed by a second PET at 4–8 weeks later; if progression is followed by PMR or SMD, the bar is reset |
CMR, complete metabolic response; CPMD, confirmed progressive metabolic disease; CR, complete remission; iCPD, immune confirmed progressive disease; iCR, immune complete remission; imPERCIST, immunotherapy-modified PERCIST; iPR, immune partial remission; irCPD, immune-related confirmed progressive disease; irCR, immune-related complete response; iRECIST, immuno-RECIST; irPD, immune-related progressive disease; irPR, immune-related partial response; irRC, immune-related response criteria; irRECIST, immune-related RECIST; irSD, immune-related stable disease; iSD, immune stable disease; iUPD, immune unconfirmed progressive disease; PD, progressive disease; PERCIST, PET response criteria in solid tumors; PET, positron emission tomography; PMD, progressive metabolic disease; PMR, partial metabolic response; PR, partial remission; RECIST, response evaluation criteria in solid tumors; SD, stable disease; SAD, short axis diameter; SMD, stable metabolic disease; SUL, standardized uptake value normalized by lean body mass; TL, target lesion; UPMD, unconfirmed progressive metabolic disease.
Currently, specific radiological criteria, including two-dimensional immune-related response criteria (irRC), immune-related RECIST (irRECIST), and immune RECIST (iRECIST), were developed and used to define patients with atypical response patterns because conventional RECIST 1.1 is not sufficient to capture PsPD and can cause underestimation of the clinical benefit from the treatment of ICIs (Table 2).23 –25 A monocentric retrospective analysis showed that 11% of progressive NSCLC patients had an underestimated benefit of PD-1 or PD-L1 inhibitor therapy based on the RECIST 1.1 criteria. 16 DR, however, is not clearly defined in the immune-related radiological criteria. According to the iRECIST criteria, the overall response evaluation for an individual patient with DR could be iPR, iSD, iUPD, or iCPD. Thus, DR could be misclassified as true progression by RECIST, as well as iRECIST criteria.
PERCIST and imPERCIST
In general, it is difficult to capture DR by conventional RECIST 1.1 criteria based on primary CT analyses. Deep analyses of CT images are required. In addition, some investigators prefer to define DR by PET that provides the advantage of a highly sensitive whole-body functional examination. Nuclear medicine imaging seems to be superior to conventional CT by showing a metabolic tumor feature and has been well described to identify immune-related PsPD. 26 Metabolic response criteria such as PET RECIST (PERCIST) may demonstrate higher rate of accurate prediction in comparison with CT in patients with NSCLC and unresectable recurrent malignant pleural mesothelioma.26,27
DR, however, could also be included in the category of metabolic PD by PERCIST. Immunotherapy-modified PERCIST (imPERCIST) was established by which the appearance of new lesions alone did not result in metabolic PD and was included in the sum of standardized uptake value normalized by lean body mass peak (SULpeak) if they showed higher uptake than existing target lesions or if fewer than five target lesions were detected on the baseline analysis. 28 As dual-time-point evaluation scale, imPERCIST introduces a need to confirm a PD such as the updated radiological criteria, but such a control may limit the risk of a false-positive PET scan. 29 The DR case presented in Figure 1 was classified as metabolic PD by PERCIST because of the appearance of a new metabolic lesion in the proximal right humerus but a partial metabolic response by imPERCIST. Thus, DR should not simply be included in the category of progressive, stable, or responsive disease. No consensus, however, exists regarding the assessment of DR by PET. A recently suggested DR definition inspired by PERCIST is a concomitant relative metabolic decrease >30% in the metabolism of some target lesions and a relative metabolic increase >30% in the remaining lesions (and/or new metabolic lesions). 6 Early total metabolic tumor volume evolution on 18FDG-PET/CT, an interesting canulated parameter that can be easily (semi)-automatically determined, was found to be associated with long-term outcome in advanced melanoma patients with pembrolizumab treatment. 30 Evaluation by PET, however, has some limitations. First, the specificity can be low for a multifunctional radiotracer 18 FDG whose uptakes concern tumor and some inflammatory cells. Second, an immune activation induced by immunotherapy can be observed in tumor-draining lymph nodes with an18 FDG uptake and be misinterpreted as disease progression. 31 Third, compared with other medical imaging modalities such as CT or MRI, nuclear medicine imaging PET as a mode of disease reevaluation is not feasible due to financial reasons. Finally, PET requires radiation protection and its spatial resolution is not good compared with CT and MRI.
Overall, the morphological and metabolic features of the tumor response should be incorporated into a new consensus on the definition of immune-related DR that comprehensively considers selected target lesions and new lesions. DR assessment with all radiological techniques may result in different degrees of bias because of difficulty in discriminating between pseudoprogressive and real progressive lesions of DR and a lack of pathological confirmation. Vaflard et al. 18 reported that the frequency of DR on CT scans was 10.0% in several cancer types with an ICI either alone or in combination with another ICI. The occurrence of DR was more common if one target lesion was biopsied.
Furthermore, most studies defined DR on the first radiological evaluation, but DR may be observed during subsequent evaluations (3 or more months after initiative treatment). In a retrospective study investigating 50 NSCLC patients with immunotherapy, 12% and 10% of patients developed PsPD and DR, respectively. 17 A subsequent PET identified more than half of them with DR (26%) and PsPD (32%), both patterns being strongly associated with a clinical benefit of continuous immunotherapy. Thus, unlike general atypical response patterns, including PsPD and DeR, DR should be evaluated not only at the first time point of immunotherapy using radiological analysis but also at later time points of immunotherapy. A confirmation assessment must be done in subsequent 4–8 weeks after an occurrence of atypical response.
Pathological features and probable mechanisms of immune-related DR
Radiological evaluations, such as CT and PET, are clinically used to define DR, but the real lesions of PsPD could be misclassified as progressive lesions of DR, resulting in the overestimation of the frequency of DR. DR was reported to be more common when the target lesion was biopsied. 18 In the real-world clinical setting, conducting a biopsy for all progressive lesions may be impossible for suspected DR because some patients refuse to have a biopsy, or the location and size of the progressive target lesions limit the application of this invasive operation. In a recent study of five patients with nivolumab-related DR, only two patients consented to biopsies of the growing lesion, including cell block analysis of the pleural effusion and kidney biopsy. 15
Tumor-draining lymph nodes
The precise mechanism of immune-related DR remains unknown. Generally, the responsive and progressive sites in patients with DR are not specific. Target lesions with DR may be lymph nodes or solid organs. As immunologically privileged sites, metastatic tumor-draining lymph nodes (TDLNs) are the most common sites where size changes often occur because of the coexistence of original cytotoxic T cells and immunosuppressive immune cells. In murine models testing PD-1 blockade, the efficacy of treatment was abolished by the ablation or surgical resection of TDLNs or depletion of CD8+ T cells prior to anti-PD-1 treatment. 32 Targeted delivery of ICI to TDLNs alone was associated with increased antitumor immunity and therapeutic effects compared with regular systemic immunotherapy. In murine models of spontaneously metastatic breast cancer, neoadjuvant immunotherapy presented greater therapeutic efficacy than adjuvant treatment, with elevated and sustained peripheral tumor-specific immune responses. 33 These studies highlight the evidence that supports TDLNs as the likely most important sites for initiating tumor-specific immune response. 34 In fact, lymph nodes as target lesions for evaluation in immunotherapy tend to fluctuate up and down in size more frequently than solid organs and may be misleading. This is supported by a recently published study showing a specific ‘nodal immune flare’ phenomenon in which NSCLC patients demonstrate radiologically abnormal nodes due to a pathological inflammatory response after neoadjuvant ICIs (16%), but not after neoadjuvant chemotherapy (0%). 35 Such temporary inflammatory response can be observed in lymph nodes and be misinterpreted as disease progression. Although no data directly show the frequency of responding lesions being seen in lymph nodes while progressive lesions may be in other viscera, avoiding using lymph nodes as target lesions and exclusion of lymph nodes in response criteria for immunotherapy should be considered, to precisely defined patients with DR or other atypical responses.
Histological and genetic heterogeneity
Tumor heterogeneity within individual patients may be responsible for these inconsistent responses to immunotherapy. First, histological temporal heterogeneity existed between the primary and metastatic lesions. In a recent report, a patient had adenosquamous histology of the primary lesion, but kidney biopsy revealed a pathology of adenocarcinoma when the lesions in the kidney progressed. 16 Second, genetic alterations are associated with DR. Previous studies have indicated that 8.8% of lung adenocarcinoma patients with a mixed response to EGFR tyrosine kinase inhibitors exhibit intertumorally discordant EGFR mutations. 8 In the case of HPD, genetic alterations, such as MDM2/MDM4 amplification and AKT1 E17K activation, have been found in HPD patients with immune checkpoint blockade.36,37 In a novel murine synchronous melanoma model, intertumoral genetic heterogeneity contributed to changes in the tumor microenvironments for different lesions and heterogeneous lesion-specific responses. 38 In addition, differences in the tumor microenvironments between primary or metastatic lesions may be responsible. Tumors growing at different sites have distinct tumor microenvironments, which influence the responses to immunotherapy and lead to different therapeutic responses. Metastatic adrenal lesions are sensitive to therapy in NSCLC patients with atypical responses,16,39 but this was not the case for patients with microsatellite instability-high metastatic colorectal cancer, melanoma, and uterine carcinosarcoma.40,41 As a marker of the response to immunotherapy, PD-L1 expression is discordant between samples from two different sites in nearly one in four patients. 42 An atypical radiological response with ICI treatment was reported in an elderly patient with high PD-L1-expressing lung adenocarcinoma. 11 Metastatic lesions, including lymph nodes, pleural fluid, soft tissue and adrenal gland, were more frequently highly positive for PD-L1 expression than primary lesions (33.8% versus 28.4%), suggesting that discordant responses can occur among different lesions. 43
Immune microenvironment
Immune cells inside the microenvironment are likely responsible for the inconsistent responses to immunotherapy across organs. 44 Previous preclinical studies have revealed that the site of tumor growth dictates the response to immunotherapy. The antitumor response to immunotherapy comprising three specific agonist antibodies, termed tri-mAb, is markedly reduced in orthotopic tumors and visceral tumors compared with subcutaneous tumors. 44 This phenomenon could be explained by orthotopic tumors having a specific microenvironment associated with immunosuppressive M2 macrophages. Similar tissue-specific responses in different anatomical sites to other immunotherapies, including an intralymphatic mRNA vaccine comprising mRNA encoding the HPV16-E7 oncoprotein and combination treatment with anti-CTLA-4 and anti-PD-1 antibodies, have been verified in mouse models of colon cancer.45,46 The recruitment of Ly6C+ monocytes from the blood was also responsible for antibody-dependent tumor cell killing of melanoma in the skin but not in the lung. 47 Using a novel murine synchronous melanoma model, Qin et al. 38 found that intertumoral genetic differences were sufficient to generate a distinct tumor immune microenvironment that led to the independent regulation of the PD-1/PD-L1 axis. Intratumoral delivery and tumor tissue-targeted agents, including immunostimulatory monoclonal antibodies, pattern recognition receptor agonists, genetically engineered viruses, bacteria, cytokines and immune cells, are attractive strategies to increase the in situ bioavailability and efficacy of immunotherapies.48,49 The local tissue microenvironment likely determines which immune populations contribute to specific therapeutic responses or progression. Some radiological measures can be carried out to overcome tumor heterogeneity, such as including smaller lesions as target lesions and developing new radiotracers targeting specifically an immune cell receptor, or an active inflammatory signal can increase the specificity of radiological assessment.
Prognosis and clinical management for patients with immune-related DR
In contrast to PR/CR, DR is viewed as an unfavorable prognostic factor of survival for patients receiving targeted or systematic chemotherapy. 7 In nearly all studies regarding the response pattern of immune-related DR, however, patients with DR had a prolonged overall survival (OS) or increased clinical benefit compared with those who achieved true disease progression (Table 1). Sato et al. reported that advanced nivolumab-treated NSCLC patients showing DR had significantly longer OS than those showing PD (46.9 versus 8.2 months). A durable clinical benefit was observed in approximately 20–50% of patients with DR after treatment with immunotherapy. In some patients with DR captured on subsequent PET evaluation, a 6-month clinical benefit of immunotherapy was reached. 17 Furthermore, patients with DR had a longer OS than those with concordant PD (without DR), but no significant difference was found in OS between patients with concordant PR (without DR) and those with concordant SD (without DR). 15 The survival of patients with DR was comparable with that reported for patients with concordant PR or SD.12,15 These findings indicate that the clinical survival benefit of immunotherapy may be underestimated when patients have a DR by conventional radiological evaluation using RECIST.
DR may be considered a useful marker to make a clinical decision regarding whether one patient should continue or discontinue immunotherapy following the detection of progressive lesions by RECIST 1.1. Importantly, DR is not simply considered a true PD and does not represent real acquired resistance to immunotherapy with ICIs. Immediate discontinuing immunotherapy or switching to other systematic treatment, including chemotherapy or targeted therapy, may not be an optional early strategy. If a patient is initially assessed as DR, oncologists must do next assessment in subsequent 4–8 weeks, and continue on immunotherapy because patient’s prognosis is good, or consider local therapy for PD lesions and continue treatment, or move on to the next line of therapy. Deciding to keep patients on immunotherapy, however, could be based on several factors, such as the extent of disease progression, real-time patient performance status assessed by the physician in clinical practice, and risk of developing immune-related adverse events. Pathological, genetic, and clinical risk factors such as neutrophil-to-lymphocyte ratio are helpful for distinguishing between atypical responses and true PD.50,51 In some patients who exhibit DR and discontinue immunotherapy, subsequent rechallenge with ICIs alone or in combination with local therapy may be alternative strategies, like the situation for those with HPD.21,52,53 Recent retrospective studies showed that, after incomplete responses to immunotherapy, early surgical resection brought the potential benefit and remained the only definitive method to render patients free of disease, particularly for those whose adrenal gland was viewed as a potential sanctuary site of metastases.40,54,55 In another proof-of-concept study, additional ablation increased the objective response rate and prolonged OS in advanced hepatocellular carcinoma patients with SD or mixed responses to previous anti-PD-1 therapy. 56
Conclusion
DR is considered a type of mixed or heterogeneous radiological response and captured at a single time point for different target lesions that present inverse responses to immunotherapy. DR has a reported wide frequency of 3.3–47.8% in patients receiving immunotherapy based on different radiological evaluations and DR definitions. PET seems to capture more patients with DR than conventional CT scan. Frequency of DR occurrence could be overestimated because of the incorporation of stable target lesions into DR evaluation. Tumor and immune microenvironment heterogeneity within an individual cancer patient could be responsible for DR and therapeutic response to immunotherapy. The patients who exhibited DR show relatively favorable outcomes versus those with true PD. DR at the initial or subsequent radiological evaluation may be a surrogate factor in determining whether a patient could continue immunotherapy treatment. Clinicians should be familiar with DR through the interpretation of radiological, clinical, and pathological data, to better understand and manage cancer patients with immunotherapy and guarantee the best clinical benefit. The definition of DR must be confirmed and the molecular and cellular mechanism of DR should be elucidated through pathological, immunological, cellular, and molecular investigations. The iRECIST could be considered to analyze DR. Novel immune-related therapeutic evaluation criteria based on the modified morphological and new metabolic features of tumor response and biomarker assessments that can accurately predict response to immunotherapy and represent the clinical benefit of patients with DR should be established. Furthermore, specific clinical treatment options, including continuous immunotherapy, additional local therapy, and intratumoral or tumor tissue-targeted immunotherapies, should be developed to achieve higher functional concentrations or bioavailability of immune mediators in specific tumor tissues for individual progressive lesions in patients with DR.
Acknowledgments
We acknowledge the patient’s contribution and all health-care workers involved in the diagnosis and treatment of the patient with DR.
Footnotes
Ethics approval and consent to participate: Ethics approval was not sought for the present study because all data were public and de-identified. All patients provided informed consent in the original studies.
Consent for publication: Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author contribution(s): Yaping Guan: Formal analysis; Validation; Writing – original draft.
Dongfeng Feng: Formal analysis; Validation.
Beibei Yin: Formal analysis; Validation.
Kun Li: Formal analysis; Validation.
Jun Wang: Conceptualization; Formal analysis; Funding acquisition; Supervision; Validation; Writing – original draft; Writing – review & editing.
ORCID iD: Jun Wang  https://orcid.org/0000-0003-3941-2507
https://orcid.org/0000-0003-3941-2507
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (Grant No. 81572875), Shandong Provincial Natural Science Foundation (Grant No. ZR202102190539), CSCO-MSD Cancer Research Foundation (Grant No. Y-MSD2020-0350), CSCO-PILOT Cancer Research Foundation (Grant No. Y-2019AZMS-0440), and Wu Jieping Medical Foundation for Clinical Scientific Research (Grant No. 320.6750.2020-12-16).
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Availability of data and materials: The original contributions presented in the study are included in the article material. Further inquiries can be directed to the corresponding author.
Contributor Information
Yaping Guan, Department of Oncology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Jinan, China Shandong Lung Cancer Institute, Jinan, China; Shandong Key Laboratory of Rheumatic Disease and Translational Medicine, Jinan, China.
Dongfeng Feng, Department of Oncology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Jinan, China Shandong Lung Cancer Institute, Jinan, China; Shandong Key Laboratory of Rheumatic Disease and Translational Medicine, Jinan, China.
Beibei Yin, Department of Oncology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Jinan, China Shandong Lung Cancer Institute, Jinan, China; Shandong Key Laboratory of Rheumatic Disease and Translational Medicine, Jinan, China.
Kun Li, Department of PET/CT, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Jinan, China.
Jun Wang, Department of Oncology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, No. 16766, Jingshi Road, Jinan 250014, China; Shandong Lung Cancer Institute, Jinan, China; Shandong Key Laboratory of Rheumatic Disease and Translational Medicine, Jinan, China.
References
- 1. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011; 331: 1565–1570. [DOI] [PubMed] [Google Scholar]
- 2. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015; 33: 1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mushti SL, Mulkey F, Tang S, et al. Immune response evaluation and treatment with immune checkpoint inhibitors beyond clinical progression: response assessments for cancer immunotherapy. Curr Oncol Rep 2020; 22: 116. [DOI] [PubMed] [Google Scholar]
- 4. Borcoman E, Kanjanapan Y, Champiat S, et al. Novel patterns of response under immunotherapy. Ann Oncol 2019; 30: 385–396. [DOI] [PubMed] [Google Scholar]
- 5. Champiat S, Ferrara R, Massard C, et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol 2018; 15: 748–762. [DOI] [PubMed] [Google Scholar]
- 6. Humbert O, Chardin D. Dissociated response in metastatic cancer: an atypical pattern brought into the spotlight with immunotherapy. Front Oncol 2020; 10: 566297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dong ZY, Zhai HR, Hou QY, et al. Mixed responses to systemic therapy revealed potential genetic heterogeneity and poor survival in patients with non-small cell lung cancer. Oncologist 2017; 22: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen ZY, Zhong WZ, Zhang XC, et al. EGFR mutation heterogeneity and the mixed response to EGFR tyrosine kinase inhibitors of lung adenocarcinomas. Oncologist 2012; 17: 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee Y, Kim HY, Lee SH, et al. Clinical significance of heterogeneity in response to retreatment with epidermal growth factor receptor tyrosine kinase inhibitors in patients with lung cancer acquiring secondary resistance to the drug. Clin Lung Cancer 2014; 15: 145–151. [DOI] [PubMed] [Google Scholar]
- 10. de Velasco G, Krajewski KM, Albiges L, et al. Radiologic heterogeneity in responses to anti-PD-1/PD-L1 therapy in metastatic renal cell carcinoma. Cancer Immunol Res 2016; 4: 12–17. [DOI] [PubMed] [Google Scholar]
- 11. Teixidor E, Sais E, Vásquez CA, et al. Immune-related adverse events and atypical radiological response with checkpoint inhibitor immunotherapy in an elderly patient with high PD-L1 expressing lung adenocarcinoma. Oncotarget 2018; 9: 33043–33049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tozuka T, Kitazono S, Sakamoto H, et al. Dissociated responses at initial computed tomography evaluation is a good prognostic factor in non-small cell lung cancer patients treated with anti-programmed cell death-1/ligand 1 inhibitors. BMC Cancer 2020; 20: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong A, Vellayappan B, Cheng L, et al. Atypical response patterns in renal cell carcinoma treated with immune checkpoint inhibitors-navigating the radiologic potpourri. Cancers (Basel) 2021; 13: 1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernard-Tessier A, Baldini C, Castanon E, et al. Patterns of progression in patients treated for immuno-oncology antibodies combination. Cancer Immunol Immunother 2021; 70: 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sato Y, Morimoto T, Hara S, et al. Dissociated response and clinical benefit in patients treated with nivolumab monotherapy. Invest New Drugs 2021; 39: 1170–1178. [DOI] [PubMed] [Google Scholar]
- 16. Tazdait M, Mezquita L, Lahmar J, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer 2018; 88: 38–47. [DOI] [PubMed] [Google Scholar]
- 17. Humbert O, Cadour N, Paquet M, et al. 18FDG PET/CT in the early assessment of non-small cell lung cancer response to immunotherapy: frequency and clinical significance of atypical evolutive patterns. Eur J Nucl Med Mol Imaging 2020; 47: 1158–1167. [DOI] [PubMed] [Google Scholar]
- 18. Vaflard P, Paoletti X, Servois V, et al. Dissociated responses in patients with metastatic solid tumors treated with immunotherapy. Drugs R D 2021; 21: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou H, Sun Y, Xiu W, et al. Overall survival benefit of continuing immune checkpoint inhibitors treatment post dissociated response in patients with advanced lung cancer. J Cancer Res Clin Oncol 2020; 146: 2979–2988. [DOI] [PubMed] [Google Scholar]
- 20. Martin-Romano P, Castanon E, Ammari S, et al. Evidence of pseudoprogression in patients treated with PD1/PDL1 antibodies across tumor types. Cancer Med 2020; 9: 2643–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feng D, Guan Y, Liu M, et al. Excellent response to atezolizumab after clinically defined hyperprogression upon previous treatment with pembrolizumab in metastatic triple-negative breast cancer: a case report and review of the literature. Front Immunol 2021; 12: 608292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ippolito D, Maino C, Ragusi M, et al. Immune response evaluation criteria in solid tumors for assessment of atypical responses after immunotherapy. World J Clin Oncol 2021; 12: 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hodi FS, Ballinger M, Lyons B, et al. Immune-modified response evaluation criteria in solid tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol 2018; 36: 850–858. [DOI] [PubMed] [Google Scholar]
- 24. Nishino M, Giobbie-Hurder A, Gargano M, et al. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res 2013; 19: 3936–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017; 18: e143–e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beer L, Hochmair M, Haug AR, et al. Comparison of RECIST, iRECIST, and PERCIST for the evaluation of response to PD-1/PD-L1 blockade therapy in patients with non-small cell lung cancer. Clin Nucl Med 2019; 44: 535–543. [DOI] [PubMed] [Google Scholar]
- 27. Kitajima K, Maruyama M, Yokoyama H, et al. Response to immune checkpoint inhibitor therapy in patients with unresectable recurrent malignant pleural mesothelioma shown by FDG-PET and CT. Cancers (Basel) 2021; 13: 1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldfarb L, Duchemann B, Chouahnia K, et al. Monitoring anti-PD-1-based immunotherapy in non-small cell lung cancer with FDG PET: introduction of iPERCIST. EJNMMI Res 2019; 9: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ayati N, Lee ST, Zakavi SR, et al. Response evaluation and survival prediction following PD-1 immunotherapy in patients with non-small-cell lung cancer: comparison of assessment methods. J Nucl Med 2021; 62: 926–933. [DOI] [PubMed] [Google Scholar]
- 30. Vermeulen S, Awada G, Keyaerts M, et al. Early reassessment of total metabolic tumor volume on FDG-PET/CT in advanced melanoma patients treated with pembrolizumab predicts long-term outcome. Curr Oncol 2021; 28: 1630–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fransen MF, Schoonderwoerd M, Knopf P, et al. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight 2018; 3: e124507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chamoto K, Wakita D, Narita Y, et al. An essential role of antigen-presenting cell/T-helper type 1 cell-cell interactions in draining lymph node during complete eradication of class II-negative tumor tissue by T-helper type 1 cell therapy. Cancer Res 2006; 66: 1809–1817. [DOI] [PubMed] [Google Scholar]
- 33. Liu J, Blake SJ, Yong MC, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov 2016; 6: 1382–1399. [DOI] [PubMed] [Google Scholar]
- 34. Goode EF, Roussos Torres ET, Irshad S. Lymph node immune profiles as predictive biomarkers for immune checkpoint inhibitor response. Front Mol Biosci 2021; 8: 674558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cascone T, Weissferdt A, Godoy MCB, et al. Nodal immune flare mimics nodal disease progression following neoadjuvant immune checkpoint inhibitors in non-small cell lung cancer. Nat Commun 2021; 12: 5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 2017; 23: 4242–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu Z, Chen L, Zheng L, et al. Hyperprogressive disease in cervical small cell carcinoma treated by immune checkpoint inhibitor. Onco Targets Ther 2019; 12: 8873–8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Qin SS, Han BJ, Williams A, et al. Intertumoral genetic heterogeneity generates distinct tumor microenvironments in a novel murine synchronous melanoma model. Cancers (Basel) 2021; 13: 2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nishino M, Giobbie-Hurder A, Manos MP, et al. Immune-related tumor response dynamics in melanoma patients treated with pembrolizumab: identifying markers for clinical outcome and treatment decisions. Clin Cancer Res 2017; 23: 4671–4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cohen R, Jonchère V, De La, Fouchardière C, et al. Adrenal gland as a sanctuary site for immunotherapy in patients with microsatellite instability-high metastatic colorectal cancer. J Immunother Cancer 2021; 9: e001903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nguyen MC, Shah MH, Liebner DA, et al. The adrenal gland as a sanctuary site of metastases after pembrolizumab treatment: a case series. J Natl Compr Canc Netw 2018; 16: 1279–1283. [DOI] [PubMed] [Google Scholar]
- 42. Wang H, Agulnik J, Kasymjanova G, et al. The metastatic site does not influence PD-L1 expression in advanced non-small cell lung carcinoma. Lung Cancer 2019; 132: 36–38. [DOI] [PubMed] [Google Scholar]
- 43. Moutafi MK, Tao W, Huang R, et al. Comparison of programmed death-ligand 1 protein expression between primary and metastatic lesions in patients with lung cancer. J Immunother Cancer 2021; 9: e002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oliver AJ, Lau PKH, Unsworth AS, et al. Tissue-dependent tumor microenvironments and their impact on immunotherapy responses. Front Immunol 2018; 9: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Devaud C, Westwood JA, John LB, et al. Tissues in different anatomical sites can sculpt and vary the tumor microenvironment to affect responses to therapy. Mol Ther 2014; 22: 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bialkowski L, van Weijnen A, Van der Jeught K, et al. Intralymphatic mRNA vaccine induces CD8 T-cell responses that inhibit the growth of mucosally located tumours. Sci Rep 2016; 6: 22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao X, Li L, Starr TK, et al. Tumor location impacts immune response in mouse models of colon cancer. Oncotarget 2017; 8: 54775–54787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lehmann B, Biburger M, Brückner C, et al. Tumor location determines tissue-specific recruitment of tumor-associated macrophages and antibody-dependent immunotherapy response. Sci Immunol 2017; 2: eaah6413. [DOI] [PubMed] [Google Scholar]
- 49. Melero I, Castanon E, Alvarez M, et al. Intratumoural administration and tumour tissue targeting of cancer immunotherapies. Nat Rev Clin Oncol 2021; 18: 558–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kiriu T, Yamamoto M, Nagano T, et al. Pseudo-progression and the neutrophil-to-lymphocyte ratio in non-small cell lung cancer treated with immune checkpoint inhibitors: a case-control study. Onco Targets Ther 2019; 12: 10559–10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tang Y, Cui Y, Li LL, et al. Dynamics of early serum tumour markers and neutrophil-to-lymphocyte ratio predict response to PD-1/PD-L1 inhibitors in advanced non-small-cell lung cancer. Cancer Manag Res 2021; 13: 8241–8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fujita K, Yamamoto Y, Kanai O, et al. Retreatment with anti-PD-1 antibody in non-small cell lung cancer patients previously treated with anti-PD-L1 antibody. Thorac Cancer 2020; 11: 15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Otani Y, Mori K, Morikawa N, et al. Rechallenge of anti-PD-1/PD-L1 antibody showed a good response to metastatic breast cancer: a case report. Immunotherapy 2021; 13: 189–194. [DOI] [PubMed] [Google Scholar]
- 54. Puza CJ, Bressler ES, Terando AM, et al. The emerging role of surgery for patients with advanced melanoma treated with immunotherapy. J Surg Res 2019; 236: 209–215. [DOI] [PubMed] [Google Scholar]
- 55. Vaishampayan U, Shah H, Asad MF, et al. Adrenal metastases as sanctuary sites in advanced renal cancer. J Kidney Cancer VHL 2020; 7: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lyu N, Kong Y, Li X, et al. Ablation reboots the response in advanced hepatocellular carcinoma with stable or atypical response during PD-1 therapy: a proof-of-concept study. Front Oncol 2020; 10: 580241. [DOI] [PMC free article] [PubMed] [Google Scholar]