Abstract
Interleukin-27 is a pleiotropic cytokine that is involved in tissue responses to infection, cell stress, neuronal disease, and tumors. Recent studies in various tissues indicate that interleukin-27 has complex activating and inhibitory properties in innate and acquired immunity. The availability of recombinant interleukin-27 protein and mice with genetic deletions of interleukin-27, its receptors and signaling mediators have helped define the role of interleukin-27 in neurodegenerative diseases. Interleukin-27 has been well-characterized as an important regulator of T cell activation and differentiation that enhances or suppresses T cell responses in autoimmune conditions in the central nervous system. Evidence is also accumulating that interleukin-27 has neuroprotective activities in the retina and brain. Interleukin-27 is secreted from and binds to infiltrating microglia, macrophage, astrocytes, and even neurons and it promotes neuronal survival by regulating pro- and anti-inflammatory cytokines, neuroinflammatory pathways, oxidative stress, apoptosis, autophagy, and epigenetic modifications. However, interleukin-27 can have the opposite effect and induce inflammation and cell death in certain situations. In this review, we describe the current understanding of regulatory activities of interleukin-27 on cell survival and inflammation and discuss its mechanisms of action in the brain, spinal cord, and retina. We also review evidence for and against the therapeutic potential of interleukin-27 for dampening harmful neuroinflammatory responses in central nervous system diseases.
Key Words: central nervous system, cytokine, inflammatory, interleukin-27, neuroprotection, retina
Interleukin-27 and Its Receptors
Interleukin-27 (IL-27) is a heterodimeric cytokine composed of a 28 kDa IL-27p28 subunit and the 24 kDa Epstein Barr Virus-induced gene 3 (EBI3) protein. IL-27 subunits are induced primarily in antigen-presenting cells after stimulation of Toll-like receptors, CD40, complement, and interferon (IFN) receptors, which positions IL-27 as a central responder to numerous inflammatory signals. Examination of publicly available single-cell RNA sequencing databases from mouse retina and brain indicates that EBI3 and IL-27p28 are detected in multiple glial and neuronal cell subtypes (singlecell.broadinstitute.org). Immunodetection and transcript analyses also confirmed IL-27 expression in non-inflammatory cells in the central nervous system (CNS), including spinal cord neurons, retinal ganglion cells, and photoreceptors (Amadi-Obi et al., 2007).
IL-27 belongs to the IL-6/IL-12 cytokine superfamily that shares receptors and has overlapping inflammatory functions (Yoshida and Hunter, 2015). Secreted IL-27 binds to a heterodimeric receptor complex composed of gp130 and interleukin-27 receptor alpha (IL-27Rα) (also known as WSX-1 or TCCR) (Luo et al., 2021). IL-27Rα is specific for IL-27 and is primarily expressed on the cell surface of inflammatory cells, including macrophages, microglia, mast cells, B cells, dendritic cells, natural killer cells, naive CD4+ and CD8+ T cells, as well as neurons and radial glia. The gp130 receptor is expressed widely and is found on most inflammatory cells as well as retinal Muller glia, neurons, and endothelial cells (singlecell.broadinstitute.org). Gp130 also interacts with several other ligands, including cytokines and growth factors such as IL-6, LIF, OSM, and CNTF. The expression of IL-27 and its receptor are rapidly induced after neuronal damage. For example, EBI3, IL-27p28, and IL-27Rα transcripts are up-regulated in inflammatory cells in the CNS at the peak of pathology in an experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis (MS) (Li et al., 2005), and IL-27 expression is increased during intraocular inflammation (uveitis) (Lee et al., 2011). Although both receptor subunits are typically required for IL-27 signaling, homodimers of IL-27Rα are functional in certain cell types, such as transformed hematopoietic cells (Pradhan et al., 2007). Furthermore, IL-27Rα interacts with other receptor complexes, including ciliary neurotrophic factor receptor and IL-6Rα, which expands the number of potential cellular pathways stimulated by IL-27. Interestingly, a soluble form of IL-27Rα was identified in CD4+ and CD8+ T cells, B cells, monocytes, human sera, and COS7 cells that binds to and inhibits IL-27 signaling, indicating an additional route of IL-27 regulation (Dietrich et al., 2014), although the conditions that promote its formation are not yet understood.
IL-27 signals through the JAK-STAT pathway, which is a well-described pathway that mediates many cellular processes, including cellular differentiation, proliferation, and immunologic responses (Burrell et al., 2020). JAKs are receptor-associated tyrosine kinases that are activated when bound to ligands including cytokines, growth factors, and interferons. The gp130 component of the IL-27 receptor engages JAK1 or JAK2, and WSX-1 binds to Tyk2, which catalyzes tyrosine phosphorylation and creates a binding site for SH2 domain-containing STAT proteins. STATs are a family of transcription factors with varied tissue expression, promoter binding affinity, and responses to extracellular signaling. STATs are phosphorylated in the cytosol by activated JAKs, leading to STAT dimerization and activation. STAT dimers are then transported to the nucleus where they induce transcription of target genes. The anti-inflammatory and cytoprotective activities of IL-27 have been linked to STAT1 or STAT3 activation in microglia, dendritic cells, and astrocytes (Amadi-Obi et al., 2007; Lee et al., 2011; Luo et al., 2021), described below. The involvement of JAK-STAT pathways in numerous cellular processes in the CNS increases the potential roles for IL-27 throughout the nervous system, suggests points of cross-talk between IL-27 and other extracellular stimuli, and provides additional regulatory pathways that potentially enhance or suppress IL-27 signaling.
Cell Types Mediating the Effects of Interleukin-27
The role of IL-27 in regulating T cell responses that prevent immune hyperactivity has been extensively characterized and IL-27 has been investigated as a possible therapeutic for chronic inflammatory conditions with excessive T cell activation, such as MS and rheumatoid arthritis. IL-27 antagonizes differentiation and function of type 1 effector cells (Th1), type 2 effector cells (Th2), and IL-17 producing helper T-cells (Th17), inhibits Th9 cell differentiation and function, and regulates B cell responses. In contrast, IL-27 has pro-inflammatory functions by promoting the clonal expansion of naive CD4+ T-cells, promoting Treg and CD8+ T cell survival, and stimulating tumor-specific cytotoxic T cell responses (Kim et al., 2019). IL-27 also regulates anti-viral responses of CD4+ T-cells by controlling cell number and effector function (Wehrens et al., 2018), and it promotes the expression of inhibitory receptors on T cells, including PD-L1, LAG-3, TIGIT, and TIM-3, which limits the immune response during parasitic infection (DeLong et al., 2019).
Although the anti-inflammatory activities of IL-27 are mostly associated with regulation of T cell responses, evidence from recent studies indicate that it also induces anti-inflammatory signaling through other cell types in the CNS such as macrophages, microglia, and dendritic cells (Baker et al., 2010; Sowrirajan et al., 2017). These cell types compose a major portion of the neuroinflammatory response to CNS injury, and fine-tuning their coordinated response to damage may limit further neuronal death. Macrophages, dendritic cells and other antigen-presenting cells secrete IL-27 and express IL-27 receptors, suggesting that IL-27 may function independently of its effects on T cells (Lee et al., 2011; Sowrirajan et al., 2017). The expression of both IL-27 and its receptors on the same cells also indicates the potential for positive and negative feedback loops. Furthermore, IL-27 increases the expression of its receptor, suggesting it strengthens its signaling as a positive feedback response (Zhao et al., 2017).
Several studies confirmed that the expression of IL-27 and its receptors were induced in innate immunity cell types during disease, including microglia, macrophage, and dendritic cells. Elevated IL-27 levels were demonstrated in CD11b+ microglia/macrophage in the EAE mouse model of MS and were associated with the therapeutic reversal of disease symptoms (Chu et al., 2020). Similarly, in a mouse model of neuropathic pain, expression of IL-27 and its receptor were increased in resident macrophage, microglia, and astrocytes within the spinal cord and dorsal root ganglion (Fonseca et al., 2019). Exogenous IL-27 stimulated these immune cells adjacent to the sensory ganglia and spinal cord after peripheral nerve injury, leading to reduced pain hypersensitivity in this animal model (Fonseca et al., 2019).
IL-27 and IL-27Rα were detected in astrocytes, microglia, and macrophages in MS post-mortem brain tissue (Senecal et al., 2016; Lalive et al., 2017). In human astrocyte cultures, inflammatory cytokines induced IL-27 and upregulated class 1 MHC, whereas M1-polarized myeloid cells induced IL-27 in microglia and macrophage (Senecal et al., 2016). Therefore, IL-27 may help coordinate the local neuroinflammatory response via astrocytes and monocytes, which could limit further neuronal death (Senecal et al., 2016). However, different macrophage cell types vary in their responses to IL-27. For example, IL-27 was pro-inflammatory in peritoneal macrophages stimulated with LPS, leading to nuclear factor kappa B (NF-κB) and MAPK activation (Shimizu et al., 2013). Further study is needed to precisely define the effects of IL-27 in macrophages from different tissues and activation states.
Neutrophils are also involved in mediating the effects of IL-27 in the brain after injury. In an interesting study by Zhao et al., induction of intracerebral hemorrhage in rat and mouse models lead to rapidly elevated expression of IL-27p28 and EBI3 in the brain, CSF, serum, and spleen within an hour after injury (Zhao et al., 2017). Furthermore, IL-27 promoted neutrophil maturation within the bone marrow and reduced neutrophil expression of pro-inflammatory genes, such as MMP-9 and NOX2, and increased anti-inflammatory genes, such as lactoferrin and other iron scavengers. (Zhao et al., 2017). Characterization of IL-27-mediated signaling in neutrophils within the CNS, and examining cross-talk between neutrophils and other neuroinflammatory cells, will help clarify the role of IL-27 in neuroprotection after brain injury.
Furthermore, a study using the EAE mouse model of MS demonstrated that IL-27 signaling in Foxp3+ Tregs was required for suppressing autoimmune inflammation. IL-27 delivered by a subcutaneous osmotic minipump led to reduced disease severity, lower inflammatory cytokines, and increased survival only when Treg cells were present. Additionally, transfer of Tregs obtained from mice lacking IL-27Rα or Lag3 prevented the protective effect of IL-27, demonstrating that the neuroprotective effects of IL-27 in this model depend on T cells, and not macrophage or astrocytes (Kim et al., 2019).
Roles of Interleukin-27 in the Central Nervous System
IL-27 increases neuronal survival in the CNS in various diseases and injuries. For example, intraperitoneal injections of IL-27 reduced ischemia-reperfusion injury in a murine stroke model, decreased infarct volume, and improved neurological function, which was associated with reduced pro-inflammatory proteins TNFα, IL-1β and MCP-1 and increased IL-10 and TGF-β (Luo et al., 2021). Additionally, in a mouse model of intracerebral hemorrhage, treatment with an IL-27 neutralizing antibody reduced endogenous IL-27 by over 80% and increased neurological deficits, compared with IgG control antibody. Recombinant IL-27 significantly decreased neurological deficits and brain pathology. Furthermore, IL-27 binding to receptors on neutrophils promoted a protective neutrophil phenotype, characterized by lactoferrin expression and reduced free iron in the brain, and was required for the beneficial effects of IL-27 (Zhao et al., 2017).
Neuroprotective activity of IL-27 was also demonstrated in the retina. Elevated IL-27 was associated with increased photoreceptor survival in the rd10 mouse model of inherited retinal degeneration injected with a MyD88 inhibitor (Garces et al., 2020). Furthermore, ocular injections of recombinant IL-27 reduced photoreceptor death in rd10 mice, confirming the neuroprotective function of IL-27 in the retina (Nortey et al., manuscript in preparation).
The activity of IL-27 in neurons may augment its effect on inflammatory cells. For example, IL-27 reduced apoptosis by acting directly on cultured neurons (Luo et al., 2021). Additionally, Lee et al. (2011) demonstrated that retinal ganglion cells and photoreceptors express basal levels of IL-27 and that IL-27 expression is upregulated in these neurons and microglia in a mouse uveitis model. Furthermore, photoreceptor and Muller glia cultures express IL-27 receptors, and IL-27 incubation led to STAT1-dependent induction of IL-10 and SOCS1. The authors concluded that IL-27 produced by retinal neurons and glia contributes to suppressing intraocular inflammation and preserving the retina (Lee et al., 2011). Amadi-Obi et al. (2007) also demonstrated that IL-27 and EBI3 are constitutively expressed in the neural retina in retinal ganglion cells, inner retina, Muller glia, and RPE. IL-27 inhibited proliferation of uveitogenic T cells in a mouse model of uveitis, IL-27 was induced by IFN-gamma in retinal cells, and retinal cultures secreted IL-27 and inhibited the expansion of Th17 cells when tested in a coculture system. These findings suggest that IL-27 expression in retinal cells, which was elevated during uveitis, may suppress additional neuronal damage. Further studies using neuron-specific or glia-specific IL-27 and IL-27R knockout mice will be needed to determine the precise involvement of neuronal-targeted IL-27.
IL-27 is also induced by other pro-survival molecules and potentially contributes to their cytoprotective functions. In the mouse MPTP-toxicity induced Parkinson’s disease model, protection of dopaminergic neurons by administration of granulocyte macrophage colony-stimulating factor (GM-CSF), a cytokine required for autoimmunity in the CNS, was associated with upregulation of IL-27 and IL-10R, increased Treg cells, and decreased microgliosis (Kosloski et al., 2013). In contrast, the protective effect of IL-27 in the EAE mouse model involved fewer infiltrating GM-CSF+ T cells into the brain and spinal cord and decreased GM-CSF expression in CD4+ T cells (Casella et al., 2017). Furthermore, reduced GM-CSF by IL-27 resulted in PD-L1 induction in myeloid cells, leading to reduced neuroinflammation and suppressed autoimmunity (Casella et al., 2017).
In a study that investigated the therapeutic properties of matrine in the EAE mouse model, elevated IFN-β and IL-27 were associated with reduced symptoms (Chu et al., 2020). Blocking IFN-β activity reduced both IL-27 and the therapeutic effect of matrine, indicating a neuroprotective role of IFN-β/IL-27 signaling. In contrast, in the SOD1-G93A mouse model of amyotrophic lateral sclerosis (ALS) treated with a P2X7 antagonist, serum IL-27, IFN-β, and IL-10 levels were elevated despite lack of a therapeutic effect of the antagonist, indicating IL-27 did not promote protection (Ly et al., 2020). It is possible that IL-27 levels may be elevated as an intrinsic neuroprotective response in the ALS model that is ultimately insufficient, or IL-27 may promote disease progression due to its pro-inflammatory properties (see below). Further studies on the role of IL-27 in ALS should be performed, including analysis of serum IL-27 levels in patients with ALS.
A link between IL-27 and autophagy has also been demonstrated, with opposite effects noted in different studies. IL-27 promotes survival of Mycobacterium tuberculosis-infected macrophages by inhibiting autophagy in an mTOR and Mcl-1-dependent manner (Sharma et al., 2014). In contrast, IL-27 promotes autophagy in stimulated primary human macrophages, which was not associated with mTOR phosphorylation or LC3 lipidation (Laverdure et al., 2021). Autophagy is critical to many neuronal diseases and the role of IL-27 in autophagy regulation in the CNS should be further investigated.
Several studies suggest that IL-27 levels may represent a biomarker for neurodegenerative disease progression. Patients with Parkinson’s disease (PD) had lower serum IL-27 levels and higher TNFα levels compared with age-matched controls, and although total IL-27 levels were lower, they correlated with the severity of PD symptoms (Kouchaki et al., 2018). Increased IL-27 expression in more severe PD may be a compensatory mechanism to reduce excessive inflammation. Similarly, higher IL-27 levels were measured in the CSF of patients with MS compared with control individuals, although serum IL-27 levels did not differ between patients and controls (Lalive et al., 2017). The differences in CSF and serum levels of IL-27 with respect to disease pathology may indicate different roles and cellular interactions of IL-27 at the site of injury compared with the periphery. Additionally, higher serum levels of IL-27 also correlated with cardiopulmonary failure from Enterovirus in children with neurological complications from infection, without an association between IL-27 levels and viral load, suggesting its use as a predictor of disease severity in these patients (Huang et al., 2016). However, several studies indicated no correlation between IL-27 and disease severity, perhaps representing differences in the timing of disease pathogenesis, patient populations or other factors (Kouchaki et al., 2018; Ly et al., 2020).
Molecular Mechanisms of Interleukin-27-Induced Cytoprotection
IL-10
Studies in animal models of disease and cultured cells demonstrated that multiple signaling pathways contribute to the anti-inflammatory effects of IL-27 (Figure 1). In addition to suppressing T cell differentiation and function, IL-27 induces secretion of the anti-inflammatory cytokine IL-10 from macrophages and activated CD4+ T cells (Fonseca et al., 2019). In the CNS, IL-10 limits neuroinflammation by reducing pro-inflammatory cells and reactive astrogliosis and promotes neuroprotective microglial phenotypes (Burmeister and Marriott, 2018). IL-10 binds to microglia and astrocytes where it activates JAK1/STAT and NF-κB and suppresses the release of inflammatory cytokines. IL-10 also binds to IL-10R on the surface of several neuronal types, such as retinal ganglion cells (RGCs), cortical neurons, and spinal cord neurons. In the latter, IL-10 induces STAT3/AKT signaling, leading to increased transcription of the anti-apoptotic genes Bcl-2 and Bcl-XL and increased survival of cultured neurons exposed to the elevated glutamate (Zhou et al., 2009). IL-10 also leads to inactivation of GSK3β, raising the possibility that IL-27/IL-10 signaling could stimulate the neuroprotective Wnt pathway (Garcia et al., 2018). Furthermore, the anti-inflammatory effect of IL-27 is lost or reduced in animals lacking IL-10 (Fonseca et al., 2019), indicating the importance of IL-10 signaling to IL-27 function. However, IL-27 induced IL-10 expression in CD4+ T cells in vitro but not in vivo in a mouse model of MS (Kim et al., 2019), indicating that factors that mediate IL-27/IL-10 signaling are not yet fully understood.
Figure 1.
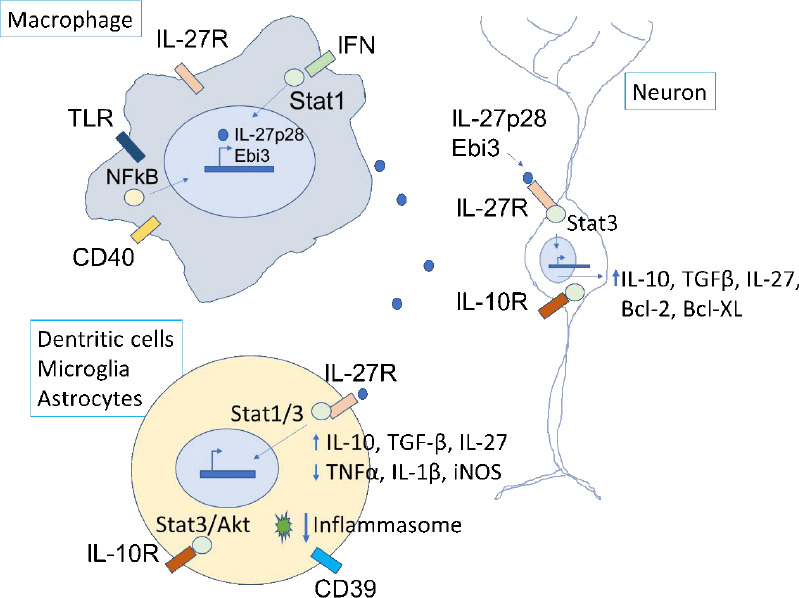
Potential mechanisms of neuronal protection from IL-27 acting on innate immune cells and neurons.
Macrophages produce IL-27 after stimulation of TLR, CD40, IFN, and other receptors, via NF-κB or STAT1. The secreted IL-27 binds to receptors on microglia, dendritic cells, and astrocytes, which activates STAT1/3 signaling pathways, induction of anti-inflammatory proteins, reduction of pro-inflammatory proteins, and inhibition of the NRLP3 inflammasome. CD39 expression in dendritic cells is required for inflammasome inhibition in dendritic cells. IL-10 released from microglia binds to IL-10 receptors on neurons and stimulates anti-apoptotic pathways (Bcl-2, Bcl-XL). IL-27 has direct effects on neurons by binding to IL-10 receptors and stimulating anti-expression of IL-10 and TGFβ. IL-27 is expressed in microglia, astrocytes, and neurons, suggesting positive-feedback pathways to sustain anti-inflammatory/cytoprotective signaling during sustained neuroinflammatory injury. The contribution of T cells and other IL-27-responsive cell types is not shown. IFN: Interferon; IL: interleukin; IL-27R: interleukin-27 receptor; NF-κB: nuclear factor kappa B; TGFβ: tumor growth factor-beta; TLR: Toll-like receptor.
Cytokine expression
IL-27 reduces the expression of multiple inflammatory cytokines, and the combined effects of suppressed pro-inflammatory cytokines and elevated anti-inflammatory IL-10 may tip the balance to promoting an immunosuppressive and neuroprotective tissue environment. A study by Mascanfroni et al. using the EAE model showed that IL-27 reduced effector Th1 and Th17 cells and decreased EAE severity. IL-27 induced CD39 (ENTPD1) in dendritic cells (DC) in the EAE model and CD39 was required for IL-27-induced immunosuppression because CD39 knock-out mice had increased EAE severity. Furthermore, IL-27 reduced ATP levels, decreased ATP-dependent activation of the NLRP3 inflammasome, and reduced inflammatory signaling (Mascanfroni et al., 2013). DCs treated with IL-27 had lower levels of IL-6, IL-12, and IL-23 cytokines, lower antigen-presenting cell function, and increased anti-inflammatory cytokines IL-10 and TGF-β1. Microarray analysis demonstrated that IL-27 increased expression of the NF-κB inhibitors Tnip3 and Tnfaip3 in DCs, indicating a transcriptional mechanism for reducing NF-κB inflammatory signaling (Mascanfroni et al., 2013).
In contrast, IL-27 did not alter IL-10 or SOCS-3 levels in cultured microglia co-treated with oncostatin M (OSM) but reduced TNFα and iNOS and inhibited NF-κB activation (Baker et al., 2010). Lower levels of inflammatory cytokines by IL-27 treatment were associated with decreased OSM-induced death of cultured mouse primary cortical neurons (Baker et al., 2010). The mechanism by which IL-27 inhibits NF-κB in these cells is unclear but it may involve altered binding to the gp130 receptor that IL-27 and OSM both use (Baker et al., 2010). Interestingly, IL-27 was shown to induce epigenetic changes in microglia by reducing binding of coactivator p300, decreased acetylation of histone H3 in TNFα and iNOS promoters, and increased binding of the transcriptional corepressor HDAC1 (Baker et al., 2010). Additionally, IL-27 induces several transcription factors, including c-Maf, Egr-2, IRF1, and BATF, which alter chromatin accessibility at promoter regions and induce transcription of target genes that mediate the effects of IL-27, such as IL-10 and genes involved in T cell function (Karwacz et al., 2017). Therefore, these studies suggest that IL-27 is a component of the tissue response that restores homeostatic levels of inflammation.
STAT signaling
Similar to other protective growth factors and cytokines, cytoprotection from IL-27 is mediated through STAT1 or STAT3 signaling. Activation of STAT1 and STAT3 by IL-27 links this pathway to a number of downstream neuroprotective mechanisms including interferon regulatory factors IRF1, IRF8, suppressor of cytokine signaling proteins (SOCS) and IL-10. In a murine stroke model, IL-27-induced neuronal protection depended on STAT3 signaling (Luo et al., 2021). Signaling through STAT1, 3 and 5 has also been implicated in IL-27-induced survival and proliferation of alloreactive splenocytes and confirmed by blocking the protective effects of IL-27 using a JAK/STAT inhibitor (Zhao et al., 2020).
Pro-Inflammatory and Apoptotic Activities of Interleukin-27
Although the majority of studies indicate anti-inflammatory and anti-apoptotic effects of IL-27 in the CNS, developing the IL-27 cytokine as a potential neuroprotective therapy may be limited by its pro-inflammatory and pro-death activities observed in certain cell types and disease models. Although some of these pro-death activities are beneficial and limit an overactive immune response, the mechanism by which IL-27 induces cell death is not entirely understood. A recent study demonstrated that IL-27/STAT3 signaling in the EAE mouse model induced apoptosis of CD4+ T cells, which occurs by inducing PD-L1 on monocyte-derived dendritic cells that bind to programmed cell death protein 1 on pathogenic T cells (Casella et al., 2020). Furthermore, using the streptozotocin-induced mouse model of Alzheimer’s disease, Salem et al. (2021) demonstrated that treatment with the phosphodiesterase 5 inhibitor tadalafil and anti-inflammatory naturally derived product bergapten resulted in reduced IL-27 and IL-23 levels that were associated with decreased neuroinflammation, reduced neuronal loss, and improved cognitive function. These findings raise the possibility that IL-27 promotes degeneration in this mouse model and is suppressed by neuroprotective treatments.
The role of IL-27 in neuroinflammation in the BTBR mouse model of autism was investigated (Ahmad et al., 2017). Inhibiting adenosine A2a receptor signaling in this mouse model led to reduced inflammation, and was associated with lower levels of splenic CD14+IL-27+ cells and other pro-inflammatory molecules, and decreased IL-27 expression in the CNS (Ahmad et al., 2017). These results suggest that IL-27 may be involved in microglial and neuroinflammatory responses underlying adenosine A2a receptor-regulated behaviors.
IL-27 induces apoptosis as a component of its anti-tumor function. For example, IL-27 induced apoptosis of B cells from patients with chronic lymphocyte leukemia, although did not induce apoptosis in B cells from control subjects (Manouchehri-Doulabi et al., 2020). Furthermore, IL-27 promoted apoptosis of several tumor cell types, including enhancing sensitivity to the chemotherapy drug cisplatin in a lung cancer cell line (Jiang et al., 2021) and increasing sensitivity to poly(I:C)-induced cell death in a prostate tumor cell line (Kourko et al., 2019). Evidence for its anti-tumor effects in vivo was shown using mouse models of colon, lung, and breast tumors, in which IL-27 over-expression led to improved survival by depleting Tregs through Stat1 signaling and CD25 downregulation and increasing IL-2 expression (Zhu et al., 2018). How the IL-27/STAT1 and STAT3 signaling pathways lead to survival or death in different cell types remains to be elucidated.
IL-27 also mediates pro-inflammatory effects by promoting proliferation and survival of CD4+ T cells, differentiation, and effector function of Th1/Tfh and inhibition of Treg cells (Wehrens et al., 2018). Several direct and indirect mechanisms have been described for these activities, including CD3 and TCR binding and downregulation of FasL and cFLIP. IL-27 also upregulates pro-inflammatory chemokines, such as Ccl2, Ccl3, and Ccl4 in non-neuronal tissue (Liu et al., 2019), although this has not been reported in the CNS. Furthermore, exogenous IL-27 induced macrophage polarization to a pro-inflammatory phenotype and enhanced liver cell apoptosis in a murine liver injury model (Fan et al., 2021). The effect of IL-27 on inflammation may be influenced by the timing of the immune response because evidence suggests that IL-27 is pro-inflammatory early in the disease course (Zhu et al., 2018), although other factors are surely involved, including specific signaling pathways induced by IL-27 and the co-expression of other cytokines and inflammatory mediators.
Conclusions and Future Directions
Precise control of neuroinflammatory signals is essential to restore tissue homeostasis after CNS injury — too little inflammation would reduce protective immune responses that remove dying neurons and limit damage, whereas too much inflammation impairs neuronal survival and worsens tissue damage. Research into the roles of IL-27 in regulating innate and adaptive immunity pathways that protect the CNS from neurodegeneration is still in its early stages. Several studies show that delivering functional IL-27 to the retina or brain reduces excessive neuroinflammation through several mechanisms and decreases neuronal death. IL-27 also directly stimulates STAT3-mediated survival pathways upon binding to IL-27Rα on neurons. It is important to note that while the majority of studies indicate anti-inflammatory and anti-apoptotic effects of IL-27 in the CNS, the pro-inflammatory functions observed in certain disease models must be properly understood before developing IL-27 as a potential therapy. Key questions to be investigated are: (1) how IL-27 expression and activity are regulated after acute and chronic injury, (2) what is the role of IL-27 in tissues with “immune privilege”, such as the retina, (3) how IL-27 secretion recruits the activity of other immune cells within the CNS in the immediate stages after injury, (4) identification of the signaling pathways that regulate the protective effects of IL-27, (5) what is the contribution of IL-27 signaling within other CNS cell types, such as radial glia, astrocytes, and neurons, to the overall tissue response, and (6) what downstream signals determine whether IL-27 is anti-apoptotic, as seen in neuronal tissue, or pro-apoptotic, as seen in tumor cells. In conclusion, further investigation of the promising cytoprotective and immunomodulatory properties of the IL-27 cytokine is warranted and may ultimately lead to the development of novel therapeutic strategies for diseases of the CNS and retina.
Search Strategy and Selection Criteria
The following search terms were used in PubMed for preparing this narrative review: “IL-27”, “Ebi3”, “WSX-1”, “IL-10” and “IL-27R” in combination with “CNS”, “neuron”, “brain”, “retina”, “spinal cord”, “neurodegenerative disease”, “neurodegeneration”, “apoptosis”, “survival”, or specific diseases (“Alzheimer’s disease”, “Parkinson’s disease”, “multiple sclerosis”, etc.), or in combination with specific cell types (“microglia”, “macrophage”, “dendritic cell”, “neuron”, “neutrophil”, “astrocyte”, “T cell”) or in combination with specific mechanisms (“autophagy”, “JAK/STAT”, “apoptosis”, etc). All years were chosen in the search. These searches were performed between July and September 2021.
Footnotes
Funding: Financial support for this work for ASH was from National Eye Institute R01 EY026546 and an NEI Center Core Grant EY014801.
Conflicts of interest: There are no conflicts of interest.
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Ahmad SF, Ansari MA, Nadeem A, Bakheet SA, Al-Ayadhi LY, Attia SM. Toll-like receptors, NF-kappaB, and IL-27 mediate adenosine A2A receptor signaling in BTBR T(+) Itpr3(tf)/J mice. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:184–191. doi: 10.1016/j.pnpbp.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 2.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 3.Baker BJ, Park KW, Qin H, Ma X, Benveniste EN. IL-27 inhibits OSM-mediated TNF-alpha and iNOS gene expression in microglia. Glia. 2010;58:1082–1093. doi: 10.1002/glia.20989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burmeister AR, Marriott I. The interleukin-10 family of cytokines and their role in the CNS. Front Cell Neurosci. 2018;12:458. doi: 10.3389/fncel.2018.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burrell JA, Boudreau A, Stephens JM. Latest advances in STAT signaling and function in adipocytes. Clin Sci. 2020;134:629–639. doi: 10.1042/CS20190522. [DOI] [PubMed] [Google Scholar]
- 6.Casella G, Finardi A, Descamps H, Colombo F, Maiorino C, Ruffini F, Patrone M, Degano M, Martino G, Muzio L, Becher B, Furlan R. IL-27, but not IL-35, inhibits neuroinflammation through modulating GM-CSF expression. Sci Rep. 2017;7:16547. doi: 10.1038/s41598-017-16702-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casella G, Rasouli J, Thome R, Descamps HC, Vattikonda A, Ishikawa L, Boehm A, Hwang D, Zhang W, Xiao D, Park J, Zhang GX, Alvarez JI, Rostami A, Ciric B. Interferon-gamma/interleukin-27 axis induces programmed death ligand 1 expression in monocyte-derived dendritic cells and restores immune tolerance in central nervous system autoimmunity. Front Immunol. 2020;11:576752. doi: 10.3389/fimmu.2020.576752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu YJ, Ma WD, Thome R, Ping JD, Liu FZ, Wang MR, Zhang ML, Zhang G, Zhu L. Matrine inhibits CNS autoimmunity through an IFN-beta-dependent mechanism. Front Immunol. 2020;11:569530. doi: 10.3389/fimmu.2020.569530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLong JH, O'Hara Hall A, Rausch M, Moodley D, Perry J, Park J, Phan AT, Beiting DP, Kedl RM, Hill JA, Hunter CA. IL-27 and TCR stimulation promote T cell expression of multiple inhibitory receptors. Immunohorizons. 2019;3:13–25. doi: 10.4049/immunohorizons.1800083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietrich C, Candon S, Ruemmele FM, Devergne O. A soluble form of IL-27Ralpha is a natural IL-27 antagonist. J Immunol. 2014;192:5382–5389. doi: 10.4049/jimmunol.1303435. [DOI] [PubMed] [Google Scholar]
- 11.Fan J, He M, Wang CJ, Zhang M. Gadolinium chloride inhibits the production of liver interleukin-27 and mitigates liver injury in the CLP mouse model. Mediators Inflamm. 2021;2021:2605973. doi: 10.1155/2021/2605973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonseca MM, Davoli-Ferreira M, Santa-Cecilia F, Guimaraes RM, Oliveira FFB, Kusuda R, Ferreira DW, Alves-Filho JC, Cunha FQ, Cunha TM. IL-27 counteracts neuropathic pain development through induction of IL-10. Front Immunol. 2019;10:3059. doi: 10.3389/fimmu.2019.03059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garces K, Carmy T, Illiano P, Brambilla R, Hackam AS. Increased neuroprotective microglia and photoreceptor survival in the retina from a peptide inhibitor of myeloid differentiation factor 88 (MyD88) J Mol Neurosci. 2020;70:968–980. doi: 10.1007/s12031-020-01503-0. [DOI] [PubMed] [Google Scholar]
- 14.Garcia AL, Udeh A, Kalahasty K, Hackam AS. A growing field:the regulation of axonal regeneration by Wnt signaling. Neural Regen Res. 2018;13:43–52. doi: 10.4103/1673-5374.224359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang M, Du W, Liu J, Zhang H, Cao L, Yang W, Zhang H, Wang Z, Wei P, Wu W, Huang Z, Fang Y, Lin Q, Qin X, Zhang Z, Zhou K, Zeng J. Interleukin-27 as a novel biomarker for early cardiopulmonary failure in enterovirus 71-infected children with central nervous system involvement. Mediators Inflamm. 2016;2016:4025167. doi: 10.1155/2016/4025167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang B, Shi W, Li P, Wu Y, Li Y, Bao C. The mechanism of and the association between interleukin-27 and chemotherapeutic drug sensitivity in lung cancer. Oncol Lett. 2021;21:14. doi: 10.3892/ol.2020.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karwacz K, Miraldi ER, Pokrovskii M, Madi A, Yosef N, Wortman I, Chen X, Watters A, Carriero N, Awasthi A, Regev A, Bonneau R, Littman D, Kuchroo VK. Critical role of IRF1 and BATF in forming chromatin landscape during type 1 regulatory cell differentiation. Nat Immunol. 2017;18:412–421. doi: 10.1038/ni.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D, Le HT, Nguyen QT, Kim S, Lee J, Min B. Cutting edge:IL-27 attenuates autoimmune neuroinflammation via regulatory T cell/Lag3-dependent but IL-10-independent mechanisms in vivo. J Immunol. 2019;202:1680–1685. doi: 10.4049/jimmunol.1800898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosloski LM, Kosmacek EA, Olson KE, Mosley RL, Gendelman HE. GM-CSF induces neuroprotective and anti-inflammatory responses in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxicated mice. J Neuroimmunol. 2013;265:1–10. doi: 10.1016/j.jneuroim.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kouchaki E, Kakhaki RD, Tamtaji OR, Dadgostar E, Behnam M, Nikoueinejad H, Akbari H. Increased serum levels of TNF-alpha and decreased serum levels of IL-27 in patients with Parkinson disease and their correlation with disease severity. Clin Neurol Neurosurg. 2018;166:76–79. doi: 10.1016/j.clineuro.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Kourko O, Smyth R, Cino D, Seaver K, Petes C, Eo SY, Basta S, Gee K. Poly(I:C)-mediated death of human prostate cancer cell lines is induced by interleukin-27 treatment. J Interferon Cytokine Res. 2019;39:483–494. doi: 10.1089/jir.2018.0166. [DOI] [PubMed] [Google Scholar]
- 22.Lalive PH, Kreutzfeldt M, Devergne O, Metz I, Bruck W, Merkler D, Pot C. Increased interleukin-27 cytokine expression in the central nervous system of multiple sclerosis patients. J Neuroinflammation. 2017;14:144. doi: 10.1186/s12974-017-0919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laverdure S, Wang Z, Yang J, Yamamoto T, Thomas T, Sato T, Nagashima K, Imamichi T. Interleukin-27 promotes autophagy in human serum-induced primary macrophages via an mTOR- and LC3-independent pathway. Sci Rep. 2021;11:14898. doi: 10.1038/s41598-021-94061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YS, Amadi-Obi A, Yu CR, Egwuagu CE. Retinal cells suppress intraocular inflammation (uveitis) through production of interleukin-27 and interleukin-10. Immunology. 2011;132:492–502. doi: 10.1111/j.1365-2567.2010.03379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Gran B, Zhang GX, Rostami A, Kamoun M. IL-27 subunits and its receptor (WSX-1) mRNAs are markedly up-regulated in inflammatory cells in the CNS during experimental autoimmune encephalomyelitis. J Neurol Sci. 2005;232:3–9. doi: 10.1016/j.jns.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Li S, Jin J, Zhu T, Xu K, Liu C, Zeng Y, Mao R, Wang X, Chen Z. Preventative tracheal administration of interleukin-27 attenuates allergic asthma by improving the lung Th1 microenvironment. J Cell Physiol. 2019;234:6642–6653. doi: 10.1002/jcp.27422. [DOI] [PubMed] [Google Scholar]
- 27.Luo C, Li B, Chen L, Zhao L, Wei Y. IL-27 protects the brain from ischemia-reperfusion injury via the gp130/STAT3 signaling pathway. J Mol Neurosci. 2021;71:1838–1848. doi: 10.1007/s12031-021-01802-0. [DOI] [PubMed] [Google Scholar]
- 28.Ly D, Dongol A, Cuthbertson P, Guy TV, Geraghty NJ, Sophocleous RA, Sin L, Turner BJ, Watson D, Yerbury JJ, Sluyter R. The P2X7 receptor antagonist JNJ-47965567 administered thrice weekly from disease onset does not alter progression of amyotrophic lateral sclerosis in SOD1(G93A) mice. Purinergic Signal. 2020;16:109–122. doi: 10.1007/s11302-020-09692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manouchehri-Doulabi E, Abbaspour S, Rostami S, Faranoush M, Ghahramanfard F, Pak F, Barati M, Kokhaei P, Momtazi-Borojeni AA. Evaluating the mechanism underlying antitumor effect of interleukin 27 on B cells of chronic lymphocytic leukemia patients. J Cell Physiol. 2020;235:9424–9431. doi: 10.1002/jcp.29747. [DOI] [PubMed] [Google Scholar]
- 30.Mascanfroni ID, Yeste A, Vieira SM, Burns EJ, Patel B, Sloma I, Wu Y, Mayo L, Ben-Hamo R, Efroni S, Kuchroo VK, Robson SC, Quintana FJ. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol. 2013;14:1054–1063. doi: 10.1038/ni.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pradhan A, Lambert QT, Reuther GW. Transformation of hematopoietic cells and activation of JAK2-V617F by IL-27R, a component of a heterodimeric type I cytokine receptor. Proc Natl Acad Sci U S A. 2007;104:18502–18507. doi: 10.1073/pnas.0702388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salem MA, Budzynska B, Kowalczyk J, El Sayed NS, Mansour SM. Tadalafil and bergapten mitigate streptozotocin-induced sporadic Alzheimer's disease in mice via modulating neuroinflammation, PI3K/Akt, Wnt/beta-catenin, AMPK/mTOR signaling pathways. Toxicol Appl Pharmacol. 2021;429:115697. doi: 10.1016/j.taap.2021.115697. [DOI] [PubMed] [Google Scholar]
- 33.Senecal V, Deblois G, Beauseigle D, Schneider R, Brandenburg J, Newcombe J, Moore CS, Prat A, Antel J, Arbour N. Production of IL-27 in multiple sclerosis lesions by astrocytes and myeloid cells:modulation of local immune responses. Glia. 2016;64:553–569. doi: 10.1002/glia.22948. [DOI] [PubMed] [Google Scholar]
- 34.Sharma G, Dutta RK, Khan MA, Ishaq M, Sharma K, Malhotra H, Majumdar S. IL-27 inhibits IFN-gamma induced autophagy by concomitant induction of JAK/PI3 K/Akt/mTOR cascade and up-regulation of Mcl-1 in Mycobacterium tuberculosis H37Rv infected macrophages. Int J Biochem Cell Biol. 2014;55:335–347. doi: 10.1016/j.biocel.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu M, Ogura K, Mizoguchi I, Chiba Y, Higuchi K, Ohtsuka H, Mizuguchi J, Yoshimoto T. IL-27 promotes nitric oxide production induced by LPS through STAT1, NF-kappaB and MAPKs. Immunobiology. 2013;218:628–634. doi: 10.1016/j.imbio.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 36.Sowrirajan B, Saito Y, Poudyal D, Chen Q, Sui H, DeRavin SS, Imamichi H, Sato T, Kuhns DB, Noguchi N, Malech HL, Lane HC, Imamichi T. Interleukin-27 enhances the potential of reactive oxygen species generation from monocyte-derived macrophages and dendritic cells by induction of p47(phox) Sci Rep. 2017;7:43441. doi: 10.1038/srep43441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wehrens EJ, Wong KA, Gupta A, Khan A, Benedict CA, Zuniga EI. IL-27 regulates the number, function and cytotoxic program of antiviral CD4 T cells and promotes cytomegalovirus persistence. PLoS One. 2018;13:e0201249. doi: 10.1371/journal.pone.0201249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annu Rev Immunol. 2015;33:417–443. doi: 10.1146/annurev-immunol-032414-112134. [DOI] [PubMed] [Google Scholar]
- 39.Zhao X, Ting SM, Liu CH, Sun G, Kruzel M, Roy-O'Reilly M, Aronowski J. Neutrophil polarization by IL-27 as a therapeutic target for intracerebral hemorrhage. Nat Commun. 2017;8:602. doi: 10.1038/s41467-017-00770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Z, Peng X, Insolera R, Fink DJ, Mata M. Interleukin-10 provides direct trophic support to neurons. J Neurochem. 2009;110:1617–1627. doi: 10.1111/j.1471-4159.2009.06263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu J, Liu JQ, Shi M, Cheng X, Ding M, Zhang JC, Davis JP, Varikuti S, Satoskar AR, Lu L, Pan X, Zheng P, Liu Y, Bai XF. IL-27 gene therapy induces depletion of Tregs and enhances the efficacy of cancer immunotherapy. JCI Insight. 2018;3:e98745. doi: 10.1172/jci.insight.98745. [DOI] [PMC free article] [PubMed] [Google Scholar]


