Abstract
We investigated the potential synergy between two cell wall-active agents, the echinocandin FK463 (FK) and the chitin synthase inhibitor nikkomycin Z (NZ), against 16 isolates of filamentous fungi. Susceptibility testing was performed with a broth macrodilution procedure by NCCLS methods. The median minimal effective concentration (MEC) of FK against all Aspergillus species was 0.25 μg/ml (range, 0.05 to 0.5 μg/ml). For Fusarium solani and Rhizopus oryzae, MECs of FK were >512 μg/ml. The median MEC of NZ against Aspergillus fumigatus was 32 μg/ml (range, 8 to 64 μg/ml), and that against R. oryzae was 0.5 μg/ml (range, 0.06 to 2 μg/ml); however, for the other Aspergillus species, as well as F. solani, MECs were >512 μg/ml. A checkerboard inhibitory assay demonstrated synergy against A. fumigatus (median fractional inhibitory concentration index = 0.312 [range, 0.15 to 0.475]). The effect was additive to indifferent against R. oryzae and indifferent against other Aspergillus spp. and F. solani. We further investigated the pharmacodynamics of hyphal damage by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay and examined the time-sequenced changes in hyphal ultrastructure. Significant synergistic hyphal damage was demonstrated with the combination of NZ (2 to 32 μg/ml) and FK (0.03 to 0.5 μg/ml) over a wide range of concentrations (P < 0.001). The synergistic effect was most pronounced after 12 h of incubation and was sustained through 24 h. Time-sequenced light and electron microscopic studies demonstrated that structural alterations of hyphae were profound, with marked transformation of hyphae to blastospore-like structures, in the presence of FK plus NZ, while fungi treated with a single drug showed partial recovery at 24 h. The methods used in this study may be applicable to elucidating the activity and interaction of other cell wall-active agents. In summary, these two cell wall-targeted antifungal agents, FK and NZ, showed marked time-dependent in vitro synergistic activity against A. fumigatus.
The frequency of life-threatening fungal infections caused by Aspergillus species and other filamentous fungi has increased dramatically in the past several years. The antifungal agents approved for treatment of invasive filamentous fungal infections are limited. The two currently used antifungal drugs have a variety of associated problems. Amphotericin B can cause serious side effects due to its nephrotoxicity (6). While lipid formulations of amphotericin B have reduced nephrotoxicity, renal impairment is still observed and infusion-related toxicity may be debilitating. Itraconazole may not be reliably absorbed in sufficiently high quantities to be therapeutic and may interact adversely with a wide spectrum of drugs (8). Furthermore, the overall efficacy of either drug is limited, as evidenced by the high mortality associated with aspergillosis and other filamentous fungal infections (2).
The rise in serious fungal infections over the past decade has prompted the development of new antifungal agents with novel modes of action. Owing to their eukaryotic nature, fungal cells have only a restricted set of specific targets that do not overlap with their mammalian counterparts. The fungal cell wall is a structure that is essential for the fungus and absent from the mammalian host, and it consequently presents an attractive target for new antifungals. With considerable variation among different species, the gross macromolecular components of the cell walls of most fungi include chitin, α- or β-linked glucans, and a variety of mannoproteins. The dynamics of the fungal cell wall are closely coordinated with cell growth and cell division, and its predominant function is to control the internal turgor pressure of the cell. Disruption of the cell wall structure leads to osmotic instability and, ultimately, lysis of the fungal cell (1).
Nikkomycin Z (NZ), a nucleoside-peptide, is a competitive inhibitor of chitin synthase of the fungal cell wall. FK463 (FK), a new echinocandin derivative, is an inhibitor of 1,3-β-d-glucan synthase. We hypothesized that the combination of these two compounds may produce a synergistic inhibition of cell wall biosynthesis. In this study, we investigated the in vitro activity and potential synergy of NZ and FK against different medically important filamentous fungi. We further sought to elucidate the pharmacodynamics and time-sequenced ultrastructural changes that occur in Aspergillus fumigatus after exposure to this potentially synergistic combination.
(This work was presented in part at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, 18 September 2000.)
MATERIALS AND METHODS
Organisms.
Three strains of A. fumigatus, Aspergillus flavus, Aspergillus terreus, Fusarium solani, four strains of Rhizopus oryzae, and one strain of Aspergillus niger were studied. The isolates used in this study were identified at the Microbiology Laboratory of the Warren Grant Magnuson Clinical Center, National Institutes of Health (Bethesda, Md.). Cultures were maintained on the surfaces of potato dextrose agar slants (Remel, Lenexa, Kans.) at −70°C.
Antifungal drugs.
The echinocandin derivative FK was synthesized by Fujisawa Pharmaceutical Co. Ltd., Osaka, Japan. FK is a semisynthetic echinocandin derived from the fungus Coleophoma empedri via enzymatic cleavage of FR901370, a natural product of the fungus. It is a water-soluble hexapeptide with a fatty N-acyl side chain (17). FK powder was dissolved in normal saline at a concentration of 5,120 μg/ml.
NZ was obtained as a powder from Shaman Pharmaceuticals, South San Francisco, Calif. NZ is a peptide-nucleoside compound that is produced by Streptomyces tendae. Its structure is similar to that of UDP-N-acetylglucosamine, the precursor substrate of chitin synthase (7). NZ powder was dissolved in RPMI medium (adjusted to pH 6) at a concentration of 5,120 μg/ml.
Antifungal susceptibility testing.
A spectrophotometric method was used for inoculum preparation (4). Briefly, fresh mature isolates on potato dextrose agar slants were flooded with 7 ml of saline and gently scraped with a sterile transfer pipette. Heavy particles were allowed to settle, and the supernatant was transferred to sterile tubes. The turbidities were measured with a spectrophotometer at 530 nm. The inoculum was adjusted with saline to achieve a starting inoculum concentration of 1 × 106 to 5 × 106 CFU/ml. The suspensions were diluted 100-fold in test medium to yield a final inoculum of 1 × 104 to 5 × 104 CFU/ml.
Susceptibility tests were conducted in RPMI 1640 medium with l-glutamine, without sodium bicarbonate, and buffered with 0.165 M morphlinepropanesulfonic acid (MOPS). The pH of the medium was adjusted to 6 with 1 M HCl, as NZ is more stable under acidic conditions (18). The range of concentrations used for FK was 0.0375 to 512 μg/ml, and that for NZ was 0.25 to 512 μg/ml. The MICs of FK and NZ were determined by a broth macrodilution method according to NCCLS guidelines (14). The MICs of each drug against all isolates were determined at least in triplicate, and determinations were repeated at least three times to ensure reproducibility. Each growth control was assigned a value of 4+ (100%), and the turbidity of each tube was classified as follows in comparison with the control: 0, optically clear; 1+, slightly hazy; 2+, 50% reduction of growth; 3+, 25% reduction of growth; and 4+, growth equal to that of the control tubes. The minimal effective concentration (MEC) was used as the end point for FK and NZ against Aspergillus spp. and Fusarium. It was defined as the lowest concentration of drug producing a substantial reduction of growth (2+) and the presence of microcolonies (11). For Rhizopus spp., the end point was defined as the point of 2+ growth, since Rhizopus did not form any microcolonies. Cultures for determination of MECs for Aspergillus spp. and Fusarium spp. were incubated for 48 h, and cultures for determination of MICs for Rhizopus spp. was incubated for 24 h.
Combination studies (i) Inhibitory studies by checkerboard assay.
A two-dimensional checkerboard macrodilution technique was used to characterize interactions between FK and NZ. At least six experiments were performed for each of the isolates described above. Inocula and drugs were prepared similarly to those for susceptibility testing with single drugs. Drugs were diluted in serial twofold dilutions, and concentrations ranged from those for several tubes below to several tubes above the MEC of each drug for each organism. The fractional inhibitory concentration (FIC) index (FICI) was used to define the interaction between the two drugs (10). The FICI is the sum of the FICs of each of the drugs. The FIC was calculated as follows: MIC (MEC) of the drug tested in combination/MIC (MEC) of the drug tested alone. The interaction was defined as synergistic if the FICI was ≤0.5, as additive if the FICI was >0.5 to 1.0, as indifferent if the FICI was >1.0 to 2.0, and as antagonistic if the FICI was >2.0.
(ii) Hyphal damage assay with MTT.
In order to assess hyphal damage in organisms demonstrating synergy in the checkerboard assay, a colorimetric assay using the tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma Chemical Co. St. Louis, Mo.) was performed (12). Aspergillus conidia were harvested with phosphate-buffered saline with 0.025% Tween, filtered, washed twice with phosphate-buffered saline, and centrifuged at room temperature at 2,100 × g for 15 min. The conidia were counted in a hemacytometer and suspended in yeast nitrogen broth (2% [wt/vol] glucose and 0.67% [wt/vol] YNB [National Institutes of Health Media Department]) to a final concentration of 105 CFU/ml. A quantity of 1 ml of this suspension was placed in each well of a 24-well plate (Costar, Cambridge, Mass.) and incubated at 35°C for 16 h. After the supernatant was discarded, the wells were filled with 800 μl of RPMI medium and 100 μl of each drug. Dilutions of FK and NZ were prepared in RPMI medium and added to the wells in a checkerboard manner. Appropriate volumes of solvent were added to the drug-free controls and to wells containing only one drug to a final volume of 1.0 ml. After 4, 8, 12, or 24 h of incubation at 35°C, the supernatant was discarded and the fungi were washed three times with 800 μl of sterile distilled water. The hyphae were then incubated for another 3 h at 35°C in an MTT suspension (2.5 mg of MTT powder suspended in 50 ml of RPMI 1640 without phenol red). After removal of the MTT suspension, the MTT formazan crystals were extracted from the hyphae with 200 μl of isopropanol, and 150 μl of this was transferred to a flat-bottom 96-well plate. Absorbance (A) was measured on a multiscan-enzyme-linked immunosorbent assay reader (Titertek MCC/340; Labsystems, Helsinki, Finland) at a dual wavelength of 570 and 690 nm. Percent hyphal damage was calculated by the following equation: percent hyphal damage = 1 − ([A570 −, A690 with drugs]/[A570 −, A690 without drugs]) × 100. The formazan of MTT is measured at a dual wavelength of 570 and 690 nm. Absorbance is adjusted for nonspecific absorption by subtracting absorbance at 690 nm from absorbance at 570 nm.
Effects on hyphal structure. (i) Light microscopy.
The effects of FK and NZ alone and in combination on the light microscopic morphology of A. fumigatus also were studied. Organisms from drug-free controls, as well as organisms incubated with serial dilutions of each drug and combination (FK, 0.03 to 0.5 μg/ml; NZ, 2 to 32 μg/ml) were stained serially with lactophenol cotton blue (Remel) after 4, 8, 12, and 24 h of incubation. Lactophenol cotton blue was applied directly to organisms in each well of the flat-bottom 24-well plates. Photomicrographs were taken with phase contrast at a magnification of ×400. All isolates of A. fumigatus (isolates 4215, 972025, and 972350) were examined by microscopy and found to be similar in light microscopic and electron microscopic features. For consistency, A. fumigatus isolate 4215 is shown in all figures, including the light and electron micrographs.
(ii)Electron microscopy.
After incubation of conidia and drug(s) (FK at 0.5 μg/ml and NZ at 32 μg/ml) in RPMI medium in 12-well plates (Costar) for 4, 8, 12, and 24 h, all fluid and hyphal elements were suctioned from the wells and placed in a 12-ml conical tube. These tubes were placed in a centrifuge for 10 min at 3,000 × g. This resulted in the formation of a loose pellet. The supernatant was suctioned and discarded. The pellet was resuspended in 1 ml of 4% formaldehyde and 1% gluteraldehyde and shaken gently to ensure that the entire sample was exposed to the preservative. The resuspended pellet was then placed in a 1.8-ml tube for storage at 4°C.
Gluteraldehyde-fixed specimens were processed for transmission electron microscopy. The specimens were rinsed three times in 0.1 M Na-cacodylate with 0.2 M sucrose buffer and then postfixed in buffered 1% OsO4 for 3 h. Next, the specimens were en block stained for 2 h. Specimens then were dehydrated in serial ethanol concentrations for a total of 2 h. Afterward, the specimens were exposed to propylene oxide and then embedded in epoxy overnight. The epoxy with embedded specimens was allowed to polymerize at 65°C for 48 h. The epoxy was then trimmed and cut into thick sections. After processing, the samples were examined and photographed under a JEM 1200EX electron microscope.
Statistical methods.
MECs were expressed as medians and ranges. Comparisons of mean percentages of hyphal damage were analyzed by the Wilcoxon rank sum test. A two-sided P value ≤0.05 was considered to be significant.
RESULTS
Antifungal susceptibility testing.
Table 1 shows the MECs of FK and NZ for each isolate studied. FK was active against all of the Aspergillus spp; however, it had no effect on F. solani and R. oryzae. On the other hand, NZ was highly active against R. oryzae, moderately active against A. fumigatus, and inactive against the non-A. fumigatus species of Aspergillus as well as F. solani.
TABLE 1.
Median MECs of FK and NZ against different filamentous fungi
| Species and isolate | FK
|
NZ
|
||
|---|---|---|---|---|
| Median MEC or MIC (μg/ml)a (range) | No. of expts | Median MEC or MIC (μg/ml)a (range) | No. of expts | |
| A. fumigatus | ||||
| 4215 | 0.25 (0.06–0.5) | 12 | 16 (8–32) | 15 |
| 972025 | 0.25 (0.125–0.5) | 12 | 32 (16–64) | 12 |
| 972350 | 0.25 (0.125–0.5) | 12 | 16 (16–64) | 12 |
| A. flavus | ||||
| 50 | 0.25 (0.125–0.5) | 12 | >512 | 12 |
| 51 | 0.25 (0.125–0.5) | 12 | >512 | 12 |
| 52 | 0.25 (0.125–0.5) | 12 | >512 | 12 |
| A. terreus | ||||
| 53 | 0.25 (0.125–0.5) | 12 | >512 | 12 |
| 54 | 0.25 (0.125–0.5) | 12 | >512 | 12 |
| 55 | 0.25 (0.125–0.5) | 12 | >512 | 12 |
| A. niger 13 | 0.25 (0.06–0.5) | 9 | >512 | 9 |
| F. solani | ||||
| 26 | >512 | 9 | >512 | 9 |
| 27 | >512 | 9 | >512 | 9 |
| 28 | >512 | 9 | >512 | 9 |
| R. oryzae | ||||
| 17 | >512 | 12 | 1 (0.06–2) | 12 |
| 18 | >512 | 12 | 0.5 (0.06–1) | 12 |
| 19 | >512 | 12 | 1 (0.03–4) | 12 |
| 20 | >512 | 12 | 0.125 (0.03–0.5) | 12 |
The MEC was determined for A. fumigatus, A. flavus, A. terreus, and F. solani, and the MIC was determined for R. oryzae.
Combination studies. (i) Checkerboard assay.
The median FICIs of checkerboard macrodilution assays for the filamentous fungi studied are shown in Table 2. Synergistic effects were observed in three different isolates of A. fumigatus. An indifferent effect was found with A. flavus, A. terreus, A. niger, and F. solani. An indifferent to additive effect was observed with R. oryzae.
TABLE 2.
Median FICIs for the combination of FK and NZ against different filamentous fungi
| Species and isolate | FICI
|
No. of expts | |
|---|---|---|---|
| Median | Range | ||
| A. fumigatus | |||
| 4215 | 0.25 | 0.15–0.475 | 12 |
| 972025 | 0.3125 | 0.185–0.475 | 12 |
| 972350 | 0.375 | 0.185–0.5 | 12 |
| A. flavus | |||
| 50 | 2 | 2 | 15 |
| 51 | 2 | 2 | 15 |
| 52 | 2 | 2 | 15 |
| A. terreus | |||
| 53 | 2 | 2 | 15 |
| 54 | 2 | 2 | 12 |
| 55 | 2 | 2 | 15 |
| A. niger 13 | 2 | 2 | 6 |
| F. solani | |||
| 26 | 2 | 2 | 6 |
| 27 | 2 | 2 | 6 |
| 28 | 2 | 2 | 6 |
| R. oryzae | |||
| 17 | 1.75 | 0.8–2 | 9 |
| 18 | 1.25 | 1–2 | 9 |
| 19 | 1.25 | 0.75–2 | 9 |
| 20 | 1.5 | 1–2 | 9 |
(ii) MTT assay.
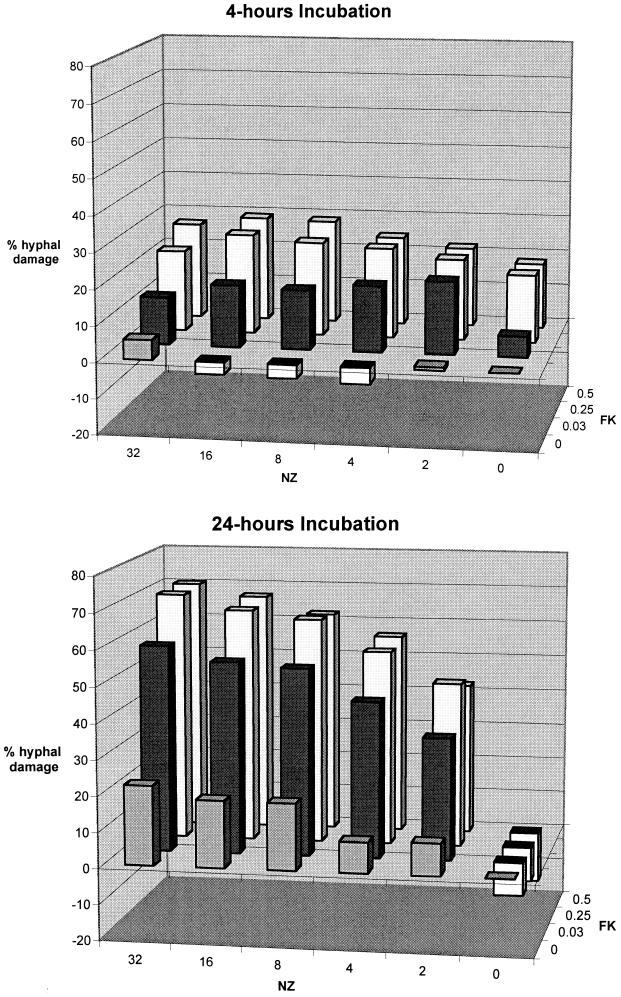
The in vitro pharmacodynamics of hyphal damage as determined by checkerboard MTT assays (n = 14) were measured at 4, 8, 12, and 24 h. Figure 1 shows the percent hyphal damage measured by checkerboard MTT assays after 4 and 24 h of incubation of A. fumigatus with FK and NZ alone and in combination. Synergistic hyphal damage was observed over a wide range of concentrations of FK and NZ, particularly at 12 h (not shown in Fig. 1) and 24 h (P < 0.001). The results represent the means from experiments with three isolates of A. fumigatus (4215, 972025, and 972350). There was no significant interstrain variation in the results of MTT assay.
FIG. 1.
Percent hyphal damage measured by checkerboard MTT assays after 4 and 24 h of incubation of A. fumigatus (isolates 4215, 972025, and 972350) with FK (0.03, 0.25, and 0.5 μg/ml) and NZ (2 to 32 μg/ml) alone and in combination.
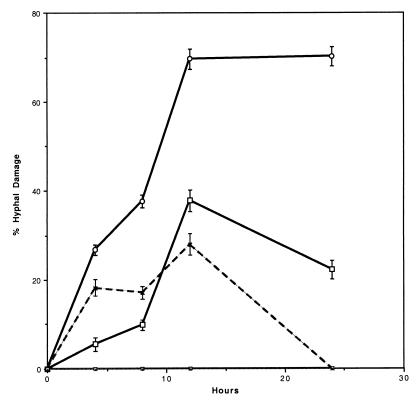
Figure 2 shows the percent hyphal damage of A. fumigatus determined by checkerboard MTT assay in the presence of FK (0.5 μg/ml) and NZ (32 μg/ml) over time. Synergistic hyphal damage increased over time with the combination of FK and NZ, reaching a maximum effect at 12 h and being sustained through 24 h. The percent hyphal damage with the combination of FK and NZ was greater than that with either drug alone at 12 and 24 h (P < 0.001). The metabolic damage due to single-agent FK and NZ was not sustained beyond 12 h.
FIG. 2.
Percent hyphal damage of A. fumigatus (isolates 4215, 972025, and 972350) in MTT checkerboard assay in the presence of NZ alone (32 μg/ml) (□), FK alone (0.5 μg/ml) (×), or the combination of NZ and FK (○) over time. ⊡, control. Error bars indicate standard error of the mean.
Effects on hyphal structure. (i) Light microscopy.
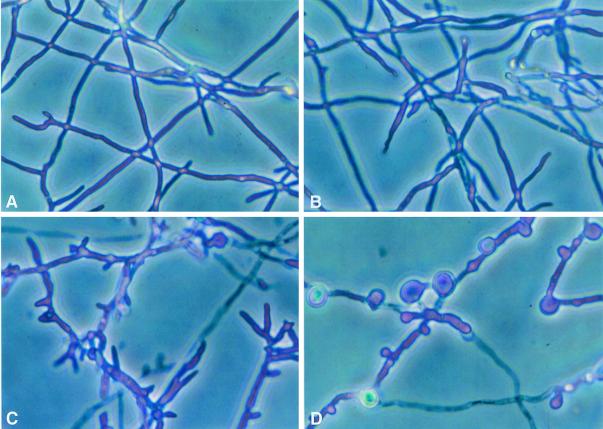
Figures 3 and 4 demonstrate the effects of FK and NZ alone and in combination on the microscopic morphology of A. fumigatus after 4 and 24 h, respectively, of incubation. At 4 h, there was virtually no change in the NZ-treated organisms; however, the FK-treated organisms became truncated, branched, and shortened. The organisms treated with both agents demonstrated focal dilatations along the hyphal elements.
FIG. 3.
Time-sequenced light microscopic changes in hyphal structure of A. fumigatus (isolate 4215) with FK and NZ alone and in combination after 4 h of incubation. (A) Drug-free control; (B) NZ alone (32 μg/ml); (C) FK alone (0.5 μg/ml); (D) FK (0.5 μg/ml) plus NZ (32 μg/ml).
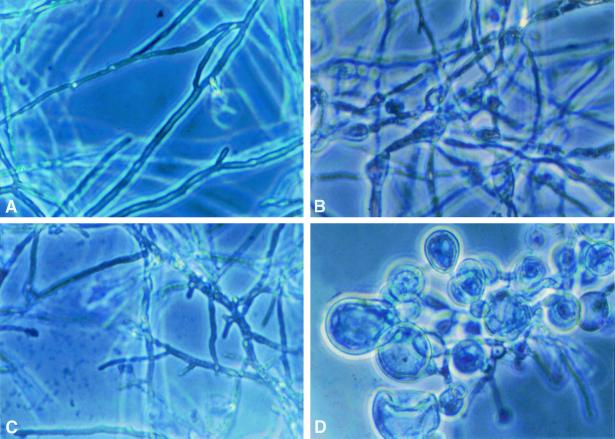
FIG. 4.
Time-sequenced light microscopic changes in hyphal structure of A. fumigatus (isolate 4215) with FK and NZ alone and in combination after 24 h of incubation. (A) Drug-free control; (B) NZ alone (32 μg/ml); (C) FK alone (0.5 μg/ml); (D) FK (0.5 μg/ml) plus NZ (32 μg/ml).
At 8 h, scattered intercalary dilatations along the hyphal elements were present in NZ-treated organisms. The effects of FK were similar to those observed at 4 h but were more prominent. Organisms treated with FK plus NK demonstrated an increased number and size of blastospore-like structures at 8 h (not shown).
At 12 h, the changes in hyphal structures exposed to single agents were similar to those at 8 h. However, the structural changes in organisms treated with FK plus NZ after 12 h were striking: blastospore-like structures almost entirely replaced the normal hyphal elements (not shown). This effect of the combination of FK plus NZ was sustained through 24 h. However, at 24 h, there appeared to be recovery of normal hyphae with either FK or NZ alone.
(ii)Electron microscopy.
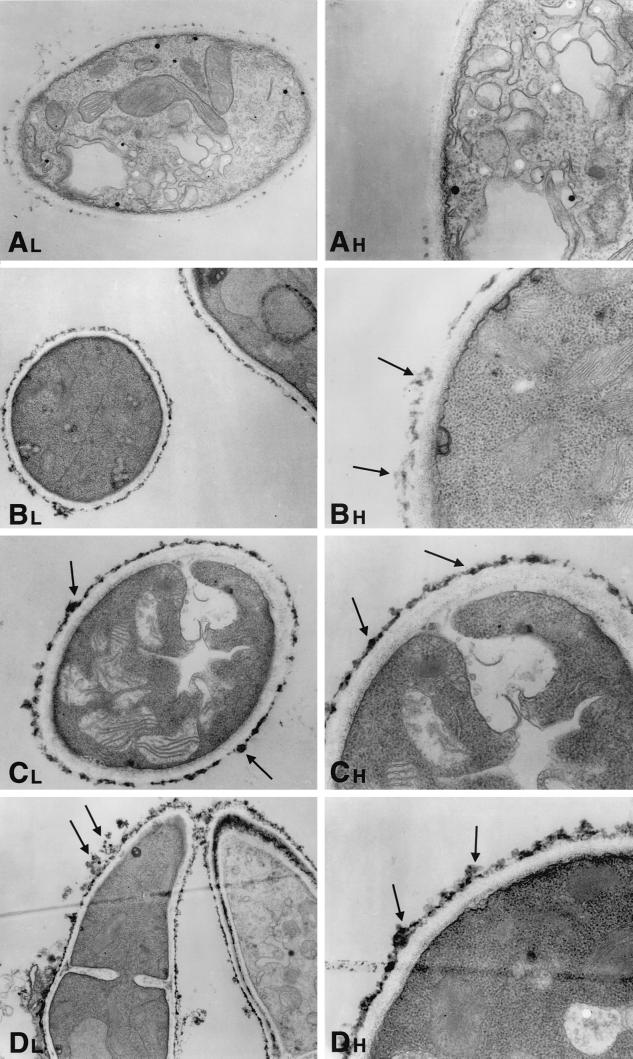
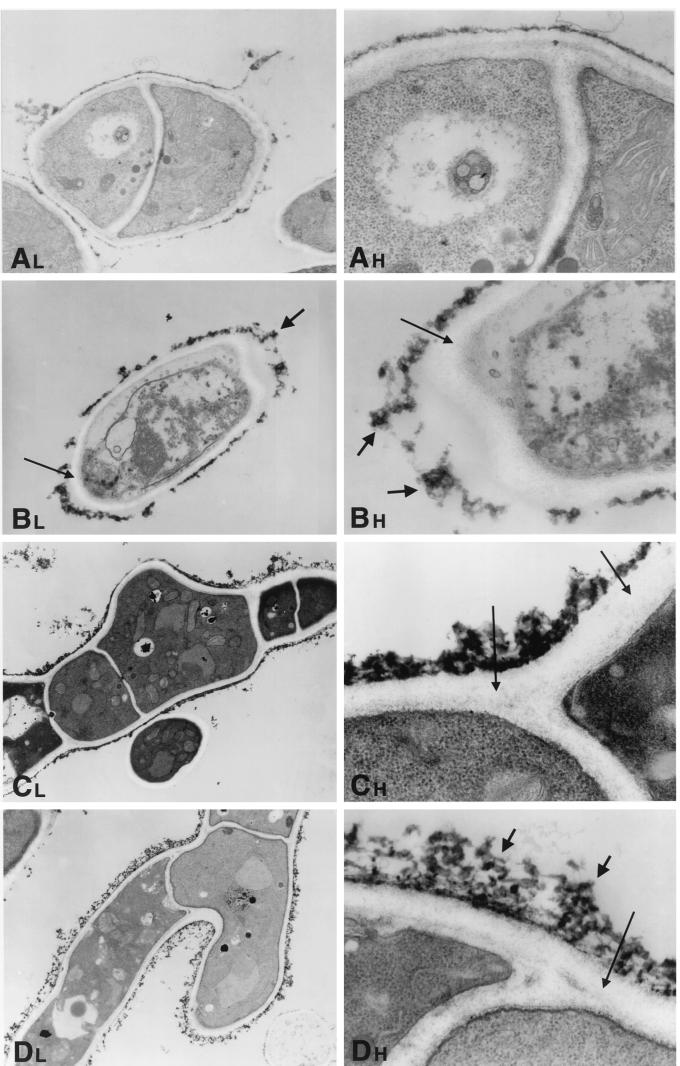
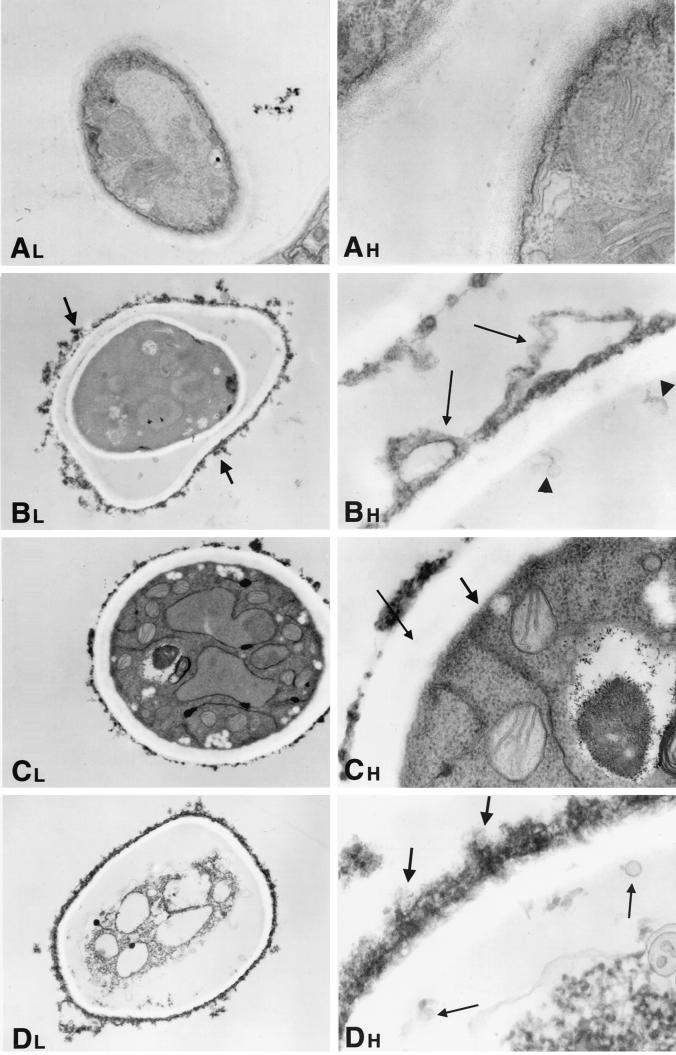
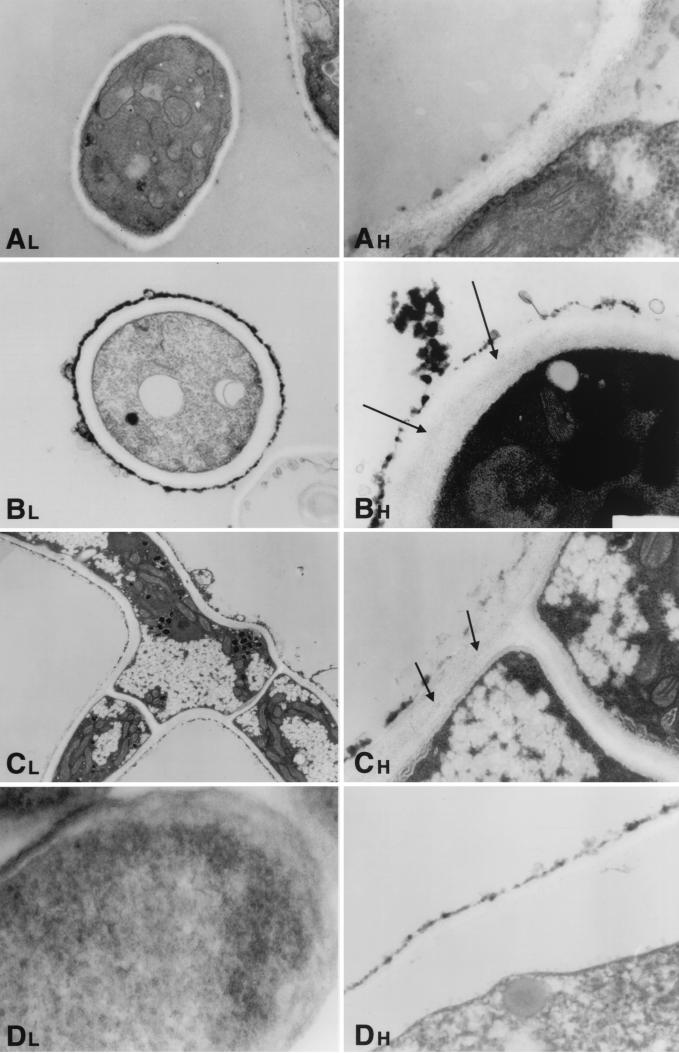
Hyphae of A. fumigatus as a normal growth control demonstrated cells with distinct, clearly identifiable organelles (Fig. 5A, 6A, 7A, and 8A). Normal mitochondria were apparent by their linear cristae. The fungal cytoplasmic membrane appeared as a sharp, electron-dense, lipid bilayer structure. The inner fibrillar layer of the cell wall appeared as a finely granular, electron-dense region just external to the cell membrane. The outer fibrillar layer displayed a sparsely distributed, coarse, electron-dense layer on the cell wall surface.
FIG. 5.
Time-sequenced electron microscopic changes of A. fumigatus (isolate 4215) after 4 h of incubation with NZ (32 μg/ml) and FK (0.5 μg/ml) alone and in combination. L, lower magnifications; H, higher magnifications. (A) Normal growth control of A. fumigatus hyphae.Magnifications, ×1,100 and ×3,100. (B) NZ alone. Scattered reticular aggregates appear on the outer surface of the fungal cell wall (arrows). Magnifications, ×620 and ×2,900. (C) FK alone. These photomicrographs demonstrate an increase in electron-dense aggregates on the outer fibrillar layer of the cell wall (arrows). Magnifications, ×620 and ×2,900. (D) NZ plus FK. The cell wall has increased reticular aggregations on the outer fibrillar layer (arrows). Magnifications, ×760 and ×2,900.
FIG. 6.
Time-sequenced electron microscopic changes of A. fumigatus (isolate 4215) after 8 h of incubation with NZ (32 μg/ml) and FK (0.5 μg/ml) alone and in combination. L, lower magnifications; H, higher magnifications. (A) Normal growth control of A. fumigatus hyphae. Magnifications, ×1,100 and ×3,100. (B) NZ alone. The cell membrane has undergone significant changes, with loss of the electron-dense lipid bilayer structure. Small microvesicles may be seen in the area of the cell membrane. The inner fibrillar layer of the cell wall has lost its characteristic distinct, fine granularity. This layer is also much wider than that of controls (thin arrows). The outer fibrillar layer of the cell wall is aggregated and thicker than in the controls (thick arrows). In other areas, the outer fibrillar layer appears to be detached from the cell itself. Magnifications, ×900 and ×3,100. (C) FK alone. The outer fibrillar layer is focally thicker than that of the controls, and there are aggregates similar to those seen in the NZ-treated cells. The granularity of the inner fibrillar layer is relatively preserved, but it is not as fine as in the controls (arrows). Magnifications, ×620 and ×4,800. (D) NZ plus FK. The outer fibrillar layer has a lattice-like structure that is loose and thready (thick arrows). The inner fibrillar layer is not uniformly visible at these magnifications (thin arrow). Magnifications, ×620 and ×4,800.
FIG. 7.
Time-sequenced electron microscopic changes of A. fumigatus (isolate 4215) after 12 h of incubation with NZ (32 μg/ml) and FK (0.5 μg/ml) alone and in combination. L, lower magnifications; H, higher magnifications. (A) Normal growth control of A. fumigatus hyphae. Magnifications, ×1,100 and ×3,100. (B) NZ alone. The lower magnification (×620) shows a substantial disruption and loss of cellular organelles. The higher magnification (×4,800) shows that the cell membrane has lost its characteristic electron-dense structure. The architecture of the inner fibrillar layer has been obliterated. In its place there is an irregular distended structure that appears to be pulling away from the cell interior (thin arrows). There also appear to be some microvesicles (along the cell membrane) (arrowheads). The outer fibrillar layer is irregularly thickened and aggregated (thick arrows). (C) FK alone. The lower magnification (×620) demonstrates an increased number of cytoplasmic vacuoles. The higher magnification (×5,200) shows that the double-layer structure of the cytoplasmic membrane has been obliterated (thick arrow) and the granular architecture of the inner fibrillar layer is absent (thin arrow). The width of cell wall is increased, and the outer fibrillar layer is now thickened. (D) NZ plus FK. The internal structure is completely obliterated, with no sign of any distinct organelles. The cytoplasm is filled with vacuoles of various sizes. The cell membrane has become irregular, with loss of the bilayer structure and microvesicles budding from its outer surface. The severely disrupted and widened inner fibrillar layer lacks inner architecture and contains microvesicles (thin arrows). The outer fibrillar layer of the cell wall is uniformly thickened along the circumference of the cells with focal reticular aggregates (thick arrows). Magnifications, ×620 and ×4,800.
FIG. 8.
Time-sequenced electron microscopic changes of A. fumigatus (isolate 4215) after 24 h of incubation with NZ (32 μg/ml) and FK (0.5 μg/ml) alone and in combination. L, lower magnifications; H, higher magnifications. (A) Normal growth control of A. fumigatus hyphae. Magnifications ×1,100 and ×3,100. (B) NZ alone. The fine granularity of the inner fibrillar layer appears to have been partially reestablished in comparison to the 12-h time point (arrows). The outer fibrillar layer is again irregularly thickened and aggregated, while microvesicles are visible at various points along the cell surface. Magnifications, ×620 and ×3,100. (C) FK alone. There are numerous small cytoplasmic vacuoles and small electron-dense vacuoles. There is some restoration of the normal inner fibrillar layer, as evidenced by new fine granular electron-dense particles between the inner and outer layers (arrows). Magnifications, ×620 and ×3,100. (D) NZ plus FK. The lower magnification (×620) shows that the internal cytoplasmic architecture is obliterated. The higher magnification (×4,800) shows that the cell wall architecture is markedly disrupted, with loss of the inner layer and marked attenuation of the outer fibrillar layer.
When A. fumigatus was incubated with NZ alone, the electron micrographs demonstrated over 12 h that the inner fibrillar layer of the cell wall became increasingly distorted, pulling away from the cell membrane with loss of granularity, widening of the cell wall, and loss of cell wall architecture (Fig. 5B, 6B, 7B, and 8B). Microvesicular-like structures formed between the cell wall and the cytoplasmic membrane. The outer portion of the cell wall at a very early stage acquired irregularly thickened aggregates, which became increasingly electron dense and irregular. By 24 h, the inner fibrillar layer showed restoration of a fine granularity, consistent with new cell wall biosynthesis. During the course of exposure to NZ, the cytoplasmic membrane also lost its electron-dense lipid bilayer structure.
The cell wall of A. fumigatus under the influence of FK alone lost its inner fibrillar layer by 12 h but appeared to begin to recover some of that fine granularity by 24 h, consistent with new cell wall biosynthesis (Fig. 5C, 6C, 7C, and 8C). There was an increase in outer layer aggregates by 8 h, which returned to a virtually normal architecture by 24 h. In contrast to the case with NZ, however, there was cytoplasmic vacuolization over the entire time course, with particularly striking deposition of small cytoplasmic vacuoles by 24 h.
In contrast to these subtle changes induced by the individual agents, there were striking changes in cell wall, cell membrane, and cytoplasm under the influence of the combination of NZ and FK (Fig. 5D, 6D, 7D, and 8D). The inner granular layer of the cell wall was severely disrupted, and it was completely lost by 12 and 24 h. Aggregates of the outer surface of the cell wall progressively appeared over the course of 4 to 12 h, followed by a marked disruption of the integrity of the outer cell wall, with only a thin residual layer remaining by 24 h. By 24 h the cell walls of the A. fumigatus cells had been virtually eliminated. Concomitant with these marked cell wall changes, there were striking changes in the cytoplasm. Large cytoplasmic vacuoles formed under the influence of the combination by 12 h. At 24 hours, there were no distinct cytoplasmic organelles, indicating severe damage to cytoplasmic structures. Mitochondria were progressively destroyed, such that by 12 and 24 h there were no distinct mitochondria visible. The cytoplasmic membrane also was severely disrupted, with complete loss of the lipid bilayer and only a tenuously thin electron-dense layer remaining by 12 and 24 h. Thus, the effects of the combination of the echinocandin and NZ were markedly more striking in the cell wall as well as the cytoplasmic membrane and organelles than those observed for either single agent.
DISCUSSION
Little is known about the pharmacodynamics of these novel cell wall-targeted agents (FK and NZ) alone or in combination against filamentous fungi. Synergistic hyphal damage was demonstrated against A. fumigatus in the presence of NZ plus FK by serial two-dimensional checkerboard inhibitory assays, serial MTT assays, and time-sequenced light and electron microscopy. To our knowledge, this is the first report to elucidate the in vitro pharmacodynamics and time-sequenced ultrastructural changes of an echinocandin and a nikkomycin alone and in combination.
Echinocandins inhibit 1,3-β-glucan synthase, a key enzyme necessary for synthesis of β-glucan, a major structural component of the cell walls of Aspergillus spp. and Candida spp. (9). Matsumoto et al. demonstrated that FK given at dosages to achieve concentrations of ≥0.55 μg/ml in plasma significantly reduced the number of viable fungal organisms in mice infected with A. fumigatus (13). This finding agreed with our observation that the median MEC for Aspergillus species was 0.25 to 0.5 μg/ml.
Chitin is a linear polymer of β-(1,4)-linked N-acetylglucosamine residues that is synthesized on the cytoplasmic surface of the plasma membrane by chitin synthase (7). NZ acts as a competitive inhibitor of chitin synthase because it has a higher affinity for the enzyme than does the natural substrate. Our studies demonstrated moderate activity of NZ against A. fumigatus but not against other species of Aspergillus or F. solani. The MIC of NZ against different isolates of R. oryzae was surprisingly low and suggests a therapeutic potential against this pathogen. As high contents of chitin in Rhizopus species have recently been demonstrated (L. Edebo and H. Hjorth, Abstr. 14th Congr. Int. Soc. Human Animal Mycol., abstr. 48, 2000), it is reasonable to speculate that the rich content of chitin contributed to the efficacy of NZ against R. oryzae.
We observed a strong synergistic effect in A. fumigatus with the checkerboard method and further corroborated this finding by MTT assay. Notably, the antifungal effect of either single drug or the combination showed a time-dependent trend. For FK-treated organisms and NZ-treated organisms, the percent hyphal damage was highest at 12 h, while at 24 h, the hyphal elements appeared to recover from this metabolic injury. However, while ultrastructural studies showed some restoration of normal cell wall architecture, residual cell damage was still apparent in the presence of a single agent. By comparison, the hyphal damage as determined by MTT assay with the combination of FK and NZ was significantly greater, particularly at ≥12 h, and was sustained through 24 h.
Hyphal damage was measured by serial MTT assays and by time-sequenced electron microscopy to measure metabolic and structural injury, respectively. MTT has been shown to be useful as a viability test for individual hyphae in Aspergillus (12). Fungi need to be metabolically active to reduce MTT. The tetrazolium salt MTT is cleaved by metabolically active fungi to its purple formazan derivative. The purple crystals of MTT formazan can be extracted from the fungus by alcohol, allowing spectrophotometric quantification. We suggest that the methods of serial pharmacodynamic two-dimensional MTT checkerboard assay with FICI analysis and time-sequenced ultrastructural studies may be useful tools by which to measure the antifungal properties and interactions between echinocandins and other compounds against filamentous fungi.
While the exact mechanism of synergy between these two drugs is unknown, inhibition of glucan synthesis has been shown to increase the chitin content in the cell walls of Candida albicans, suggesting a compensatory effect to stabilize cell wall structure (8). Moreover, knockouts of the fks1 gene in Saccharomyces cerevisiae resulted in an increase in chitin synthase activity, leading to high rates of chitin synthesis (5). Conversely, Elorza et al. found that nikkomycin inhibition of chitin synthesis in protoplasts of C. albicans resulted in new formation of cell wall enriched in alkali-soluble glucan (3). The combination of FK and NZ may prevent compensatory biosynthesis of chitins and glucans, therefore resulting in a net loss of structural integrity of the cell wall. The striking ultrastructural changes imply that A. fumigatus does not have the ability to resist the simultaneous loss of two important cell wall components.
Our initial findings were consistent with those of Perfect and colleagues, who demonstrated the synergistic interaction between NZ and cilofungin (an echinocandin no longer in clinical trials) against A. fumigatus, Cunninghamella bertholletiae, and Fusarium spp. at a single time point by microdilution inhibitory assay (15). Based upon these 24-h time point observations, we then proceeded to investigate the in vitro pharmacodynamics and to perform time-sequenced structural studies of NZ plus FK against A. fumigatus. These synergistic effects were observed at achievable concentrations of FK and NZ in plasma. More recently, Stevens reported synergistic interaction between NZ and LY303366 (now known as anidulafungin) against A. fumigatus, Coccidioides immitis, and, to a lesser extent, Rhizopus species and C. albicans using macrodilution inhibitory and fungicidal assays at a single time point (16).
The ultrastructural changes demonstrate a time-dependent effect of alteration of cell wall and cytoplasmic structures. Furthermore, the effects of the combination of FK and NZ were markedly more striking in both the cell wall and the cytoplasmic membrane and organelles than those of either agent alone. The organism appeared to partially recover from the effect of these single agents, as evidenced by new formation of the inner granular layer by 24 h. These findings suggest reversible binding of the drugs to the target enzymes (1,3-β-glucan synthase and chitin synthase) or high turnover of these cell wall biosynthesis enzymes. An alternative hypothesis may be a reciprocal upregulation of chitin synthase when 1,3-β-glucan synthase is inhibited and vice versa. When both enzymes are inhibited, no recovery of cell wall biosynthesis may occur. Instead, there appears to be a progressive alteration and inexorable obliteration of organized cell wall structure. In vitro degradation of FK and NZ may also contribute to this recovery but would not necessarily explain the phenomenon at high concentrations, where levels well above the MECs would be expected to persist for 24 h. Finally, there appears to be a distinction between metabolic hyphal injury, from which the organisms may recover to near baseline levels, and structural injury, from which the organisms only partially recovered as evidenced by residual ultrastructural damage at 24 h after single-agent therapy.
The severe structural injury to hyphal elements of A. fumigatus under the effect of the echinocandin plus NZ could markedly alter its pathogenesis for blood vessel and tissue invasion during invasive pulmonary aspergillosis. The binding that the effects of the combination of the echinocandin and NZ occur over a wide range of concentrations suggests that these in vitro events may be potentially translated to in vivo systems.
REFERENCES
- 1.Chiou C C, Groll A H, Walsh T J. New drugs and novel targets for treatment of invasive fungal infections in patients with cancer. Oncologist. 2000;5:120–135. doi: 10.1634/theoncologist.5-2-120. [DOI] [PubMed] [Google Scholar]
- 2.Denning D W. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23:608–615. doi: 10.1093/clinids/23.3.608. [DOI] [PubMed] [Google Scholar]
- 3.Elorza M V, Murgui A, Rico H, Miragall F, Sentandreu R. Formation of a new cell wall by protoplasts of Candida albicans: effect of papulacandin B, tunicamycin and Nikkomycin. J Gen Microbiol. 1987;133:2315–2325. doi: 10.1099/00221287-133-8-2315. [DOI] [PubMed] [Google Scholar]
- 4.Espinel-Ingroff A, Kerkering T M. Spectrophotometric method of inoculum preparation for the in vitro susceptibility testing of filamentous fungi. J Clin Microbiol. 1991;29:393–394. doi: 10.1128/jcm.29.2.393-394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Rodriguez L J, Trilla J A, Castro C, Valdivieso M H, Duran A, Roncero C. Characterization of the chitin biosynthesis process as a compensatory mechanism in the fks1 mutant of Saccharomyces cerevisiae. FEBS Lett. 2000;28:84–88. doi: 10.1016/s0014-5793(00)01835-4. [DOI] [PubMed] [Google Scholar]
- 6.Groll A H, Piscitelli S C, Walsh T J. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv Pharmacol. 1998;44:343–500. doi: 10.1016/s1054-3589(08)60129-5. [DOI] [PubMed] [Google Scholar]
- 7.Hector R F. Compounds active against cell walls of medically important fungi. Clin Microbiol Rev. 1993;6:1–21. doi: 10.1128/cmr.6.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hector R F, Braun P C. Synergistic action of nikkomycins X and Z with papulacandin B on whole cells and regenerating protoplasts of Candida albicans. Antimicrob Agents Chemother. 1986;29:389–394. doi: 10.1128/aac.29.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda F, Wakai Y, Matsumoto S, Maki K, Watabe E, Tawara S, Goto T, Wantanabe Y, Matsumoto F, Kuwahara S. Efficacy of FK 463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis. Antimicrob Agents Chemother. 2000;44:614–618. doi: 10.1128/aac.44.3.614-618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koneman E W, Allen S D, Janda W M, Schreckenberger P C, Winn W C. Antimicrobial susceptibility testing. In: Koneman E W, Allen S D, Janda W M, Schreckenberger P C, Winn W C, editors. Diagnostic microbiology. 4th ed. Philadelphia, Pa: Lippincott; 1992. pp. 609–668. [Google Scholar]
- 11.Kurtz M B, Heath I B, Marrinan J, Dreikorn S, Onishi J, Douglas C. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-β-d-glucan synthase. Antimicrob Agents Chemother. 1994;38:1480–1489. doi: 10.1128/aac.38.7.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levitz S M, Diamond R D. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J Infect Dis. 1985;152:938–945. doi: 10.1093/infdis/152.5.938. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto S, Wakai Y, Naka T, Hatano K, Ushitani T, Ikeda F, Tawara S, Goto T, Matsumoto F, Kuwahara S. Efficacy of FK 463, a new lipopeptide antifungal agent, in mouse models of pulmonary aspergillosis. Antimicrob Agents Chemother. 2000;44:619–621. doi: 10.1128/aac.44.3.619-621.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard M38-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 15.Perfect J R, Wright K A, Hector R F. Synergistic interaction of nikkomycin and cilofungin against diverse fungi. In: Yamaguchi H, Kobayashi G S, Takahashi H, editors. Recent progress in antifungal chemotherapy. New York, N.Y: Marcel Dekker, Inc; 1993. [Google Scholar]
- 16.Stevens D A. Drug interaction studies of a glucan synthase inhibitor (LY 303366) and a chitin synthase inhibitor (Nikkomycin Z) for inhibition and killing of fungal pathogens. Antimicrob Agents Chemother. 2000;44:2547–2548. doi: 10.1128/aac.44.9.2547-2548.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tawara S, Ikeda F, Maki K, Morishita Y, Otomo K, Teratani N, Goto T, Tomishima M, Ohki H, Yamada A, Kawabata K, Takasugi H, Sakane K, Tanaka H, Matsumoto F, Kuwahara S. In vitro activities of new lipopeptide antifungal agent, FK 463, against a variety of clinically important fungi. Antimicrob Agents Chemother. 2000;44:57–62. doi: 10.1128/aac.44.1.57-62.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tokumura T, Horie T. Kinetics of nikkomycin Z degradation in aqueous solution and in plasma. Biol Pharm Bull. 1997;20:577–580. doi: 10.1248/bpb.20.577. [DOI] [PubMed] [Google Scholar]