Abstract
Previous studies on the mechanisms of peripheral nerve injury (PNI) have mainly focused on the pathophysiological changes within a single injury site. However, recent studies have indicated that within the central nervous system, PNI can lead to changes in both injury sites and target organs at the cellular and molecular levels. Therefore, the basic mechanisms of PNI have not been comprehensively understood. Although electrical stimulation was found to promote axonal regeneration and functional rehabilitation after PNI, as well as to alleviate neuropathic pain, the specific mechanisms of successful PNI treatment are unclear. We summarize and discuss the basic mechanisms of PNI and of treatment via electrical stimulation. After PNI, activity in the central nervous system (spinal cord) is altered, which can limit regeneration of the damaged nerve. For example, cell apoptosis and synaptic stripping in the anterior horn of the spinal cord can reduce the speed of nerve regeneration. The pathological changes in the posterior horn of the spinal cord can modulate sensory abnormalities after PNI. This can be observed in cases of ectopic discharge of the dorsal root ganglion leading to increased pain signal transmission. The injured site of the peripheral nerve is also an important factor affecting post-PNI repair. After PNI, the proximal end of the injured site sends out axial buds to innervate both the skin and muscle at the injury site. A slow speed of axon regeneration leads to low nerve regeneration. Therefore, it can take a long time for the proximal nerve to reinnervate the skin and muscle at the injured site. From the perspective of target organs, long-term denervation can cause atrophy of the corresponding skeletal muscle, which leads to abnormal sensory perception and hyperalgesia, and finally, the loss of target organ function. The mechanisms underlying the use of electrical stimulation to treat PNI include the inhibition of synaptic stripping, addressing the excessive excitability of the dorsal root ganglion, alleviating neuropathic pain, improving neurological function, and accelerating nerve regeneration. Electrical stimulation of target organs can reduce the atrophy of denervated skeletal muscle and promote the recovery of sensory function. Findings from the included studies confirm that after PNI, a series of physiological and pathological changes occur in the spinal cord, injury site, and target organs, leading to dysfunction. Electrical stimulation may address the pathophysiological changes mentioned above, thus promoting nerve regeneration and ameliorating dysfunction.
Key Words: axonal transport, brain-derived neurotrophic factor, dorsal horn stimulation, dorsal root ganglion stimulation, electrical stimulation, nerve regeneration, neuropathic pain, peripheral nerve injury, spinal cord dorsal stimulation
Introduction
Peripheral nerve injury (PNI) can lead to severe sensorimotor impairment and chronic neurogenic pain (Li et al., 2021; Xing et al., 2021), and is a prevalent cause of disability worldwide (Martínez-Marcos and Sañudo, 2019). Sunderland (1951) classified PNI into five grades according to the extent of injury and loss of function. The first grade describes a conduction block, which is the physiological interruption of nerve conduction along the axon at the site of injury, with an intact nerve structure and no Wallerian degeneration. Self-repair is expected in these cases. The second grade is characterized by axonal interruption, an intact endoneurium, and Wallerian degeneration. In these cases, nerve self-repair is observed with a speed of 1 mm/d. The third, fourth, and fifth grades involve injury to the endoneurial tubes, perineurium, and epineurium, respectively. Although self-repair is expected in the third grade, this is usually slow and incomplete. Thus, surgical intervention may eventually be required. Patients with grades four and five PNI are not expected to exhibit self-repair capacity, and surgical intervention is often necessary (Davis, 2020). The last decade has brought many surgical innovations in the treatment of PNI. However, there have been no significant improvements in the success of rehabilitation, as patients continue to experience somatosensory disorders. Moreover, 57% of patients with PNI are between 16 and 35 years of age, and 25% of patients with upper limb PNI are unable to return to work within 1.5 years post-operation (Kouyoumdjian et al., 2017). Therefore, PNI is associated with long-term disability and financial difficulties.
Treatments for PNI range from conservative approaches to surgical interventions. Conservative approaches include pharmacological treatments, cell-based therapies, and physical therapy. Pharmacological treatments have been found to improve axonal regeneration. Commonly used neurotrophic drugs include B vitamins, methylcobalamin, and exogenous neurotrophic factors (Ehmedah et al., 2019; Karagyaur et al., 2020; Sawangjit et al., 2020). However, because local drug concentrations in the peripheral blood tend to be low, pharmacological treatments often fail to achieve sustained clinical benefits. Recent years have seen tremendous progress in cell-based therapies such as those employing Schwann cells (SCs), mesenchymal stem cells (MSCs), bone marrow stromal stem cells, and skin precursor cells (Kubiak et al., 2020). Among these, MSCs can secrete various growth factors and support both nerve cells and SCs. A recent study indicated that exosomes from MSCs may be useful in a novel therapeutic strategy for PNI, as they can promote neurite outgrowth in dorsal root ganglion (DRG) and cortical neurons (Dong et al., 2019). However, difficulties associated with obtaining and sustaining such materials severely limit the clinical applications of cell therapy. Common physical therapies include phototherapy, magnetic therapy, acupuncture, and functional training. Despite the large variety of options, the treatment effects are not satisfactory. When spontaneous recovery is not observed in clinical practice, surgical interventions are often necessary. However, it is difficult to determine the optimal time for surgical intervention (Midha and Grochmal, 2019).
Compared with conservative approaches and surgical treatment, electrical stimulation (ES) is a safe and effective treatment option that can be applied in the vast majority of patients. Generally, patients without malignant tumors, high fever, coma, active bleeding, skin damage, or acute suppurative inflammation are eligible to receive ES. A previous study showed that ES can cause twisting of the axons and cytoarchitecture, leading to edema (Martellucci, 2015). However, recent studies reported that ES is efficacious in promoting axonal regeneration and functional rehabilitation in PNI patients (Gordon, 2016; Barber et al., 2018; Power et al., 2020). In addition, ES can be used to treat neuropathic pain after PNI. However, the physiological changes underlying PNI and the therapeutic effects of ES are complex. In this review, we summarize the physiological changes associated with PNI and the specific mechanisms of ES treatment at three levels: the cell body, the site of injury, and the target organ, to reflect a holistic view. Our goal was to generate theoretical guidance for further clinical ES applications.
Search Strategy and Selection Criteria
We used PubMed (https://www.ncbi.nlm.nih.gov/pubmed) to collect relevant papers published from inception to 2021 for inclusion in this narrative review. Our search keywords were: “peripheral nerve injury”, “electrical stimulation”, “peripheral nerve regeneration”, “spinal cord dorsal stimulation”, “dorsal horn stimulation”, “dorsal root ganglion stimulation”, “neuropathic pain”, “brain-derived neurotrophic factor”, “axonal transport”, “transcutaneous electrical nerve stimulation”, and “skeletal muscle stimulation”. After eliminating duplicates from the retrieved studies, we read the titles and abstracts of each article as a preliminary screening process, and then read the full texts to eliminate studies that did not cover the mechanisms of peripheral nerve injury and stimulation. The literature search process was primarily completed by author XLC.
The Mechanisms of Peripheral Nerve Injury
PNI is a common neurological condition that may cause motor and sensory disturbances as well as neuropathic pain. Pathological changes occur not only at the site of direct injury, but also throughout the affected regions of the central nervous system. In addition, target organs can be affected due to prolonged denervation. Therefore, the pathological mechanisms are complex, necessitating a holistic view. Changes at different levels are discussed in the following sections (Table 1).
Table 1.
Summary of the basic mechanism of peripheral nerve injury
| The first to study this field | The greatest contributions to this field | The affected site | Main mechanism |
|---|---|---|---|
| Adams et al., 1966 | Shen et al., 2019 | Skeletal muscle | Oxidative stress and inflammatory reactions results in skeletal muscle atrophy |
| Bray and Aguayo, 1974 | Mackinnon and Dellon, 1992 | Peripheral nerve axons | Wallerian degeneration and axonal staggered regeneration |
| Schmalbruch, 1988 | Navarro et al., 2007 | Spinal cord ventral horn | Motor neuron apoptosis and synaptic stripping |
| Sato and Perl, 1991 | Duraku et al., 2012 | Sensory receptors | The thresholds of thermoreceptors and nociceptors are decreased |
| Kajander et al., 1992 | Hussain et al., 2020 | Spinal cord dorsal horn | Excessive discharge of dorsal root ganglion neurons caused by inflammatory response; the numbers of microglia and morphological change |
| Rabinovsky et al., 1992 | McGregor and English, 2018 | The inner tube of nerve fibers and growth factors | The inner tube of nerve fibers narrows gradually and the ability of express growth factor decreases gradually |
Neurophysiological changes associated with PNI-induced spinal cord injury
Motor neuron apoptosis and synaptic stripping in the ventral horn of the spinal cord
After PNI, motor neurons in the anterior horn of the spinal cord often undergo apoptosis. Current research suggests that there are two explanations for motor neurons apoptosis after PNI (Hart et al., 2008). The first is oxidative stress, and the second is significant upregulation of the expression of apoptosis-related genes Caspase-3, Caspase-8, tumor necrosis factor-related apoptosis-inducing ligand receptor, tumor necrosis factor receptor, and Fas after PNI. Consistent with the abovementioned mechanism, injection of a Bcl-12-expressing vector 1 week prior to root avulsion was found to increase the survival of lesioned motor neurons by 50% (Natsume et al., 2002).
Synaptic stripping is another cause of functional loss after PNI. PNI usually causes activation and proliferation of microglia in the spinal cord. Upon activation, microglia proliferate and migrate toward the axotomized ventral horn, interposing themselves between ventral horn cell bodies and synapses undergoing detachment. The phenomenon in which microglia ‘lift’ these synapses has been termed “synaptic stripping” (Salvany et al., 2021). Synaptic stripping can induce Ia axons and synapses to withdraw from the ventral horn. Although motor and Ia axons can reinnervate muscles, Ia axons cannot return to the ventral horn. Consequently, individuals with this type of PNI show deficits during high force-related motor tasks due to the absence of Ia axons (Lyle et al., 2017).
Given this information, we hypothesized that motor neuron apoptosis can cause the dendritic tree to shrink, thereby creating a need for reactive microglia to scavenge debris. After neuronal apoptosis, microglia aggregate around neuronal debris. Thus, immune surveillance appears to play an important role in the injured nervous system during neuronal cell death.
Ectopic DRG activity in the dorsal horn of the spinal cord
The DRG, also called the sensory ganglia, is a gathering place for the syncytium of most primary sensory neurons. Most DRG neurons are pseudounipolar, and are located in the intervertebral foramen between the vertebrae. Every cell body has two axons: one peripheral axon and one central axon that transmits electrical signals from the peripheral to the dorsal horn of the spinal cord (Esposito et al., 2019). Morphologically, DRG neurons can be divided into three types: large, medium, and small, according to diameter size. Large DRG neurons are associated with proprioceptive sensation, and play an important role in transmitting non-nociceptive sensation and inhibiting nociceptive sensation. Medium neurons relay tactile information, while small neurons are implicated in sensations of pain and touch, and often form unmyelinated C-fiber sensory nerves. Therefore, the DRG is closely associated with sensory abnormalities and pain (Esposito et al., 2019). Excessive neuronal firing is believed to be a critical factor in neuropathic pain (Moutal et al., 2019), and the inflammatory response induced by PNI commonly causes DRG neurons to fire excessively. A large number of inflammatory cells, including leucocytes, glial cells, T lymphocytes, SCs, and histocompatibility complex class II macrophages have been detected in the DRG following PNI. Even if the original peripheral nerve is restored, these inflammatory cells continue to deliver excitatory cytokines, and thus contribute to extended pain.
The relationship between microglia and pain has received widespread attention. Animal studies have shown a number of spinal dorsal horn microglia and morphological changes following PNI, and these are considered to underlie the pathogenesis of neuropathic pain (Inoue and Tsuda, 2018). PNI rapidly activates NK-κB in the DRG, and colony stimulating factor 1 (CSF1) is transported anterogradely along axons to the spinal dorsal horn. A DAP12-dependent pathway via CSF1R contributes to the local expansion of resident microglia via proliferation in the spinal dorsal horn. These microglia convert from having normal morphology to over-reactive morphology in response to inflammatory factors. Cell morphological analyses have revealed that PNI induces microglia to change their phenotype to a reactive hypertrophic shape, for instance, that with a reduced process length and complexity, and an increased volume (Batti et al., 2016; Gu et al., 2016). Several studies have reported that activated microglia produce and release a variety of bioactive diffusible factors in response to extracellular ligands via their cognate receptors, and that these factors can influence spinal dorsal horn neuronal function. Furthermore, reciprocal microglia appear to play a causal role in spinal dorsal horn neuronal signaling related to neuropathic pain (Tsuda, 2016; Inoue and Tsuda, 2018). Such signaling can lead to the secretion of brain-derived neurotrophic factor (BDNF) by activated microglial cells, which can enhance excitatory synaptic transmission to excitatory neurons.
A study conducted using a neuropathic pain model indicated that greater neuropathological damage may be correlated with changes in nerve growth factor (NGF), neurotrophin 3, and insulin-like growth factor, while vascular endothelial growth factor may attenuate pain behavior and prevent neuronal stress by influencing transient receptor potential ankyrin 1 activity (Hulse et al., 2015). These changes can increase the excitability of the DRG (Hilz et al., 2000; Simmons and Feldman, 2002; Generaal et al., 2016). The T-junction, which is located between the axon and cell soma, is also regarded as a key region in aberrant neuronal activity. The T-junction acts as a low-pass filter that limits the rate at which peripheral signals can be transported centrally. Previous studies have shown that the T-junction in DRG neurons permits an increased amount of high-frequency burst firing after PNI. This alteration in the filtering action of the T-junction may also cause hyperexcitability following PNI (Liem et al., 2016).
The injury site
After PNI, axons sprout from the broken ends of proximal axons. The speed of axon regeneration is affected by axonal transportation and neurotrophic growth factors, which are secreted by SCs in the reconstructed basement membrane tube.
Staggered axonal regeneration
Wallerian degeneration is initiated immediately after PNI in the distal nerve stumps, suggesting that these axons denature and disintegrate. Thus, one aim of therapeutic treatments is to create a microenvironment that is conducive to axonal regrowth and reinnervation (Conforti et al., 2014). During neuronal degeneration, macrophages play a main role in clearing the resulting myelin debris. Studies have suggested that non-neuronal cells and axotomized neuronal cell bodies express chemokines that are targeted at macrophages. In the early stage of injury, these signals appear to mainly recruit M1 macrophages, which secrete pro-inflammatory cytokines. This can thereby enhance inflammatory reactions and tissue necrosis (Zigmond and Echevarria, 2019).
SCs are activated and involved in the entire process of injury and regeneration. After Wallerian degeneration, the proximal section begins axonal regeneration by forming a growth cone. The proliferating SCs form Bungner bands, which guide the growth of newly sprouting axons. If a growth cone reaches the endoneurial tube, it has a better chance of reaching the target organ (Gordon, 2020). NGF is upregulated in SCs during injury, which promotes the growth and proliferation of SCs and provides trophism to the outgrowing axon. In theory, the speed of axonal regeneration is 1–3 mm/d, but this speed is much slower through damaged regions. In reality, it takes 8–10 weeks for axons to regenerate by 25 mm, which is much longer than the theoretical time (Al-Majed et al., 2000b; Gordon, 2016).
Unfortunately, exhausted nerve growth factors may limit the degree to which SCs can sustain regenerated axons. Liu et al. explored the factors associated with long nerve recovery durations, and found that SC atrophy and changes in the endoneurial microenvironment were responsible for delayed nerve recovery (Reinhold and Rittner, 2017; Liu et al., 2018). Ronchi et al. (2017) found that prolonged denervation led to the atrophy of SCs, which downregulated the expression of factors that sustain nerve regeneration (such as glial fibrillary acidic protein, a SC-specific cytoskeleton constituent expressed by nonmyelinating SCs) and upregulated the expression of molecules that inhibit axon regeneration [such as low-affinity nerve growth factor receptor (p75), a member of the tumor necrosis factor receptor family]. These changes had an overall effect on nerve regeneration. Furthermore, endothelial cells form a physical barrier called the blood-nerve barrier. The inflammatory reactions induced by PNI can break this barrier and disrupt the stable internal microenvironment, which can also contribute to slow axonal regeneration (Yi et al., 2017).
Growth factors and the inner tube of nerve fibers
Progressive decreases in the levels of growth factors are another reason for postponed neuronal regeneration after PNI. The secretion and expression of growth factors by SCs peaks 15 days after nerve injury, and returns to normal levels 35 days later (McGregor and English, 2018). In cases of prolonged nerve injury, the ability of SCs to express growth factors in the distal region of injured axons gradually decreases. As it is difficult to maintain the proliferation of supporting axons for a long duration, the rate of axonal growth may gradually decrease.
The state of the nerve fiber tube is an important factor affecting the recovery of peripheral nerve function. As macrophages engulf and clear away the fragments of the degenerated axon and myelin sheath, it may be a long time before new axon buds enter the inner tube of nerve fibers (Cattin and Lloyd, 2016). This long absence of tube components can decrease the tube pressure, which can lead to collapse or a progressive decrease in the caliber of endoneurium tubes. In addition, the absence of tube components may enable the number of collagen fibers in the endoneurium to slowly increase, which can lead to thickening of the endoneurium tube and decreased ease of growth for new axial buds (Krarup et al., 2017).
In summary, after axonal injury, axons near the injury site begin to produce new axon buds. However, staggered axonal growth may lead to a progressive prolongation of the amount of time required for the axons to pass through the injury site. This increase in time can weaken the proliferation-promoting ability of SCs at the far end of the injury. Cumulative narrowing of the inner neural membrane tube and nonspecific reinnervation can also exacerbate the passage of injured axons.
Target organs
Atrophy of skeletal muscles
PNI often leads to impaired sensory motor function. Skeletal muscles, which control human movement, are innervated by the nervous system. All voluntary movements in daily life are made possible by muscle contractions. Compared with the atrophy of skeletal muscles, that of denervated muscle has received more attention from researchers. Once a nerve is transected, target muscles lose their ability to “pump” muscles due to the loss of nerve innervation. This leads to relatively reduced perfusion of the target muscle, which can result in skeletal muscle atrophy. A previous study indicated that PNI causes the cross-sectional width of skeletal muscles to decrease by 70% within 2 months (Willand et al., 2015).
During skeletal muscle atrophy, a series of biochemical and physiological alterations occur in atrophic muscle, which trigger changes in gene expression. Shen et al. (2019) identified thousands of genes that were differentially expressed in the anterior tibial muscle at different times after sciatic nerve transection via cDNA microarray. They divided the period encompassing the 28 days after nerve injury into four transcriptional phases, and examined the activation of different functional genes and signaling pathways within each phase. PNI has been found to induce four transcriptional phases: the “oxidative stress stage” (0–12 hours), “inflammation stage” (12 hours–3 days), “atrophy stage” (3–14 days), and “atrophic fibrosis stage” (14–28 days) (Mancinelli et al., 2019). The oxidative stress stage is characterized by an increase in cytochrome P450 enzymes, which are responsible for the production of reactive oxygen species (ROS). This can be interpreted to mean that a large number of ROS are produced due to oxidative stress in transcription phase 1 (He et al., 2017). Hypoxia-inducible factor 1 (HIF-1) signaling pathways are activated to eliminate ROS, and thus avoid cellular damage (Eyrich et al., 2019). Then, in the inflammation stage, inflammation-related genes, such as tumor necrosis factor (TNF) and transforming growth factor-beta, are triggered by ROS.
Inflammatory proteins are activated by upstream signals and increases in the expression of cachexia-related genes. ROS and inflammatory proteins persistently damage skeletal muscles, causing the activation of proteasome signaling pathways. The proteasome signaling pathways promote protein degradation, leading to muscle atrophy. Inactivation of insulin can also activate the ubiquitin proteolytic system, causing skeletal muscle hypertrophy (O’Neill et al., 2016). Furthermore, the metabolic shift from glycolytic to oxidative processes can lead fast fibers to gradually transition to slow fibers. For example, a study by Ma et al. (2019) confirmed that the ratio of MyHC II-positive fibers in the soleus rose significantly after denervation, suggesting that denervation leads to the slow transformation of skeletal muscle fibers into fast skeletal muscle fibers in the soleus muscle. Hence, oxidative stress and inflammatory reactions are both associated with denervated skeletal muscle atrophy. Accordingly, antioxidant and anti-inflammatory therapy may be an important strategy for preventing the initial atrophy of denervated skeletal muscle.
Sensory perception degeneration and nociception overactivity
Initially, changes in skeletal muscle strength are a primary concern for patients. However, their attention may shift to positive sensory symptoms as motor function is gradually regained. Sensory receptors can be divided into mechanoreceptors, thermoreceptors, and nociceptors. A mechanoreceptor is a sensory neuron that responds to mechanical pressure or distortion. PNI results in the loss of mechanical pressure because of the absence of mechanoreceptors. In clinical settings, neuropathic pain is measured in terms of cold intolerance. Aδ and C fibers transmit the sensation of temperature, and have free nerve endings that terminate in the skin. A main difference between the two fibers is that Aδ fibers are myelinated while the C fibers are not myelinated. Aδ fibers mainly convey sensitivity to cold and pain (rapid pain, “pinprick” sensations), and carry this information from the peripheral to the dorsal horn of the spinal cord. C fibers receive and transmit information primarily related to heat and pain (slow pain, “burning” sensations) (Sène, 2018). Both fibers contain neurotransmitter receptors in the skin that are known as transient receptor potential (TRP) channels. TRP channels are important mediators of sensory signals, and have a strong impact on cellular function and signaling pathways. As “cellular sensors”, they respond to changes in temperature, pH, stretch/pressure, and chemicals in the cellular environment (Sakaguchi and Mori, 2020). Kambiz et al. (2014) found that thermosensitive TRP channels in the skin contribute to thermal intolerance via three mechanisms. Specifically, thermal intolerance can result from 1) an increase in the expression of TRP channels on nerve fibers and keratinocytes, 2) a reduction in the threshold of TRP channels, leading the receptors to be activated by lower intensity stimuli, or 3) sprouting from non-injured nerve fibers. For example, Duraku et al. (2012) observed reinnervation that occurred in transected rat tibial and peroneal nerves due to sprouting of uninjured saphenous and sural nerves. Upon PNI, cell damage-related mediators increased nerve fiber excitation by activating TRP channels directly. This manifested as mechanical and cold hypersensitivity. This viewpoint was further confirmed by Mickle et al. (2016), who blocked TRP channels individually and found that peripheral fiber excitation was reduced.
The nociceptive system modulates peripheral pain signal transduction. Peripheral nociceptive neurons are initially excited when free nerve endings (Aδ and C fibers) receive noxious stimuli or undergo injury. Over time, primary afferent Aδ- and C-nociceptors in the injured nerve area start to respond to noxious stimulation in an amplified way, leading to the development of hyperexcitability and spontaneous activity. This can be explained by the dramatically reduced firing thresholds of the Aδ fibers. Previous studies have explored the underlying mechanisms by which primary nociceptors are remodeled by the inflammatory effects of chemicals that infiltrate the injured site. Generally speaking, primary nociceptors are more likely to fire following an injury. Inflammatory factors can cause changes in the genetic and molecular composition of nociceptors, leading to an increase in primary nociceptor excitability (Bjorgen et al., 2018).
The peripheral nerve contains sensory and motor fibers. Previous studies have suggested that injured sensory fibers are responsible for neuropathic pain. However, recent evidence has indicated that motor fiber injury is essential to neuropathic pain. The expression of voltage-gated sodium channels is altered in DRGs after PNI. This change forms the basis of ectopic discharges, and eventually leads to neuropathic pain. Chen et al. (2011) reported that the selective injury of motor fibers can lead to the upregulation of voltage-gated sodium channels in DRGs, and that this process might be mediated by the over-production of TNF-α in bilateral DRGs. In their follow-up study, they showed that the overexpression of TNF-α induced hyperalgesia via calpain-2, which activated satellite glia to produce extra NGF. This, in turn, enhanced nociceptor excitability, resulting in apparent mirror-image pain. In addition, Liu et al. (2016) reported that injury to motor fibers may induce long-term potentiation at spinal C-fiber synapses, indicating that noxious inputs from muscle afferents induce long-lasting central sensitization. Hence, in contrast to sensations at the skin level, changes in muscle innervation should be investigated to fully understand neuropathic pain.
Summary of the basic mechanisms of PNI
The above-mentioned data indicate that regenerated axons cannot effectively reinnervate target-end organs. Therefore, a large number of patients with PNI fail to completely recover normal function. The reasons are listed as follows:
Sensory changes affect the peripheral nerves, spinal cord organization, and plasticity of the brain, and all three can affect early regeneration and later neuropathic pain. The slow speed of regeneration delays the rate at which the proximal end crosses the surgical gap. Furthermore, nonspecific reinnervation reduces the chance of regeneration. Generally, SCs support axonal regeneration after injury in the peripheral nervous system. However, SCs gradually lose regeneration-supporting features and eventually die. The denervation of skeletal muscle cells and sensory abnormalities can lead to early loss of function. Furthermore, decreases in the activation threshold of thermoreceptors and nociceptors can gradually lead to neuropathic pain. The slow rate of nerve regeneration may account for negative sensory symptoms. However, the negative sensory symptoms that can recover the remaining positive sensory symptoms associated with PNI are difficult to treat. Nociceptor excitability can increase as the result of decreased firing thresholds. Furthermore, common noxious stimuli are still likely to cause hyperexcitability in nociceptors. Hence, treatments are needed for the positive symptoms of PNI.
The Mechanisms of Electrical Stimulation for the Treatment of Peripheral Nerve Injury
As stated above, PNI can trigger a series of pathophysiological changes at the level of the cell body, at the site of injury, and in the target organ, thus reflecting the overall structure of the nervous system. ES has received much attention from PNI researchers as an effective treatment for nerve regeneration. Several studies have examined the effects of ES on the cell body and target organ in models of PNI, including the stimulation of sites aside from the sites of injury, and most have reported a good outcome. In the sections that follow, we discuss the mechanisms of ES for the treatment of PNI in terms of changes in the cell body, local sites, and end organs (Table 2).
Table 2.
Summary of the studies regarding neuron electrostimulation in PNI
| The first to study this field | The greatest contributions to this field | The stimulation site | The main mechanism of stimulation |
|---|---|---|---|
| Kosman et al., 1948 | Salmons, 2009 | Skeletal muscle electrical stimulation | Promote skeletal muscle regeneration and prevent skeletal muscle atrophy |
| Taub et al., 1974 | Linderoth and Foreman, 1999 | Spinal cord stimulation | Inhibit apoptosis and synaptic stripping |
| Burton, 1976 | Johnson and Tabasam, 2003 | Transcutaneous electrical nerve stimulation | Mediate decreased local inflammatory mediators and elevated pain thresholds |
| Kadekaro et al., 1985 | Schmidt, 2019 | Dorsal root ganglion stimulation | Suppress the excitability of the dorsal root ganglion |
| Gybels and Vancalenbergh, 1990 | Gordon, 2016 | Peripheral nerve stimulation | Promote axon regeneration and the exactness of axon growth; activated Schwann cells secrete glutamate and exosomes to enhance the ability of regeneration and inhibit apoptosis |
| Leem et al., 1995 | Wang et al., 2019 | Subcutaneous electrical stimulation | Reduce inflammatory response and neuronal apoptosis and activate Aβ and Aδ fibers to relieve pain |
Cell body arrangement in the dorsal horn of the spinal cord
Most previous studies have prioritized ES of the site of injury for treating PNI. However, we anticipate that proximal neuronal cell bodies will be important PNI therapeutic targets in the future. Previous studies have confirmed that activity of the wide-dynamic range neurons of the dorsal horn is decreased by spinal cord stimulation (SCS). However, even after SCS is switched off, the alleviating effects last for a long time (Jensen and Brownstone, 2019). A clinical trial indicated that SCS can produce coordinated spinal motor output and facilitate the restoration of the sensorimotor network of the spinal cord (Formento et al., 2018). Apoptosis and synaptic stripping are the reasons for the functional loss following PNI. Accordingly, a previous study investigated whether SCS can promote functional recovery by inhibiting apoptosis and synaptic stripping (Pei et al., 2015). The researchers found that SCS could reduce the Ca2+ influx and stabilize the intracellular environment, which led to reduced motor neuron apoptosis in the ventral horn of the spinal cord (Pei et al., 2015).
La et al. (2019) showed that an electric field targeting the spinal cord also increased the expression of anti-apoptotic Bcl-2 and decreased the expression of the apoptosis-related Bax gene after sciatic nerve transection. Glial cells are also involved in the effects of ES. Some studies have indicated that, if directly applied to the spinal cord, ES will have a robust effect on gene expression (Sun et al., 2017; Stephens et al., 2018). For instance, levels of glial cell-related proteins glial fibrillary acidic protein and cFBJ osteosarcoma oncogene increased after ES in a PNI mouse model (Tilley et al., 2017; Shinoda et al., 2020). This suggests that glial cells may directly respond to ES and therefore contribute to motor recovery. As we discussed above, neuroglia can play an important role in PNI.
Accordingly, we speculate that ES can accelerate the speed of functional recovery and reduce neuropathic pain in cases of PNI. However, complications from SCS have been reported to occur in 30% to 40% of patients, such as electrode migration, infection, and wound breakdown (Sakaguchi and Mori, 2020). For uninsured patients, typical out-of-pocket costs for SCS are $15,000–$50,000 or more. Thus, the potential complications and cost of SCS limit its use in PNI. Many studies have explored easy-to-perform and safe techniques for stimulating the spinal cord. For example, electrical acupuncture and interference electrotherapy may be alternatives to SCS in promoting functional recovery after PNI.
Suppressing DRG excitability
SCS relieves pain by retraining primary sensory neurons. However, DRG stimulation has a similar effect. ES can directly suppress the excitability of the DRG, which is also a site of pain pathogenesis, and can bring about changes in DRG neuronal activity by altering activity in the T-junction. DRG stimulation blocks nociceptive signals from the periphery and enhances the filtering properties of the T-junction (Schmidt, 2019).
Koopmeiners et al. (2013) used neurophysiological techniques to measure the excitability parameters of uninjured cultured DRG cells after the application of ES. After ES, they found that fewer neurons could produce bursts of multiple action potentials, and that the conduction velocity was greatly reduced. This suggests that ES can lead to reduced neuronal excitability.
Computational modeling analyses have revealed stimulation-induced failure of action potentials traveling from the periphery in nociceptive neurons after PNI. Low-pass filtering of afferent pain signals occurs at the T-junction, and takes place via hyperpolarization of the soma and the mismatch in impedance between the peripheral stem and central axons. When the filter is attenuated, pain signals can pass through the T-junction at a faster frequency. Elevated T-junction filtering can be explained by the subsequent enhancement of Ca2+-dependent K conductivity. The activated K channels produce a sustained somatic hyperpolarization offset in the stem axon and T-junction. Hyperpolarization of the T-junction increases the degree of change in the transmembrane potential, which is essential to the propagation of action potentials. When the pain signals are generated from the periphery, T-junction filtering is not amplified due to the absence of Ca2+ and K channels.
One study compared over 500 DRG stimulator and 2000 spinal cord stimulator implants over a 1-year period. The researchers reported that DRG stimulation had a remarkable safety profile, with fewer adverse events compared with SCS. A pooled analysis further verified the effectiveness and safety of DRG stimulators (Huygen et al., 2020).
Local sites
Staggered axonal regeneration
Recent studies indicating that ES can enhance reinnervation after PNI have consistently focused on ES of a proximal nerve at the injury site. However, the exact cellular mechanisms by which ES accelerates nerve regeneration are still unclear. The intracellular Ca2+ wave, which is initiated at the site of axotomy induced by ES, plays a key role in nerve regeneration. The intracellular Ca2+ wave can travel along the axon to the neuronal body. In the neuronal soma, increased Ca2+ induces upregulation of BDNF and its receptor tropomyosin receptor kinase B (TrkB). The overexpression of BDNF can inhibit the phosphodiesterases that degrade cyclic adenosine monophosphate (cAMP), leading to sustained elevated levels of cAMP (Al-Majed et al., 2000a).
Raised cAMP levels can increase the expression of regeneration-associated genes such as Tα1 tubulin and growth-associated protein-43. Cytoskeletal assembly is enhanced through activation of the cAMP response element binding (CREB) protein, regulation of Tα1 tubulin, and the inhibition of Rho, which is a protein in the p75 NgR receptor (p75-NgR) pathway (McGregor and English, 2018). CREB activation is induced by the mitogen-activated protein kinase (MAPK) pathway. When a specific p38 MAPK inhibitor is implemented, CREB activation and neurite outgrowth are also suppressed. Hence, it is believed that ES-induced activation of the p38 MAPK pathway may play an important role in promoting neurite outgrowth (Kawamura and Kano, 2019). To further confirm that the potential effect of ES is produced by the neuron cell body, Al-Majed et al. (2000b) inhibited propagation from the injury site to the axon, and found no therapeutic effects on PNI. However, in addition to BDNF and TrkB, reports have examined other possible pathways. In PC12 mutant cells with injured NGF-induced neurite outgrowth, ES can also enhance neurite outgrowth through p38 mitogen activation (Huang et al., 2010). As explained earlier, ES of the proximal nerve at the injury site can produce substantial therapeutic effects via the cell body (Figure 1).
Figure 1.
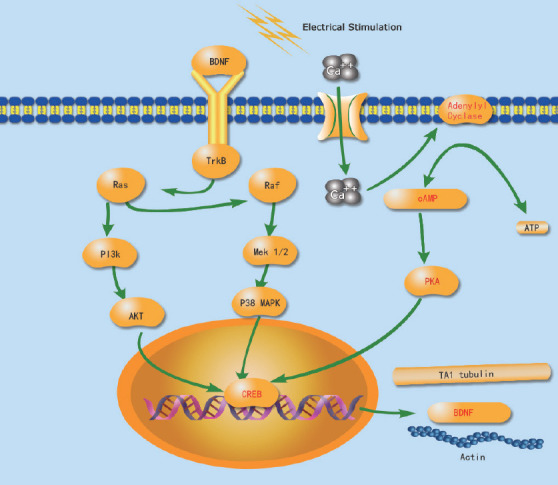
Electrical stimulation elevates peripheral nerve regeneration-associated genes within the neuronal cell body.
ES increased brain-derived neurotrophic factor (BDNF) and tropomyosin receptor kinase B (TrkB), which upregulates the expression of cyclic AMP (cAMP) through a Ca2+-dependent mechanism. The signal Ras-mitogen activated protein kinase (Ras/MAPK) pathway, which is essential for the neurophin-induced differentiation of neuronal cells, is activated by BDNF as well. Ras, which is stimulated by the trkB receptor, also causes activation of kinases phosphatidylinositol 3-kinase/Akt (PI3K/Akt). The different neurotrophin signaling pathways activated by cAMP response element-binding protein (CREB) converge at the level of transcription in the nucleus. CREB induces increased expression of regeneration-associated genes such as growth-associated protein-43 (GAP-43), which promotes axonal sprouting and prevents growth cone collapse, and Tα1 tubulin, which is an integral protein for cytoskeletal assembly. See the text in the section “Staggered axonal regeneration” for a detailed description.
In recent years, ES at the injury site has gradually attracted increased attention. The long and variable delays in the regeneration process can inhibit regenerating axons from successfully crossing the surgical gap and entering the distal nerve stumps. Axonal regeneration can be accelerated by 1 hour of ES (Gordon, 2000). Brushart et al. (2002) also found that 1 hour of 20-Hz stimulation could temporally compress staggered regeneration. Furthermore, they found that ES could synchronize distal sump reinnervation. The number of regenerated axons that cannot cross the transected nerve increases after ES. This is because ES facilitates the onset of motor axon regeneration without accelerating its speed. Moreover, continuous 20-Hz ES of the axons proximal to the repair site can remarkably accelerate referential reinnervation of the muscle nerve.
Correct reinnervation is regarded as a reason for the therapeutic effects of ES. Al-Majed et al. (2000) found that 1 hour of stimulation not only accelerated the speed of neuroregeneration, but also reduced staggered regeneration in a mouse model of PNI. Further, the stimulation lead to a similar distribution of neurons in PNI mice and control mice. This occurs by redirecting neurons back to the tissue they served originally, rather than by redistributing them (Brushart et al., 2005). Selective motor axon regeneration can be promoted by guidance factors expressed by the regenerating axons and distal targets. Franz et al. (2008) found that brief periods of ES could increase polysialic acid expression in regenerating axons. This could promote increases in collateral sprouting and in the size of arborization fields, which improve the exactness of axon growth.
SC activation
SCs are also believed to be involved in proximal ES therapy. ES can stimulate SCs to secrete glutamate, which can increase SC-derived exosomes and intracellular Ca2+ concentrations (Lopez-Leal and Court, 2016). Since cAMP and BDNF are Ca2+-dependent, increases in Ca2+-dependent protein may result from ES-induced glutamate secretion.
The role of SCs in vesicular transfer has recently received increased attention. Indeed, crucial proregenerative molecules delivered by SCs might contribute to axonal extension. As mRNAs are deposited on the distal axon in a dormant state, the transfer of mRNA from SCs to axons may supply transcripts for translation induced by electrical stimulation (Rigoni and Negro, 2020). SC-derived exosomes include mRNA, miRNA, and protein cargoes, which can promote damaged axonal regeneration, as verified via in vitro and in vivo studies. P75NTR is present in SC-derived exosomes, and is also found in abundance in the injury site. This protein can modulate growth from filopodia via the regulation of RhoA. SCs have previously been found to secrete exosomes that can be selectively internalized by axons in vitro and in vivo. Furthermore, SC-secreted exosomes, but not fibroblast-derived exosomes, markedly increase axonal regeneration by acting locally on axons and decreasing RhoA GTPase activation in growth cones (Sohn et al., 2020). Wang et al. (2020) demonstrated in vivo that the regenerative capabilities of injured sciatic nerves can be greatly enhanced by delivering SC-derived exosomes to axons. In addition, Zhou et al. (2018) verified that while cyclic mechanical strain affected the proliferation of DRG cells, SC-derived exosomes could enhance the proliferation of injured DRG cells and decrease the rate of positive staining for SA-β-gal, which is a cytochemical biomarker for senescent cells. Hence, SC-derived exosomes inhibited apoptosis and the senescence of DGR cells in vitro, providing support for a novel therapeutic strategy.
A recent study reported that ES could shift the macrophage phenotype from a proinflammatory to a pro-repair phenotype (McLean and Verge, 2016). In this way, ES could rapidly remove myelin debris. As SCs restore the nonreactive myelinating state, they maybe be useful in remyelinating nerves. Remyelination has a protective effect on nerves, and could enhance functional recovery after PNI. ES briefly decreases inflammatory reactions and increases neuronal activity, favorably altering the immune microenvironment in demyelinated nerves. Thus, ES may have beneficial therapeutic effects when applied to other pathologies.
The major drawbacks of ES include the need for surgery and the risk of possible complications at the electrode implantation site, such as infection and wire movement. Most ES of the injury site for the treatment of PNI is based on animal experiments. However, there is a significant difference in nerve length and width between humans and animals. Power et al. (2020) applied ES in a clinical setting by placing two electrodes proximal to the site of the injured nerve. They observed that ES enhanced muscle reinnervation and promoted functional recovery after surgery for cubital tunnel syndrome. Wong et al. (2015) also affirmed that ES of a proximal nerve at the injury site promoted the recovery of sensory function in a randomized controlled trial. Although these clinical studies have demonstrated the efficacy of this method, the optimal electrode arrangement and treatment parameters are still unclear. In addition, according to the molecular mechanisms, the major therapeutic effects of ES are mediated by the cell body. Thus, SCS and DRG, which have direct effects on the cell body, may be more effective than stimulation at the injury site. However, spinal cord-related simulation does not facilitate the promotion of local SCs or correct regeneration across the gap. Hence, combined stimulation of the spinal cord and injury site may be the best treatment option. Given the complicated causes of PNI, it is difficult to determine whether implanted electrodes are ideal in terms of costs and benefits. Currently, some physicians have implanted electrodes under ultrasound guidance, which reduces the risk and cost of the procedure. However, further investigations are needed to identify the optimal treatment approach.
The end organs
The peripheral nerve is responsible for providing sensory and motor innervation to the skin, muscles, and subcutaneous tissue. ES can be divided into skeletal muscle stimulation, transcutaneous stimulation, and subcutaneous stimulation, according to the targeted layer. ES based on electrode position can be divided into three broad categories: transcutaneous electrical nerve stimulation, skeletal muscle stimulation, and subcutaneous electrical stimulation. In transcutaneous electrical nerve stimulation, pads are attached directly to the skin. The method of placing electrodes within the subcutaneous space is called subcutaneous electrical stimulation. Electroacupuncture (EA) is the most common form of subcutaneous electrical stimulation. When an electrode is implanted into skeletal muscle, electricity is directly delivered to the nerves.
Skeletal muscle electrical stimulation
Many studies have shown that skeletal muscle ES is beneficial to motor function (Willand et al., 2016; Fu et al., 2020). Muscle stem cells, such as satellite cells, are responsible for the growth and repair of skeletal muscle. They can be activated by myogenic precursor cells (MPCs), and thereby promote skeletal muscle regeneration. To further investigate the underlying mechanisms of this process, a study examined whether skeletal muscle stimulation (SMS) could affect MPCs in healthy older adult subjects. The in vitro study revealed that SMS could increase the fusion of adult stem cells with the existing myofibers by increasing cytoplasmic free Ca2+ concentration and the gene expression of MYOD, as well as that of MYOG on MPCs. As the regenerative capacity of skeletal muscle was affected by myogenic precursor cell proliferation, the oxidative status of these MPCs was also evaluated. The researchers found that SMS greatly reduced O2– production, and tended to reduce super oxide dismutase activity. Thus, SMS promoted skeletal muscle regeneration through the reduction of oxidative status in the satellite cells of healthy older adult subjects (Di Filippo et al., 2017).
Another study found that ES led to heightened myotube hypertrophy and elevated mTORC1 and ERK1/2 activity in a human skeletal muscle model (Khodabukus et al., 2019). Increased glucose consumption and reduced acetyl carnitine in myobundles observed after ES indicate that SMS can enhance changes in glycolytic and fatty acid metabolic processes. This is because ES increases the transport of glucose transporter type 4 (GLUT-4) to the plasma membrane.
Muscle contractions are known to activate AMP-activated protein kinase (AMPK), which increases glucose uptake (Nedachi et al., 2008). Thus, ES may trigger the phosphorylation of AMPK, increasing GLUT-4 protein levels. However, when the AMPK kinase cascade is inhibited, the effects of SMS are not altered. One study found that ES releases ATP (Christensen et al., 2015), and ATP was observed to increase GLUT-4 transmission by activating P2γ purinergic receptors and AKT phosphorylation (Osorio-Fuentealba et al., 2013).
Although ES increases the consumption of ATP, like exercise, it does not increase oxidative stress in muscle. However, the relationship between inflammation and ES is still unclear. Lambernd et al. (2012) found that ES decreased the protein levels of IKKβ/NF-κB, implying an anti-inflammatory effect. However, Scheler et al. (2013) reported increased activation of NF-κB after ES. Time may be the major influential factor. Whitham et al. (2012) confirmed that ES in an in vitro model activated the IKKβ/NF-kB signaling pathway within 4 hours. However, the activated signaling pathways returned to normal after 4 hours of ES. In addition, measurements of oxidative stress status and inflammation state have not been adapted for ES, especially in the short term. Mancinelli et al. (2019) demonstrated that oxidative stress and inflammation began immediately after PNI and ended after 3 days.
When denervated skeletal muscle reaches the atrophy and atrophic fibrosis stage, ES increases insulin activation by inhibiting the ubiquitin proteolytic and lysosomal hydrolysis systems. Therefore, the application of ES may be optimal 3 days after PNI.
Subcutaneous electrical stimulation
Recent studies have confirmed that EA is effective for the treatment of PNI. Animal experiments have indicated that EA can improve facial muscle function by reducing inflammatory responses and neuronal apoptosis. In rabbits, increases in glial cell line-derived neurotrophic factor and N-cadherin expression in facial motoneurons are considered major cellular mechanisms of EA (Fei et al., 2019). The type 2 cannabinoid receptor-mediated activation of microglia induced by EA may also be involved in the repair of PNI (Wang et al., 2019).
When a metal EA needle is inserted into the body, it usually penetrates skeletal muscle, passing the skin and subcutaneous tissue. Thus, EA combines the effects of transcutaneous electrical nerve stimulation (TENS), SMS, and subcutaneous electrical stimulation (SQS). While EA is proficient in terms of affecting tissue via depth, this is not the case for width. The selection of EA electrode targets usually depends on traditional Chinese medicine, and does not consider the properties of electrical transmission. Accordingly, EA does not usually have a large influence on the local area. Further work is needed to facilitate the selection of optimal parameters to form matrix arrangements that lead to enhanced treatment.
Transcutaneous electrical nerve stimulation
PNI-induced sensory function injury ranges from the early loss of cutaneous sensation to late hyperpathia. However, ES can dramatically increase tactile discrimination and pressure detection scale scores (Wong et al., 2015), and lead to cutaneous regeneration of DRG neurons (Koetsier et al., 2020). EA is not only a promising form of complementary medicine for PNI, but also for neuropathic pain. It can be used to activate Aβ and Aδ fibers, thus relieving pain by preventing nociceptive signals from entering the central nervous system and releasing analgesic opioid peptides (Huo et al., 2020).
The nociceptive system controls pain modulation in the peripheral nervous system. When a noxious stimulus is encountered or tissue injury occurs, primary nociceptive neurons in the periphery with free nerve endings (Aδ and C fibers) respond. Nociceptive signals then travel into the spinal cord, where they synapse with second-order neurons in the gray matter of the dorsal horn. TENS only inhibits the transmission of the second-order neurons, because it only excites Aβ fibers, namely non-nociceptive afferent nerve fibers (Chakravarthy et al., 2016).
The analgesic effect of TENS is produced by mediation of the suppression of the descending system. Nociception from the descending nerve is inhibited during heavy noxious stimulation when endogenous opioids are released and diffuse in the rostral ventral medulla. These analgesic effects have been termed “diffuse noxious inhibitory control phenomena” (Ploner et al., 2017; Wutz et al., 2018). TENS may directly reduce the regional number of biochemical mediators inducing pain reactions. Results from a study with human volunteers confirmed that TENS curbs pain neurotransmission by mediating decreased local inflammatory mediators and elevating pain thresholds (Chakravarthy et al., 2016). The electrical field induced by TENS might be able to reach more superficial Aδ fibers and release a greater amount of opioids than that induced by SQS because the electrodes are on the surface of the skin. It is also advantageous in treating superficial nerve injury because it is noninvasive.
In contrast, SQS might activate a large number of Aβ fibers because the electrodes are positioned in the subcutaneous space. SQS electrodes are inserted percutaneously into the muscle to avoid impedance resistance from fat. Hence, SQS appears to be better than TENS for treating PNI, especially for damage of deep nerves in the underlying skeletal muscle. One study indicated that SQS and TENS might recruit different numbers of fibers (Vera-Portocarrero et al., 2013). Hence, SQS and TENS may be individually selected or combined according to the activated fibers and injured nerves.
Limitations
There are several limitations to this review. First, we did not consider PNI-induced changes in activity in the brain, which is the highest level of the nervous system. Moreover, although there are multiple causes of PNI and different degrees of nerve injury, we did not consider these variables. The parameters of electrical stimulation are an important consideration in the effects of electrical stimulation for PNI. Although most studies used a treatment strategy including 20 Hz of ES for 1 hour, identification of the optimal parameters will require further exploration. Finally, due to limited space, time, and energy, some relevant studies may have been excluded.
Future Prospects and Conclusions
Many researchers have examined the basic mechanisms of PNI, as well as potential treatment via electrical stimulation. However, previous studies have focused primarily on cellular and molecular changes in single sites after PNI, especially those in the injury site. Recently, an increasing number of studies have observed cellular and molecular changes at the level of the central nervous system, including at the site of injury and in the target organs following PNI. To the best of our knowledge, this is the first review of the existing literature to summarize and discuss changes after PNI and the mechanisms of ES from three different levels. As discussed, changes after PNI have been discovered at the level of the cell body (dorsal and ventral root), the site of injury, and in the target organs (Figure 2).
Figure 2.
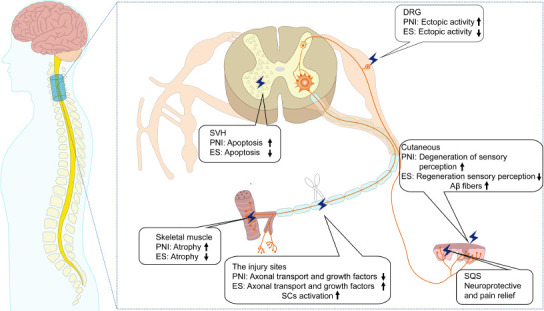
Following PNI, several molecular and cellular changes are observed at the local site of injury and the target organs.
The effects of ES therapy on peripheral neurogenesis vary according to the position of stimulation. See the text for a detailed description. DRG: Dorsal root ganglion; ES: electrical stimulation; PNI: peripheral nerve injury; SCs: Schwann cells; SDH: spinal cord dorsal horn; SQS: subcutaneous electrical stimulation; SVH: spinal cord ventral horn.
PNI mainly involves the skin, skeletal muscles, spinal cord, and brain. It implicates a nervous system injury axis that includes the peripheral nerves, corticospinal tract, and spinothalamic tract. The effects of ES therapy on peripheral neurogenesis vary according to the position of stimulation (Table 3).
Table 3.
Summary of trails about different electrical stimulation sites for PNI
| Study | Paradigm frequency/pulse width/pulse form/time/threshold | Nerve injury condition | Function/mechanism | Stimulation site | The type of study | Main viewpoints and guidance significance |
|---|---|---|---|---|---|---|
| Pei et al., 2015 | 15 Hz/NM*/NM*/30 min/6.5 mA | Sciatic nerve transection injury and its proximal and distal ends were inverted and sutured | Protects sensory neurons and anterior horn | Placed in the epidural space of spinal cord (T10 and L3) | Animal experiments | ES of the neuronal cell bodies can protect motor and sensory neurons in spinal cord after PNI. In addition, it promotes the regeneration of myelinated nerve fibers to repair injured peripheral nerve. |
| Tilley et al., 2016 | 50 Hz/NM*/charge-balanced square pulse/72 h/70% motor threshold, 0.3–10 mA. | Tibial and common peroneal nerves distal injury | cFOS and 5HT3ra and GABAbr1/ attenuate the neuroinflammatory response to relieve pain | Epidural space of spinal cord (L1–L5) | Animal experiments | SCS could relieve pain after PNI by regulation of relevant ion channels and gene expression. |
| Willand et al., 2016 | 100 Hz/ 200–400 μs/charge balanced/1 h/visible muscle contraction, 2–3 mA | Tibial nerve transection injury; immediate repair | Elevated muscle-derived GDNF | Implanted in the gastrocnemius muscle | Animal experiments | The levels of nutrient factor mRNA were increased and peripheral nerve regeneration was promoted by targeting ES of skeletal muscle. |
| Jiang et al., 2018 | 20 Hz/0.1 ms/NM*/1 h/0.3 mA, subthreshold | Ensory and motor branches of the pudendal nerve bilaterally; crush injury | Neuro regeneration through upregulating BDNF and βII-tubulin | Pudendal nerve | Animal experiments | ES of pudendal nerve can promote neuroregenerative response by upregulating BDNF to promote neuromuscular continence mechanism recovery. |
| Mendez et al., 2018 | 20 Hz/100 μs/NM*/1 h/1.5 mA, right ear flutter | Facial nerve crush injury | Improved facial nerve specific pathway regeneration | Facial nerve | Animal experiments | This is the first study to apply an implantable device to BES for facial nerve injury, accelerating functional recovery and induction of motor neuronal path-specific regeneration. |
| Nicolas et al., 2018 | 60 Hz/250 μs/NM*/15 min/3 V | Sciatic nerve transection injury; implementation of microsurgical epineural sutures | Nerve regeneration and muscle reinnervation | Epidural space of motor cortex in the brain | Animal experiments | ES of the motor cortex induces a higher rate of nerve re-innervation and a faster functional recovery than electrical stimulation of peripheral nerves after PNI. |
| Senger et al., 2018 | 20 Hz/0.1 ms/balanced biphasic pulses/1 h | Common peroneal nerve transection injury; sutured with a two-layer closure | RAG/increased the length of nerve regeneration and regenerating axons | Common peroneal nerve | Animal experiments | It is the first time to apply conditioning ES to an in vivo model of peripheral nerve regeneration. Findings from this study demonstrate that this treatment strategy accelerates nerve regeneration. |
| Senger et al., 2019 | 20 Hz/0.1 ms/balanced biphasic pulses/1 h/visible twitch in the lower limb flexors | Tibial nerve transection injury; immediate microsurgical repair surgery | Neuro regeneration and functional recovery | Tibial nerve | Animal experiments | Conditioning ES can promote the regeneration of target nerves and the recovery of functional activity, which can be given preoperatively. |
| Fu et al., 2020 | 100 Hz/200 μs/on: off = 1:2/30 min/visible toe and foot movement | Sciatic nerve transection injury; implementation of repair surgery | Axon regeneration through promoting autophagy flux in the distal nerve segments | Skin | Animal experiments | ES of denervated muscle can increase cell autophagy flux level in the nerve distal to injury, which is conducive to nerve regeneration. |
5HT3ra: 5-Hydroxytryptamine (serotonin) receptor 3a; BDNF: brain-derived neurotrophic factor; BES: brief electrical stimulation; cFOS: c FBJ osteosarcoma oncogene; ES: electrical stimulation; GABAbr1: γ-aminobutyric acid B receptor 1; GDNF: glial cell line-derived neurotrophic factor; NM*: not mentioned in the text; PNI: peripheral nerve injury; RAG: regeneration-associated gene; SCS: spinal cord stimulation.
SCS can block microglial activation and synaptic stripping-related excitability of the DRG, and subsequently dampen the pro-inflammatory cytokine response. This can delay the synaptic rearrangement of the dorsal horn. However, SCS treatment is expensive and challenging to implement, and many patients may find it difficult to cover the costs of medical care. ES of local sites is commonly applied to promote nerve regeneration after PNI, but the effects are mainly mediated by the cell body. TENS, which has a direct effect on the cell body, may be more effective in theory. However, its effects are less than those of SCS, especially for neuropathic pain. Currently, implanted electrodes are the main method of local ES, and this is associated with considerable risks for patients. End organ ES is usually conducted with exterior and needle electrodes. It is used to stimulate skeletal muscle, subcutaneous tissue, or skin to relieve neuropathic pain and skeletal muscle denervation. However, single-point electrical stimulation cannot restore PNI-induced changes in the peripheral nervous and central nervous system. Hence, a combination of ES and SCS may be better than ES alone in treating PNI. This consideration warrants further research.
Although ES therapies are useful for treating PNI, they also have side effects. ES can lead to twisting of the axon and cytoarchitecture, leading to edema (Martellucci, 2015). In addition, extended periods of ES can lead to reduced skeletal muscle excitability and abnormalities in the neuromuscular junction. Furthermore, stimulation of innervated skeletal muscles can have adverse effects in surviving asynchronous nerves. If the stimulated nerves connect with the muscle in an asynchronous way, ES can compromise functional reinnervation (Hussain et al., 2018, 2020). Thus, more research is needed to address the side effects of ES and identify the optimal stimulation regimen.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 81801787 (to XZS); China Postdoctoral Science Foundation, No. 2018M640238 (to XZS); the Natural Science Foundation of Tianjin, No. 20JCQNJC01690 (XLC).
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Editor note: XSG is an Editorial Board member of Neural Regeneration Research. He was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal’s standard procedures, with peer review handled independently of this Editorial Board member and their research groups.
C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Koke S, Song LP; T-Editor: Jia Y
References
- 1.Adams JP, Fee N, Kenmore PI. Tear-gas injuries. A clinical study of hand injuries and an experimental study of its effects on peripheral nerves and skeletal muscles in rabbits. J Bone Joint Surg Am. 1966;48:436–442. [PubMed] [Google Scholar]
- 2.Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000a;12:4381–4390. [PubMed] [Google Scholar]
- 3.Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000b;20:2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber B, Seikaly H, Ming Chan K, Beaudry R, Rychlik S, Olson J, Curran M, Dziegielewski P, Biron V, Harris J, McNeely M, O'Connell D. Intraoperative Brief Electrical Stimulation of the Spinal Accessory Nerve (BEST SPIN) for prevention of shoulder dysfunction after oncologic neck dissection:a double-blinded, randomized controlled trial. J Otolaryngol Head Neck Surg. 2018;47:7. doi: 10.1186/s40463-017-0244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batti L, Sundukova M, Murana E, Pimpinella S, De Castro Reis F, Pagani F, Wang H, Pellegrino E, Perlas E, Di Angelantonio S, Ragozzino D, Heppenstall PA. TMEM16F regulates spinal microglial function in neuropathic pain states. Cell Rep. 2016;15:2608–2615. doi: 10.1016/j.celrep.2016.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjorgen H, Koppang EO, Gunnes G, Hordvik I, Moldal T, Kaldhusdal M, Dale OB. Ectopic epithelial cell clusters in salmonid intestine are associated with inflammation. J Fish Dis. 2018;41:1031–1040. doi: 10.1111/jfd.12780. [DOI] [PubMed] [Google Scholar]
- 7.Bray GM, Aguayo AJ. Regeneration of peripheral unmyelinated nerves. Fate of the axonal sprouts which develop after injury. J Anat. 1974;117:517–529. [PMC free article] [PubMed] [Google Scholar]
- 8.Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002;22:6631–6638. doi: 10.1523/JNEUROSCI.22-15-06631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton C. Transcutaneous electrical nerve stimulation to relieve pain. Postgrad Med. 1976;59:105–108. doi: 10.1080/00325481.1976.11714390. [DOI] [PubMed] [Google Scholar]
- 10.Cattin AL, Lloyd AC. The multicellular complexity of peripheral nerve regeneration. Curr Opin Neurobiol. 2016;39:38–46. doi: 10.1016/j.conb.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Chakravarthy K, Nava A, Christo PJ, Williams K. Review of recent advances in peripheral nerve stimulation (PNS) Curr Pain Headache Rep. 2016;20:60. doi: 10.1007/s11916-016-0590-8. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Pang RP, Shen KF, Zimmermann M, Xin WJ, Li YY, Liu XG. TNF-αenhances the currents of voltage gated sodium channels in uninjured dorsal root ganglion neurons following motor nerve injury. Exp Neurol. 2011;227:279–286. doi: 10.1016/j.expneurol.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Christensen CS, Christensen DP, Lundh M, Dahllof MS, Haase TN, Velasquez JM, Laye MJ, Mandrup-Poulsen T, Solomon TP. Skeletal muscle to pancreatic beta-cell cross-talk:the effect of humoral mediators liberated by muscle contraction and acute exercise on beta-cell apoptosis. J Clin Endocrinol Metab. 2015;100:E1289–E1298. doi: 10.1210/jc.2014-4506. [DOI] [PubMed] [Google Scholar]
- 14.Conforti L, Gilley J, Coleman MP. Wallerian degeneration:an emerging axon death pathway linking injury and disease. Nat Rev Neurosci. 2014;15:394–409. doi: 10.1038/nrn3680. [DOI] [PubMed] [Google Scholar]
- 15.Davis GA. Reflections on the history of nerve repair-sir sydney sunderland's final presentation to the neurosurgical society of australasia. Neurosurgery. 2020;87:E373–e382. doi: 10.1093/neuros/nyaa059. [DOI] [PubMed] [Google Scholar]
- 16.Di Filippo ES, Mancinelli R, Marrone M, Doria C, Verratti V, Toniolo L, Dantas JL, Fulle S, Pietrangelo T. Neuromuscular electrical stimulation improves skeletal muscle regeneration through satellite cell fusion with myofibers in healthy elderly subjects. J Appl Physiol (1985) 2017;123:501–512. doi: 10.1152/japplphysiol.00855.2016. [DOI] [PubMed] [Google Scholar]
- 17.Dong R, Liu Y, Yang Y, Wang H, Xu Y, Zhang Z. MSC-derived exosomes-based therapy for peripheral nerve injury:a novel therapeutic strategy. Biomed Res Int. 2019;2019:6458237. doi: 10.1155/2019/6458237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duraku LS, Hossaini M, Hoendervangers S, Falke LL, Kambiz S, Mudera VC, Holstege JC, Walbeehm ET, Ruigrok TJ. Spatiotemporal dynamics of re-innervation and hyperinnervation patterns by uninjured CGRP fibers in the rat foot sole epidermis after nerve injury. Mol Pain. 2012;8:61–73. doi: 10.1186/1744-8069-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehmedah A, Nedeljkovic P, Dacic S, Repac J, Draskovic Pavlovic B, Vucevic D, Pekovic S, Bozic Nedeljkovic B. Vitamin B complex treatment attenuates local inflammation after peripheral nerve injury. Molecules. 2019;24:4615. doi: 10.3390/molecules24244615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esposito MF, Malayil R, Hanes M, Deer T. Unique characteristics of the dorsal root ganglion as a target for neuromodulation. Pain Med. 2019;20:S23–S30. doi: 10.1093/pm/pnz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eyrich NW, Potts CR, Robinson MH, Maximov V, Kenney AM. Reactive oxygen species signaling promotes hypoxia-inducible factor 1αstabilization in sonic hedgehog-driven cerebellar progenitor cell proliferation. Mol Cell Biol. 2019;39:e00268–00218. doi: 10.1128/MCB.00268-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fei J, Gao L, Li HH, Yuan QL, Li LJ. Electroacupuncture promotes peripheral nerve regeneration after facial nerve crush injury and upregulates the expression of glial cell-derived neurotrophic factor. Neural Regen Res. 2019;14:673–682. doi: 10.4103/1673-5374.247471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Formento E, Minassian K, Wagner F, Mignardot JB, LeG off-Mignardot CG, Rowald A, Bloch J, Micera S, Capogrosso M, Courtine G. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat Neurosci. 2018;21:1728–1741. doi: 10.1038/s41593-018-0262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franz CK, Rutishauser U, Rafuse VF. Intrinsic neuronal properties control selective targeting of regenerating motoneurons. Brain. 2008;131:1492–1505. doi: 10.1093/brain/awn039. [DOI] [PubMed] [Google Scholar]
- 25.Fu T, Jiang L, Peng Y, Li Z, Liu S, Lu J, Zhang F, Zhang J. Electrical muscle stimulation accelerates functional recovery after nerve injury. Neuroscience. 2020;426:179–188. doi: 10.1016/j.neuroscience.2019.10.052. [DOI] [PubMed] [Google Scholar]
- 26.Generaal E, Milaneschi Y, Jansen R, Elzinga BM, Dekker J, Penninx BW. The brain-derived neurotrophic factor pathway, life stress, and chronic multi-site musculoskeletal pain. Mol Pain. 2016;12:1–9. doi: 10.1177/1744806916646783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon T. Electrical stimulation to enhance axon regeneration after peripheral nerve injuries in animal models and humans. Neurotherapeutics. 2016;13:295–310. doi: 10.1007/s13311-015-0415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon T. Peripheral nerve regeneration and muscle reinnervation. Int J Mol Sci. 2020;21:8652–8676. doi: 10.3390/ijms21228652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu N, Eyo UB, Murugan M, Peng J, Matta S, Dong H, Wu LJ. Microglial P2Y12 receptors regulate microglial activation and surveillance during neuropathic pain. Brain Behav Immun. 2016;55:82–92. doi: 10.1016/j.bbi.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gybels J, Vancalenbergh F. The treatment of pain due to peripheral-nerve injury by electrical-stimulitation of the injured nerve. Adv Pain Res Ther. 1990;13:217–222. [Google Scholar]
- 31.Hart AM, Terenghi G, Wiberg M. Neuronal death after peripheral nerve injury and experimental strategies for neuroprotection. Neurol Res. 2008;30:999–1011. doi: 10.1179/174313208X362479. [DOI] [PubMed] [Google Scholar]
- 32.He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44:532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- 33.Hilz MJ, Marthol H, Neundörfer B. Diabetic somatic polyneuropathy. Pathogenesis, clinical manifestations and therapeutic concepts. Fortschr Neurol Psychiatr. 2000;68:278–288. doi: 10.1055/s-2000-11535. [DOI] [PubMed] [Google Scholar]
- 34.Huang J, Ye Z, Hu X, Lu L, Luo Z. Electrical stimulation induces calcium-dependent release of NGF from cultured Schwann cells. Glia. 2010;58:622–631. doi: 10.1002/glia.20951. [DOI] [PubMed] [Google Scholar]
- 35.Hulse RP, Beazley-Long N, Ved N, Bestall SM, Riaz H, Singhal P, Ballmer Hofer K, Harper SJ, Bates DO, Donaldson LF. Vascular endothelial growth factor-A165b prevents diabetic neuropathic pain and sensory neuronal degeneration. Clin Sci (Lond) 2015;129:741–756. doi: 10.1042/CS20150124. [DOI] [PubMed] [Google Scholar]
- 36.Huo R, Han SP, Liu FY, Shou XJ, Liu LY, Song TJ, Zhai FJ, Zhang R, Xing GG, Han JS. Responses of primary afferent fibers to acupuncture-like peripheral stimulation at different frequencies:characterization by single-unit recording in rats. Neurosci Bull. 2020;36:907–918. doi: 10.1007/s12264-020-00509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hussain G, Wang J, Rasul A, Anwar H, Qasim M, Zafar S, Aziz N, Razzaq A, Hussain R, de Aguilar JG, Sun T. Current status of therapeutic approaches against peripheral nerve injuries:a detailed story from injury to recovery. Int J Biol Sci. 2020;16:116–134. doi: 10.7150/ijbs.35653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussain G, Zhang L, Rasul A, Anwar H, Sohail MU, Razzaq A, Aziz N, Shabbir A, Ali M, Sun T. Role of plant-derived flavonoids and their mechanism in attenuation of alzheimer's and parkinson's diseases:an update of recent data. Molecules. 2018;23:814. doi: 10.3390/molecules23040814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huygen F, Kallewaard JW, Nijhuis H, Liem L, Vesper J, Fahey ME, Blomme B, Morgalla MH, Deer TR, Capobianco RA. Effectiveness and safety of dorsal root ganglion stimulation for the treatment of chronic pain:a pooled analysis. Neuromodulation. 2020;23:213–221. doi: 10.1111/ner.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inoue K, Tsuda M. Microglia in neuropathic pain:cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci. 2018;19:138–152. doi: 10.1038/nrn.2018.2. [DOI] [PubMed] [Google Scholar]
- 41.Jensen MP, Brownstone RM. Mechanisms of spinal cord stimulation for the treatment of pain:still in the dark after 50 years. Eur J Pain. 2019;23:652–659. doi: 10.1002/ejp.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang HH, Song QX, Gill BC, Balog BM, Juarez R, Cruz Y, Damaser MS. Electrical stimulation of the pudendal nerve promotes neuroregeneration and functional recovery from stress urinary incontinence in a rat model. Am J Physiol Renal Physiol. 2018;315:F1555–1564. doi: 10.1152/ajprenal.00431.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson MI, Tabasam G. An investigation into the analgesic effects of interferential currents and transcutaneous electrical nerve stimulation on experimentally induced ischemic pain in otherwise pain-free volunteers. Phys Ther. 2003;83:208–223. [PubMed] [Google Scholar]
- 44.Kadekaro M, Crane AM, Sokoloff L. Differential effects of electrical stimulation of sciatic nerve on metabolic activity in spinal cord and dorsal root ganglion in the rat. Proc Natl Acad Sci U S A. 1985;82:6010–6013. doi: 10.1073/pnas.82.17.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kajander KC, Wakisaka S, Bennett GJ. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neurosci Lett. 1992;138:225–228. doi: 10.1016/0304-3940(92)90920-3. [DOI] [PubMed] [Google Scholar]
- 46.Kambiz S, Duraku LS, Holstege JC, Hovius SE, Ruigrok TJ, Walbeehm ET. Thermo-sensitive TRP channels in peripheral nerve injury:a review of their role in cold intolerance. J Plast Reconstr Aesthet Surg. 2014;67:591–599. doi: 10.1016/j.bjps.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Karagyaur M, Rostovtseva A, Semina E, Klimovich P, Balabanyan V, Makarevich P, Popov V, Stambolsky D, Tkachuk V. A bicistronic plasmid encoding brain-derived neurotrophic factor and urokinase plasminogen activator stimulates peripheral nerve regeneration after injury. J Pharmacol Exp Ther. 2020;372:248–255. doi: 10.1124/jpet.119.261594. [DOI] [PubMed] [Google Scholar]
- 48.Kawamura K, Kano Y. Electrical stimulation induces neurite outgrowth in PC12m3 cells via the p38 mitogen-activated protein kinase pathway. Neurosci Lett. 2019;698:81–84. doi: 10.1016/j.neulet.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 49.Khodabukus A, Madden L, Prabhu NK, Koves TR, Jackman CP, Muoio DM, Bursac N. Electrical stimulation increases hypertrophy and metabolic flux in tissue-engineered human skeletal muscle. Biomaterials. 2019;198:259–269. doi: 10.1016/j.biomaterials.2018.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koetsier E, Franken G, Debets J, Heijmans L, van Kuijk SMJ, Linderoth B, Joosten EA, Maino P. Mechanism of dorsal root ganglion stimulation for pain relief in painful diabetic polyneuropathy is not dependent on GABA release in the dorsal horn of the spinal cord. CNS Neurosci Ther. 2020;26:136–143. doi: 10.1111/cns.13192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koopmeiners AS, Mueller S, Kramer J, Hogan QH. Effect of electrical field stimulation on dorsal root ganglion neuronal function. Neuromodulation. 2013;16:304–311. doi: 10.1111/ner.12028. [DOI] [PubMed] [Google Scholar]
- 52.Kosman AJ, Wood EC, Osborne SL. Effect of electrical stimulation upon atrophy of partially denervated skeletal muscle of the rat. Am J Physiol. 1948;154:451–454. doi: 10.1152/ajplegacy.1948.154.3.451. [DOI] [PubMed] [Google Scholar]
- 53.Kouyoumdjian JA, Graca CR, Ferreira VFM. Peripheral nerve injuries:a retrospective survey of 1124 cases. Neurol India. 2017;65:551–555. doi: 10.4103/neuroindia.NI_987_16. [DOI] [PubMed] [Google Scholar]
- 54.Krarup C, Rosén B, Boeckstyns M, Ibsen Sørensen A, Lundborg G, Moldovan M, Archibald SJ. Sensation, mechanoreceptor, and nerve fiber function after nerve regeneration. Ann Neurol. 2017;82:940–950. doi: 10.1002/ana.25102. [DOI] [PubMed] [Google Scholar]
- 55.Kubiak CA, Grochmal J, Kung TA, Cederna PS, Midha R, Kemp SWP. Stem-cell-based therapies to enhance peripheral nerve regeneration. Muscle Nerve. 2020;61:449–459. doi: 10.1002/mus.26760. [DOI] [PubMed] [Google Scholar]
- 56.La G, Zhou M, Lim JY, Oh S, Xing H, Liu N, Yang Y, Liu X, Zhong L. Proteomics and transcriptomics analysis reveals clues into the mechanism of the beneficial effect of electrical stimulation on rat denervated gastrocnemius muscle. Cell Physiol Biochem. 2019;52:769–786. doi: 10.33594/000000054. [DOI] [PubMed] [Google Scholar]
- 57.Lambernd S, Taube A, Schober A, Platzbecker B, Gorgens SW, Schlich R, Jeruschke K, Weiss J, Eckardt K, Eckel J. Contractile activity of human skeletal muscle cells prevents insulin resistance by inhibiting pro-inflammatory signalling pathways. Diabetologia. 2012;55:1128–1139. doi: 10.1007/s00125-012-2454-z. [DOI] [PubMed] [Google Scholar]
- 58.Leem JW, Park ES, Paik KS. Electrophysiological evidence for the antinociceptive effect of transcutaneous electrical stimulation on mechanically evoked responsiveness of dorsal horn neurons in neuropathic rats. Neurosci Lett. 1995;192:197–200. doi: 10.1016/0304-3940(95)11644-c. [DOI] [PubMed] [Google Scholar]
- 59.Li C, Liu SY, Pi W, Zhang PX. Cortical plasticity and nerve regeneration after peripheral nerve injury. Neural Regen Res. 2021;16:1518–1523. doi: 10.4103/1673-5374.303008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liem L, van Dongen E, Huygen FJ, Staats P, Kramer J. The dorsal root ganglion as a therapeutic target for chronic pain. Reg Anesth Pain Med. 2016;41:511–519. doi: 10.1097/AAP.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 61.Linderoth B, Foreman RD. Physiology of spinal cord stimulation:review and update. Neuromodulation. 1999;2:150–164. doi: 10.1046/j.1525-1403.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- 62.Liu Q, Wang X, Yi S. Pathophysiological changes of physical barriers of peripheral nerves after injury. Front Neurosci. 2018;12:597. doi: 10.3389/fnins.2018.00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu XG, Pang RP, Zhou LJ, Wei XH, Zang Y. Neuropathic pain:sensory nerve injury or motor nerve injury? Adv Exp Med Biol. 2016;904:59–75. doi: 10.1007/978-94-017-7537-3_5. [DOI] [PubMed] [Google Scholar]
- 64.Lopez-Leal R, Court FA. Schwann cell exosomes mediate neuron-glia communication and enhance axonal regeneration. Cell Mol Neurobiol. 2016;36:429–436. doi: 10.1007/s10571-015-0314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lyle MA, Nichols TR, Kajtaz E, Maas H. Musculotendon adaptations and preservation of spinal reflex pathways following agonist-to-antagonist tendon transfer. Physiol Rep. 2017;5:e13201. doi: 10.14814/phy2.13201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma W, Zhang R, Huang Z, Zhang Q, Xie X, Yang X, Zhang Q, Liu H, Ding F, Zhu J, Sun H. PQQ ameliorates skeletal muscle atrophy, mitophagy and fiber type transition induced by denervation via inhibition of the inflammatory signaling pathways. Ann Transl Med. 2019;7:440. doi: 10.21037/atm.2019.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mackinnon SE, Dellon AL. Reinnervation of distal sensory nerve environments by regenerating sensory axons. Neuroscience. 1992;46:595–603. doi: 10.1016/0306-4522(92)90147-t. [DOI] [PubMed] [Google Scholar]
- 68.Mancinelli R, Toniolo L, Di Filippo ES, Doria C, Marrone M, Maroni CR, Verratti V, Bondi D, Maccatrozzo L, Pietrangelo T, Fulle S. Neuromuscular electrical stimulation induces skeletal muscle fiber remodeling and specific gene expression profile in healthy elderly. Front Physiol. 2019;10:1459. doi: 10.3389/fphys.2019.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martellucci J. Italy: Springer International Publishing Switzerland; 2015. Electrical stimulation for pelvic floor disorders. [Google Scholar]
- 70.Martínez-Marcos A, Sañudo JR. Cranial nerves:morphology and clinical relevance. Anat Rec (Hoboken) 2019;302:555–557. doi: 10.1002/ar.24106. [DOI] [PubMed] [Google Scholar]
- 71.McGregor CE, English AW. The role of BDNF in peripheral nerve regeneration:activity-dependent treatments and Val66Met. Front Cell Neurosci. 2018;12:522. doi: 10.3389/fncel.2018.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McLean NA, Verge VM. Dynamic impact of brief electrical nerve stimulation on the neural immune axis-polarization of macrophages toward a pro-repair phenotype in demyelinated peripheral nerve. Glia. 2016;64:1546–1561. doi: 10.1002/glia.23021. [DOI] [PubMed] [Google Scholar]
- 73.Mendez A, Hopkins A, Biron VL, Seikaly H, Zhu LF, Côté DWJ. Brief electrical stimulation and synkinesis after facial nerve crush injury:a randomized prospective animal study. J Otolaryngol Head Neck Surg. 2018;47:20. doi: 10.1186/s40463-018-0264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mickle AD, Shepherd AJ, Mohapatra DP. Nociceptive TRP channels:sensory detectors and transducers in multiple pain pathologies. Pharmaceuticals (Basel) 2016;9:72. doi: 10.3390/ph9040072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Midha R, Grochmal J. Surgery for nerve injury:current and future perspectives. J Neurosurg. 2019;130:675–685. doi: 10.3171/2018.11.JNS181520. [DOI] [PubMed] [Google Scholar]
- 76.Moutal A, Luo S, Largent-Milnes TM, Vanderah TW, Khanna R. Cdk5-mediated CRMP2 phosphorylation is necessary and sufficient for peripheral neuropathic pain. Neurobiol Pain. 2019;5:100022. doi: 10.1016/j.ynpai.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Natsume A, Mata M, Wolfe D, Oligino T, Goss J, Huang S, Glorioso J, Fink DJ. Bcl-2 and GDNF delivered by HSV-mediated gene transfer after spinal root avulsion provide a synergistic effect. J Neurotrauma. 2002;19:61–68. doi: 10.1089/089771502753460240. [DOI] [PubMed] [Google Scholar]
- 78.Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 79.Nedachi T, Fujita H, Kanzaki M. Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. Am J Physiol Endocrinol Metab. 2008;295:E1191–1204. doi: 10.1152/ajpendo.90280.2008. [DOI] [PubMed] [Google Scholar]
- 80.Nicolas N, Kobaiter-Maarrawi S, Georges S, Abadjian G, Maarrawi J. Motor cortex stimulation regenerative effects in peripheral nerve injury:an experimental rat model. World Neurosurg. 2018;114:e800–808. doi: 10.1016/j.wneu.2018.03.090. [DOI] [PubMed] [Google Scholar]
- 81.O'Neill BT, Lee KY, Klaus K, Softic S, Krumpoch MT, Fentz J, Stanford KI, Robinson MM, Cai W, Kleinridders A, Pereira RO, Hirshman MF, Abel ED, Accili D, Goodyear LJ, Nair KS, Kahn CR. Insulin and IGF-1 receptors regulate FoxO-mediated signaling in muscle proteostasis. J Clin Invest. 2016;126:3433–3446. doi: 10.1172/JCI86522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Osorio-Fuentealba C, Contreras-Ferrat AE, Altamirano F, Espinosa A, Li Q, Niu W, Lavandero S, Klip A, Jaimovich E. Electrical stimuli release ATP to increase GLUT4 translocation and glucose uptake via PI3Kgamma-Akt-AS160 in skeletal muscle cells. Diabetes. 2013;62:1519–1526. doi: 10.2337/db12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pei BA, Zi JH, Wu LS, Zhang CH, Chen YZ. Pulsed electrical stimulation protects neurons in the dorsal root and anterior horn of the spinal cord after peripheral nerve injury. Neural Regen Res. 2015;10:1650–1655. doi: 10.4103/1673-5374.167765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ploner M, Sorg C, Gross J. Brain rhythms of pain. Trends Cogn Sci. 2017;21:100–110. doi: 10.1016/j.tics.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Power HA, Morhart MJ, Olson JL, Chan KM. Postsurgical electrical stimulation enhances recovery following surgery for severe cubital tunnel syndrome:a double-blind randomized controlled trial. Neurosurgery. 2020;86:769–777. doi: 10.1093/neuros/nyz322. [DOI] [PubMed] [Google Scholar]
- 86.Rabinovsky ED, Smith GM, Browder DP, Shine HD, McManaman JL. Peripheral nerve injury down-regulates CNTF expression in adult rat sciatic nerves. J Neurosci Res. 1992;31:188–192. doi: 10.1002/jnr.490310124. [DOI] [PubMed] [Google Scholar]
- 87.Reinhold AK, Rittner HL. Barrier function in the peripheral and central nervous system-a review. Pflugers Arch. 2017;469:123–134. doi: 10.1007/s00424-016-1920-8. [DOI] [PubMed] [Google Scholar]
- 88.Rigoni M, Negro S. Signals orchestrating peripheral nerve repair. Cells. 2020;9:1768. doi: 10.3390/cells9081768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ronchi G, Cillino M, Gambarotta G, Fornasari BE, Raimondo S, Pugliese P, Tos P, Cordova A, Moschella F, Geuna S. Irreversible changes occurring in long-term denervated Schwann cells affect delayed nerve repair. J Neurosurg. 2017;127:843–856. doi: 10.3171/2016.9.JNS16140. [DOI] [PubMed] [Google Scholar]
- 90.Sakaguchi R, Mori Y. Transient receptor potential (TRP) channels:biosensors for redox environmental stimuli and cellular status. Free Radic Biol Med. 2020;146:36–44. doi: 10.1016/j.freeradbiomed.2019.10.415. [DOI] [PubMed] [Google Scholar]
- 91.Salmons S. Adaptive change in electrically stimulated muscle:a framework for the design of clinical protocols. Muscle Nerve. 2009;40:918–935. doi: 10.1002/mus.21497. [DOI] [PubMed] [Google Scholar]
- 92.Salvany S, Casanovas A, Piedrafita L, Tarabal O, Hernández S, Calderó J, Esquerda JE. Microglial recruitment and mechanisms involved in the disruption of afferent synaptic terminals on spinal cord motor neurons after acute peripheral nerve injury. Glia. 2021;69:1216–1240. doi: 10.1002/glia.23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science. 1991;251:1608–1610. doi: 10.1126/science.2011742. [DOI] [PubMed] [Google Scholar]
- 94.Sawangjit R, Thongphui S, Chaichompu W, Phumart P. Efficacy and safety of mecobalamin on peripheral neuropathy:a systematic review and meta-analysis of randomized controlled trials. J Altern Complement Med. 2020;26:1117–1129. doi: 10.1089/acm.2020.0068. [DOI] [PubMed] [Google Scholar]
- 95.Scheler M, Irmler M, Lehr S, Hartwig S, Staiger H, Al-Hasani H, Beckers J, de Angelis MH, Haring HU, Weigert C. Cytokine response of primary human myotubes in an in vitro exercise model. Am J Physiol Cell Physiol. 2013;305:C877–886. doi: 10.1152/ajpcell.00043.2013. [DOI] [PubMed] [Google Scholar]
- 96.Schmalbruch H. The effect of peripheral nerve injury on immature motor and sensory neurons and on muscle fibres. Possible relation to the histogenesis of Werdnig-Hoffmann disease. Rev Neurol (Paris) 1988;144:721–729. [PubMed] [Google Scholar]
- 97.Schmidt GL. The use of spinal cord stimulation/neuromodulation in the management of chronic pain. J Am Acad Orthop Surg. 2019;27:e401–407. doi: 10.5435/JAAOS-D-17-00829. [DOI] [PubMed] [Google Scholar]
- 98.Sène D. Small fiber neuropathy:diagnosis, causes, and treatment. Joint Bone Spine. 2018;85:553–559. doi: 10.1016/j.jbspin.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 99.Senger JL, Chan KM, Macandili H, Chan AWM, Verge VMK, Jones KE, Webber CA. Conditioning electrical stimulation promotes functional nerve regeneration. Exp Neurol. 2019;315:60–71. doi: 10.1016/j.expneurol.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 100.Senger JLB, Verge VMK, Macandili HSJ, Olson JL, Chan KM, Webber CA. Electrical stimulation as a conditioning strategy for promoting and accelerating peripheral nerve regeneration. Exp Neurol. 2018;302:75–84. doi: 10.1016/j.expneurol.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 101.Shen Y, Zhang R, Xu L, Wan Q, Zhu J, Gu J, Huang Z, Ma W, Shen M, Ding F, Sun H. Microarray analysis of gene expression provides new insights into denervation-induced skeletal muscle atrophy. Front Physiol. 2019;10:1298. doi: 10.3389/fphys.2019.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shinoda M, Fujita S, Sugawara S, Asano S, Koyama R, Fujiwara S, Soma K, Tamagawa T, Matsui T, Ikutame D, Ando M, Osada A, Kimura Y, Kobayashi K, Yamamoto T, Kusama-Eguchi K, Kobayashi M, Hayashi Y, Iwata K. Suppression of superficial microglial activation by spinal cord stimulation attenuates neuropathic pain following sciatic nerve injury in rats. Int J Mol Sci. 2020;21:2390. doi: 10.3390/ijms21072390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Simmons Z, Feldman EL. Update on diabetic neuropathy. Curr Opin Neurol. 2002;15:595–603. doi: 10.1097/00019052-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 104.Sohn EJ, Park HT, Shin YK. Exosomes derived from differentiated Schwann cells inhibit Schwann cell migration via microRNAs. Neuroreport. 2020;31:515–522. doi: 10.1097/WNR.0000000000001435. [DOI] [PubMed] [Google Scholar]
- 105.Stephens KE, Chen Z, Sivanesan E, Raja SN, Linderoth B, Taverna SD, Guan Y. RNA-seq of spinal cord from nerve-injured rats after spinal cord stimulation. Mol Pain. 2018;14:1744806918817429. doi: 10.1177/1744806918817429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun L, Tai L, Qiu Q, Mitchell R, Fleetwood-Walker S, Joosten EA, Cheung CW. Endocannabinoid activation of CB(1) receptors contributes to long-lasting reversal of neuropathic pain by repetitive spinal cord stimulation. Eur J Pain. 2017;21:804–814. doi: 10.1002/ejp.983. [DOI] [PubMed] [Google Scholar]
- 107.Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74:491–516. doi: 10.1093/brain/74.4.491. [DOI] [PubMed] [Google Scholar]
- 108.Taub A, Collins WF, Venes J. Partial, reversible, functional spinal cord transection. A complication of dorsal column stimulation for the relief of pain. Arch Neurol. 1974;30:107–108. doi: 10.1001/archneur.1974.00490310109019. [DOI] [PubMed] [Google Scholar]
- 109.Tilley DM, Cedeño DL, Kelley CA, Benyamin R, Vallejo R. Spinal cord stimulation modulates gene expression in the spinal cord of an animal model of peripheral nerve injury. Reg Anesth Pain Med. 2016;41:750–756. doi: 10.1097/AAP.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 110.Tilley DM, Cedeño DL, Kelley CA, DeMaegd M, Benyamin R, Vallejo R. Changes in dorsal root ganglion gene expression in response to spinal cord stimulation. Reg Anesth Pain Med. 2017;42:246–251. doi: 10.1097/AAP.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 111.Tsuda M. Microglia in the spinal cord and neuropathic pain. J Diabetes Investig. 2016;7:17–26. doi: 10.1111/jdi.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vera-Portocarrero LP, Cordero T, Billstrom T, Swearingen K, Wacnik PW, Johanek LM. Differential effects of subcutaneous electrical stimulation (SQS) and transcutaneous electrical nerve stimulation (TENS) in rodent models of chronic neuropathic or inflammatory pain. Neuromodulation. 2013;16:328–335. doi: 10.1111/ner.12037. [DOI] [PubMed] [Google Scholar]
- 113.Wang L, Chopp M, Szalad A, Lu X, Zhang Y, Wang X, Cepparulo P, Lu M, Li C, Zhang ZG. Exosomes derived from schwann cells ameliorate peripheral neuropathy in type 2 diabetic mice. Diabetes. 2020;69:749–759. doi: 10.2337/db19-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang L, Zhang Y, Wang X, Ye Z. Electroacupuncture-induced cannabinoid receptor expression in repair of abducens nerve. Int J Neurosci. 2019;129:923–929. doi: 10.1080/00207454.2019.1593980. [DOI] [PubMed] [Google Scholar]
- 115.Whitham M, Chan MH, Pal M, Matthews VB, Prelovsek O, Lunke S, El-Osta A, Broenneke H, Alber J, Bruning JC, Wunderlich FT, Lancaster GI, Febbraio MA. Contraction-induced interleukin-6 gene transcription in skeletal muscle is regulated by c-Jun terminal kinase/activator protein-1. J Biol Chem. 2012;287:10771–10779. doi: 10.1074/jbc.M111.310581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Willand MP, Chiang CD, Zhang JJ, Kemp SW, Borschel GH, Gordon T. Daily Electrical muscle stimulation enhances functional recovery following nerve transection and repair in rats. Neurorehabil Neural Repair. 2015;29:690–700. doi: 10.1177/1545968314562117. [DOI] [PubMed] [Google Scholar]
- 117.Willand MP, Rosa E, Michalski B, Zhang JJ, Gordon T, Fahnestock M, Borschel GH. Electrical muscle stimulation elevates intramuscular BDNF and GDNF mRNA following peripheral nerve injury and repair in rats. Neuroscience. 2016;334:93–104. doi: 10.1016/j.neuroscience.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 118.Wong JN, Olson JL, Morhart MJ, Chan KM. Electrical stimulation enhances sensory recovery:a randomized controlled trial. Ann Neurol. 2015;77:996–1006. doi: 10.1002/ana.24397. [DOI] [PubMed] [Google Scholar]
- 119.Wutz A, Melcher D, Samaha J. Frequency modulation of neural oscillations according to visual task demands. Proc Natl Acad Sci U S A. 2018;115:1346–1351. doi: 10.1073/pnas.1713318115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xing XX, Zheng MX, Hua XY, Ma SJ, Ma ZZ, Xu JG. Brain plasticity after peripheral nerve injury treatment with massage therapy based on resting-state functional magnetic resonance imaging. Neural Regen Res. 2021;16:388–393. doi: 10.4103/1673-5374.290912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yi S, Tang X, Yu J, Liu J, Ding F, Gu X. Microarray and qPCR analyses of wallerian degeneration in rat sciatic nerves. Front Cell Neurosci. 2017;11:22. doi: 10.3389/fncel.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou M, Hu M, He S, Li B, Liu C, Min J, Hong L. Effects of RSC96 Schwann cell-derived exosomes on proliferation, senescence, and apoptosis of dorsal root ganglion cells in vitro. Med Sci Monit. 2018;24:7841–7849. doi: 10.12659/MSM.909509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zigmond RE, Echevarria FD. Macrophage biology in the peripheral nervous system after injury. Prog Neurobiol. 2019;173:102–121. doi: 10.1016/j.pneurobio.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


