Abstract
Use of non-invasive brain stimulation methods (NIBS) has become a common approach to study social processing in addition to behavioural, imaging and lesion studies. However, research using NIBS to investigate social processing faces challenges. Overcoming these is important to allow valid and reliable interpretation of findings in neurotypical cohorts, but also to allow us to tailor NIBS protocols to atypical groups with social difficulties. In this review, we consider the utility of brain stimulation as a technique to study and modulate social processing. We also discuss challenges that face researchers using NIBS to study social processing in neurotypical adults with a view to highlighting potential solutions. Finally, we discuss additional challenges that face researchers using NIBS to study and modulate social processing in atypical groups. These are important to consider given that NIBS protocols are rarely tailored to atypical groups before use. Instead, many rely on protocols designed for neurotypical adults despite differences in brain function that are likely to impact response to NIBS.
Keywords: non-invasive brain stimulation, social perception, social cognition, state-dependent TMS, Autism Spectrum Disorder
Introduction
Non-invasive brain stimulation (NIBS) refers to a range of techniques, including transcranial magnetic stimulation (TMS), transcranial electric stimulation (tES) and focussed ultrasound stimulation (tFUS), used to modulate brain excitability. Use of NIBS has increased significantly in recent years. This has enhanced our understanding of cognitive and perceptual processes (Miniussi et al., 2012; Miniussi and Ruzzoli, 2013; Parkin et al., 2015; Taylor, 2018) and enabled a new stream of intervention research (Rossi et al., 2009; Miniussi and Vallar, 2011; Miniussi et al., 2012; Perera et al., 2016). Whilst of clear utility, this increasing experimental and applied research focus has been accompanied by questions regarding study design and generalisability of findings (Parkin et al., 2015). In response, the field of brain stimulation has made efforts to strengthen experimental design. For example, several recent articles provide guidance on how to conduct well-controlled brain stimulation experiments (transcranial direct current stimulation [tDCS]—Ferrucci et al., 2015; Woods et al., 2016; TMS—Sandrini et al., 2011; TMS-electroencephalography—Ilmoniemi and Kičić, 2010; Miniussi and Thut, 2010). In addition, there is increasing interest in understanding null results in NIBS studies and the mechanisms underlying NIBS effects (de Graaf and Sack, 2018; Thut et al., 2018). One area of research that has benefitted from the use of brain stimulation techniques is social processing. Here, we review examples of the application of NIBS in this area of research and outline several key contributions of NIBS research to our understanding of social processing and its neural correlates; specifically, face processing, mirror responses and self–other processing. Whilst this review is not exhaustive, it highlights the utility of NIBS methods to study social processing.
Addressing more nuanced challenges facing social processing research using NIBS methods is important to allow for reliable interpretation of findings in neurotypical cohorts. It also allows us to tailor NIBS protocols to atypical groups with social difficulties. Therefore, we highlight several methods and techniques that may help to support the use of NIBS in both typical and atypical groups. Note, we assume that the reader has a working knowledge of commonly used NIBS techniques, but there are several useful reviews for a more detailed introduction (Walsh and Cowey, 2000; Walsh and Pascual-Leone, 2003; Wassermann et al., 2008; Parkin et al., 2015; Reed and Kadosh, 2018).
How have NIBS studies contributed to understanding of social processing?
Facial identity processing
One domain where NIBS has been used to explore social perception is the study of facial identity processing. Here, work has utilised both TMS and tES to explore this ability (e.g. Lafontaine et al., 2013; Renzi et al., 2013; Romanska et al., 2015; Barbieri et al., 2016). We specifically highlight the work elucidating the role of the occipital face area (OFA) in facial identity processing as a clear example of how using TMS can extend and support previous findings in facial identity research. Whilst beyond the scope of the current review, we also acknowledge the extensive body of work using NIBS to investigate processing of facial expressions (see Atkinson and Adolphs, 2011; Pitcher, 2019 for reviews in this area).
Influential models of face processing suggest the OFA contributes to early visual processing of faces (Haxby et al., 2000; Calder and Young, 2005), with further processing relying on a distributed network of brain regions (Rossion, 2014). This model is supported by a combination of functional magnetic resonance imaging (fMRI), lesion and animal work (Atkinson and Adolphs, 2011; Rossion, 2014), but has been extended and tested through the use of NIBS methods (Atkinson and Adolphs, 2011; Pitcher et al., 2011; Pitcher, 2019). Work by Pitcher et al. (2007) demonstrated the importance of the right occipital face area (rOFA) in processing facial features (Figure 1). Disruption of face discrimination abilities was observed after stimulation to the rOFA when facial features were varied, but not when the spacing between features was varied, suggesting a role for the rOFA in featural but not holistic face processing (Pitcher et al., 2009; Solomon-Harris et al., 2013). Furthermore, using double-pulse TMS (two single pulses of TMS applied close together in time), the authors demonstrated the time course of rOFA involvement. Specifically, rOFA TMS reduced face discrimination accuracy only when delivered 60 and 100 ms after stimulus onset. Ambrus et al. (2017a) extended these findings using TMS to explore the role of the rOFA in recognising different images of the same identity (Ambrus et al., 2017b).
Fig. 1 .
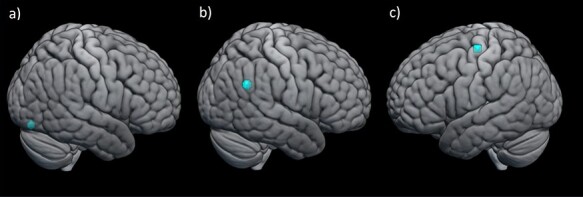
Commonly targeted stimulation sites in studies investigating face processing, self–other control and mirror responses. (a) right occipital face area; coordinates taken from Pitcher et al. (2007), (b) right temporoparietal junction; coordinates taken from Young et al. (2010), (c) left primary motor hand area; coordinates taken from Maegherman et al. (2019).
Collectively, these findings validate and extend models of face processing implicating the OFA in early face processing (Haxby et al., 2000; Calder and Young, 2005). The work builds on fMRI studies by demonstrating a causal relationship between OFA activity and face processing (Rossion, 2014). It also supports findings from lesion studies that disruption to the OFA can impair face processing, whilst overcoming limitations of such studies (such as non-localised lesions making it difficult to infer site-specific effects, or cortical reorganisation following trauma limiting generalisability to a healthy brain). This work also builds on fMRI and lesion studies by demonstrating the time course of OFA involvement in face processing. Finally, the work demonstrates task, site and temporal specificity of brain stimulation effects. It is clear, therefore, that the use of NIBS has provided an important contribution to our understanding of the role of the OFA in face processing.
Mirror responses
In the action domain, mirror neurons fire both when performing an action and when observing another agent performing the same, or a similar, action (Gallese et al., 1996). It has been suggested that this ability to map observed movements onto the observer’s own motor representations may assists in understanding another’s actions (Rizzolatti and Sinigaglia, 2010), although a re-analysis of available data suggests mirror neurons instead respond to socially contingent actions (e.g. imitation; Cook et al., 2014), with a potential role in action perception (Thompson et al., 2019).
Research into mirror responses provides another example where NIBS studies have complemented animal, imaging and lesion studies to further understanding of the neural basis of social processing (Keysers et al., 2018). Research in non-human primates identified mirror neurons in area F5 (homologue of ventral premotor cortex in humans) and inferior parietal regions (Casile, 2013). In humans, fMRI revealed increased activation in these regions during action observation and execution (Caspers et al., 2010). In NIBS studies, mirror responses are indexed by measuring muscle responses to single-pulse TMS delivered over the primary motor cortex (motor evoked potentials [MEPs]; Figure 1). Changes in MEP amplitudes are thought to index motor cortex excitability, with larger amplitudes indicative of greater excitability (Fadiga et al., 1995). Strafella and Paus (2000) demonstrated a muscle-specific increase in excitability to observation of different actions, coupled with a muscle-specific reduction in cortical inhibition and facilitation (indexed by reduced response to short intracortical inhibition and intracortical facilitation, respectively). By demonstrating the muscle-specific nature of mirror responses, these findings go beyond what had previously been demonstrated using fMRI. Subsequently, extensive NIBS work perturbing different brain regions has demonstrated the anatomical specificity and functional role of brain regions involved in producing mirror responses (Keysers et al., 2018).
NIBS studies have also shed light on connectivity patterns between regions involved in mirror responses and their likely origin. For example, Catmur et al. (2011) showed that connectivity between mirror response regions can be altered through associative learning. Initially, a conditioning pulse applied to either the dorsal or ventral premotor cortex facilitated MEP responses from M1 representations of index and little finger muscles after observation of index or little finger actions, respectively. After counter-mirror training to alter learned associations between observed and executed actions (where participants move their index finger in response to observed little finger movements and vice versa; Catmur et al., 2007), mirror responses were significantly reduced. This reduction was amplified following conditioning pulses to the premotor cortex, supporting the idea that the mirror system can adapt through associative learning (Cook et al., 2014) and demonstrating the role of premotor–M1 connections in such associations.
Collectively, these NIBS studies demonstrate the causal role of a group of brain regions, and connectivity between these regions, in mirror responses and lend support to key theories such as associative learning accounts of mirror response origin. These studies also demonstrate muscle-specific responses to action observation, and hence mirror responses, more directly than is possible using neuroimaging.
Self–other processing
During social interaction it can be important to enhance representation of another person and suppress representation of the self (e.g. in order to represent another’s beliefs when they differ from your own). Conversely, it can also be beneficial to suppress representation of another and enhance representation of the self (e.g. to inhibit imitation of another). This ability to selectively modulate representations of the self and the other is known as self–other control and is thought to play a key role in several social processes including empathy, perspective taking and theory of mind (Ward and Banissy, 2015; de Guzman et al., 2016). The medial prefrontal cortex (mPFC) and the temporoparietal junction (TPJ) have been linked to this process through a body of fMRI work (e.g. Brass et al., 2009). The use of NIBS has allowed the causal link between the TPJ and self–other control to be established. For example, Costa et al. (2008) and Young et al. (2010) both showed that 1 Hz repetitive TMS (rTMS) to the right TPJ (rTPJ; Figure 1) disrupts performance on theory of mind task. Similarly, Wang et al. (2016) showed that double-pulse TMS to the right posterior TPJ also disrupted performance on a perspective-taking task. Furthermore, rTMS delivered at a theta frequency (6 Hz) relative to alpha (10 Hz) facilitated embodied perspective taking, highlighting the role of theta oscillations in this process (Gooding-Williams et al., 2017).
Studies have also employed tDCS to investigate the role of the TPJ in self–other control. For example, Santiesteban et al. (2012) demonstrated that anodal tDCS to the rTPJ selectively improved performance on tasks requiring self–other control (imitation–inhibition and perspective taking) relative to a task requiring self-referential processing. No differences in task performance were found between cathodal stimulation and sham. This effect of improved self–other control following anodal tDCS to the rTPJ was subsequently replicated by Santiesteban et al. (2015), who also showed a similar pattern of results for left TPJ stimulation (Hogeveen et al., 2014). Collectively, these findings highlight the role of the TPJ in self–other control. In addition, they demonstrate that modulation of social processing can be achieved, and replicated, using tDCS methods (see Sellaro et al., 2016 for review on tDCS in social processing research).
Whilst NIBS research has clearly enhanced understanding of the role of the TPJ, further research is needed to understand the role of the mPFC. It is commonly thought that ventral regions of the mPFC are involved in self-referential processing, whereas dorsal regions are involved in representing others (see van der Meer et al., 2010; Denny et al., 2012 for meta-analyses). However, Nicolle et al. (2012) suggested that the mPFC is organised with respect to task-relevance, thus challenging prevailing accounts of mPFC organisation (also see Cook, 2014). They argued that ventral regions of the mPFC keep track of task-relevant information (e.g. information about the self during a self-relevant trial), whereas more dorsal regions of the mPFC keep track of task-irrelevant information (e.g. information about the self during an other-relevant trial). Use of more focal NIBS techniques (such as TMS) is one way to test contrasting accounts of brain function in social processing. However, we are generally limited to stimulating areas near the cortical surface. Targeting deeper regions often requires higher intensity stimulation, which impacts focality of the electric field. Thus, in order to test accounts regarding the role of deeper or less accessible brain structures in social processing (e.g. mPFC), we must first overcome several challenges associated with using NIBS in social processing research.
Challenges using NIBS to study social processing
Whilst the above examples highlight successes of using NIBS to modulate social processing, there are also a number of challenges. The remainder of this paper will discuss key challenges facing researchers using NIBS to study social processing in neurotypical and atypical populations. This section is not an exhaustive list of limitations, but rather highlights several challenges that are particularly problematic.
Depth of regions of interest
With most brain stimulation methods, we are only able to target shallow cortical regions (Kammer, 1998; Roth et al., 2007). This can be problematic for many areas of study, but is particularly challenging when investigating social processing that relies on networks encompassing subcortical regions. For example, processing of facial emotions requires a distributed network including cortical regions such as the ventromedial prefrontal cortex and somatosensory cortex, less accessible structures such as the fusiform gyrus, and subcortical regions such as the amygdala and insula (Adolphs, 2002; Fairhall and Ishai, 2006). If we could reliably target deeper regions, we may be able to further understand the role of, and connectivity between, different regions within networks responsible for social processing. With TMS, it is possible to stimulate subcortically using alternative coil types to the commonly used figure-of-eight coil. However, the increased current spread makes approaches like this unsuitable for most studies as it reduces the focality of stimulation. Unintended cortical surface stimulation is also a problem with such techniques. Collectively, these issues make it difficult to make inferences regarding the function of more specific, deeper brain regions (for comparison of induced electric field, see Deng et al., 2013; Lu and Ueno, 2017). Therefore, methods that allow focal stimulation of deeper regions would be very useful in social processing research.
Currently, it may be possible to overcome this issue using an indirect stimulation protocol (see Wang et al., 2014; Kim et al., 2018 for examples of network stimulation effects in associative and episodic memory). Many studies have shown that the effects of TMS can alter activity in non-targeted areas of a network activated during a given task (see Ruff et al., 2009 for review). This approach has been used to modulate interoceptive processing through direct stimulation of cortical regions implicated in the interoception network (dorsolateral prefrontal cortex) that results in indirect activation of subcortical regions in the network (anterior insula; Mai et al., 2019). Similar network effects have been shown in face processing whereby stimulating the rOFA alters fusiform face area activity, and stimulating the posterior superior temporal sulcus (pSTS) alters amygdala activity (Pitcher et al., 2014, 2017). Thus, it may be possible to exploit such effects to modulate activity in less accessible brain areas (i.e. targeting cortical sites to indirectly modulate less accessible regions). Whilst useful, this potential for indirect effects of NIBS can also make it difficult to interpret regional involvement in a given process (Coll et al., 2017).
One thing that several of these studies have in common is the use of imaging methods to verify change in subcortical network activation. Use of imaging methods is important to ensure that indirect stimulation protocols are indeed modulating these less accessible regions. This may not always be the case when targeting cortical regions that are implicated in several networks. The flexible hub theory (Cole et al., 2013) posits that brain areas are involved in multiple networks and that brain state will determine whether interaction with one network is privileged over another. Regions can flexibly interact with different brain networks depending on the nature of a participant’s task. Thus, if a brain region is part of more than one functional network (e.g. involved in both perception and memory networks), caution is required to ensure that tasks used capture the role of the region in the specific functional process of interest. In such cases, confirmation of network effects with neuroimaging would permit stronger inferences to be drawn.
In addition to indirect effects of NIBS, it may be possible to target deeper regions in the future using two emerging techniques. First, low-intensity tFUS is a form of NIBS relying on pressure produced by ultrasound waves to modulate brain activity (Tyler et al., 2018; Darrow, 2019; di Biase et al., 2019). Importantly, this technique is thought to be able to stimulate subcortically whilst preserving spatial focality. This is because the acoustic focus (where the acoustic energy is greatest) can be steered towards deep sites whilst keeping the size of the stimulated area as small as possible (Legon et al., 2018; Folloni et al., 2019). Accordingly, this also reduces the degree of unintended cortical stimulation (i.e. stimulation of superficial sites when targeting less accessible regions). Thus, tFUS provides a useful alternative to other deep NIBS methods (e.g. deep TMS using H- or double-cone coils), which suffer from a depth-focality trade off (Deng et al., 2013; Lu and Ueno, 2017). Preliminary data in humans have shown that tFUS can alter unilateral thalamic activity (Legon et al., 2018). In addition, tFUS over the primary somatosensory cortex modulates somatosensory evoked potentials and behavioural performance on a sensory discrimination task (Legon et al., 2014), thus highlighting the potential of tFUS techniques to modulate behaviour in humans. However, tFUS is still in its infancy and more research into safety thresholds and mechanisms of action is needed prior to use in social processing research (Pasquinelli et al., 2019). Once better understood, tFUS may provide a useful tool to modulate deeper regions in social brain networks.
Transcranial temporal interference stimulation (tTIS; Grossman et al., 2017) may also overcome unintended cortical stimulation whilst being able to target less accessible regions. This method applies two different high-frequency electrical fields to the brain via surface electrodes. Applying current at such high frequencies (in the kHz range) is not thought to modulate neural oscillations (Hutcheon and Yarom, 2000). However, at the point where the frequencies overlap, an amplitude-modulated field is created. This waveform oscillates at a slower frequency, the rate of which is equal to the difference between the frequencies generated by the two surface electrode pairs. Depending on surface electrode placement, it may be possible for this overlap to occur in deeper brain regions, thus modulating activity of deeper areas. Importantly, because the waveforms are not overlapping on the cortical surface, activity of more superficial areas is unaffected. This method may therefore be useful for modulating deeper areas of social brain networks. tTIS has been shown to modulate focal cortical and subcortical regions in rats (Grossman et al., 2017), and feasibility of this technique in humans has recently been addressed using computational modelling approaches (Grossman et al., 2018; Rampersad et al., 2019). However, more work is needed to understand the mechanisms of action, feasibility and safety of this approach in humans.
Overlapping and neighbouring brain regions
When using NIBS, it can be difficult to dissociate the role of a region of interest in task performance from the role of other neighbouring regions. This is due to both network activation and current spread to other neighbouring regions. For example, different regions of the TPJ are involved in different cortical networks. The anterior TPJ shows connectivity with the ventral attention network (Corbetta and Shulman, 2002) and is implicated in both social and non-social processing, whereas the posterior TPJ shows connectivity with the social cognition network and is primarily implicated in social processing (Mars et al., 2011; see Krall et al., 2015 for meta-analysis). Whilst associated with different processes, these regions are topographically close. Thus, targeting just one with NIBS techniques becomes challenging. As such, it is important to ensure that when investigating the effects of brain stimulation on regions involved in social processing, we do not use tasks that also rely on alternative networks that include anatomically close regions. Conversely, it is also possible to use control tasks that may differentially activate these alternative networks. For example, Santiesteban et al. (2017) demonstrated that domain-general attentional processes, rather than implicit mentalising, were modulated by rTMS to the rTPJ. By investigating both domain-general and domain-specific effects of rTPJ stimulation, the authors were able to shed light on rTPJ involvement in social processing. It can be difficult to design tasks that allow for this dissociation, but it is essential if we are to understand how modulation to an area affects social processing specifically, rather than more general processing.
It may also be possible to account for anatomical specificity of an effect by stimulating the region of interest and other anatomically close control regions. If task behaviour is modulated by stimulation to one site but not another nearby site, this would provide stronger evidence that modulation of the region of interest, rather than neighbouring regions, is driving the effect (subtractive inference; Walsh and Cowey, 2000). Coupling such protocols with imaging methods would further enhance our knowledge of anatomical specificity. It is also possible to record network activation following plasticity-inducing NIBS protocols (e.g. network activation recorded prior to and following a theta-burst TMS protocol). Whilst this does not overcome the issue of stimulating overlapping or neighbouring regions, it does allow for regional and network changes in activity to be detected.
One way to potentially overcome this issue is to exploit state-dependent effects of NIBS. Brain stimulation effects are influenced by the state of the brain at the time of stimulation (Silvanto et al., 2007). For example, in visual perception, researchers have been able to selectively influence the behavioural outcomes of brain stimulation by altering the brain state at the time of stimulation (e.g. Cattaneo and Silvanto, 2008; Silvanto et al., 2008; Silvanto and Muggleton, 2008). Silvanto et al. (2008) showed that priming area V5 of the visual cortex (vs the vertex) with 1 Hz inhibitory rTMS resulted in facilitation of motion detection performance when receiving online TMS. In contrast, online TMS to area V5 disrupted motion detection performance when activity in this area was not suppressed (offline rTMS delivered to vertex control site). This study shows that it is possible to change the nature of the effects of stimulation by influencing the brain state at the time of stimulation (Cattaneo and Silvanto, 2008; Silvanto and Muggleton, 2008). Endogenous baseline activity has also been shown to partially explain variability in response to TMS (Pasley et al., 2009; for theoretical framework see Silvanto and Cattaneo, 2017). Silvanto and Pascual-Leone (2008) described the potential utility of exploiting state-dependent effects of NIBS in perceptual studies to selectively target specific brain networks. It may be possible to apply a similar approach to social processing research. In theory, this approach may provide a way to selectively activate networks involved in social processing whilst limiting modulation of other contiguous networks that may otherwise be influenced by NIBS (Figure 2).
Fig. 2 .
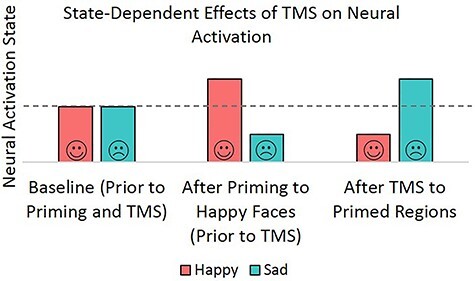
Theoretical approach to exploiting state-dependent effects of NIBS in social processing research. Left to right: neural activation of representations of different facial emotions is initially at a baseline level. Activation of neurons coding for a particular facial emotion is then manipulated through use of priming. Following subsequent TMS, activity of the primed neurons (i.e. those coding for happy faces) may be inhibited compared to baseline, whereas activity of unprimed neurons (coding for sad faces) may be facilitated. This theoretical pattern of results is in line with empirical evidence in the visual perception domain whereby TMS facilitates activity of less active neural populations (Silvanto et al., 2008).
One example comes from Mazzoni et al. (2017) who exploited state-dependent effects of TMS to investigate areas involved in representing affective body kinematics (using point-light displays). Working on the premise that single-pulse TMS facilitates less active/excitable neural populations (Silvanto and Pascual-Leone, 2008), Mazzoni et al. (2017) used an adaptation paradigm where participants were exposed to happy or fearful adapters prior to a judgement task. During the judgement task, participants indicated whether a target display was happy, fearful or neutral. Participants were faster to respond to adapter-incongruent targets when receiving no TMS, TMS to an active control site or TMS to the pSTS. However, this effect was abolished for fearful displays only when receiving TMS to the anterior intraparietal sulcus (aIPS). This suggests that neural populations in the aIPS code affective (fearful) kinematic profiles and highlights the utility of state-dependent effects of TMS in social processing research (see also Cattaneo et al., 2010, 2011; Jacquet and Avenanti, 2015 for state-dependent studies of action observation; and Ambrus et al., 2017b, 2019 for state-dependent studies of face processing). Thus, state manipulations may provide a useful method to understand the role of regions/networks in social processing, and to overcome limitations associated with stimulation of overlapping/neighbouring regions.
Use of NIBS in autism and other atypical groups
There is a growing body of work assessing the potential use of NIBS in clinical disorders (for reviews see Machado et al., 2008; Kim et al., 2009; Wassermann and Zimmermann, 2012; Schulz et al., 2013). Several studies have also shown promising results using NIBS to modulate social processing in atypical groups (see Boggio et al., 2015 for review). However, the research in this area is limited. It is also important to consider that, in addition to the key challenges mentioned in the previous section, there are several additional challenges facing NIBS studies of social processing in atypical groups. These are important to consider given that NIBS protocols are rarely tailored to atypical groups. Instead, research in atypical cohorts often relies on protocols shown to be effective in neurotypical groups. In the next section, we discuss challenges facing studies of social processing in atypical groups using NIBS. We will use the case of Autism Spectrum Disorder (hereafter ‘autism’) as an example throughout.
Autism is a neurodevelopmental disorder characterised by social difficulties and rigid and repetitive behaviours (American Psychiatric Association, 2013). In addition to these core symptoms, people with autism often exhibit motor control difficulties (Gowen and Hamilton, 2013) and have significantly higher rates of neuropsychiatric disorders such as depression and anxiety (Hollocks et al., 2019). Research investigating ways to ameliorate social difficulties associated with autism or co-occurring disorders and traits (e.g. social anxiety, alexithymia) is therefore an important area of study for researchers investigating social processing and for the autistic community (Pellicano et al., 2014).
Atypical groups may also benefit greatly from social interventions in neurotypical participants. For example, many autistic individuals find social situations challenging due to difficulties interpreting social cues of others. However, social situations may also be challenging due to a failure of neurotypical controls to interpret social cues of their autistic peers (Brewer et al., 2016; Edey et al., 2016). Thus, interventions must account for both autistic and neurotypical difficulties in order to improve social interactions across these cohorts. NIBS techniques may provide a useful tool to understand and ameliorate social difficulties in both typical and atypical populations. However, use of such techniques in people with autism and other disorders should be approached with caution (Wassermann and Lisanby, 2001; Bersani et al., 2013; Kuo et al., 2014; Oberman et al., 2015).
Stimulation protocols in typical and atypical populations
Network recruitment and connectivity
Multiple papers highlight high variability in response to brain stimulation in neurotypical adults and the need to individualise or tailor protocols to achieve maximal gain in both typical and atypical groups (for review see Krause and Cohen Kadosh, 2014). However, in practice, many studies investigating social perception in atypical groups are reliant on findings from the neuroptyical literature to inform protocols. This is problematic as it assumes that what holds in a neurotypical population will directly apply to atypical populations (Walsh and Pascual-Leone, 2003). This is important when considering the use of NIBS in atypical groups such as those with autism. Hanson et al. (2013) found that autistic participants showed different connectivity patterns during social processing tasks relative to neurotypical controls. This is consistent with other findings suggesting general atypical connectivity in autistic cohorts (Rubenstein and Merzenich, 2003; Assaf et al., 2010). Importantly, this difference was not uniform across tasks. Participants with autism showed similar connectivity patterns to neurotypical controls when face processing networks were recruited, but not when theory of mind or action understanding networks were recruited (Hanson et al., 2013). Collectively, these findings highlight different network recruitment and connectivity patterns in participants with autism relative to neurotypical controls. NIBS studies investigating social processing in these groups should, therefore, take this into account when selecting target sites or when designing paradigms to investigate connectivity patterns in participants with autism. Importantly, we cannot assume that stimulation to target sites shown to modulate social processing in neurotypical adults will modulate social processing in the same way in atypical groups.
Neurotransmitters
Atypical inhibition in the brain has been proposed as a common candidate endophenotype for a range of disorders (Marín, 2012). In autism, atypical GABAergic activity in the brain is observed due to a multitude of factors including reduced γ-aminobutyric acid (GABA) synthesis and reduced number of GABAergic receptors (for reviews see Rubenstein and Merzenich, 2003; Blatt and Fatemi, 2011). Atypical inhibition in autism may also results from atypical N-methyl-D-aspartate (NMDA) receptor activity (Lee et al., 2015). In line with the above, atypical plasticity profiles have been observed across a range of disorders including schizophrenia and autism (e.g. Bourgeron, 2015; Hall et al., 2015; Forrest et al., 2018). These findings are important given that several NIBS techniques are thought to work by influencing NMDA and GABAergic activity and increasing plasticity in targeted regions (Liebetanz et al., 2002; Huang et al., 2007; Stagg et al., 2009; Bachtiar et al., 2015). Therefore, modulating these systems in the atypical brain may not have the same outcome as in a neurotypical brain. Indeed, atypical plasticity following rTMS in participants with autism has been observed (Oberman et al., 2010, 2012). Thus, whilst interventions targeting these neurotransmitters in atypical groups may be useful, it is important to first tailor such interventions to the intended cohort.
One way to achieve this is through testing physiological and behavioural responses to NIBS techniques in atypical cohorts. This can be done by borrowing protocols from studies addressing this in neurotypical controls (Walsh and Cowey, 2000; Jacobson et al., 2012; Krause and Cohen Kadosh, 2014; Parkin et al., 2015; Reed and Kadosh, 2018). Ideally, this should be done prior to attempts to induce long-term changes in atypical groups using NIBS. A good example of work in atypical groups comes from Hoy et al. (2014) who showed dose-dependent effects of tDCS on working memory in participants with schizophrenia. Such work is important to ensure the safety of participants undergoing interventions and to increase the likelihood that participation is worthwhile for these groups. NIBS interventions can span months and require regular lab visits. Regular visits may be draining for atypical groups for many reasons (e.g. unknown social situation, anxiety when using public transport, etc.). Therefore, the time and energy cost to the participant must be taken into account when engaging atypical groups in interventions. Understanding how NIBS affects these groups, prior to undertaking longer-term interventions, is one way to address this. Thus, whilst this work does not explicitly relate to investigating social processing in atypical groups, it is a necessary precursor.
Stimulus properties
Several studies have used NIBS methods to investigate social processing in autism (e.g. Théoret et al., 2005; Enticott et al., 2012). For example, Théoret et al. (2005) demonstrated a reduced MEP response to observed actions in participants with autism relative to neurotypical controls. One explanation for these results may be that participants with autism show a reduced mirror response to observed actions. However, this reduced response may also be due to the type of stimuli used. Specifically, if stimuli presented do not adequately map onto motor representations in the brains of participants with autism, this may also present as a reduced MEP response. One reason for this may be that participants with autism move differently to neurotypical controls. For example, participants with autism have a different kinematic profile when executing intransitive movements compared to neurotypical controls (Cook et al., 2013). Considering such differences when designing stimuli is important to allow stronger inferences to be drawn. In the case of action observation, this could simply involve inclusion of movements made by autistic and non-autistic individuals, as well as several movements made by the participant themselves.
Understanding NIBS–medication interactions
Many cognitive studies using NIBS typically exclude participants taking psychotropic medications based on safety criteria from Rossi et al. (2009). In neurotypical adults, this is important to reduce noise in the data and to ensure participant safety. However, this approach is less straightforward in atypical groups. It is common to decide on exclusion based on contraindications to NIBS by assessing the cost/benefit ratio of participant involvement in the study. Whilst this may be a good approach for therapeutic interventions targeting treatment-resistant disorders, it does limit inclusion of participants in research investigating atypical groups. Approximately 60% of participants with autism are taking one or more psychotropic medications (Buck et al., 2014). Therefore, we need to understand safety and efficacy of NIBS in combination with these drugs to prevent sampling bias when testing atypical groups. Due to high heterogeneity, ensuring that study findings reflect the wider cohort is essential in order to interpret cognitive processes in atypical groups.
This is particularly important when investigating social processing, as people may be on medication to ameliorate social deficits. Excluding such participants from studies investigating social processing can therefore bias the sample tested. McLaren et al. (2018) reviewed the interaction between medications and tDCS effects over M1 in neurotypical adults. Among others, interactions between drugs that alter neurotransmitter concentrations (e.g. GABA and dopamine) and the effects of tDCS were observed. The authors highlight the use of such drugs in treating neuropsychiatric conditions (e.g. anxiety and schizophrenia), and, therefore, the importance of considering such interactions when translating tDCS protocols to atypical cohorts. However, the authors also stress caution when applying such findings to an atypical cohort, given differences in brain structure and function relative to neurotypical controls as well as potential differences in response to a given drug. Support for this cautious approach comes from work by Ajram et al. (2017), who showed that participants with autism showed a different neural response to a GABA- and glutamate-acting drug compared to neurotypical controls. Thus, it is important to consider the way in which a drug works in an atypical group, as well as potential (differential) NIBS–medication interactions. This will be a challenging line of research requiring data beyond that collected in neurotypical controls, and such research is currently in its infancy.
One way to inform design of such studies is to use existing data from atypical groups taking part in clinical trials using NIBS. An increasing number of studies are being conducted using NIBS in participants with psychiatric disorders either as a treatment for core symptoms or to treat co-occurring disorders (e.g. for treatment of depression in participants with schizophrenia or autism). Many of these participants are also on psychotropic medications, and so, whilst not the primary aim of the research, some of these studies also include analyses looking at NIBS–drug interactions (e.g. Hoffman et al., 2000). Using findings from this literature, and literature assessing NIBS–drug interactions in neurotypical participants (e.g. Rumi et al., 2005; Herwig et al., 2007; Liu et al., 2014; McLaren et al., 2018), may help us to begin to identify common interactions and safety limits of NIBS use in an atypical brain. Once these are better understood, we can then use these findings to inform the design of studies investigating other areas of cognition such as social processing.
Conclusions
It is clear that NIBS methods have improved understanding of social processing. However, many challenges still face research into social processing in typical and atypical groups. Promising techniques (e.g. targeting deeper structures using tFUS) are emerging, and it may be possible to exploit existing knowledge of NIBS techniques (e.g. state-dependent effects of TMS) to refine methodology. Research into NIBS in typical groups can also be used to inform NIBS protocol in atypical groups when combined with advances in understanding of brain stimulation effects in different cohorts. Along with growing understanding of NIBS mechanisms in typical and atypical cohorts, advances in our understanding of social processing have brought behavioural paradigms in the field to a stage where they are accessible both conceptually and anatomically to NIBS research.
Funding
This work was supported by a doctoral studentship from the Medical Research Council (MR/M50175X/1 to T.P.), the Leverhulme Trust (grant number PLP-2015-019 to C.C.), the Economic and Social Research Council (ES/R007527/1 to G.B. and M.J.B.) and the Baily Thomas Charitable Trust (3751-6536 to G.B.).
Conflict of interest: None declared.
Contributor Information
Tegan Penton, Department of Psychology, Goldsmiths, University of London, London SE14 6NW, UK.
Caroline Catmur, MRC Social, Genetic and Developmental Psychiatry Centre, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 8AF, UK.
Michael J Banissy, Department of Psychology, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 8AF, UK.
Geoffrey Bird, Department of Experimental Psychology, University of Oxford, Oxford OX1 3PH, UK.
Vincent Walsh, Institute of Cognitive Neuroscience, University College London, London WC1N 3AR, UK.
References
- Adolphs, R. (2002). Neural systems for recognizing emotion. Current Opinion in Neurobiology, 12(2), 169–77. [DOI] [PubMed] [Google Scholar]
- Ajram, L.A., Horder, J., Mendez, M.A., et al. (2017). Shifting brain inhibitory balance and connectivity of the prefrontal cortex of adults with autism spectrum disorder. Translational Psychiatry, 7(5), e1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrus, G.G., Windel, F., Burton, A.M., Kovács, G. (2017a). Causal evidence of the involvement of the right occipital face area in face-identity acquisition. NeuroImage, 148, 212–8. [DOI] [PubMed] [Google Scholar]
- Ambrus, G.G., Dotzer, M., Schweinberger, S.R., Kovacs, G. (2017b). The occipital face area is causally involved in the formation of identity-specific face representations. Brain Structure & Function, 222, 4271–82. [DOI] [PubMed] [Google Scholar]
- Ambrus, G.G., Amado, C., Krohn, L., Kovacs, G. (2019). TMS of the occipital face area modulates cross-domain identity priming. Brain Structure & Function, 224, 149–57. [DOI] [PubMed] [Google Scholar]
- APA (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th edn, Washington, DC: American Psychiatric Association. [Google Scholar]
- Assaf, M., Jagannathan, K., Calhoun, V.D., et al. (2010). Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. NeuroImage, 53, 247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson, A.P., Adolphs, R. (2011). The neuropsychology of face perception: beyond simple dissociations and functional selectivity. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1571), 1726–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtiar, V., Near, J., Johansen-Berg, H., Stagg, C.J. (2015). Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. eLife, 4, e08789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri, M., Negrini, M., Nitsche, M.A., Rivolta, D. (2016). Anodal-tDCS over the human right occipital cortex enhances the perception and memory of both faces and objects. Neuropsychologia, 81, 238–44. [DOI] [PubMed] [Google Scholar]
- Bersani, F.S., Minichino, A., Enticott, P.G., et al. (2013). Deep transcranial magnetic stimulation as a treatment for psychiatric disorders: a comprehensive review. European Psychiatry, 28(1), 30–9. [DOI] [PubMed] [Google Scholar]
- di Biase, L., Falato, E., Di Lazzaro, V. (2019). Transcranial focused ultrasound (tFUS) and transcranial unfocused ultrasound (tUS) neuromodulation: from theoretical principles to stimulation practices. Frontiers in Neurology, 10, 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt, G.J., Fatemi, S.H. (2011). Alterations in GABAergic biomarkers in the autism brain: research findings and clinical implications. The Anatomical Record, 294(10), 1646–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio, P.S., Asthana, M., Costa, T.L., Valasek, C.A., Osório, A.A.C. (2015). Promoting social plasticity in developmental disorders with non-invasive brain stimulation techniques. Frontiers in Neuroscience, 9, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeron, T. (2015). From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nature Reviews Neuroscience, 16(9), 551. [DOI] [PubMed] [Google Scholar]
- Brass, M., Ruby, P., Spengler, S. (2009). Inhibition of imitative behaviour and social cognition. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1528), 2359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer, R., Biotti, F., Catmur, C., et al. (2016). Can neurotypical individuals read autistic facial expressions? Atypical production of emotional facial expressions in autism spectrum disorders. Autism Research, 9(2), 262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck, T.R., Viskochil, J., Farley, M., et al. (2014). Psychiatric comorbidity and medication use in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(12), 3063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder, A.J., Young, A.W. (2005). Understanding the recognition of facial identity and facial expression. Nature Reviews. Neuroscience, 6, 641–51. [DOI] [PubMed] [Google Scholar]
- Casile, A. (2013). Mirror neurons (and beyond) in the macaque brain: an overview of 20 years of research. Neuroscience Letters, 540, 3–14. doi: 10.1016/j.neulet.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Caspers, S., Zilles, K., Laird, A.R., Eickhoff, S.B. (2010). ALE meta-analysis of action observation and imitation in the human brain. NeuroImage, 50, 1148–67. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catmur, C., Walsh, V., Heyes, C. (2007). Sensorimotor learning configures the human mirror system. Current Biology, 17(17), 1527–31. [DOI] [PubMed] [Google Scholar]
- Catmur, C., Mars, R.B., Rushworth, M.F., Heyes, C. (2011). Making mirrors: premotor cortex stimulation enhances mirror and counter-mirror motor facilitation. Journal of Cognitive Neuroscience, 23(9), 2352–62. [DOI] [PubMed] [Google Scholar]
- Cattaneo, L., Sandrini, M., Schwarzbach, J. (2010). State-dependent TMS reveals a hierarchical representation of observed acts in the temporal, parietal, and premotor cortices. Cerebral Cortex, 20, 2252–8. [DOI] [PubMed] [Google Scholar]
- Cattaneo, L., Barchiesi, G., Tabarelli, D., Arfeller, C., Sato, M., Glenberg, A.M. (2011). One’s motor performance predictably modulates the understanding of others’ actions through adaptation of premotor visuo-motor neurons. Social Cognitive and Affective Neuroscience, 6, 301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo, Z., Silvanto, J. (2008). Investigating visual motion perception using the transcranial magnetic stimulation-adaptation paradigm. Neuroreport, 19(14), 1423–7. [DOI] [PubMed] [Google Scholar]
- Cole, M.W., Reynolds, J.R., Power, J.D., Repovs, G., Anticevic, A., Braver, T.S. (2013). Multi-task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience, 16(9), 1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll, M.P., Penton, T., Hobson, H. (2017). Important methodological issues regarding the use of transcranial magnetic stimulation to investigate interoceptive processing: a comment on Pollatos et al. (2016). Philosophical Transactions of the Royal Society B: Biological Sciences, 372(1721), 20160506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, J.L., Blakemore, S.J., Press, C. (2013). Atypical basic movement kinematics in autism spectrum conditions. Brain, 136(9), 2816–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, J.L. (2014). Task-relevance dependent gradients in medial prefrontal and temporoparietal cortices suggest solutions to paradoxes concerning self/other control. Neuroscience & Biobehavioral Reviews, 42, 298–302. [DOI] [PubMed] [Google Scholar]
- Cook, R., Bird, G., Catmur, C., Press, C., Heyes, C. (2014). Mirror neurons: from origin to function. Behavioral and Brain Sciences, 37(2), 177–92. [DOI] [PubMed] [Google Scholar]
- Corbetta, M., Shulman, G.L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201. [DOI] [PubMed] [Google Scholar]
- Costa, A., Torriero, S., Oliveri, M., Caltagirone, C. (2008). Prefrontal and temporo-parietal involvement in taking others’ perspective: TMS evidence. Behavioural Neurology, 19(1–2), 71–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow, D.P. (2019). Focused ultrasound for neuromodulation. Neurotherapeutics, 16(1), 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Z.D., Lisanby, S.H., Peterchev, A.V. (2013). Electric field depth–focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimulation, 6(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny, B.T., Kober, H., Wager, T.D., Ochsner, K.N. (2012). A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience, 24(8), 1742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edey, R., Cook, J., Brewer, R., Johnson, M.H., Bird, G., Press, C. (2016). Interaction takes two: typical adults exhibit mind-blindness towards those with autism spectrum disorder. Journal of Abnormal Psychology, 125(7), 879. [DOI] [PubMed] [Google Scholar]
- Enticott, P.G., Kennedy, H.A., Rinehart, N.J., et al. (2012). Mirror neuron activity associated with social impairments but not age in autism spectrum disorder. Biological Psychiatry, 71(5), 427–33. [DOI] [PubMed] [Google Scholar]
- Fadiga, L., Fogassi, L., Pavesi, G., Rizzolatti, G. (1995). Motor facilitation during action observation: a magnetic stimulation study. Journal of Neurophysiology, 73(6), 2608–11. [DOI] [PubMed] [Google Scholar]
- Fairhall, S.L., Ishai, A. (2006). Effective connectivity within the distributed cortical network for face perception. Cerebral Cortex, 17(10), 2400–6. [DOI] [PubMed] [Google Scholar]
- Ferrucci, R., Cortese, F., Priori, A. (2015). Cerebellar tDCS: how to do it. The Cerebellum, 14(1), 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folloni, D., Verhagen, L., Mars, R.B., et al. (2019). Manipulation of subcortical and deep cortical activity in the primate brain using transcranial focused ultrasound stimulation. Neuron, 101(6), 1109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest, M.P., Parnell, E., Penzes, P. (2018). Dendritic structural plasticity and neuropsychiatric disease. Nature Reviews Neuroscience, 19(4), 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese, V., Fadiga, L., Fogassi, L., Rizzolatti, G. (1996). Action recognition in the premotor cortex. Brain, 119(Pt 2), 593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gooding-Williams, G., Wang, H., Kessler, K. (2017). THETA-rhythm makes the world go round: dissociative effects of TMS Theta versus alpha entrainment of right pTPJ on embodied perspective transformations. Brain Topography, 30(5), 561–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen, E., Hamilton, A. (2013). Motor abilities in autism: a review using a computational context. Journal of Autism and Developmental Disorders, 43(2), 323–44. [DOI] [PubMed] [Google Scholar]
- de Graaf, T.A., Sack, A.T. (2018). When and how to interpret null results in NIBS: a taxonomy based on prior expectations and experimental design. Frontiers in Neuroscience, 12, 915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman, N., Bono, D., Dedic, N., et al. (2017). Noninvasive deep brain stimulation via temporally interfering electric fields. Cell, 169(6), 1029–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman, N., Okun, M.S., Boyden, E.S. (2018). Translating temporal interference brain stimulation to treat neurological and psychiatric conditions. JAMA Neurology, 75(11), 1307–8. [DOI] [PubMed] [Google Scholar]
- de Guzman, M., Bird, G., Banissy, M.J., Catmur, C. (2016). Self–other control processes in social cognition: from imitation to empathy. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1686), 20150079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, J., Trent, S., Thomas, K.L., O’Donovan, M.C., Owen, M.J. (2015). Genetic risk for schizophrenia: convergence on synaptic pathways involved in plasticity. Biological Psychiatry, 77(1), 52–8. [DOI] [PubMed] [Google Scholar]
- Hanson, C., Hanson, S.J., Ramsey, J., Glymour, C. (2013). Atypical effective connectivity of social brain networks in individuals with autism. Brain Connectivity, 3(6), 578–89. [DOI] [PubMed] [Google Scholar]
- Haxby, J.V., Hoffman, E.A., Gobbini, M.I. (2000). The distributed human neural system for face perception. Trends in Cognitive Sciences, 4, 223–33. doi: 10.1016/S1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Herwig, U., Fallgatter, A.J., Höppner, J., et al. (2007). Antidepressant effects of augmentative transcranial magnetic stimulation: randomised multicentre trial. The British Journal of Psychiatry, 191(5), 441–8. [DOI] [PubMed] [Google Scholar]
- Hoffman, R.E., Boutros, N.N., Hu, S., Berman, R.M., Krystal, J.H., Charney, D.S. (2000). Transcranial magnetic stimulation and auditory hallucinations in schizophrenia. The Lancet, 355(9209), 1073–5. [DOI] [PubMed] [Google Scholar]
- Hogeveen, J., Obhi, S.S., Banissy, M.J., et al. (2014). Task-dependent and distinct roles of the temporoparietal junction and inferior frontal cortex in the control of imitation. Social Cognitive and Affective Neuroscience, 10(7), 1003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollocks, M.J., Lerh, J.W., Magiati, I., Meiser-Stedman, R., Brugha, T.S. (2019). Anxiety and depression in adults with autism spectrum disorder: a systematic review and meta-analysis. Psychological Medicine, 49(4), 559–72. [DOI] [PubMed] [Google Scholar]
- Hoy, K.E., Arnold, S.L., Emonson, M.R., Daskalakis, Z.J., Fitzgerald, P.B. (2014). An investigation into the effects of tDCS dose on cognitive performance over time in patients with schizophrenia. Schizophrenia Research, 155(1–3), 96–100. [DOI] [PubMed] [Google Scholar]
- Huang, Y.Z., Chen, R.S., Rothwell, J.C., Wen, H.Y. (2007). The after-effect of human theta burst stimulation is NMDA receptor dependent. Clinical Neurophysiology, 118(5), 1028–32. [DOI] [PubMed] [Google Scholar]
- Hutcheon, B., Yarom, Y. (2000). Resonance, oscillation and the intrinsic frequency preferences of neurons. Trends in Neurosciences, 23(5), 216–22. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi, R.J., Kičić, D. (2010). Methodology for combined TMS and EEG. Brain Topography, 22(4), 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson, L., Koslowsky, M., Lavidor, M. (2012). tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Experimental Brain Research, 216(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Jacquet, P.O., Avenanti, A. (2015). Perturbing the action observation network during perception and categorization of actions’ goals and grips: state dependency and virtual lesion TMS effects. Cerebral Cortex, 25, 598–608. [DOI] [PubMed] [Google Scholar]
- Kammer, T. (1998). Phosphenes and transient scotomas induced by magnetic stimulation of the occipital lobe: their topographic relationship. Neuropsychologia, 37(2), 191–8. [DOI] [PubMed] [Google Scholar]
- Keysers, C., Paracampo, R., Gazzola, V. (2018). What neuromodulation and lesion studies tell us about the function of the mirror neuron system and embodied cognition. Current Opinion in Psychology, 24, 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D.R., Pesiridou, A., O’Reardon, J.P. (2009). Transcranial magnetic stimulation in the treatment of psychiatric disorders. Current Psychiatry Reports, 11(6), 447–52. [DOI] [PubMed] [Google Scholar]
- Kim, S., Nilakantan, A.S., Hermiller, M.S., Palumbo, R.T., VanHaerents, S., Voss, J.L. (2018). Selective and coherent activity increases due to stimulation indicate functional distinctions between episodic memory networks. Science Advances, 4(8), eaar2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall, S.C., Rottschy, C., Oberwelland, E., et al. (2015). The role of the right temporoparietal junction in attention and social interaction as revealed by ALE meta-analysis. Brain Structure and Function, 220(2), 587–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause, B., Cohen Kadosh, R. (2014). Not all brains are created equal: the relevance of individual differences in responsiveness to transcranial electrical stimulation. Frontiers in Systems Neuroscience, 8, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, M.F., Paulus, W., Nitsche, M.A. (2014). Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. NeuroImage, 85, 948–60. [DOI] [PubMed] [Google Scholar]
- Lafontaine, M.P., Théoret, H., Gosselin, F., Lippé, S. (2013). Transcranial direct current stimulation of the dorsolateral prefrontal cortex modulates repetition suppression to unfamiliar faces: an ERP study. PLoS One, 8(12), e81721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, E.J., Choi, S.Y., Kim, E. (2015). NMDA receptor dysfunction in autism spectrum disorders. Current Opinion in Pharmacology, 20, 8–13. [DOI] [PubMed] [Google Scholar]
- Legon, W., Sato, T.F., Opitz, A., et al. (2014). Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nature Neuroscience, 17(2), 322. [DOI] [PubMed] [Google Scholar]
- Legon, W., Bansal, P., Tyshynsky, R., Ai, L., Mueller, J.K. (2018). Transcranial focused ultrasound neuromodulation of the human primary motor cortex. Scientific Reports, 8, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetanz, D., Nitsche, M.A., Tergau, F., Paulus, W. (2002). Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain, 125(10), 2238–47. [DOI] [PubMed] [Google Scholar]
- Liu, B., Zhang, Y., Zhang, L., Li, L. (2014). Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry, 14(1), 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, M., Ueno, S. (2017). Comparison of the induced fields using different coil configurations during deep transcranial magnetic stimulation. PLoS One, 12(6), e0178422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado, S., Bittencourt, J., Minc, D., et al. (2008). Therapeutic applications of repetitive transcranial magnetic stimulation in clinical neurorehabilitation. Functional Neurology, 23(3), 113. [PubMed] [Google Scholar]
- Maegherman, G., Nuttall, H.E., Devlin, J.T., Adank, P. (2019). Motor imagery of speech: the involvement of primary motor cortex in manual and articulatory motor imagery. Frontiers in Human Neuroscience, 13, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai, S., Braun, J., Probst, V., Kammer, T., Pollatos, O. (2019). Changes in emotional processing following interoceptive network stimulation with rTMS. Neuroscience, 406, 405–19. [DOI] [PubMed] [Google Scholar]
- Marín, O. (2012). Interneuron dysfunction in psychiatric disorders. Nature Reviews Neuroscience, 13(2), 107. [DOI] [PubMed] [Google Scholar]
- Mars, R.B., Sallet, J., Schüffelgen, U., Jbabdi, S., Toni, I., Rushworth, M.F. (2011). Connectivity-based subdivisions of the human right “temporoparietal junction area”: evidence for different areas participating in different cortical networks. Cerebral Cortex, 22(8), 1894–903. [DOI] [PubMed] [Google Scholar]
- Mazzoni, N., Jacobs, C., Venuti, P., Silvanto, J., Cattaneo, L. (2017). State-dependent TMS reveals representation of affective body movements in the anterior intraparietal cortex. Journal of Neuroscience, 37(30), 7231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren, M.E., Nissim, N.R., Woods, A.J. (2018). The effects of medication use in transcranial direct current stimulation: a brief review. Brain Stimulation, 11(1), 52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer, L., Costafreda, S., Aleman, A., David, A.S. (2010). Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neuroscience & Biobehavioral Reviews, 34(6), 935–46. [DOI] [PubMed] [Google Scholar]
- Miniussi, C., Thut, G. (2010). Combining TMS and EEG offers new prospects in cognitive neuroscience. Brain Topography, 22(4), 249. [DOI] [PubMed] [Google Scholar]
- Miniussi, C., Vallar, G. (2011). Brain stimulation and behavioural cognitive rehabilitation: a new tool for neurorehabilitation? Neuropsychological Rehabilitation, 21(5), 553–9. [DOI] [PubMed] [Google Scholar]
- Miniussi, C., Paulus, W., Rossini, P.M. (Eds.). (2012). Transcranial Brain Stimulation, Florida, USA: CRC Press. [Google Scholar]
- Miniussi, C., Ruzzoli, M. (2013). Transcranial stimulation and cognition. In: Lozano, A. M., & Hallett, M. (Eds.) Handbook of Clinical Neurology, (Vol. 116, pp. 739–750). Oxford, UK: Elsevier. [DOI] [PubMed] [Google Scholar]
- Nicolle, A., Klein-Flügge, M.C., Hunt, L.T., Vlaev, I., Dolan, R.J., Behrens, T.E. (2012). An agent independent axis for executed and modeled choice in medial prefrontal cortex. Neuron, 75(6), 1114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman, L.M., Ifert-Miller, F., Najib, U., et al. (2010). Transcranial magnetic stimulation provides means to assess cortical plasticity and excitability in humans with fragile X syndrome and autism spectrum disorder. Frontiers in Synaptic Neuroscience, 2, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman, L., Eldaief, M., Fecteau, S., Ifert-Miller, F., Tormos, J.M., Pascual-Leone, A. (2012). Abnormal modulation of corticospinal excitability in adults with Asperger’s syndrome. European Journal of Neuroscience, 36(6), 2782–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman, L.M., Rotenberg, A., Pascual-Leone, A. (2015). Use of transcranial magnetic stimulation in autism spectrum disorders. Journal of Autism and Developmental Disorders, 45(2), 524–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin, B.L., Ekhtiari, H., Walsh, V.F. (2015). Non-invasive human brain stimulation in cognitive neuroscience: a primer. Neuron, 87(5), 932–45. [DOI] [PubMed] [Google Scholar]
- Pasley, B.N., Allen, E.A., Freeman, R.D. (2009). State-dependent variability of neuronal responses to transcranial magnetic stimulation of the visual cortex. Neuron, 62(2), 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquinelli, C., Hanson, L.G., Siebner, H.R., Lee, H.J., Thielscher, A. (2019). Safety of transcranial focused ultrasound stimulation: a systematic review of the state of knowledge from both human and animal studies. Brain Stimulation. 12(6), 1367–1380. [DOI] [PubMed] [Google Scholar]
- Pellicano, E., Dinsmore, A., Charman, T. (2014). What should autism research focus upon? Community views and priorities from the United Kingdom. Autism, 18(7), 756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera, T., George, M.S., Grammer, G., Janicak, P.G., Pascual-Leone, A., Wirecki, T.S. (2016). The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimulation, 9(3), 336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher, D., Walsh, V., Yovel, G., Duchaine, B. (2007). TMS evidence for the involvement of the right occipital face area in early face processing. Current Biology, 17(18), 1568–73. [DOI] [PubMed] [Google Scholar]
- Pitcher, D., Charles, L., Devlin, J.T., Walsh, V., Duchaine, B. (2009). Triple dissociation of faces, bodies, and objects in extrastriate cortex. Current Biology, 19(4), 319–24. [DOI] [PubMed] [Google Scholar]
- Pitcher, D., Walsh, V., Duchaine, B. (2011). The role of the occipital face area in the cortical face perception network. Experimental Brain Research, 209(4), 481–93. [DOI] [PubMed] [Google Scholar]
- Pitcher, D., Duchaine, B., Walsh, V. (2014). Combined TMS and fMRI reveal dissociable cortical pathways for dynamic and static face perception. Current Biology, 24(17), 2066–70. [DOI] [PubMed] [Google Scholar]
- Pitcher, D., Japee, S., Rauth, L., Ungerleider, L.G. (2017). The superior temporal sulcus is causally connected to the amygdala: a combined TBS-fMRI study. Journal of Neuroscience, 37(5), 1156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher, D. (2019). Face processing and TMS. doi: 10.31234/osf.io/h86xz. [DOI]
- Rampersad, S., Roig-Solvas, B., Yarossi, M., et al. (2019). Prospects for transcranial temporal interference stimulation in humans: a computational study. bioRxiv, 602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, T., Kadosh, R.C. (2018). Transcranial electrical stimulation (tES) mechanisms and its effects on cortical excitability and connectivity. Journal of Inherited Metabolic Disease, 41(6), 1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzi, C., Schiavi, S., Carbon, C.C., Vecchi, T., Silvanto, J., Cattaneo, Z. (2013). Processing of featural and configural aspects of faces is lateralized in dorsolateral prefrontal cortex: a TMS study. NeuroImage, 74, 45–51. [DOI] [PubMed] [Google Scholar]
- Rizzolatti, G., Sinigaglia, C. (2010). The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nature Reviews. Neuroscience, 11, 264–74. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Romanska, A., Rezlescu, C., Susilo, T., Duchaine, B., Banissy, M.J. (2015). High-frequency transcranial random noise stimulation enhances perception of facial identity. Cerebral Cortex, 25(11), 4334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, S., Hallett, M., Rossini, P.M., Pascual-Leone, A., Safety of TMS Consensus Group (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12), 2008–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion, B. (2014). Understanding face perception by means of prosopagnosia and neuroimaging. Frontiers in Bioscience, 6(258), e307. [DOI] [PubMed] [Google Scholar]
- Roth, Y., Amir, A., Levkovitz, Y., Zangen, A. (2007). Three-dimensional distribution of the electric field induced in the brain by transcranial magnetic stimulation using figure-8 and deep H-coils. Journal of Clinical Neurophysiology, 24(1), 31–8. [DOI] [PubMed] [Google Scholar]
- Rubenstein, J.L.R., Merzenich, M.M. (2003). Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, Brain, and Behavior, 2, 255–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff, C.C., Driver, J., Bestmann, S. (2009). Combining TMS and fMRI: from ‘virtual lesions’ to functional-network accounts of cognition. Cortex, 45(9), 1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumi, D.O., Gattaz, W.F., Rigonatti, S.P., et al. (2005). Transcranial magnetic stimulation accelerates the antidepressant effect of amitriptyline in severe depression: a double-blind placebo-controlled study. Biological Psychiatry, 57(2), 162–6. [DOI] [PubMed] [Google Scholar]
- Sandrini, M., Umiltà, C., Rusconi, E. (2011). The use of transcranial magnetic stimulation in cognitive neuroscience: a new synthesis of methodological issues. Neuroscience & Biobehavioral Reviews, 35(3), 516–36. [DOI] [PubMed] [Google Scholar]
- Santiesteban, I., Banissy, M.J., Catmur, C., Bird, G. (2012). Enhancing social ability by stimulating right temporoparietal junction. Current Biology, 22(23), 2274–7. [DOI] [PubMed] [Google Scholar]
- Santiesteban, I., Banissy, M.J., Catmur, C., Bird, G. (2015). Functional lateralization of temporoparietal junction–imitation inhibition, visual perspective-taking and theory of mind. European Journal of Neuroscience, 42(8), 2527–33. [DOI] [PubMed] [Google Scholar]
- Santiesteban, I., Kaur, S., Bird, G., Catmur, C. (2017). Attentional processes, not implicit mentalizing, mediate performance in a perspective-taking task: evidence from stimulation of the temporoparietal junction. NeuroImage, 155, 305–11. [DOI] [PubMed] [Google Scholar]
- Schulz, R., Gerloff, C., Hummel, F.C. (2013). Non-invasive brain stimulation in neurological diseases. Neuropharmacology, 64, 579–87. [DOI] [PubMed] [Google Scholar]
- Sellaro, R., Nitsche, M.A., Colzato, L.S. (2016). The stimulated social brain: effects of transcranial direct current stimulation on social cognition. Annals of the New York Academy of Sciences, 1369(1), 218–39. [DOI] [PubMed] [Google Scholar]
- Silvanto, J., Muggleton, N.G., Cowey, A., Walsh, V. (2007). Neural activation state determines behavioral susceptibility to modified theta burst transcranial magnetic stimulation. European Journal of Neuroscience, 26(2), 523–8. [DOI] [PubMed] [Google Scholar]
- Silvanto, J., Cattaneo, Z., Battelli, L., Pascual-Leone, A. (2008). Baseline cortical excitability determines whether TMS disrupts or facilitates behavior. Journal of Neurophysiology, 99(5), 2725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto, J., Muggleton, N.G. (2008). Testing the validity of the TMS state-dependency approach: targeting functionally distinct motion-selective neural populations in visual areas V1/V2 and V5/MT+. NeuroImage, 40(4), 1841–8. [DOI] [PubMed] [Google Scholar]
- Silvanto, J., Pascual-Leone, A. (2008). State-dependency of transcranial magnetic stimulation. Brain Topography, 21(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto, J., Cattaneo, Z. (2017). Common framework for “virtual lesion” and state-dependent TMS: the facilitatory/suppressive range model of online TMS effects on behavior. Brain and Cognition, 119, 32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon-Harris, L.M., Mullin, C.R., Steeves, J.K. (2013). TMS to the “occipital face area” affects recognition but not categorization of faces. Brain and Cognition, 83(3), 245–51. [DOI] [PubMed] [Google Scholar]
- Stagg, C.J., Wylezinska, M., Matthews, P.M., et al. (2009). Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. Journal of Neurophysiology, 101(6), 2872–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella, A.P., Paus, T. (2000). Modulation of cortical excitability during action observation: a transcranial magnetic stimulation study. Neuroreport, 11(10), 2289–92. [DOI] [PubMed] [Google Scholar]
- Taylor, P.C.J. (2018). Combining NIBS with EEG: what can it tell us about normal cognition? Current Behavioral Neuroscience Reports, 5(2), 165–9. [Google Scholar]
- Théoret, H., Halligan, E., Kobayashi, M., Fregni, F., Tager-Flusberg, H., Pascual-Leone, A. (2005). Impaired motor facilitation during action observation in individuals with autism spectrum disorder. Current Biology, 15(3), R84–5. [DOI] [PubMed] [Google Scholar]
- Thompson, E.L., Bird, G., Catmur, C. (2019). Conceptualizing and testing action understanding. Neuroscience & Biobehavioral Reviews 105, 106–114. [DOI] [PubMed] [Google Scholar]
- Thut, G., Miniussi, C., Cecere, R., Sauseng, P., Benwell, C.S.Y., Veniero, D., editors. (2018). Non-invasive brain stimulation effects on cognition and brain activity: positive lessons from negative findings [special issue], Frontiers in Neuroscience, 11. [Google Scholar]
- Tyler, W.J., Lani, S.W., Hwang, G.M. (2018). Ultrasonic modulation of neural circuit activity. Current Opinion in Neurobiology, 50, 222–31. [DOI] [PubMed] [Google Scholar]
- Walsh, V., Cowey, A. (2000). Transcranial magnetic stimulation and cognitive neuroscience. Nature Reviews Neuroscience, 1(1), 73. [DOI] [PubMed] [Google Scholar]
- Walsh, V., Pascual-Leone, A. (2003). Transcranial Magnetic Stimulation: A Neurochronometrics of Mind, London, UK: MIT Press. [Google Scholar]
- Wang, H., Callaghan, E., Gooding-Williams, G., McAllister, C., Kessler, K. (2016). Rhythm makes the world go round: an MEG-TMS study on the role of right TPJ theta oscillations in embodied perspective taking. Cortex, 75, 68–81. [DOI] [PubMed] [Google Scholar]
- Wang, J.X., Rogers, L.M., Gross, E.Z., et al. (2014). Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science, 345(6200), 1054–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, J., Banissy, M.J. (2015). Explaining mirror-touch synesthesia. Cognitive Neuroscience, 6(2–3), 118–33. [DOI] [PubMed] [Google Scholar]
- Wassermann, E.M., Lisanby, S.H. (2001). Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clinical Neurophysiology, 112(8), 1367–77. [DOI] [PubMed] [Google Scholar]
- Wassermann, E., Epstein, C., Ziemann, U., Walsh, V., Paus, T., Lisanby, S., editors (2008). Oxford Handbook of Transcranial Stimulation, Oxford, UK: Oxford University Press. [Google Scholar]
- Wassermann, E.M., Zimmermann, T. (2012). Transcranial magnetic brain stimulation: therapeutic promises and scientific gaps. Pharmacology & Therapeutics, 133(1), 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, A.J., Antal, A., Bikson, M., et al. (2016). A technical guide to tDCS, and related non-invasive brain stimulation tools. Clinical Neurophysiology, 127(2), 1031–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, L., Camprodon, J.A., Hauser, M., Pascual-Leone, A., Saxe, R. (2010). Disruption of the right temporoparietal junction with transcranial magnetic stimulation reduces the role of beliefs in moral judgments. Proceedings of the National Academy of Sciences, 107(15), 6753–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


