Abstract
The general immune landscape of nasopharyngeal carcinoma (NPC) renders immunotherapy suitable for patients with NPC. Immune checkpoint inhibitors (ICIs) based on programmed death-1/programmed death ligand-1 (PD-1/PD-L1) blockade have made a breakthrough with the approval of PD-1 inhibitor for refractory recurrence and/or metastatic (R/M NPC) and the approval of PD-1 inhibitor in combination with gemcitabine and cisplatin as first line for R/M NPC in 2021 in China. The incorporation of ICIs into the treatment paradigms of NPC has become a clinical hot spot and many prospective clinical studies are ongoing. In this review, we provide a comprehensive overview of the rationale for immunotherapy in NPC and current status, advances and challenges of immunotherapy in NPC based on published clinical data, and ongoing trials. We focus on the clinical application and advances of PD-1 inhibitor monotherapy and its combination with chemotherapy and summarize the clinical explorations of other immunotherapy approaches, for example, combination of PD-1/PD-L1 inhibitors with antiangiogenic inhibitor with molecular targeted agents, cancer vaccines, adaptive immunotherapy, and new ICI agents beyond PD-1/PD-L1 inhibitors in R/M NPC. We also describe the clinical studies’ status and challenges of ICIs-based immunomodulatory strategies in local advanced NPC and pay attention to the biomarker application for personalized immunotherapy of NPC in the hope to provide insights for clinical practice and future clinical studies.
Keywords: Immune checkpoint inhibitors, immunotherapy, nasopharyngeal carcinoma
Introduction
Nasopharyngeal carcinoma (NPC) has a unique geographical and ethical distribution, which is attributed to both genetic and environmental factors. 1 Based on GLOBOCAN estimates, in 2018, there were 129,079 new cases and 72,987 deaths due to NPC. 2 NPC occurs more frequently in Southeast Asia than in the rest of the world and is particularly observed in Southern China, which has incidence of >20 cases per 100,000 people and a mortality risk of 6 cases per 100,000 people. 3 The non-keratinizing subtype is the most common pathological subtype of NPC and accounts for >95% of cases in endemic areas, which is predominantly associated with Epstein–Barr virus (EBV) infection, greater response to radiation therapy, and better survival than the keratinizing NPC subtype.4–7
For non-metastatic NPC, radiotherapy is the primary curative treatment. The strategy of combining chemotherapy with radiotherapy is a pivotal advancement for locally advanced NPC (LANPC). Approximately 5–11% of patients present with de novo metastatic disease, whereas 15–30% of patients who are treated for locally advanced disease develop local recurrence and/or metastatic (R/M) NPC.8,9 Platinum-based doublet chemotherapeutic regimens, preferentially gemcitabine plus cisplatin (GP), are generally considered the standard first line of care for patients with R/M NPC. 10 For patients with R/M NPC receiving first-line chemotherapy, their estimated median overall survival (OS) is 15.7 months (95% CI, 12.3–19.1) and median progression-free survival (PFS) is 7.6 months (95% CI, 6.2–9.0). 11 For the patients undergoing second- or later-line therapies, the estimated median OS is approximately 11.5 months and median PFS is 5.4 months (95% CI, 3.8–7.0). 11 No consensus has been reached regarding the treatment following disease progression after the first-line therapy. Current conventional treatments, including radiotherapy, chemotherapy, and surgical resection, are often associated with significant adverse effects and limited efficacy. 12 Thus, there is an urgent need for novel treatment strategies to further improve the outcomes of patients with NPC.
In recent years, immunotherapy has prompted a revolution in the clinical management of many cancers. Immune checkpoint inhibitors (ICIs) based on programmed death-1/programmed death ligand-1 (PD-1/PD-L1) blockade have made a breakthrough and were approved for the treatment of R/M NPC in 2021 in China. The incorporation of ICIs into the current treatment paradigms of LANPC is a clinical hot spot, and many prospective phase II or III clinical studies are ongoing. This review provides a comprehensive overview of the current landscape of immunotherapy in NPC by presenting the rationale of immunotherapy, updated status and challenges of immunotherapy in R/M NPC and LANPC based on the published clinical trial data and ongoing trials data. We also described and analyzed the current status and controversies of different combinatorial strategies and biomarkers application for personalized NPC immunotherapy in the hope to provide insights for clinical practice and future clinical studies.
Rationale for immunotherapy in NPC
NPC is regarded as a highly immune-inflamed tumor due to its unique immune landscape. Close associations with chronic EBV infection, abundant lymphocytic infiltrations, and high expression of PD-L1, and several key immune molecular regulating the activation of T-cells (CD40, CD70, CD80, and CD86) are often observed in EBV-induced NPC. 10 We profiled the rationale supporting immunotherapy in NPC by analyzing the basic immunobiological foundations, including tumor antigenicity, tumor microenvironment (TME), and tumor immunogenicity. The biological steps required to achieve an effective immune response in NPC is depicted Figure 1. 13
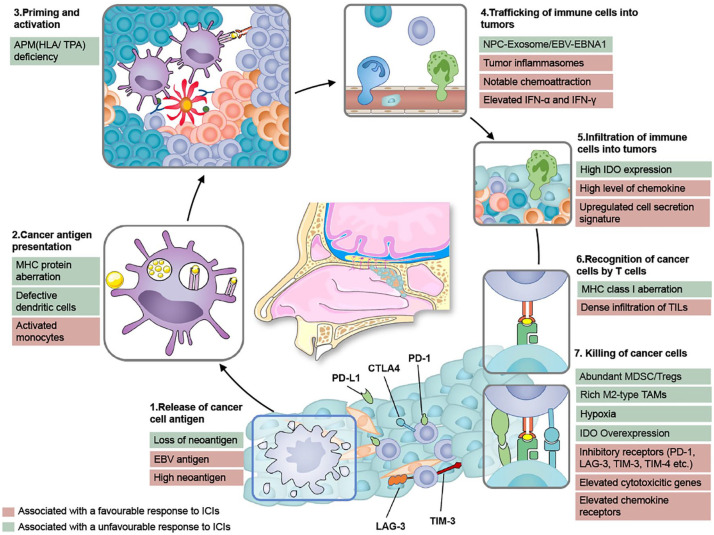
Figure 1.
The biological steps to achieve an effective immune response in NPC. This figure is based on the original cancer–immunity cycle proposed by Chen and Mellman. 13 The steps and factors affecting the response of ICIs are edited to reflect the specific immunobiology of NPC. The release of NPC-specific antigens (1) and their subsequent presentation by dendritic cells (DCs) and other antigen-presenting cells (APCs) (2), is followed by T-cell priming and activation (3), trafficking into tumors (4), tumor infiltration (5), and recognition and killing of tumor cells (6 and 7). Notable NPC-specific characteristics of this cycle favoring antitumor immunity include the release of NPC-specific antigens (including EBV-derived antigens and a large number of neoantigens), activated monocytes which could also be considered as a kind of APCs, promoting the trafficking of immune cells into tumors [via tumor inflammasomes, increased chemoattraction, notable interferon-α (IFN-α) and IFN-γ], dense infiltration of immune cells into tumors, high expression of inhibitory receptors, elevated cytotoxicity genes, and chemokine receptors. Conversely, antitumor immunity could be impeded by the loss of NPC-specific neoantigens, major histocompatibility complex protein (MHC) aberration, defective DCs or antigen-processing machinery components (APM) deficiency, high epithelial indoleamine 2,3-dioxygenase, abundant myeloid-derived suppressor cells (MDSC) or regulatory T-cells (Tregs), rich M2-type tumor-associated macrophages (TAMs), and several features typically associated with T-cell exhaustion.
Antigenicity is the initial stimulus that activates the tumor immune response and depends on the quality and quantity of tumor antigens, and the antigen’s successful presentation. Neoantigens are the main antigens that induce antitumor activity.14,15 Compared with other cancers in the cancer genome atlas, a large number of neoantigens have been detected in NPC. 14 Since EBV infection is a driving factor for NPC, EBV antigens, such as latent membrane protein (LMP-1, LMP-2) and nuclear antigen-1 (EBNA1), are regarded as surrogate neoantigens.4,16,17 In addition to having efficient antigens, the interferon response is elevated in NPC and is related to a higher proportion of antigen-presenting cells. 18 However, the aberration of major histocompatibility complex protein (MHC) or APM components impede the successful antigen presentation in NPC and leads to the invasion of immune surveillance.19–24 Subsequently, immune cell trafficking and infiltration of the TME play an essential role in activating an immune response.25,26
Distinct features of tumor immunogenicity to overcome an immunosuppressive microenvironment and eventually kill tumor cells provide more potential for efficient immunotherapy in NPC. Infiltrating lymphocytes (TILs), including CD4 + T, CD8 + T, and CD57 + NK cells, are abundant in tumor tissues.27–30 However, upregulated MDSC,31–34 overexpression of PD-L1, and various co-stimulators associated with T-cell exhaustion and dysfunction in NPC include cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), lymphocyte activation gene 3 (LAG-3), T-cell immunoglobulin and ITIM domain (TIGIT), T-cell immunoglobulin and mucin domain 3 (TIM3), and CD276, which participate in the blockade of immune response and could be considered as targets for immune therapy.18,29,30,35–41 The general immune texture of NPC renders the patients potentially appropriate for immunotherapy, especially for ICIs, although some immunosuppressive elements warrant further study (i.e. loss of neoantigen, MHC protein aberration, defective DCs, and abundance of Tregs). When ICIs are used to stop an immune response cascade, a strong antitumor immune activity would be elicited to kill tumor cells, which could pave a novel and promising treatment for NPC. The approval of toripalimab and camrelizumab supported the establishment of a standard treatment setting for patients with refractory R/M NPC.
PD-1/PD-L1 inhibitors in R/M NPC
Many PD-1/PD-L1 inhibitors have been evaluated in R/M NPC. Toripalimab and camrelizumab monotherapy were approved for refractory R/M NPC by National Medical Products Administration (NMPA) in 2021. The promising antitumor activity and manageable safety profile of PD-1/PD-L1 inhibitors have promoted the investigation of various combination approaches, including chemotherapy, targeted therapy, other immunotherapeutic agents, or radiotherapy. Camrelizumab combined with GP regimen as first-line setting was approved for R/M NPC by NMPA in June 2021 based on the results of CAPTAIN study. 42 Toripalimab combined with GP regimen as first-line setting was approved for R/M NPC by NMPA in November 2021 based on the results of JUPITER-02 study. Here, we focus on the clinical application and advance of PD-1 inhibitor monotherapy and its combination with chemotherapy and summarize the clinical explorations of other combination strategies, for example, combination of PD-1/PD-L1 inhibitors with antiangiogenic inhibitor, radiotherapy, or new ICI agents in R/M NPC
PD-1/PD-L1 inhibitor monotherapy in R/M NPC
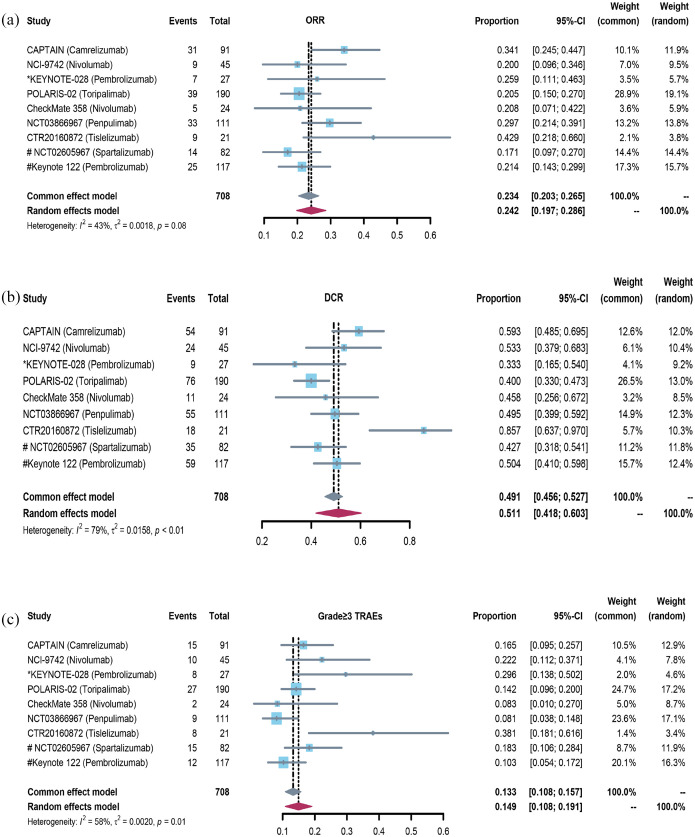
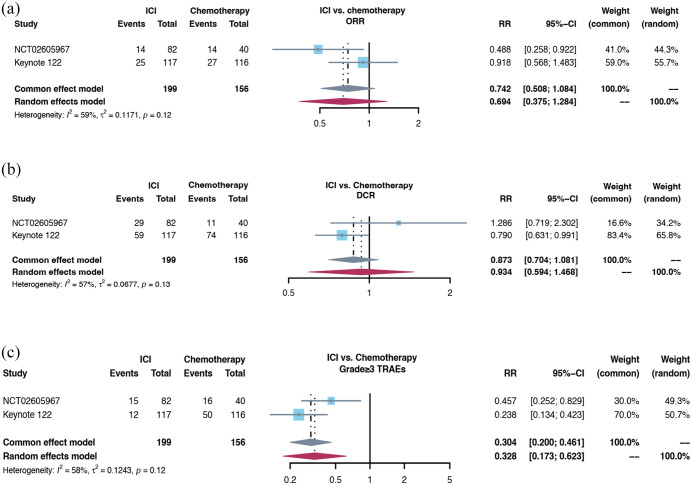
For the second- or later-line setting, 10 clinical trials with 1163 R/M NPC patients were included. All patients progressed during or after platinum-based chemotherapy and then received PD-1/PD-L1 inhibitor monotherapy. The pooled objective response rate (ORR) was 22.5% (95% CI 18.3–26.7%) [Figure 2(a)] and disease control rate (DCR) was 52.1% (95% CI 43.4–60.9%) [Figure 2(b)]. The forest plots of grade ⩾3 treatment-related adverse effects (TRAEs) incidence was 14.9% (95% CI 10.8–19.1%) [Figure 2(c)]. Both NCT02605967 study and KEYNOTE-122 study were randomized clinical trials which compared the efficacy and safety between ICIs monotherapy and chemotherapy based on physician’s choice. The pooled ORR and DCR had no significant difference [Figure 3(a) and (b)], the incidence of grade ⩾3 TRAEs was significantly lower in ICIs group [Figure 3(c)]. For unselected refractory R/M NPC patients, the antitumor activity of PD-1 antibody monotherapy was moderate, which was similar to chemotherapy.
Figure 2.
The forest plot of ORR, DCR, and grade ⩾3 TRAEs incidence in ICI monotherapy for R/M NPC: (a) the forest plot of ORR in ICI monotherapy for R/M NPC. (b) The forest plot of DCR in ICI monotherapy for R/M NPC. (c) The forest plot of grade ⩾3 TRAEs incidence in ICI monotherapy for R/M NPC.
DCR, disease control rate; ORR, objective response rate; TRAE, treatment-related adverse effect.
Figure 3.
The forest plot of ORR, DCR, and grade ⩾3 TRAEs incidence in comparison between ICI monotherapy and chemotherapy for R/M NPC: (a) the forest plot of ORR in comparison between ICI monotherapy and chemotherapy for R/M NPC. (b) The forest plot of DCR in comparison between ICI monotherapy and chemotherapy for R/M NPC. (c) The forest plot of grade ⩾3 TRAEs incidence in the comparison between ICI monotherapy and chemotherapy for R/M NPC.
DCR, disease control rate; ORR, objective response rate; TRAE, treatment-related adverse effect.
POLARIS-02 study is a single-arm and multicenter phase II study which evaluated the efficacy, safety, and correlative biomarkers of toripalimab in previously treated R/M NPC and enrolled 190 patients. The ORR was 20.5% (39/190), median duration of response (DOR) was 12.8 months, and median OS was 17.4 months. The ORR and DOR of 92 patients who failed at least two lines of systemic chemotherapy were 23.9% and 21.5 months, respectively. However, 27 (14.2%) patients experienced grades 3–5 TRAEs. Hypothyroidism, hyperthyroidism, and abnormal liver function were the main immune-related adverse events. 43
CAPTAIN study is also a single-arm and multicenter phase II study which evaluated the efficacy and safety of camrelizumab in previously treated R/M NPC and enrolled 93 patients. The ORR was 34.0% (31/91) and DCR was 59.0% (54/91). The median PFS was 5.6 months with a median follow-up time of 9.9 months. The incidence of grade 3 or 4 TRAEs was 16% (15/91), and the most common adverse events were stomatitis, anemia, and abnormal liver function. 43
The NCT02605967 study is the first randomized phase II study to compare spartalizumab (PD-1 monoclonal antibody) with chemotherapy in R/M NPC patients who progressed on or after platinum-based chemotherapy. The patients were randomized to receive either spartalizumab (n = 82) or chemotherapy (n = 40). Chemotherapy was administered according to the investigator’s choice and one-third of the patients received chemotherapy combinations with two or more drugs. Spartalizumab showed a safety profile consistent with that of other anti-PD-1 antibodies. The most common TRAEs were fatigue (10.3%) and pruritus (9.3%). The primary endpoint of median PFS was not met. The median PFS in spartalizumab group was 1.9 versus 6.6 months in chemotherapy group (p = 0.915), and the ORR in spartalizumab group was lower (17.1% versus 35.0%). However, the median OS (25.2 versus 15.5 months) and median DOR (10.2 versus 5.7 months) were longer in spartalizumab group than in chemotherapy group. 44
The KEYNOTE-122 study (NCT02611960) was the first randomized phase III study comparing pembrolizumab with single-agent standard chemotherapy based on the investigator’s choice (capecitabine, gemcitabine, or docetaxel) in patients with platinum-pretreated R/M NPC. The results were first reported in 2021 European Society of Medical Oncology (ESMO). There were 117 patients in pembrolizumab group and 116 patients in chemotherapy arm. The study failed to meet the primary endpoint of OS. The median OS in pembrolizumab arm was 17.2 and 15.3 months in chemotherapy group (p = 0.226). The median PFS in pembrolizumab group was 4.1 versus 5.5 months in chemotherapy group. The ORR was similar (21.4% versus 23.3%). The incidence of grades 3–5 TRAEs was 10.3% in pembrolizumab group versus 43.8% in chemotherapy group. Although the study did not meet the primary endpoint, pembrolizumab showed a trend of better OS and a lower incidence of treatment-related adverse events. Examination of Kaplan–Meier OS curves for pembrolizumab versus chemotherapy showed an early favorable trend toward chemotherapy. Meanwhile, the separation in favor of pembrolizumab was sustained after 13 months, indicating a long-term survival benefit with pembrolizumab.
In these two randomized controlled studies, a crossover of the survival curves was observed. With the extension of follow-up time, we can see further improvements of OS. This might reflect the time taken by ICI to induce an effective antitumor immune response and greater durability of benefit from immunotherapy in patients who achieved response or prolonged stable disease.
These phenomena have been observed in studies of PD-1 or PD-L1 inhibitors in unselected patients with other refractory metastatic solid tumor types, such as non-small cell lung cancer (KEYNOTE-010), 45 head and neck squamous cell carcinoma (KEYNOTE-040), 46 esophageal cancer (KEYNOTE-181), 47 and gastric cancer (KEYNOTE-061 or KEYNOTE-062).48,49 This violation of the proportional hazard assumption supports the idea that alternative statistical approaches for addressing changes in risk over time will be necessary for future clinical studies evaluating the delayed onset of benefit typically observed with immunotherapies.
Coupled with these results, it could be inferred that both chemotherapy and PD-1 inhibitor monotherapy could be choices for R/M NPC patients who progressed during or after platinum-based chemotherapy. However, compared with chemotherapy, the TRAEs incidence is significantly lower in ICIs group with good tolerance. A subset of patients responding to anti-PD-1 antibody could benefit from long-term survival. The clinically meaningful survival prolongation and favorable safety profile of ICIs supported the prioritized recommendations of ICIs, especially for those patients with heavy-treated R/M NPC who had limited treatment options and poorer tolerance. Given to only a small subset of patients benefiting from PD-1 immunotherapy, molecular biomarkers will further help to identify the patients who benefit more from PD-1 inhibitors. The novel ICIs is a hot spot in the future research. Further studies regarding ICI as monotherapy or as part of combination therapy in earlier stage settings should be considered.
Combination of PD-1/PD-L1 inhibitor and chemotherapy in R/M NPC
The combination of ICIs and chemotherapy, which can eliminate or modulate immune suppressive cells in the TME, has shown promising synergy.50–52 The immunosuppression states could be unleashed by chemotherapy (including gemcitabine, platinum, and 5-fluorouracil) by promoting antigen presentation, enhancing T-cell responses, and relieving immunosuppression in TME.41,50,53–59 Given the promising results of ICI monotherapy and preclinical evidence of the synergy between chemotherapy and ICIs, clinical exploration of the combination of ICIs with chemotherapy is required to promote the management of R/M NPC (Table 1).
Table 1.
Completed clinical trial of PD-1/PD-L1 inhibitors monotherapy in recurrent and/or metastatic (R/M) NPC.
| Trial | Design | Population | Previous lines | Treatment and dosage | ORR (%) | DCR (%) | Median PFS (95% CI), months | Median OS (95% CI), months | DOR (95% CI), months | Grade ⩾ 3 TRAEs (%) | The most common Grade ⩾ 3 TRAEs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CAPTAIN | Phase I | 91, Asian (100%) | 37.0% patients ⩾ 3 | Camrelizumab: anti-PD-1 IgG4 mAb bridging dose of 200 mg every 2 weeks |
34.0 | 59.0 | 5.6 (3.3–7.9) | NA | NR (6.3–NR) | 16.0 | Anemia (2.0%); ALT elevation (2.0%); AST elevation (2.0%); bilirubin elevation (2.0%) |
| NCI-9742 | Phase II | 45, Asian (82.2%) | 61.0% patients ⩾ 3 | Nivolumab: anti-PD-1 IgG4 mAb 3 mg/kg every 2 weeks |
20.5 | 54.5 | 2.8 (1.8–7.4) | 17.1 (10.9–NR) | NA | 22.0 | Hepatitis, diarrhea, fatigue, hyponatremia ALT elevation, neutropenia |
| a KEYNOTE-028 | Phase Ib | 27, Asian (63%) | 70.4% patients ⩾ 3 | Pembrolizumab: anti-PD-1 IgG4 mAb 10 mg/kg every 2 weeks |
25.9 | 37.0 | 3.7 (2.1–13.4) | 16.5 (10.1–NR) | 17.1 (4.8–22.1) | 29.0 | Hepatitis (7.4%); pneumonitis (7.4%) |
| POLARIS-02 | Phase II | 190, Asian (100%) | 48.4% patients ⩾ 2 | Toripalimab: anti-PD-1 IgG4 mAb 3 mg/kg once every 2 weeks |
20.5 | 40.0 | 1.9 (1.8–3.5) | 17.4 (11.7–22.9) | 12.8 (9.4–NR) | 14.0 | Anemia (1.1%); Asthenia (1.1%) Neutropenia (0.5%) |
| CheckMate 358 | Phase I/II | 24, European (88%) | All patients ⩽ 2 79.2% patients ⩾ 1 |
Nivolumab: anti-PD-1 IgG4 mAb 240 mg every 2 weeks |
20.8 | 45.8 | 2.4 (1.5–NR) | NR | NR | 8.3 | NA |
| NCT03866967 | Phase I | 130 were included, 111 in FAS, Asian (100%) | All patients ⩾ 2 | Penpulimab: anti-PD-1 IgG4 mAb with less binding to FcγR; 240 mg every 2 weeks |
29.7 | 49.5 | 3.7 (1.9–6.6) | 18.6 (14.1–NR) | NR | 8.5 | Hepatic function abnormal (2.3%); rash (2.3%) |
| CTR20160872 | Phase I/II | 300 patients with solid tumor including 21 R/M NPCs | NA | Tislelizumab: anti-PD-1 IgG4 mAb with less binding to FcγR; 200 mg every 3 weeks | 43.0 | 86.0 | 4.0 (2.1–8.1) | NA | 8.3 (3.9–NR) | 40.0 | GGT elevation (4%); Anemia (3.0%); AST elevation (3.0%); ALT elevation (3.0%) |
| b NCT02605967 | Phase II | 113 (2:1) in spartalizumab arm versus CT arm | All patients ⩾ 2 | Spartalizumab: anti-PD-1 IgG4 mAb 400 mg every 4 weeks, CT decided by investigators |
17.1 versus 35.0 | 35.0 versus 28.0 | 1.9 versus 6.6 | 25.2 versus 15.5 | 10.2 versus 5.7 | 18.4 versus 40.5 | NA |
| b KEYNOTE-122 | Phase III | 233 (1:1) in pembrolizumab arm versus CT arm |
All patients ⩾ 2 | Pembrolizumab: anti-PD-1 IgG4 mAb 200 mg every 3 weeks, CT decided by investigators |
21.4 versus 23.3 | 50.4 versus 63.8 | 4.1 versus 5.5 | 17.2 versus 15.3 | 12.0 versus 13.1 | 10.3 versus 43.8 | NA |
| NCT02825940 | Phase I | 20, Asian (100%) | NA | Atezolizumab: anti-PD-L1 IgG4 mAb 1200 mg every 3 weeks |
10.0 | 65.0 | NA | NA | NA | NA | Hyponatremia (5%); lipase increased (3%); aspartate aminotransferase (3%) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; CT, chemotherapy; DCR, disease control rate; DOR, duration of response; GGT, Gamma-glutamyl transferase; mAb, monoclonal antibody; N/A, not available; NPC, nasopharyngeal carcinoma; NR, not reached; ORR, objective response rate; OS, overall survival; PD-1, programmed death-1; PD-L1, programmed death ligand-1; PFS, progression-free survival; R/M, recurrence and/or metastatic; TRAE, treatment-related adverse effect; FAS, full analysis set.
In the study of KEYNOTE-028, all R/M NPC patients had PD-L1 expression in 1% or more of tumor cells or tumor-infiltrating lymphocytes.
In the study of spartalizumab and KEYNOTE-122, the data of efficacy and safety are shown as anti-PD-1 antibody arm versus CT arm.
The combination of camrelizumab with the GP regimen has shown promising antitumor activity as first-line setting for R/M NPC in a phase I trial. 42 The CAPTAIN-1st study is a randomized, double-blind, phase III trial that explored the addition of camrelizumab to the GP regimen as a first-line therapy. In total, 263 eligible patients were randomly assigned to the camrelizumab group (n = 134) or placebo group (n = 129). At the prespecified interim analysis (15 June 2020), the independent review committee-assessed median PFS was significantly longer in the camrelizumab group [HR = 0.54, 9.7 versus 6.9 months, HR = 0.54, p = 0.0002)]. In the camrelizumab group, 94% (126/134) and 91% (118/129) of patients in the placebo group had grade ⩾3 TRAEs, of which a decrease in white blood cell count, decreased neutrophil count and anemia, and decreased platelet count were the most common (Table 2). 60
Table 2.
Completed clinical trial of PD-1/PD-L1 inhibitors combination in R/M NPC.
| Trial | Design | Population | Treatment | ORR (%), ICI arm versus placebo arm | DCR (%), ICI arm versus placebo arm | Median PFS (months), ICI arm versus placebo arm | Median OS, ICI arm versus placebo arm | DOR (months), ICI arm versus placebo arm | 1-year PFS rate (%), ICI arm versus placebo arm |
Grade ⩾ 3 TRAEs (%) ICI arm versus placebo arm |
The most common grade ⩾ 3 TRAEs in ICI arm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| JUPITER-02 | Phase III | 289 R/M NPC (1:1) without prior CT | Toripalimab 240 mg or placebo (day 1), gemcitabine 1 g/m2
(Days 1 and 8), and cisplatin 80 mg/m2 (day 1) every 3 weeks |
77.4 versus 66.4 p = 0.0335 |
87.7 versus 79.7 p = 0.0650 |
11.7 versus 8.0 p = 0.0003 |
Immature | 10.0 versus 5.7 p < 0.005 |
49.4 versus 27.9 | 89.0 versus 89.5 p > 0.05 |
Neutropenia (63.6%) Leukopenia (58.0%) |
| CAPTAIN-1st | Phase III | 263 R/M NPC (1:1) without prior CT | Camrelizumab 200 mg or placebo (day 1), gemcitabine 1 g/m2
(Days 1 and 8), and cisplatin 80 mg/m2 (day 1) every 3 weeks |
87.3 versus 80.6 p > 0.05 |
96.3 versus 94.6 p > 0.05 |
9.7 versus 6.9 p = 0.0002 |
Immature | 8·5 versus 5.6 | N/A | 94.0 versus 91.0 p > 0.05 |
Decreased WBC count (66.0%); Neutropenia (64.0%); anemia (40.0%) |
| RATIONALE 309 | Phase III | 263 R/M NPC (1:1) without prior CT | Tislelizumab 200 mg or placebo (day 1), gemcitabine 1 g/m2
(Days 1 and 8), and cisplatin 80 mg/m2 (day 1) every 3 weeks |
69.5 versus 55.3 | 89.3 versus 84.8 | 9.2 versus 7.4 p < 0.0001 |
Immature | 8.5 versus 6.1 | 35.7 versus 12.2 | 80.9 versus 81.8 | Neutropenia (30.0%); anemia (25.0%) |
| NCT03121716 | Phase I | 22 R/M NPC without prior CT | Camrelizumab 200 mg (day 1), gemcitabine 1 g/m2
(Days 1 and 8), and cisplatin 80 mg/m2 (day 1) every 3 weeks |
91.0 | 100 | NR | NA | NR | 61.0 | 13 (57.0%) | Neutropenia (53.0%); anemia (48.0%) |
CT, chemotherapy; DCR, disease control rate; DOR, duration of response; ICI, immune checkpoint inhibitors; N/A, not available; NPC, nasopharyngeal carcinoma; NR, not reached; ORR, objective response rate; OS, overall survival; PD-1, programmed death-1; PD-L1, programmed death ligand-1; PFS, progression-free survival; R/M, recurrence and/or metastatic; TRAE, treatment-related adverse effect; WBC, white blood cell.
The JUPITER-02 study was a multinational, randomized, double-blind, phase III study conducted in mainland China, Taiwan, and Singapore that compared the GP regimen in combination with either toripalimab or placebo in patients with R/M NPC as the first-line setting. 61 A total of 289 patients were randomized to either toripalimab or placebo in combination with GP every 3 weeks for up to six cycles, followed by maintenance therapy with toripalimab or placebo. The interim analysis showed that median PFS in toripalimab group was significantly superior to that in placebo group (HR = 0.52, 11.7 versus 8.0 months). Significant improvements of 1-year PFS rates and ORR were observed in toripalimab group. In toripalimab group, 89.0% developed grade 3 or worse adverse events, similar to those in the placebo arm (89.5%) (Table 2).
The RATIONALE 309 study, a phase III randomized clinical trial, explored the value of adding tislelizumab, an anti-human PD-1 monoclonal IgG4 antibody, or placebo to GP regimen for R/M NPC as first-line setting. 62 A total of 263 patients from Asia were enrolled. The interim analysis showed that tislelizumab in combination with chemotherapy demonstrated a statistically significant improvement in PFS compared with chemotherapy alone (9.2 versus 7.4 months). Clinical trials using other anti-PD-1 inhibitors, including nivolumab, penpulimab, and SHR-1701, in combination with chemotherapy are ongoing (Table 3).
Table 3.
Ongoing clinical trials of ICIs in R/M NPC.
| Trial identifier | Treatment | Phase | Patient selection | Estimated enrollment | Primary endpoint | Completion date | |
|---|---|---|---|---|---|---|---|
| ICI monotherapy | |||||||
| NCT02605967 | PDR001 a | Phase II | R/M NPC at least failing to first-line chemotherapy | 122 | PFS | February 2021 | |
| NCT03866967 | AK105 a | Phase II | R/M NPC at least failing to first-line chemotherapy | 153 | ORR | December 2020 | |
| NCT02875613 | Avelumab | Phase II | EBV-related R/M NPC at least failing to first-line therapy | 6 | ORR | April 2019 | |
| NCT04221516 | Camrelizumab a | Phase II | After completion of radiotherapy for oligometastatic NPC | 52 | PFS | February 2023 | |
| NCT04220307 | AK-104 | Phase II | R/M NPC as third-line setting | 140 | ORR | November 2021 | |
| NCT03558191 | SHR-1210 | Phase II | R/M NPC as third-line setting | 155 | ORR | 30 December 2020 | |
| NCT05198531 | TQB2858 | Phase Ib | R/M NPC failed platinum-based chemotherapy and ICIs; untreated NPC | 90 | AEs | October 2022 | |
| NCT05222009 | G-CSF + PD-(L)1 antibody | Phase I | Received prior treatment with platinum agents and PD-(L)1 inhibitors in R/M NPC | 25 | ORR | November 2022 | |
| NCT03752398 | XmAb23104 | Phase I | Advanced solid tumor including NPC failure at standard therapy | 234 | AEs | July 2023 | |
| NCT04272034 | INCB099318 | Phase I | Advanced solid tumor including NPC failure at standard therapy | 100 | AEs | December 2022 | |
| ICI combination therapy | |||||||
| Combine with ICIs | NCT03849469 | XmAb22841 + pembrolizumab a | Phase I | Advanced solid tumor including NPC | 242 | Safety; AEs | November 2025 |
| NCT04945421 | Sintilimab a + IBI310 | Phase I/II | Anti-PD-1/PD-L1 resistance R/M NPC | 121 | ORR | March 2024 | |
| NCT03097939 | Ipilimumab + nivolumab a | Phase II | Advanced EBV-driven NPC | 40 | ORR | November 2021 | |
| NCT04282070 | SHR-1701 | Phase I | R/M NPC failing to first-line chemotherapy; failure at anti-PD-1 immunotherapy | 91 | AEs | December 2022 | |
| NCT05020925 | SHR-1701 + famitinib | Phase I/II | R/M NPC failing to prior platinum therapy | 30 | ORR | February 2024 | |
| NCT02834013 | Nivolumab and ipilimumab | Phase II | Rare cancer including NPC | 818 | ORR | October 2023 | |
| NCT03517488 | XmAb20717 | Phase I | Advanced solid tumors including NPC | 154 | AEs | April 2021 | |
| Combine with CT | NCT02611960 | Pembrolizumab a + GTX | Phase III | R/M NPC failing to prior platinum therapy | 233 | OS | May 2022 |
| NCT04458909 | Nivolumab a + GCP | Phase III | R/M NPC as first-line treatment | 316 | OS | May 2028 | |
| NCT03924986 | Tislelizumab a + GP | Phase III | R/M NPC as first-line treatment | 256 | PFS | June 2022 | |
| NCT04890522 | Toripalimab a + GFP | Phase II/III | Primary metastatic NPC as first-line treatment | 622 | PFS; OS; SAE | December 2028 | |
| NCT04944914 | Camrelizumab a + SBRT | Phase III | Oligometastatic NPC | 188 | PFS | June 2026 | |
| NCT04974398 | AK 105 a + GP | Phase III | R/M NPC as first-line treatment | 278 | PFS | July 2023 | |
| NCT05166577 | Nanatinostat + valganciclovir + pembrolizumab | Phase Ib/II | R/M NPC with EBV positive as second-or third-line setting | 88 | DLT; ORR | May 2024 | |
| Combine with RT | NCT03925090 | Toripalimab + CCRT | Phase II | Stage III–IVa and EBV DNA ⩾ 1500 copies/ml | 138 | PFS | October 2023 |
| Combine with CT + RT | NCT04421469 | Toripalimab a + gemcitabine + RT | Phase II | Multiple metastatic NPC | 39 | PFS | June 2023 |
| NCT04398056 | CT + subsequent RT + toripalimab | Phase II | De novo metastatic NPC | 22 | ORR | April 2021 | |
| Targeted therapy | NCT04825990 | Pembrolizumab a + olaparib | Phase II | Platinum-resistant R/M NPC | 30 | ORR | June 2027 |
| NCT04562441 | Avelumab + axitinib | Phase II | R/M NPC at least failing to first-line chemotherapy | 43 | ORR | December 2027 | |
| NCT04586088 | Camrelizumab a + apatinib | Phase II | R/M NPC failing to first-line chemotherapy | 57 | ORR | May 2022 | |
| NCT04872582 | Sintilimab a + bevacizumab | Phase II | R/M NPC failing to first-line chemotherapy | 33 | ORR | May 2022 | |
| NCT04350190 | PD-1 inhibitor + apatinib | Phase II | R/M NPC failing to one comprehensive treatment | 25 | ORR | May 2022 | |
| NCT04548271 | Camrelizumab a + apatinib | Phase II | PD-1 antagonists resistant R/M NPC failing to first-line therapy | 25 | ORR | October 2023 | |
| NCT04547088 | Camrelizumab a + Apatinib | Phase II | R/M NPC failing to first-line chemotherapy | 27 | ORR | October 2023 | |
| NCT03813394 | Pembrolizumab a + bevacizumab | Phase Ib/II | EBV-positive NPC failing to first-line chemotherapy | 48 | ORR; PFS | March 2024 | |
| NCT04736810 | AK105 a + GP + anlotinib | Phase II | R/M NPC as first-line treatment | 90 | ORR | June 2023 | |
| NCT04073784 | Toripalimab a + gemcitabine + apatinib | Phase I | R/M NPC as first-line treatment | 20 | AEs | June 2021 | |
| NCT05162872 | Niraparib and sintilimab | Phase II | R/M NPC failing to first-line therapy | 99 | ORR | October 2023 | |
| NCT03074513 | Atezolizumab and bevacizumab | Phase II | Rare solid tumors including NPC | 137 | ORR | December 2022 | |
AC, adjuvant chemotherapy; AE, adverse event; CCRT, concurrent chemoradiothrapy; CT, chemotherapy; DLTS, incidence of dose-limiting toxicities; DNA, deoxyribonucleic acid; EBV, Epstein–Barr virus; GCP, Cisplatin + Gemcitabine + Carboplatin; GFP, Cisplatin + Gemcitabine + 5-Fluorouracil; GP, Cisplatin + Gemcitabine; GTX, Capecitabine + Gemcitabine + Docetaxel; IC, induction chemotherapy; ICIs, immune checkpoint inhibitors; NPC, nasopharyngeal carcinoma; ORR, objective response rate; OS, overall survival; PD-1, programmed death-1; PD-L1, programmed death ligand-1; PFS, progression-free survival; R/M, recurrence and/or metastatic; RT, radiotherapy; SAE, serious adverse event; SBRT, stereotactic body radiation therapy.
Nanatinostat: a selective class I HDAC inhibitor which induces EBV early lytic phase protein generation, activating (val) ganciclovir to its cytotoxic form.
Represents anti-PD-1 antibody; avelumab: anti-PD-L1 antibody; INCB099318: an oral, small molecule that targets PD-L1; XmAb22841: CTLA-4/LAG-3 bispecific antibody; IBI310: anti-CTLA-4 antibody; AK-104: PD-1/CTLA-4 bispecific antibody; ipilimumab: anti-CTLA-4 antibody; SHR-1701: a bifunctional fusion protein targeting PD-L1 and TGF-β; TQB2858 injection: a bifunctional fusion protein against PD-L1 and transforming growth factor-β; XmAb23104: a bispecific antibody targeting PD-1 and ICOS; XmAb20717: a bispecific antibody targeting PD-1 and CTLA-4 bispecific antibody; BOR: best overall response rate.
The published data from three phase III studies in the first-line setting reported positive results of primary endpoint PFS with a manageable safety profile, which supported that PD-1 inhibitors plus GP regimen become a new standard of care for patients with R/M NPC in the first-line setting. However, OS events were not mature; longer follow-up is needed to assess whether or not the PFS benefit could translate into OS prolongation and long-term adverse effects. All patients enrolled were from the endemic region where the predominant histology was undifferentiated non-keratinizing carcinoma. Whether or not the combined regimen has the same clinical efficacy in regions where keratinizing carcinoma dominance is yet to be elucidated. Comprehensive analysis of potential predictive biomarkers is needed to determine the tailor treatments for individual patients.
Combination of PD-1/PD-L1 inhibitors and antiangiogenic inhibitors in R/M NPC
Angiogenesis inhibitors reshape the TME and normalize the vascularity to enhance cancer immunotherapy using four methods: depletion of the hypoxic TME, improvement of tumor perfusion, facilitation of T-effector cell infiltration, especially CD4(+) and CD8(+) T lymphocytes, CD4(+)FOXP3(+) Tregs with higher expression of PD-L1, and enhancing the differentiation toward M1-like macrophage.63–67 Combination strategy of PD-1/PD-L1 inhibitors and antiangiogenic inhibitors has been successful and obtained approval of indications in various tumors, such as pembrolizumab plus axitinib for advanced renal cell carcinoma, pembrolizumab plus lenvatinib for advanced endometrial carcinoma, atezolizumab plus bevacizumab for hepatocellular carcinoma, and so on. The combination strategies of ICIs plus angiogenesis inhibitors became attractive and receive key attentions.
Several clinical trials have tested antiangiogenic inhibitors in heavily pretreated R/M NPC patients, with variable objective response rates (ORRs) of 2.7–31.3%68–71 (Table 3). The NPC-AXEL study is a single-arm, phase II study evaluating the activity and safety of the combination of axitinib and avelumab in patients with refractory R/M NPC (NCT04562441). Phase II studies regarding the combination of apatinib and camrelizumab, or the combination of bevacizumab and pembrolizumab for patients failing to first-line therapy are currently recruiting (NCT04586088, NCT03813394).
PD-1/PD-L1 inhibitors in LANPC
PD-1/PD-L1 inhibitors in recurrent LANPC
As a consequence of the current excellent loco-regional control rates attained using the generally accepted treatment paradigms involving intensity-modulated radiotherapy for NPC, only 10–20% of patients will suffer from local and/or nodal recurrence after primary treatment. Due to the possibility of radio-resistance of tumor and the limited tolerance of adjacent normal tissues to sustain further additional treatment, such as re-irradiation or chemotherapy, the clinical management of local recurrent NPC remains great challenges. Although systemic chemotherapy is often given, the high-level evidence was lack and the potential aggravating effect of chemotherapy related to increased late toxicities should also be addressed. Ongoing studies focus on the emerging role of immunotherapy in locally recurrent NPC.
Promising results of ICIs in the second-line setting have been reported. However, all of these studies comprise small series of mixed groups with distant metastases and/or local-regional recurrence. Therefore, the exact role of immunotherapy in the management of locally recurrent NPC remains yet to be evaluated. A phase II trial using toripalimab plus intensity-modulated radiotherapy showed promising antitumor activity and satisfactory tolerance in LANPC failing to previous treatment. However, 25 patients unsuitable for surgery were included. ORR and DCR were 79.2% (19/25) and 95.8% (23/25), respectively. The 1-year PFS rate was 91.8%. The acute and late adverse events were tolerable and included blood triglyceride elevation, creatine phosphokinase elevation, skin reaction, mucositis, nasopharyngeal wall necrosis, nasal bleeding, and trismus. 72
PD-1/PD-L1 inhibitors in LANPC at initial diagnosis
About 70% of NPC patients have locally advanced disease at initial diagnosis. Concomitant radio-chemotherapy is the cornerstone of the treatment of locally advanced forms. For the locally advanced stage, the addition of induction chemotherapy has become the new standard care according to the latest international recommendations to reduce tumor volumes and act early on micro-metastases. Despite these therapeutic advances, the local and especially distant failure rate remains high. Promising efficacy results and manageable safety profile of ICIs monotherapy or in combination with chemotherapy in R/M NPC have been reported. The addition of immunotherapy in the multidisciplinary management of LANPC, specifically anti-PD1or PD-L1 antibody, needs to be evaluated.
Radiotherapy not only directly kills tumor cells but also has a synergistic effect with ICIs by improving tumor antigenicity, enhancing tumor immunogenicity, and reshaping the TME to enhance immune surveillance.73–78 Furthermore, radiotherapy in combination with ICIs could not only upregulate the expression of PD-L1 but also decrease the accumulation of MDSC to enhance antitumor activity. 79
Radiotherapy is considered as the main curative treatment for early-stage NPC. Radiotherapy not only directly kills tumor cells but also has a synergistic effect with ICIs by improving tumor antigenicity, enhancing tumor immunogenicity, and reshaping the TME to enhance immune surveillance.73–78 Furthermore, radiotherapy in combination with ICIs could not only upregulate the expression of PD-L1 but also decrease the accumulation of MDSC to enhance antitumor activity. 79 Based on preclinical evidence and promising results of ICIs in R/M NPC, clinical studies on the addition of ICIs to radiotherapy are being conducted in different settings of LANPC; specific information about ongoing clinical trials is shown in Table 4.
Table 4.
Ongoing clinical trials of ICIs in LANPC.
| Trial identifier | Phase | Treatment | Patient population | Enrollment | Primary endpoint | Completion date | |
|---|---|---|---|---|---|---|---|
| Local-regional recurrent NPC | NCT04376866 | Phase III | Concurrent and adjuvant toripalimaba or placebo plus CCRT | Local–regional recurrent NPC | 204 | OS | April 2028 |
| NCT04453813 | Phase III | Concurrent and adjuvant toripalimaba or placebo plus CCRT | Unresectable locally recurrent NPC | 226 | PFS | July 2027 | |
| NCT04143984 | Phase II | Camrelizumaba plus IC followed by CIRT | Locally recurrent NPC | 146 | PFS | December 2025 | |
| NCT04534855 | Phase II | Treprilimaba | Recurrent NPC after re-irradiation | 40 | ORR | September 2025 | |
| NCT04778956 | Phase III | Toripalimaba plus surgery versus surgery alone for resectable recurrent NPC | Resectable locally recurrent NPC | 218 | DFS | March 2025 | |
| NCT04895345 | Phase II | TQB2450 + TQB2450 plus with IMRT | Inoperable locally recurrent NPC | 25 | ORR | December 2022 | |
| NCT05011227 | Phase II | Camrelizumaba + CT + endoscopic surgery | Local recurrent NPC | 100 | OS | August 2025 | |
| LANPC without prior therapy | NCT04453826 | Phase III | Camrelizumaba or placebo plus chemoradiotherapy | Staged as II–III NPC without response or positive EBV DNA after three cycles of IC and staged as IVa | 338 | PFS | September 2028 |
| NCT03700476 | Phase III | Sintilimaba or placebo combine with IC and CCRT | Stage III–IVa LANPC | 417 | FFS | January 2025 | |
| NCT04557020 | Phase III | Toripalimaba or placebo combine with IC and CCRT | Clinical staged as T4 or N3 and without distant metastasis | 200 | PFS | March 2024 | |
| NCT04447612 | Phase II | Durvalumab combine with IC and CCRT | Locoregionally advanced stage III–IVa NPC | 25 | PFS | June 2023 | |
| NCT04447326 | Phase II | Toripalimaba and endostar plus IC and CCRT | T4 and N3 untreated NPC | 106 | PFS | June 2026 | |
| NCT04782765 | Phase II | Camrelizumaba plus neoadjuvant chemotherapy followed by chemoradiotherapy | NPC without distant metastasis | 59 | DFS rate of 3 years | March 2025 | |
| NCT03734809 | Phase II | Neoadjuvant pembrolizumaba plus chemotherapy followed by concurrent pembrolizumab–cisplatin radiation, then maintenance of pembrolizumab monotherapy | Untreated stage IVa NPC | 46 | PFS rate of 2 years | December 2023 | |
| NCT03984357 | Phase II | Whole-course concurrent and adjuvant nivolumaba plus IC followed by RT alone | High-risk LANPC | 152 | FFS | August 2026 | |
| NCT04870905 | Phase II | IC-based CCRT plus adjuvant tislelizumaba | T4 N1 or T1–4 N2–3 LANPC | 100 | FFS | May 2026 | |
| NCT03544099 | Phase II | Adjuvant therapy with pembrolizumaba | NPC with solely detectable EBV DNA after curative chemoradiation | 63 | DFS rate of 1 year | December 2024 | |
| NCT03930498 | Phase II | Toripalimaba or placebo plus chemoradiotherapy | High-risk recurrent NPC | 43 | ORR | December 2023 | |
| NCT03427827 | Phase III | Camrelizumaba versus best supportive care | Stage III–IVa LANPC after chemoradiation therapy | 442 | FFS | February 2024 | |
| NCT03267498 | Phase II | Nivolumaba plus chemoradiation | Stage II–IVb LANPC | 40 | Feasibility of treatment | December 2021 | |
| NCT04910347 | Phase II | Consolidation nivolumaba after CCRT | Stage II–IVa LANPC | 57 | PFS rate of 2 years | December 2025 | |
| NCT04072107 | Phase II | GP IC + IMRT concurrent with CT and sintilimaba | Detectable EBV DNA after three cycles of IC or with EBV DNA bounce during the induction phase | 110 | Failure-free survival | December 2022 |
CCRT, concurrent chemoradiotherapy; CIRT, carbon-ion radiotherapy; DFS, disease-free survival; DNA, deoxyribonucleic acid; EBV, Epstein–Barr virus; GP, gemcitabine plus cisplatin; IC, induction chemotherapy; IMRT, intensity-modulated radiotherapy; LANPC, local–regionally advanced nasopharyngeal carcinoma; NPC, nasopharyngeal carcinoma; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; RT, radiotherapy; SBRT, stereotactic body radiation therapy; FFS, failure free survival.
Represents anti-PD-1 antibody; Endostar: VEGFR inhibitor; Durvalumab: anti-PD-L1 antibody; TQB 2450: anti-PD-L1 antibody
For LANPC at initial diagnosis, clinical studies on the addition of ICIs to radiotherapy are being conducted in different settings of LANPC; specific information about ongoing clinical trials is shown in Table 4. The phase III clinical trials regarding the addition of toripalimab, camrelizumab, or sintilimab to induction and concurrent chemotherapy are in the recruitment stage (NCT04557020, NCT04453826, NCT03700476, NCT04557020). Interestingly, a phase III clinical trial (NCT04453826) focused on high-risk LANPC (staged as II–III without response, or EBV DNA >0 copies/ml after three cycles of chemotherapy with GP induction, or staged as IVa) are exploring the role of concurrent and adjuvant camrelizumab combined with chemoradiotherapy. The results of this trial are anticipated due to the prospect of personalized treatment based on the response to induction therapy. Phase II clinical trials using whole-course concurrent and adjuvant nivolumab, pembrolizumab, camrelizumab, toripalimab, and durvalumab (anti-PD-L1 antibody) in LANPC patients are also ongoing (Table 4).
In general, the breakthrough of immunotherapy in LANPC is worth looking forward to. The ongoing clinical trials are focusing on those patients with high-risk [detectable EBV DNA following induction chemotherapy and concurrent chemoradiothrapy (CCRT) or stage of T4 N2-3M0] and explore different schedule of ICIs (e.g. induction chemotherapy to CCRT plus ICI, CCRT plus PD1 or PD-L1 inhibitors followed by 1 year of adjuvant PD1 or PD-L1 inhibitors, induction chemotherapy to CCRT plus ICI, and so on). The multiple variants of radiotherapy synergizing ICIs also need to be considered, including the dose, volume, fractionation, and the schedule of radiotherapy. These results from different clinical trials may answer the optimal multidisciplinary management in the era of immunotherapy.
Other immunotherapy in NPC
Based on the immune landscape of NPC, there are other approaches to enhance the patients’ immunity apart from ICIs. Promoting tumor antigenicity, tumor immunogenicity, and stimulating the killing ability of T-cells are three key elements to activate an immune response. EBV-specific vaccines and autologous DCs are aimed at increasing tumor antigenicity, whereas adoptive T-cell transfer focuses on promoting tumor immunogenicity and T-cell killing ability. In addition, the immune modulator is an interesting topic for releasing the immune suppressive TME. Although challenges exist in the translation from bench to bedside, further exploration appears promising.
Cancer vaccine in NPC
As early as 2002, the first clinical study of autologous immune cell vaccine was conducted by injecting autologous DCs with human leukocyte antigen (HLA)-restricted epitopes from EBV LMP-2 into 16 patients with local R/M NPC. Nine patients demonstrated boosted CD8 + T-cell responses and two patients had partial response (PR). 80 Subsequently, a series of clinical trials of therapeutic EBV vaccine have been launched. In 2011, a Singapore clinical trial designed a new Ad-ΔLMP1-LMP2 DC vaccine without HLA restriction and enrolled 16 participants with advanced NPC. However, limited T-cell response was detected. Only one patient had PR and two SD. 81
Virus-based vaccine seems to be an effective and convenient substitute for traditional cell vaccine. An modified vaccinia Ankara EBNA1/LMP (MVA-EL) virus-based vaccine which encodes inactive EBNA1/LMP2 fusion protein was tested in two phase-I clinical trials. One trial enrolled 18 Hong Kong NPC patients; T-cell responses were detected in 15 subjects and had a positive correlation with vaccine dose. 82 In another UK trial, enhanced immune response to either specific antigen was observed in 8 of 14 patients regardless of ethnicities. 83 In addition, with improved accuracy and specificity for the detection of antigens, immunogenic peptide-based therapeutic vaccines tailored for specific immune profiles may be available soon and become a new trend. 84
Adaptive immunotherapy in NPC
Adaptive immunotherapy is a distinct approach to stimulate immune response by bypassing the antigen presentation step and direct activation of the effector cells. Various preclinical studies have explored the application of CTLs, TILs, natural killer (NK) cells, and DCs in the treatment of R/M NPC with certain clinical benefits. 85 Most clinical trials focused on CTLs, and the CTLs-related information are shown in Table 5. Corey Smith et al. conducted a phase II trial to investigate the autologous EBV-specific T-cells generated with a novel AdE1-LMPpoly vector in R/M NPC. In total, 29 patients completed therapy without significant toxicity. The median PFS was 5.5 (95% CI 2.1–9.0) months and the median OS was 38.1 months (95% CI 17.2 months to not reached). 86
Table 5.
Completed clinical trials of EBV-CTLs in NPC.
| Ref. | Status | Design | Population | Disease status | Therapy lines | Treatment | Efficacy | Grade ⩾ 3 TRAEs |
|---|---|---|---|---|---|---|---|---|
| (Chua et al.)87 | Completed | Pilot | 4 | R/M EBV (+) NPC | ⩾2 | Autologous EBV-CTLs | 3/4: EBV burden decrease; 3/4: die of PD (9–21 months after EBV-CTLs) | None |
| (Comoli et al.)88 | Completed | Preliminary | 1 | Relapsed EBV (+) NPC | ⩾2 | EBV-specific CTLs | SD | None |
| (Comoli et al.)89 | Completed | Phase I | 10 | Stage IV EBV (+) NPC | ⩾2 | LCL-stimulated CTL with low-dose IL-2 | 2/10: PR; 4/10: SD; 4/10: PD | None |
| (Straathof et al.)90 | Completed | Phase I | 10 | Stage III/IV EBV (+) NPC in remission or with R/R history |
⩾2 | EBV-specific CTLs | Four pts with previous remission: DFS: 19–27 months; 6 pts with previous R/R NPC: 2/6: CR; 1/6: PR; 1/6: SD; 2/6: no response |
None |
| (Louis et al. 2009)91 | Completed | Phase I | 8 | EBV (+) poorly differentiated or undifferentiated NPC (WHO type II/III) | ⩾2 | CTL following anti-CD45 mAb administration | 1/8: CR; 2/8: SD; 5/8: PD | None |
| (Louis et al.)92 | Completed | Phase I/II | 23 | R/R EBV (+) NPC | ⩾2 | EBV-specific CTLs | Eight pts in remission: 6/8: PFS: 17–75 months; 2/8: PD 15 pts with previous R/R NPC:5/15: CR; 2/15: PR; 3/15: SD; 5/15: PD |
None |
| (Secondino et al.)93 | Completed | Phase II | 11 | Stage IV, EBV-LMP1- and EBER-positive NPC | ⩾2 | CTL following cyclophosphamide and fludarabine CT | 2/11: PR; 1/11: minor response; 3/11: SD; 5/11: PD | 4/11: G3 neutropenia |
| Smith et al. 86 | Completed | Phase II | 29a | 9 pts with no or minimal residual disease (N/MRD) 20 pts with active recurrent/metastatic disease (ARMD) |
⩾2 | AdE1-LMPpoly vector-based CTL | ARMD:12/20: SD; 8/20: PD; N/MRD:6/9: maintain response; 3/9: PD All: mPFS: 5.5 months; mOS: 38.1 months |
2/29: G3 lung abscess |
| Chia et al. 94 | Completed | Phase II | 38b | Metastatic/locoregional EBV (+) NPC | 1 | EBV-CTL following GC CT | 3/38: CR; 22/38: PR; 11/38: SD; 1/38: PD; 1/38: N/A; 3-year OS: 37.1% | None |
| (Lutzky et al.)95 | Completed | Case report | 1 | Refractory metastatic EBV (+) NPC | ⩾2 | EBV-specific CTLs | PR | None |
| (Huang et al.)96 | Completed | Phase I/II | 21c | R/M EBV (+) NPC | ⩾2 | EBV-specific CTLs | 1/21: CR; 2/21: SD; 18/21: PD; mPFS: 2.2 months; mOS: 16.7 months | None |
CR, complete response; CT, chemotherapy; CTL, cytotoxic T-cell; DCR, disease control rate; DFS, disease-free survival; EBV, Epstein–Barr virus; GC, gemcitabine and carboplatin; LMP, latent membrane protein; N/A, not available; N/MRD, no or minimal residual disease; NPC, nasopharyngeal carcinoma; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; R/M, recurrent/metastatic; R/R, refractory or relapsed; Ref., reference; SD, stable disease; WHO, World Health Organization; IL-2, interleukin-2.
29a:1 Patient of 30 pts died after single administration due to lung abscess.
38b: 35 of 38 pts received GC and EBV-CTL.
21c: 21 of 28 enrolled pts received EBV-CTL.
A phase II clinical trial evaluated the efficacy and safety of GC regimen followed by up to six doses of EBV-CTL for R/M NPC. 87 In total, 35 patients were enrolled and achieved ORR of 71.4% (including 3 CR and 22 PR) and 2-year OS rate was 62.9%. Thereafter, a randomized phase III study (VANCE, NCT02578641) is ongoing, which aims to assess whether the GP regimen followed by adoptive T-cell therapy as first-line treatment for R/M NPC could prolong OS, compared with GP. The study is expected to enroll 330 patients from 30 hospital centers across Asia and the United States. Information of other ongoing clinical trials of EBV-CTLs in NPC is showed in Table 6.
Table 6.
Ongoing clinical trials of EBV-CTLs in NPC.
| Trial | Status | Design | Estimated enrollment | Disease status | Therapy lines | Treatment | Primary endpoint | Completion date |
|---|---|---|---|---|---|---|---|---|
|
NCT02578641 VANCE |
Ongoing | Phase III | 330 | R/M non-keratinizing and undifferentiated EBV (+) NPC | 1 | GC-CTL versus GC | Prolonging OS | January 2023 |
|
NCT00953420 CADEN |
Completed | Phase II | 20 | R/R EBV (+) NPC | ⩾2 | EBV-CTL following docetaxel–carboplatin–dexamethasone CT | ORR | July 2015 |
| NCT02065362 | Not recruiting | Phase I | 14 | R/R EBV (+) NPC | ⩾2 | DNR.NPC-specific T-cells versus DNR.NPC-specific T-cells + cyclophosphamide + fludarabine | Safety | Feburary 2033 |
|
NCT00706316 NPC-CTL |
Completed | Phase I | 5 | R/M EBV (+) NPC | ⩾2 | EBV-Specific CTLs + CD45 Mab | Safety | November 2012 |
|
NCT00516087 NATELLA |
Completed | Phase I | 23 | R/R or high risk (T3 or T4, or node positive disease) EBV (+) NPC | ⩾2 | LMP1- and LMP2-specific CTLs | Safety | July 2013 |
|
NCT00078546 CLANC |
Completed | Phase I | 12 | R/R EBV (+) NPC | ⩾2 | EBV-specific CTL Infusion following anti-CD45 monoclonal antibody | Safety | April 2007 |
|
NCT02287311 MABEL |
Recruiting | Phase I | 42 | EBV (+) lymphoma and other malignancies (including EBV (+) NPC) | ⩾2 | MABEL CTLs | Safety | August 2021 |
| NCT03769467 | Completed | Phase Ib/2 | 12 | R/M EBV (+) NPC | ⩾2 | Tabelecleucel + pembrolizumab | Safety | January 2020 |
|
NCT01447056 MALTED |
Completed | Phase I | 37 | Relapsed EBV-associated diseases | ⩾2 | LMP-specific T-cells | Safety | January 2023 |
CT, chemotherapy; CTL, cytotoxic T-cell; DNR, dominant negative receptor; EBV, Epstein–Barr virus; GC, gemcitabine and carboplatin; Mab, monoclonal antibody; MABEL, LMP, BARF-1 and EBNA1; N/A, not available; NPC, nasopharyngeal carcinoma; ORR, objective response rate; OS, overall survival; R/M, recurrent/metastatic; R/R, refractory or relapsed; Ref., reference.
Adoptive immunotherapy could be a potential and effective treatment for NPC. Notably, immune-mediated antitumor activities were observed in heavily pre-treated NPC patients. EBV-CTL showed some efficacy, but T-cell amplification technology needs to be further optimized. The results of EBV TCR-T in the treatment of NPC are worthy of expectation. The translation from bench to bedside is challenging and its current status is yet to be completely discovered. The encouraging results in refractory NPC patients promote the exploration of adaptive therapy as the first-line setting.
New ICI agents beyond PD-1/PD-L1 inhibitors in NPC
Apart from PD-1/PD-L1 pathway, there are other co-inhibitory pathways, such as CTLA4, LAG-3, TIGIT, and TIM3, which suppress immune cell activation and avoid immune surveillance in TME, and co-stimulatory pathways including CD27/CD70, CD40/CD40 L, 4-1BB/4-1BBL, and OX40/OX4, which regulate T-cell function.52–55
The dual immune checkpoint blockade strategies, including CTAL4, TIM-3, LAG-3, and TIGIT, synergized with anti-PD-1/PD-L1 antibody in enhancing TIL function and suppressing tumor growth. They are in phase I–III clinical trials, having not been approved by the FDA or NMPA. The results of these clinical trials exhibited encouraging antitumor activity even in some patients with prior ICI treatment and were tolerable. Bispecific antibody targeting two inhibitory immune checkpoints including CTLA-4 plus PD-L1, LAG-3 plus PD-L1, TIM-3 plus PD-L1, TIGIT plus PD-L1, and PD-1 plus inducible T-cell co-stimulator (ICOS) have been developed. Most bispecific antibodies achieved excellent antitumor efficacies in murine tumor models. Some bispecific antibodies have been in clinical trials, and the specific information is shown in Table 3.
Biomarkers for personalized immunotherapy in NPC
Despite the substantial advancements in clinical care, a small subset of patients with R/M NPC can obtain long-lasting clinical benefits and improved survival from immunotherapy. Identifying biomarkers that can predict response and elucidate the biological mechanisms underlying response or non-response to immunotherapy are of paramount importance. Some predictive biomarkers derived from tumor tissues or peripheral blood, such as PD-L1 expression, plasma EBV DNA, tumor mutational burden (TMB), and actionable hot spot mutations, have been investigated.42,43,61,97–99
PD-L1 expression
Tumor PD-L1 expression has been the most commonly explored biomarker for predicting the response to PD-1 and PD-L1 blockade across tumor types. However, a meta-analysis including 1836 NPC patients from 15 studies concerning PD-L1 expression did not observe a significant correlation between PD-L1 expression and OS, which indicated that the expression level of PD-L1 may not act as a useful prognostic biomarker for NPC. 100 Several studies have explored the predictive value of anti-PD-1/PD-L1 in single or combined therapy in NPC, and the specific information is shown in Table 7.
Table 7.
The PD-L1 expression and the predictive value of NPC.
| Clinical trials | Treatment | Method | Positive rates | Predictive value |
|---|---|---|---|---|
| POLARIS-02 | Toripalimab monotherapy | IHC: SP142 antibody, Ventana medical systems | TCs expressed > 1% (25.3%); TCs expressed ⩽ 1% (70.5%); TCs expressed > 25% (11.1%), TCs expressed ⩽ 25% (84.7%); ICs expressed > 1% (21.4%), ICs expressed ⩽ 1% (74.4%); PD-L1 expression unavailable (4.2%); |
(TCs expressed > 1% versus TCs expressed ⩽ 1%) ORR: 27.1% versus 19.4%, p > 0.05 (TCs expressed > 25% versus TCs expressed ⩽ 25%) ORR: 38.1% versus19.3%; PFS: 7.2 versus 1.9 months; OS: unreached versus 15.1 months; all ps > 0.05 (ICs expressed > 1% versus ICs expressed ⩽ 1%) ORR: 23.1% versus21.0%, p > 0.05 |
| NCI-9742 trial | Nivolumab monotherapy | IHC: 22 C3 antibody, pharmDx assay |
TCs expressed ⩾ 1% (40.0%); TCs expressed < 1% (53.3%); TCs expression unavailable (6.7%); ICs expressed ⩾ 1% (22.2%); ICs expressed < 1% (68.9%); ICs expression unavailable (8.9%); |
(TCs expressed ⩾ 1% versus TCs expressed < 1%) ORR: 33.0% versus 13.0%; PFS and OS without statistical difference (specific results unknown) |
| CTR20160872 | Tislelizumab monotherapy | IHC: SP263 antibody, Ventana medical systems | TCs expressed ⩾ 10% (43.0%); TCs expressed < 10% (55.0%); TCs expression unavailable (2.0%) |
(TCs expressed ⩾ 10% versus TCs expressed < 10%) ORR: 17.0% versus 19.0% |
| JUPITER-02 | Toripalimab + GP versus placebo + GP | IHC: JS311 antibody, Junshi Biosciences | ICI plus CT arm: CPS ⩾ 1% (75.0%); CPS < 1% (14.0%); CPS unavailable (11.0%) Placebo plus CT arm: CPS ⩾ 1% (76.0%); CPS < 1% (17.0%); CPS unavailable (7.0%) |
ICI plus CT arm (CPS ⩾ 1% versus CPS < 1%): PFS: 11.4 versus11.0 months; p > 0.05 Placebo plus CT arm (CPS ⩾ 1% versus CPS < 1%): PFS: 8.2 versus 6.0 months; p > 0.05 |
CPS, combined positive score; CT, chemotherapy; GP, gemcitabine plus cisplatin; ICI, immune checkpoint inhibitors; ICs, PD-L1 expression on immune cells; IHC, immunohistochemistry; ORR, objective response rate; OS, overall survival; PD-L1, programmed death ligand-1; PFS, progression-free survival; TCs, PD-L1 expression on tumor cells.
The POLARIS-02 trial showed that 21/190 (11.1%) patients treated with toripalimab had high PD-L1 expression (>25%) and were associated with a trend of higher ORR, better median PFS, and median OS than patients with low PD-L1 expression, whereas no significant differences between the two groups were observed. 43 In addition, there was no difference in ORR between PD-L1 positive (>1%) and PD-L1 negative expression on immune cells. Similarly, responses to tislelizumab were observed in R/M NPC, regardless of PD-L1 expression in a phase I/II study of tislelizumab in Chinese patients with advanced solid tumors. 101 The NCI-9742 trial evaluated the antitumor activity of nivolumab in R/M NPC. There was no significant difference between patients with PD-L1-negative and PD-Ll-positive tumors in terms of OS or PFS. However, a descriptive analysis showed that a higher proportion of patients with higher levels of PD-L1 expressing tumors responded to nivolumab than those with PD-L1-negative tumors. 97 The phase III JUPITER-02 study explored the predictive value of PD-L1 expression for PD-1 inhibitor combined chemotherapy in NPC. A clinical benefit from using toripalimab along with the GP regimen was observed regardless of PD-L1 expressions status. 61
There is significant variability in the literature regarding the prevalence and prognostic significance of PD-L1 expression in NPC. The use of PD-L1 as a predictive biomarker in NPC is limited by the lack of consensus in defining a clinically meaningful threshold, the choice of assay, and the optimal analytical method to be used. In summary, current studies did not prove that high PD-L1 expression would be the optimal biomarker to predict efficacy and prognosis in patients with R/M NPC receiving immunotherapy, but its value merits further investigation.
Plasma EBV DNA
Plasma EBV DNA has been considered as a useful biomarker for population screening, disease surveillance, and prognosis or efficacy prediction of chemotherapy and radiotherapy.102–107 The value of plasma EBV DNA as a biomarker in the era of NPC immunotherapy needs to be verified.
The predictive and prognostic effects of baseline plasma EBV DNA in NPC treated with ICIs are under-investigated. In the POLARIS-02 study, patients with baseline EBV DNA titers less than 10,000 IU/mL had numerically higher ORR from toripalimab (26.7% versus 15.4%); however, the difference was not statistically significant. Similarly, a trend toward a lower overall response in the patients with higher EBV DNA levels receiving camrelizumab monotherapy was observed, but the difference was not statistically significant.42,43,60
The dynamic change in plasma EBV DNA after immunotherapy could be a more reliable and promising biomarker for the response. The POLARIS-02 study indicated that an early decrease in plasma EBV titer during the first 4 weeks was significantly correlated with a favorable response and survival benefit to toripalimab monotherapy.43,108 The significant increase in plasma EBV DNA titer could predict the disease progression prior than radiological review. 108 Although the plasma EBV DNA clearance and the increasing or decreasing trend of EBV DNA during the first month with nivolumab monotherapy did not differ significantly in terms of efficacy, more responders showed a decreasing trend of plasma EBV DNA. The failure to demonstrate statistical significance might be due to the small number of patients investigated. 97 The CAPTAIN-1st trial demonstrated that the early clearance of plasma EBV DNA was related to the response rate of the combination of camrelizumab and the GP regimen. 60 However, the predictive value of dynamic plasma EBV DNA for survival is unclear and warrants further exploration. The change in plasma EBV DNA was not significantly related to PFS in patients who received camrelizumab or nivolumab monotherapy, whereas in a phase I study of camrelizumab in combination with GP, the patients with post-baseline undetectable EBV DNA after the first month of camrelizumab had significantly longer PFS than those with detectable post-baseline EBV DNA. 42
TMB
NPC is a tumor with low TMB, as shown by several studies, with a median TMB ranging from 0.9 to 3.3 muts/Mb.42,43,109 Both the POLARIS-02 study of toripalimab monotherapy and the CAPTAIN study of camrelizumab monotherapy had negative results regarding value of TMB.42,43 In the POLARIS-02 study, patients with low TMB had the same median PFS as those with high TMB (1.9 months) and despite having longer median OS (17.4 versus 9.2 months), no statistical difference was observed between the two groups. 43 The clinical response was not significantly related to TMB level, with a cutoff value of 2.9 muts/Mb (top 10% TMB value). In the CAPTAIN study, there was no difference in PFS or ORR between patients with low and high TMB. 42 There are no data regarding the predictive value of TMB in ICI combination treatment in R/M NPC.
Other biomarkers
Recently, researchers have attempted to identify predictors of R/M NPC responses to immunotherapy through whole-exome sequencing (WES). The POLARIS-02 study reported that patients with genomic amplification in the 11q13 region or ETV6 genomic alterations by WES had extremely poor responses to toripalimab. 43 It was also found that copy number loss in either GZMB or GZMH genes was related to the poor survival of R/M NPC patients receiving camrelizumab monotherapy. 110
Immune signatures and subtypes have been attracting attention since the development of single-cell RNA-seq revealed the genomic and immune landscape of NPC.111–113 Chen et al. 112 demonstrated the TME of NPC and identified three immune subtypes (immune-enriched subtype, evaded subtype, and active immune subtype) that could predict the prognosis and response of NPC patients from immunotherapy plus chemotherapy. A prognostic model for immunotherapy was established using the expression of nine immune checkpoints according to five features (B7-H3TAIC, IDO-1TAIC, VISTATAIC, ICOSTAIC, and LAG3TAIC). 114 The integration of four immune markers (PD-L1+, CD163+, CXCR5, and CD117) in the intratumor is useful for predicting distant metastasis in NPC, as this may also be a promising biomarker for immunotherapy. 113 Further studies are required to verify the predictive value of the immune signature in NPC to better understand their potential for suggesting appropriate immunotherapy regimens and estimating the corresponding treatments treatment outcomes in different patient subgroups through immune signature. This might be challenging in clinical practice because of the limited number of patients and hypothesis-generating studies.
Conclusion
The general immune landscape of NPC renders patients suitable for immunotherapy, especially ICIs. Clinical trials regarding R/M NPC have confirmed the safety and antitumor activity of anti-PD-1 monotherapy in late-line settings and the addition of PD-1 inhibitor to chemotherapy in first-line settings. For LANPC, a variety of clinical trials on the addition of PD-1 inhibitor to radio-chemotherapy are ongoing, and their results are worth expecting. In addition, anti-PD-1 treatment strategies, such as its combination with anti-vascular agents and other immunotherapies, are promising and explore broad spheres. EBV vaccine, adoptive T-cell therapy and other adoptive immunotherapy could be a potential and effective treatment for NPC, although their current status is challenging and remains to be completely discovered. Finally, the dynamic changes in plasma EBV DNA could be a useful and practical biomarker for immunotherapy in NPC. However, well-known biomarkers, such as PD-L1 expression and TMB, have not been proven to have a clear clinical predictive value for immunotherapy in NPC. More studies regarding biomarkers that effectively predict the response and prognosis of anti-PD-1 monotherapy or combination therapy are required to maximize the personalized treatment approaches in NPC. Thus, further investigations regarding the molecular and cellular drivers of immune escape to overcome immunotherapy resistance are of great significance and could lead to innovative treatment approaches to improve the treatment outcomes of NPC patients.
Acknowledgments
None
Footnotes
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Jian-Ying Xu: Conceptualization; Validation; Visualization; Writing – original draft.
Xiao-Li Wei: Conceptualization; Validation; Visualization; Writing – original draft.
Yi-Qin Wang: Conceptualization; Validation; Visualization.
Feng-Hua Wang: Conceptualization; Supervision; Validation; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Main data are shown in this article and additional data about this study could be obtained from the corresponding author on reasonable request.
Contributor Information
Jian-Ying Xu, Department of Medical Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, P.R. China.
Xiao-Li Wei, Department of Medical Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, P.R. China.
Yi-Qin Wang, Department of Clinical Medicine, Sun Yat-sen University, Guangzhou, P.R. China.
Feng-Hua Wang, Department of Medical Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, 651 Dong Feng Road East, Guangzhou 510060, Guangdong, P.R. China.
References
- 1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 3. Xia C, Yu XQ, Zheng R, et al. Spatial and temporal patterns of nasopharyngeal carcinoma mortality in China, 1973-2005. Cancer Lett 2017; 401: 33–38. [DOI] [PubMed] [Google Scholar]
- 4. Young LS, Dawson CW. Epstein-Barr virus and nasopharyngeal carcinoma. Chin J Cancer 2014; 33: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pathmanathan R, Prasad U, Chandrika G, et al. Undifferentiated, nonkeratinizing, and squamous cell carcinoma of the nasopharynx. Variants of Epstein-Barr virus-infected neoplasia. Am J Pathol 1995; 146: 1355–1367. [PMC free article] [PubMed] [Google Scholar]
- 6. Kim KY, Le QT, Yom SS, et al. Clinical utility of Epstein-Barr virus DNA testing in the treatment of nasopharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys 2017; 98: 996–1001. [DOI] [PubMed] [Google Scholar]
- 7. Chan KCA, Woo JKS, King A, et al. Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med 2017; 377: 513–522. [DOI] [PubMed] [Google Scholar]
- 8. Lee AW, Ma BB, Ng WT, et al. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol 2015; 33: 3356–3364. [DOI] [PubMed] [Google Scholar]
- 9. Sun XS, Liu SL, Liang YJ, et al. The role of capecitabine as maintenance therapy in de novo metastatic nasopharyngeal carcinoma: a propensity score matching study. Cancer Commun 2020; 40: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet 2016; 388: 1883–1892. [DOI] [PubMed] [Google Scholar]
- 11. Prawira A, Oosting SF, Chen TW, et al. Systemic therapies for recurrent or metastatic nasopharyngeal carcinoma: a systematic review. Br J Cancer 2017; 117: 1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Poh SS, Soong YL, Sommat K, et al. Retreatment in locally recurrent nasopharyngeal carcinoma: current status and perspectives. Cancer Commun 2021; 41: 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39: 1–10. [DOI] [PubMed] [Google Scholar]
- 14. Lin M, Zhang XL, You R, et al. Neoantigen landscape in metastatic nasopharyngeal carcinoma. Theranostics 2021; 11: 6427–6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGranahan N, Swanton C. Neoantigen quality, not quantity. Sci Transl Med 2019; 11: eaax7918. [DOI] [PubMed] [Google Scholar]
- 16. Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet 2019; 394: 64–80. [DOI] [PubMed] [Google Scholar]
- 17. Chow JC, Ngan RK, Cheung KM, et al. Immunotherapeutic approaches in nasopharyngeal carcinoma. Expert Opin Biol Ther 2019; 19: 1165–1172. [DOI] [PubMed] [Google Scholar]
- 18. Jin S, Li R, Chen MY, et al. Single-cell transcriptomic analysis defines the interplay between tumor cells, viral infection, and the microenvironment in nasopharyngeal carcinoma. Cell Res 2020; 30: 950–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li YY, Chung GT, Lui VW, et al. Exome and genome sequencing of nasopharynx cancer identifies NF-κB pathway activating mutations. Nature Commun 2017; 8: 14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Albanese M, Tagawa T, Bouvet M, et al. Epstein-Barr virus microRNAs reduce immune surveillance by virus-specific CD8+ T cells. Proc Natl Acad Sci USA 2016; 113: E6467–E6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levitskaya J, Sharipo A, Leonchiks A, et al. Inhibition of ubiquitin/proteasome-dependent protein degradation by the Gly-Ala repeat domain of the Epstein-Barr virus nuclear antigen 1. Proc Natl Acad Sci USA 1997; 94: 12616–12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Apcher S, Daskalogianni C, Manoury B, et al. Epstein Barr virus-encoded EBNA1 interference with MHC class I antigen presentation reveals a close correlation between mRNA translation initiation and antigen presentation. PLoS Pathog 2010; 6: e1001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ren YX, Yang J, Zhang LJ, et al. Downregulation of expression of transporters associated with antigen processing 1 and 2 and human leukocyte antigen I and its effect on immunity in nasopharyngeal carcinoma patients. Mol Clin Oncol 2014; 2: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao J, Guo C, Xiong F, et al. Single cell RNA-seq reveals the landscape of tumor and infiltrating immune cells in nasopharyngeal carcinoma. Cancer Lett 2020; 477: 131–143. [DOI] [PubMed] [Google Scholar]
- 25. Huo S, Luo Y, Deng R, et al. EBV-EBNA1 constructs an immunosuppressive microenvironment for nasopharyngeal carcinoma by promoting the chemoattraction of Treg cells. J Immunother Cancer 2020; 8: e001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mrizak D, Martin N, Barjon C, et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J Natl Cancer Inst 2015; 107: 363. [DOI] [PubMed] [Google Scholar]
- 27. Lu J, Chen XM, Huang HR, et al. Detailed analysis of inflammatory cell infiltration and the prognostic impact on nasopharyngeal carcinoma. Head Neck 2018; 40: 1245–1253. [DOI] [PubMed] [Google Scholar]
- 28. Wong KCW, Hui EP, Lo KW, et al. Nasopharyngeal carcinoma: an evolving paradigm. Nat Rev Clin Oncol 2021; 18: 679–695. [DOI] [PubMed] [Google Scholar]
- 29. Gong L, Kwong DL, Dai W, et al. Comprehensive single-cell sequencing reveals the stromal dynamics and tumor-specific characteristics in the microenvironment of nasopharyngeal carcinoma. Nature Commun 2021; 12: 1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen YP, Yin JH, Li WF, et al. Single-cell transcriptomics reveals regulators underlying immune cell diversity and immune subtypes associated with prognosis in nasopharyngeal carcinoma. Cell Res 2020; 30: 1024–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cai TT, Ye SB, Liu YN, et al. LMP1-mediated glycolysis induces myeloid-derived suppressor cell expansion in nasopharyngeal carcinoma. PLoS Pathog 2017; 13: e1006503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lo AK, Dawson CW, Lung HL, et al. The role of EBV-encoded LMP1 in the NPC tumor microenvironment: from function to therapy. Front Oncol 2021; 11: 640207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang B, Miao T, Shen X, et al. EB virus-induced ATR activation accelerates nasopharyngeal carcinoma growth via M2-type macrophages polarization. Cell Death Dis 2020; 11: 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang J, Luo Y, Bi P, et al. Mechanisms of Epstein-Barr virus nuclear antigen 1 favor Tregs accumulation in nasopharyngeal carcinoma. Cancer Med 2020; 9: 5598–5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen TC, Chen CH, Wang CP, et al. The immunologic advantage of recurrent nasopharyngeal carcinoma from the viewpoint of Galectin-9/Tim-3-related changes in the tumour microenvironment. Sci Rep 2017; 7: 10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guo L, Chen Y, Wang J, et al. Down-regulation of UL16-binding protein 3 mediated by interferon-gamma impairs immune killing in nasopharyngeal carcinoma. Am J Transl Res 2020; 12: 6509–6523. [PMC free article] [PubMed] [Google Scholar]
- 37. Fang W, Zhang J, Hong S, et al. EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: implications for oncotargeted therapy. Oncotarget 2014; 5: 12189–12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu P, Xie BL, Cai SH, et al. Expression of indoleamine 2,3-dioxygenase in nasopharyngeal carcinoma impairs the cytolytic function of peripheral blood lymphocytes. BMC Cancer 2009; 9: 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Larbcharoensub N, Mahaprom K, Jiarpinitnun C, et al. Characterization of PD-L1 and PD-1 expression and CD8+ tumor-infiltrating lymphocyte in Epstein-Barr virus-associated nasopharyngeal carcinoma. Am J Clin Oncol 2018; 41: 1204–1210. [DOI] [PubMed] [Google Scholar]
- 40. Ahmed MM, Gebriel MG, Morad EA, et al. Expression of immune checkpoint regulators, cytotoxic T-lymphocyte antigen-4, and programmed death-ligand 1 in Epstein-Barr virus-associated nasopharyngeal carcinoma. Appl Immunohistochem Mol Morphol 2021; 29: 401–408. [DOI] [PubMed] [Google Scholar]
- 41. Makowska A, Meier S, Shen L, et al. Anti-PD-1 antibody increases NK cell cytotoxicity towards nasopharyngeal carcinoma cells in the context of chemotherapy-induced upregulation of PD-1 and PD-L1. Cancer Immunol Immunother 2021; 70: 323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fang W, Yang Y, Ma Y, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol 2018; 19: 1338–1350. [DOI] [PubMed] [Google Scholar]
- 43. Wang FH, Wei XL, Feng J, et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: a phase II clinical trial (POLARIS-02). J Clin Oncol 2021; 39: 704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Even C, Wang HM, Li SH, et al. Phase II, randomized study of spartalizumab (PDR001), an anti-PD-1 antibody, versus chemotherapy in patients with recurrent/metastatic nasopharyngeal cancer. Clin Cancer Res 2021; 27: 6413–6423. [DOI] [PubMed] [Google Scholar]
- 45. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 46. Cohen EEW, Soulières D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019; 393: 156–167. [DOI] [PubMed] [Google Scholar]
- 47. Kojima T, Shah MA, Muro K, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol 2020; 38: 4138–4148. [DOI] [PubMed] [Google Scholar]
- 48. Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018; 392: 123–133. [DOI] [PubMed] [Google Scholar]
- 49. Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol 2020; 6: 1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu J, Waxman DJ. Immunogenic chemotherapy: dose and schedule dependence and combination with immunotherapy. Cancer Lett 2018; 419: 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yu WD, Sun G, Li J, et al. Mechanisms and therapeutic potentials of cancer immunotherapy in combination with radiotherapy and/or chemotherapy. Cancer Lett 2019; 452: 66–70. [DOI] [PubMed] [Google Scholar]
- 52. Rotte A, Jin JY, Lemaire V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol 2018; 29: 71–83. [DOI] [PubMed] [Google Scholar]
- 53. Chen YL, Chang MC, Cheng WF. Metronomic chemotherapy and immunotherapy in cancer treatment. Cancer Lett 2017; 400: 282–292. [DOI] [PubMed] [Google Scholar]
- 54. McDonnell AM, Lesterhuis WJ, Khong A, et al. Tumor-infiltrating dendritic cells exhibit defective cross-presentation of tumor antigens, but is reversed by chemotherapy. Eur J Immunol 2015; 45: 49–59. [DOI] [PubMed] [Google Scholar]
- 55. Salewski I, Henne J, Engster L, et al. Combined gemcitabine and immune-checkpoint inhibition conquers anti-PD-L1 resistance in low-immunogenic mismatch repair-deficient tumors. Int J Mol Sci 2021; 22: 5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xue Y, Gao S, Gou J, et al. Platinum-based chemotherapy in combination with PD-1/PD-L1 inhibitors: preclinical and clinical studies and mechanism of action. Expert Opin Drug Deliv 2021; 18: 187–203. [DOI] [PubMed] [Google Scholar]
- 57. Wanderley CW, Colón DF, Luiz JPM, et al. Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1 profile in a TLR4-dependent manner. Cancer Res 2018; 78: 5891–5900. [DOI] [PubMed] [Google Scholar]
- 58. Bracci L, Schiavoni G, Sistigu A, et al. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ 2014; 21: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhu L, Chen L. Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett 2019; 24: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang Y, Qu S, Li J, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2021; 22: 1162–1174. [DOI] [PubMed] [Google Scholar]
- 61. Mai HQ, Chen QY, Chen D, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med 2021; 27: 1536–1543. [DOI] [PubMed] [Google Scholar]
- 62.Ann Oncol 2021; 32(Suppl. 7): S1428–S1457, https://oncologypro.esmo.org/meeting-resources/esmo-immuno-oncology-congress/pembrolizumab-pembro-with-or-without-lenvatinib-lenva-in-first-line-metastatic-nsclc-with-pd-l1-tps-1-leap-007-a-phase-3-randomized-doub [Google Scholar]
- 63. Huang Y, Goel S, Duda DG, et al. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res 2013; 73: 2943–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huang Y, Yuan J, Righi E, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA 2012; 109: 17561–17566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Erber R, Thurnher A, Katsen AD, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J 2004; 18: 338–340. [DOI] [PubMed] [Google Scholar]
- 66. Lopes-Coelho F, Martins F, Pereira SA, et al. Anti-angiogenic therapy: current challenges and future perspectives. Int J Mol Sci 2021; 22: 3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liu XD, Hoang A, Zhou L, et al. Resistance to antiangiogenic therapy is associated with an immunosuppressive tumor microenvironment in metastatic renal cell carcinoma. Cancer Immunol Res 2015; 3: 1017–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hui EP, Ma BBY, Loong HHF, et al. Efficacy, safety, and pharmacokinetics of axitinib in nasopharyngeal carcinoma: a preclinical and phase II correlative study. Clin Cancer Res 2018; 24: 1030–1037. [DOI] [PubMed] [Google Scholar]
- 69. Li L, Kong F, Zhang L, et al. Apatinib, a novel VEGFR-2 tyrosine kinase inhibitor, for relapsed and refractory nasopharyngeal carcinoma: data from an open-label, single-arm, exploratory study. Invest New Drugs 2020; 38: 1847–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hui EP, Ma BBY, King AD, et al. Hemorrhagic complications in a phase II study of sunitinib in patients of nasopharyngeal carcinoma who has previously received high-dose radiation. Ann Oncol 2011; 22: 1280–1287. [DOI] [PubMed] [Google Scholar]
- 71. Lim WT, Ng QS, Ivy P, et al. A phase II study of pazopanib in Asian patients with recurrent/metastatic nasopharyngeal carcinoma. Clin Cancer Res 2011; 17: 5481–5489. [DOI] [PubMed] [Google Scholar]
- 72. Hua Y, You R, Wang Z, et al. Toripalimab plus intensity-modulated radiotherapy for recurrent nasopharyngeal carcinoma: an open-label single-arm, phase II trial. J Immunother Cancer 2021; 9: e003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wan S, Pestka S, Jubin RG, et al. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PLoS ONE 2012; 7: e32542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006; 203: 1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gupta A, Probst HC, Vuong V, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol 2012; 189: 558–566. [DOI] [PubMed] [Google Scholar]
- 76. Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015; 520: 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lugade AA, Moran JP, Gerber SA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 2005; 174: 7516–7523. [DOI] [PubMed] [Google Scholar]
- 78. Matsumura S, Wang B, Kawashima N, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 2008; 181: 3099–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014; 124: 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lin CL, Lo WF, Lee TH, et al. Immunization with Epstein-Barr virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Cancer Res 2002; 62: 6952–6958. [PubMed] [Google Scholar]
- 81. Chia WK, Wang WW, Teo M, et al. A phase II study evaluating the safety and efficacy of an adenovirus-ΔLMP1-LMP2 transduced dendritic cell vaccine in patients with advanced metastatic nasopharyngeal carcinoma. Ann Oncol 2012; 23: 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hui EP, Taylor GS, Jia H, et al. Phase I trial of recombinant modified vaccinia ankara encoding Epstein-Barr viral tumor antigens in nasopharyngeal carcinoma patients. Cancer Res 2013; 73: 1676–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Taylor GS, Jia H, Harrington K, et al. A recombinant modified vaccinia ankara vaccine encoding Epstein-Barr virus (EBV) target antigens: a phase I trial in UK patients with EBV-positive cancer. Clin Cancer Res 2014; 20: 5009–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Masterson L, Howard J, Gonzalez-Cruz J, et al. Immune checkpoint inhibitors in advanced nasopharyngeal carcinoma: beyond an era of chemoradiation? Int J Cancer 2020; 146: 2305–2314. [DOI] [PubMed] [Google Scholar]
- 85. Lee AZE, Tan LSY, Lim CM. Cellular-based immunotherapy in Epstein-Barr virus induced nasopharyngeal cancer. Oral Oncol 2018; 84: 61–70. [DOI] [PubMed] [Google Scholar]
- 86. Smith C, Lee V, Schuessler A, et al. Pre-emptive and therapeutic adoptive immunotherapy for nasopharyngeal carcinoma: phenotype and effector function of T cells impact on clinical response. Oncoimmunology 2017; 6: e1273311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chua D, Huang J, Zheng B, et al. Adoptive transfer of autologous Epstein-Barr virus-specific cytotoxic T cells for nasopharyngeal carcinoma. Int J Cancer 2001; 94: 73–80. [DOI] [PubMed] [Google Scholar]
- 88. Comoli P, De Palma R, Siena S, et al. Adoptive transfer of allogeneic Epstein-Barr virus (EBV)-specific cytotoxic T cells with in vitro antitumor activity boosts LMP2-specific immune response in a patient with EBV-related nasopharyngeal carcinoma. Ann Oncol 2004; 15: 113–117. [DOI] [PubMed] [Google Scholar]
- 89. Comoli P, Pedrazzoli P, Maccario R, et al. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus-targeted cytotoxic T lymphocytes. J Clin Oncol 2005; 23: 8942–8949. [DOI] [PubMed] [Google Scholar]
- 90. Straathof KC, Bollard CM, Popat U, et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus--specific T lymphocytes. Blood 2005; 105: 1898–1904. [DOI] [PubMed] [Google Scholar]
- 91. Louis CU, Straathof K, Bollard CM, et al. Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood 2009; 113: 2442–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Louis CU, Straathof K, Bollard CM, et al. Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J Immunother 2010; 33: 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Secondino S, Zecca M, Licitra L, et al. T-cell therapy for EBV-associated nasopharyngeal carcinoma: preparative lymphodepleting chemotherapy does not improve clinical results. Ann Oncol 2012; 23: 435–444. [DOI] [PubMed] [Google Scholar]
- 94. Chia WK, Teo M, Wang WW, et al. Adoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinoma. Mol Ther 2014; 22: 132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lutzky VP, Crooks P, Morrison L, et al. Cytotoxic T cell adoptive immunotherapy as a treatment for nasopharyngeal carcinoma. Clin Vaccine Immunol 2014; 21: 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Huang J, Fogg M, Wirth LJ, et al. Epstein-Barr virus-specific adoptive immunotherapy for recurrent, metastatic nasopharyngeal carcinoma. Cancer 2017; 123: 2642–2650. [DOI] [PubMed] [Google Scholar]
- 97. Ma BBY, Lim WT, Goh BC, et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an international, multicenter study of the Mayo Clinic phase 2 consortium (NCI-9742). J Clin Oncol 2018; 36: 1412–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hsu C, Lee SH, Ejadi S, et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol 2017; 35: 4050–4056. [DOI] [PubMed] [Google Scholar]
- 99. Young LS. A novel Epstein-Barr virus subtype associated with nasopharyngeal carcinoma found in South China. Cancer Commun 2020; 40: 60–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Huang ZL, Liu S, Wang GN, et al. The prognostic significance of PD-L1 and PD-1 expression in patients with nasopharyngeal carcinoma: a systematic review and meta-analysis. Cancer Cell Int 2019; 19: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Shen L, Guo J, Zhang Q, et al. Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, phase 1/2 study. J Immunother Cancer 2020; 8: e000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wang WY, Twu CW, Chen HH, et al. Long-term survival analysis of nasopharyngeal carcinoma by plasma Epstein-Barr virus DNA levels. Cancer 2013; 119: 963–970. [DOI] [PubMed] [Google Scholar]
- 103. Tang LQ, Chen QY, Guo SS, et al. The impact of plasma Epstein-Barr virus DNA and fibrinogen on nasopharyngeal carcinoma prognosis: an observational study. Br J Cancer 2014; 111: 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li JY, Huang CL, Luo WJ, et al. An integrated model of the gross tumor volume of cervical lymph nodes and pretreatment plasma Epstein-Barr virus DNA predicts survival of nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: a big-data intelligence platform-based analysis. Ther Adv Med Oncol 2019; 11: 1758835919877729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhang Y, Tang LL, Li YQ, et al. Spontaneous remission of residual post-therapy plasma Epstein-Barr virus DNA and its prognostic implication in nasopharyngeal carcinoma: a large-scale, big-data intelligence platform-based analysis. Int J Cancer 2019; 144: 2313–2319. [DOI] [PubMed] [Google Scholar]
- 106. Lai L, Chen X, Zhang C, et al. Pretreatment plasma EBV-DNA load guides induction chemotherapy followed by concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal cancer: a meta-analysis. Front Oncol 2020; 10: 610787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chan ATC, Hui EP, Ngan RKC, et al. Analysis of plasma Epstein-Barr virus DNA in nasopharyngeal cancer after chemoradiation to identify high-risk patients for adjuvant chemotherapy: a randomized controlled trial. J Clin Oncol 2018; 36: JCO2018777847. [DOI] [PubMed] [Google Scholar]
- 108. Xu JY, Wei XL, Ren C, et al. Association of plasma Epstein-Barr virus DNA with outcomes for patients with recurrent or metastatic nasopharyngeal carcinoma receiving anti-programmed cell death 1 immunotherapy. JAMA Netw Open 2022; 5: e220587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zheng H, Dai W, Cheung AK, et al. Whole-exome sequencing identifies multiple loss-of-function mutations of NF-κB pathway regulators in nasopharyngeal carcinoma. Proc Natl Acad Sci USA 2016; 113: 11283–11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ma Y, Chen X, Wang A, et al. Copy number loss in granzyme genes confers resistance to immune checkpoint inhibitor in nasopharyngeal carcinoma. J Immunother Cancer 2021; 9: e002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lu L, Cai M, Peng M, et al. miR-491-5p functions as a tumor suppressor by targeting IGF2 in colorectal cancer. Cancer Manag Res 2019; 11: 1805–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Chen YP, Lv JW, Mao YP, et al. Unraveling tumour microenvironment heterogeneity in nasopharyngeal carcinoma identifies biologically distinct immune subtypes predicting prognosis and immunotherapy responses. Mol Cancer 2021; 20: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Liu SL, Bian LJ, Liu ZX, et al. Development and validation of the immune signature to predict distant metastasis in patients with nasopharyngeal carcinoma. J Immunother Cancer 2020; 8: e000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang YQ, Zhang Y, Jiang W, et al. Development and validation of an immune checkpoint-based signature to predict prognosis in nasopharyngeal carcinoma using computational pathology analysis. J Immunother Cancer 2019; 7: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]