Abstract
The plasma pharmacokinetics and tissue distribution of the novel antifungal echinocandin-like lipopeptide micafungin (FK463) were investigated in healthy rabbits. Cohorts of three animals each received micafungin at 0.5, 1, and 2 mg/kg of body weight intravenously once daily for a total of 8 days. Serial plasma samples were collected on days 1 and 7, and tissue samples were obtained 30 min after the eighth dose. Drug concentrations were determined by validated high-performance liquid chromatographic methods. Plasma drug concentration data were fit to a two-compartment pharmacokinetic model, and pharmacokinetic parameters were estimated using weighted nonlinear least-square regression analysis. Micafungin demonstrated linear plasma pharmacokinetics without changes in total clearance and dose-normalized area under the concentration-time curve from 0 h to infinity. After administration of single doses to the rabbits, mean peak plasma drug concentrations ranged from 7.62 μg/ml at 0.5 mg/kg to 16.8 μg/ml at 2 mg/kg, the area under the concentration-time curve from 0 to 24 h ranged from 5.66 to 21.79 μg · h/ml, the apparent volume of distribution at steady state ranged from 0.296 to 0.343 liter/kg, and the elimination half-life ranged from 2.97 to 3.20 h, respectively. No significant changes in pharmacokinetic parameters and no accumulation was noted after multiple dosing. Mean tissue micafungin concentrations 30 min after the last of eight daily doses were highest in the lung (2.26 to 11.76 μg/g), liver (2.05 to 8.82 μg/g), spleen (1.87 to 9.05 μg/g), and kidney (1.40 to 6.12 μg/g). While micafungin was not detectable in cerebrospinal fluid, the concentration in brain tissue ranged from 0.08 to 0.18 μg/g. These findings indicate linear disposition of micafungin at dosages of 0.5 to 2 mg/kg and achievement of potentially therapeutic drug concentrations in plasma and tissues that are common sites of invasive fungal infections.
Micafungin (FK463) is a novel, semisynthetic antifungal echinocandin-like lipopeptide that inhibits the synthesis on 1,3β-glucan, an essential polymeric polysaccharide in the cell wall of many pathogenic fungi (12, 24). As a class, the echinocandins lack mechanism-based toxicity and have an extended spectrum of antifungal activity without cross-resistance to existing antifungal agents (4, 5, 9, 15, 18). In vitro, micafungin has demonstrated potent and broad-spectrum fungicidal activity against clinically relevant Candida spp. and potent inhibitory activity against Aspergillus spp. (21, 23, 25). The compound displayed promising antifungal efficacy in murine models of disseminated candidiasis as well as disseminated and pulmonary aspergillosis (16, 19, 20) and is currently in advanced stages of clinical development (12).
Little is still known, however, about the disposition of micafungin in plasma and tissues. Therefore, the purpose of this study was to assess the compartmental plasma pharmacokinetics and tissue distribution of micafungin at potentially therapeutic dosages in healthy laboratory animals. The information derived from this study will assist to further explore the relationships between concentration and effect of micafungin in pharmacodynamic models of disseminated candidiasis and invasive pulmonary aspergillosis in persistently neutropenic animals of the same species.
(A preliminary report of this work has been presented previously [A. H. Groll, D. Mickiene, V. Petraitis, R. Petraitiene, R. Alfaro, K. H. Ibrahim, A. Kalim, I. Bekersky, S. C. Piscitelli, and T. J. Walsh, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1688, p. 388, 2000].)
MATERIALS AND METHODS
Experimental design. (i) Study drug.
Micafungin (FK463; Fujisawa USA, Inc. Deerfield, Ill.) was provided as a 10-mg/ml solution for injection and maintained at room temperature protected from light. Prior to use, the drug was freshly diluted with sterile normal saline to a 1-mg/ml solution. Micafungin was administered at ambient temperature as a slow intravenous (IV) bolus over 4 min through the indwelling catheter.
(ii) Animals.
Healthy female New Zealand White rabbits (Hazleton, Denver, Pa.) weighing 2.8 to 3.2 kg were used in all experiments. They were individually housed and maintained with water and standard rabbit feed ad libitum according to the National Institutes of Health Guidelines for Laboratory Animal Care (2) and in fulfillment of American Association for Accreditation of Laboratory Animal Care criteria. Vascular access was established in each rabbit ≥72 h prior to experimentation by the surgical placement of a subcutaneous silastic central venous catheter as previously described (27).
(iii) Single-dose plasma pharmacokinetics.
Three groups of three animals each were studied. Animals received micafungin at 0.5, 1, or 2 mg/kg of body weight as a steady IV bolus over 4 min. Plasma samples (2.0 ml of blood) were drawn immediately before administration of the drug and then at 0.1 (maximum concentration of drug in plasma [Cmax]), 0.25, 0.5, 1, 2, 4, 6, 8, 12, 18, and 24 h after the start of the IV bolus.
(iv) Multiple-dose plasma pharmacokinetics.
After completion of single-dose pharmacokinetics, the identical three groups of three animals each continued to receive micafungin at either 0.5, 1, or 2 mg/kg of body weight once daily as a steady IV bolus over 4 min for a total of 7 days. On day 7, plasma samples (2.0 ml of blood) were drawn immediately before administration of the drug and then at 0.1 (Cmax), 0.25, 0.5, 1, 2, 4, 6, 8, 12, 18, and 24 h after the start of the IV bolus.
(v) Tissue distribution studies.
For the assessment of tissue micafungin concentrations near peak plasma micafungin levels after multiple doses, animals received one more dose of the assigned regimen on day 8. All animals were sacrificed 30 min after dosing by IV pentobarbital and brain tissue, cerebrospinal fluid (CSF), choroid, vitreous humor, aqueous humor, lung, liver, spleen, and kidney were obtained at autopsy for analysis of drug concentrations.
(vi) Assessment of tolerance.
All animals were evaluated clinically each day. Biochemical parameters of hepatic and renal toxicities were monitored in plasma samples obtained on the last day of the experiment. Values were compared to reference values established in healthy rabbits naive to prior drug exposure.
Processing of samples and analytical assay. (i) Processing of blood and tissues.
Blood samples were collected in heparinized syringes, and plasma was separated by centrifugation. All plasma, body fluid, and tissue samples were stored at −80°C until assay.
Micafungin was extracted from acidified heparinized plasma by liquid/liquid extraction with acetonitrile-based organic solvents and diluted with phosphate buffer prior to injection. Tissue specimens were thoroughly rinsed with phosphate-buffered saline and blotted to dryness with Micro Wipes (Scott Paper Company, Philadelphia, Pa.). Specimens were then weighed and homogenized twice for 30 s each time with ice-cold phosphate-buffered saline (pH 7.4) (1:4 [wt/wt]) using a high-speed tissue homogenizer (Ultra-Turrax; Tekmar, Cincinnati, Ohio) with a 10N head and placement of the sample in an ice bucket. The homogenates were incubated for 30 min at 4°C and centrifuged at 2,000 × g for 10 min. Micafungin was extracted from tissue homogenates and all other body fluids by solid-phase extraction using acetonitrile-ammonium acetate-based solvents and C8 bonded phase extraction cartridges (Varian Inc., Harbor City, Calif.) as previously described (14). Standards and quality control samples were similarly prepared by adding known amounts of micafungin to either healthy rabbit plasma (for serum and choroid; Gibco Laboratories, Grand Islands, N.Y.), commercially available CSF standards (Instrumentation Laboratories, Lexington, Mass.), Hanks' balanced salt solution (for vitreous and aqueous humor; Mediatech, Herndon, Va.), or healthy rabbit tissue homogenates. Blank samples of all matrices also were extracted to ensure the absence of interfering peaks.
(ii) Analytical assay.
Concentrations of micafungin were determined using reversed-phase high-performance liquid chromatography. For plasma, the mobile phase consisted of 20 mM KH2PO4-acetonitrile (59:41 [vol/vol]), delivered at 1 ml/min. Samples were maintained in the autosampler at room temperature in amber glass vials. The injection volume was 75 μl. Micafungin eluted at 10.3 to 13.8 min, using a 5-μm TSK-GEL silica-based analytical column (ODS80TM [150 by 4.6 mm]; TosoHaas, Montgomeryville, Pa.) maintained at 50°C in conjunction with a precolumn filter containing a 5-μm insert and fluorimetric detection (excitation wavelength, 273 nm excitation; emission wavelength 464 nm). For nonplasma body fluids and tissue homogenates, the mobile phase consisted of acetonitrile–50 mM ammonium acetate (pH 4.0) (45:55 [vol/vol]), delivered at 0.75 ml/min. Samples were maintained in the autosampler at room temperature in amber glass vials. The injection volume was 75 μl. Micafungin eluted at circa 8 min, using a 5-μm C8 analytical column (Alltech Inertsil [150 by 4.6 mm]; Alltech, Deerfield, Ill.) maintained at room temperature, and UV detection at a wavelength of 273 nm.
Quantification was based on the peak height of micafungin and the nonweighted concentration response of the external calibration standard. Eight- to ten-point standard curves (range, 0.05 to 25 μg/ml for plasma; 0.05 to 5 μg/ml for all other matrices) were linear with r2 values greater than 0.987. The lower limit of quantification (LLQ) in plasma was 0.100 μg/ml, and the LLQ in all other body fluids and tissue homogenates was 0.05 μg/ml. The methods were sensitive to at least 0.010 μg/ml. Accuracies in plasma were within 1.7 to 12.8%, and intra- and interday variability ranged from 1.2 to 6.8% (0.4 to 13% for tissues and 0.99 to 8.44% for nonplasma body fluids).
Pharmacokinetic data analysis. (i) Pharmacokinetic modeling.
Pharmacokinetic parameters for micafungin were determined using compartmental analysis. Experimental plasma micafungin concentration-versus-time profiles were fitted to a two-compartment open model with IV bolus input and linear first-order elimination from the central compartment using iterative weighted nonlinear least-squares regression with the ADAPT II computer program (3). Model selection was guided by visual inspection of the plasma drug profiles and Akaike's information criterion (28). The model fit the data well, with r2 values for the individual fits ranging from 0.954 to 1.000 (mean, 0.977). The regression lines through the plot of observed concentrations versus estimated concentrations did not differ from the line of identity, and no bias was observed. Cmaxs were determined as model-estimated concentrations 6 min after the start of the IV bolus, and the minimum concentrations of drug in plasma (Cmins) were determined as model-estimated concentrations 24 h postdosing, respectively. Area under the concentration-time curve from 0 h to infinity (AUC0–∞) was calculated from estimated 24-h plasma drug concentration profiles using the trapezoidal rule and extrapolation to infinity by standard techniques (8). Dose linearity after single and multiple doses was determined by comparison of the dose-normalized AUC0–∞ across dosage levels by analysis of variance (ANOVA) and linear regression analysis. Accumulation was assessed for each dosage level by comparing the mean AUC between doses after multiple doses as an approximation of AUC between doses at steady state with the mean AUC0–∞ after single doses. Distribution and clearance terms were normalized to body weight to allow for comparison across species.
(ii) Statistical analysis.
All values are presented as means ± standard errors of the means for three animals in each group. Differences between the means of pharmacokinetic parameters across dosage levels were evaluated by ANOVA. A two-tailed P value of <0.05 was considered statistically significant.
RESULTS
Single-dose studies.
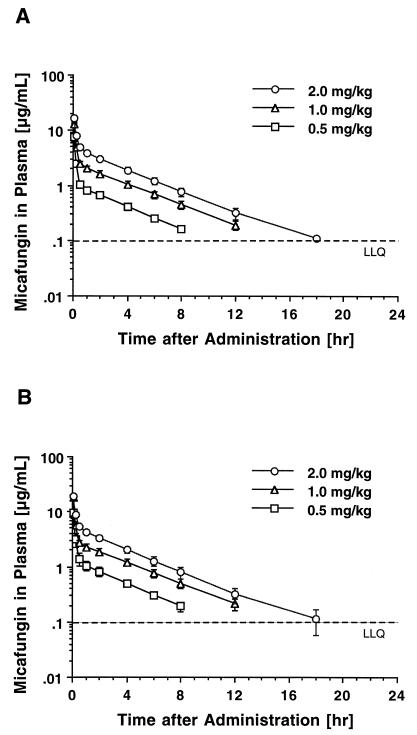
The estimated plasma drug concentration-versus-time curves following single-dose administration of micafungin are shown in Fig. 1A, and the corresponding mean compartmental pharmacokinetic parameters are listed in Table 1. IV bolus administration of micafungin at dosages of 0.5 to 2 mg/kg resulted in mean peak plasma drug levels that ranged from 7.67 ± 1.49 to 16.08 ± 1.72 μg/ml. Plasma drug concentration profiles showed a rapid initial distributive phase, followed by a slower elimination phase with an estimated elimination half-life of approximately 3 h. Mean plasma drug levels fell below the LLQ (0.1 μg/ml) in a dose-dependent manner 8, 12, and 18 h after dosing. Consistent with dose-independent, linear plasma pharmacokinetics, total plasma clearance (CLt) and dose-normalized AUC0–∞ were not different across the investigated dosage range. Similarly, the apparent volume of distribution at steady state (VSS) did not change with the dosage.
FIG. 1.
Concentration-versus-time profiles in plasma after IV bolus administration of micafungin over 4 min. (A) Single-dose profiles after administration of 0.5, 1, and 2 mg/kg. (B) Profiles after administration of 0.5, 1, and 2 mg/kg over 7 days. Each point is the mean ± SEM for three rabbits at that time point. The LLQ was 0.100 μg/ml.
TABLE 1.
Single-dose compartmental pharmacokinetic parameters of micafungin in plasmaa
| Drug dose (mg/kg) | Cmax (μg/ml) | Cmin (μg/ml) | AUC0–∞ (μg · h/ml) | Vp (liter/kg) | Vc (liter/kg) | VSS (liter/kg) | CLd (liter/h/kg) | CLt (liter/h/kg) | α-HL (h) | β-HL (h) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 7.672 ± 1.495 | <LLQ | 5.68 ± 0.43 | 0.305 ± 0.026 | 0.04 ± 0.008 | 0.301 ± 0.025 | 0.267 ± 0.047 | 0.09 ± 0.007 | 0.071 ± 0.003 | 2.974 ± 0.109 |
| 1.0 | 13.073 ± 0.204 | <LLQ | 13.50 ± 1.56 | 0.251 ± 0.02 | 0.045 ± 0.000 | 0.296 ± 0.019 | 0.316 ± 0.008 | 0.077 ± 0.01 | 0.07 ± 0.002 | 3.2 ± 0.145 |
| 2.0 | 16.087 ± 1.725 | <LLQ | 21.96 ± 1.73 | 0.254 ± 0.006 | 0.089 ± 0.018 | 0.343 ± 0.014 | 0.428 ± 0.023 | 0.089 ± 0.01 | 0.325 ± 0.22 | 3.049 ± 0.269 |
| P valueb | 0.0113 | NA | 0.0005 | 0.9060 | 0.0407 | 0.2561 | 0.0255 | 0.5282 | 0.2566 | 0.7005 |
All values represent the means ± SEMs for three rabbits. Abbreviations: Vp and Vc, volume of distribution in the peripheral and central compartment, respectively; CLd, distributional clearance; α-HL, distributional half-life; β-HL, elimination half-life; NA, not applicable. The LLQ of the analytical assay was 0.1 μg/ml.
P values for the comparison among dosage groups by ANOVA.
Multiple-dose studies.
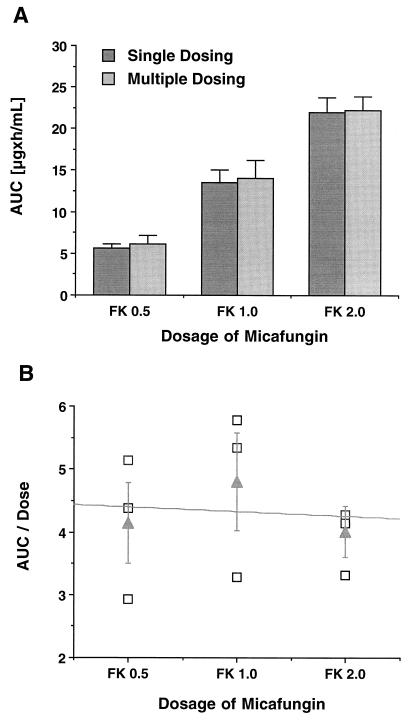
The estimated plasma micafungin concentration-versus-time profiles following multiple once-daily doses of the compound for 7 days are shown in Fig. 1B, and the corresponding mean compartmental pharmacokinetic parameters are listed in Table 2. At all three dosage levels, plasma drug concentrations immediately prior to dosing were below the LLQ. Peak plasma drug concentrations immediately after dosing were not significantly different from those observed after administration of a single dose. Similar to single-dose administration, mean plasma drug levels fell below LLQ in a dose-dependent manner 8, 12, and 18 h postdosing. There were no significant differences in AUC (Fig. 2A), VSS, CLt, and half-life compared to the values after single doses. No differences in dose-normalized AUC0–∞ across the investigated dosages were noted by ANOVA and linear regression (Fig. 2B), indicating dose-independent plasma pharmacokinetics of micafungin also after multiple doses.
TABLE 2.
Multiple-dose compartmental pharmacokinetic parameters of micafungin in plasmaa
| Drug dose (mg/kg) | Cmax (μg/ml) | Cmin (μg/ml) | AUC0–∞ (μg · h/ml) | Vp (liter/kg) | Vc (liter/kg) | VSS (liter/kg) | CLd (liter/h/kg) | CLt (liter/h/kg) | α-HL (h) | β-HL (h) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 9.443 ± 1.83 | <LLQ | 6.22 ± 0.97 | 0.226 ± 0.053 | 0.031 ± 0.004 | 0.258 ± 0.057 | 0.182 ± 0.085 | 0.075 ± 0.012 | 0.07 ± 0.006 | 2.988 ± 0.424 |
| 1.0 | 18.093 ± 1.19 | <LLQ | 14.16 ± 2.11 | 0.215 ± 0.02 | 0.029 ± 0.002 | 0.245 ± 0.02 | 0.235 ± 0.009 | 0.067 ± 0.01 | 0.063 ± 0.002 | 3.178 ± 0.192 |
| 2.0 | 19.165 ± 0.305 | <LLQ | 22.29 ± 1.76 | 0.223 ± 0.011 | 0.090 ± 0.011 | 0.313 ± 0.007 | 0.393 ± 0.043 | 0.083 ± 0.009 | 0.101 ± 0.011 | 3.347 ± 0.447 |
| P valueb | 0.0096 | NA | 0.0040 | 0.9774 | 0.0002 | 0.5479 | 0.0306 | 0.9227 | 0.0222 | 0.7995 |
All values represent the means ± SEMs of 3 rabbits each. Abbreviations: Vp and Vc, volume of distribution of the peripheral and central compartment, respectively; CLd, distributional clearance; α-HL, distributional half-life; β-HL, elimination half-life; NA, not applicable. The LLQ of the analytical assay was 0.1 μg/ml.
P values for the comparison among dosage groups by ANOVA.
FIG. 2.
(A) AUC0–∞ at the three investigated dosage levels (0.5, 1, and 2 mg) after administration of single and multiple doses of micafungin (FK). Each bar represents the mean ± SEM of three rabbits. Note the absence of drug accumulation in plasma over time after multiple once-daily doses. (B) Plot of dose-normalized AUC after multiple once-daily doses with micafungin over 7 days versus dosage. The values for individual animals (squares) and the corresponding means (triangles) ± SEM are shown. The slope of the regression line is not significantly different from zero, indicating linear disposition in plasma over the investigated dosage range.
Tissue distribution.
Mean tissue drug concentrations near peak plasma drug concentrations 30 min after the last of eight daily doses of micafungin are shown in Table 3. At this time point of the dosing interval, the highest concentrations were detected in the lung, followed by the liver, spleen, and kidney. Drug concentrations in these organs increased proportionally to the dosage and ranged from 2.26 ± 0.10 to 11.76 ± 1.40 μg/g in the lung to 1.40 ± 0.08 to 6.12 ± 0.17 in the kidney. Although micafungin was undetectable in CSF, it was detectable in brain tissue in all animals at mean concentrations ranging from 0.08 ± 0.01 to 0.18 ± 0.02 μg/g. Similar drug concentrations were measured in the choroidal layer of the eye. Concentrations of micafungin in vitreous humor were comparatively low, and the compound was undetectable in aqueous humor.
TABLE 3.
Tissue micafungin concentrations after multiple doses over 8 days
| Drug dose (mg/kg) | Micafungin concn in tissue or body fluida:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lung (μg/g) | Liver (μg/g) | Spleen (μg/g) | Kidney (μg/g) | Brain (μg/g) | CSF (μg/ml) | Choroid (μg/ml) | Vitreous humor (μg/ml) | Aqueous humor (μg/ml) | |
| 0.5 | 2.26 ± 0.10 | 2.05 ± 0.69 | 1.87 ± 0.09 | 1.40 ± 0.08 | 0.08 ± 0.01 | ND | 0.012 ± 0.014 | ND | ND |
| 1.0 | 5.59 ± 1.31 | 4.11 ± 0.15 | 4.30 ± 0.11 | 3.34 ± 0.14 | 0.10 ± 0.00 | ND | 0.061 ± 0.039 | 0.015 ± 0.025 | ND |
| 2.0 | 11.76 ± 1.40 | 8.82 ± 0.72 | 9.05 ± 0.25 | 6.12 ± 0.17 | 0.18 ± 0.02 | ND | 0.162 ± 0.096 | 0.034 ± 0.032 | ND |
All values represent the means ± SEMs for three rabbits. Tissues and body fluids were obtained 30 min after the last of eight once-daily doses of FK463. ND, not detectable.
Toxicity.
Abnormal elevations in the mean blood urea nitrogen, serum creatinine, plasma potassium, magnesium, bilirubin, alkaline phosphatase, and hepatic transaminase levels were not observed in samples determined after 8 days of treatment. Throughout the pharmacokinetic study, no apparent infusion-related toxicities or other clinical abnormalities were observed and no abnormal weight changes were noted.
DISCUSSION
The results of this study demonstrate linear plasma pharmacokinetics of micafungin across the investigated dosage range of 0.5 and 2 mg/kg as evidenced by dose-independent plasma clearance and dose-proportional increases in AUC0–∞ with increasing dosage. Plasma drug concentration data fit best to a two-compartment open pharmacokinetic model that revealed an apparent elimination half-life of approximately 3 h. Micafungin achieved sustained plasma drug concentrations that were multiple times in excess of MICs reported for opportunistic fungi known to be susceptible to the compound. No significant differences in pharmacokinetic parameters were noted between single-dose and multiple-dose administration. Tissue drug concentrations near the completion of the initial distributive phase of micafungin in plasma showed substantial disposition in lung, liver, spleen, and kidney with achievement of potentially therapeutic concentrations at these sites. Micafungin was undetectable in CSF but was found in low concentrations in brain and eye tissues of all animals. The compound was well tolerated in rabbits without evidence for clinical or laboratory toxicities.
The favorable pharmacokinetic profile of micafungin is in principle shared by caspofungin and anidulafungin, structurally and functionally similar echinocandin-like lipopeptides that are currently in clinical development. Using a dosage of 1 mg/kg for comparison, the plasma pharmacokinetics of micafungin and caspofungin in healthy rabbits appear virtually identical. After multiple once-daily doses of caspofungin over 7 days, mean Cmax, AUC0–∞, VSS, and CLt values were 16.01 ± 0.61 μg/ml, 13.15 ± 2.37 μg · h/ml, 0.299 ± 0.01 liter/kg, and 0.086 ± 0.01 liter/h/kg, respectively, and were thus not different from the values observed for micafungin following the identical dosing schedule (11). In contrast, at similar dosages and dosing schedules, anidulafungin exhibited an approximately sixfold-lower mean Cmax, a twofold-faster CLt, a twofold-lower AUC0–∞ but a fourfold-larger VSS in comparison to the values for micafungin and caspofungin (14). Whether these differences in plasma pharmacokinetics are associated with differences in pharmacodynamics remains to be investigated.
The plasma pharmacokinetics of micafungin in rabbits after single doses were similar to those obtained in mice, rats, and dogs. In these species, after a single IV bolus of 1 mg/kg, the mean AUC0–24 ranged from 11.9 to 21.2 μg · h/ml, the mean VSS ranged from 0.25 to 0.56 liter/kg, the mean CLt ranged from 0.079 to 0.046 liter/h/kg, and the half-life ranged from 4.57 to 5.34 h, (S. Suzuki, M. Terakawa, F. Yokobayashi, F. Fujiwara, and T. Hata, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F144, 1998). In healthy male human volunteers, at dosages ranging from 12.5 to 50 mg given as a 2-h infusion by the IV route, micafungin exhibited linear pharmacokinetics with mean Cmaxs ranging from 0.94 to 3.36 μg/ml and mean AUC0–∞ values of 17.11 to 60.93 μg · h/ml. The mean VSS was between 0.237 and 0.242 liter/kg, and the terminal half-life was approximately 15 h. While mean peak plasma drug concentrations in humans were four- to 13-fold lower than after bolus administration in rabbits, AUC0–∞ values were similar at the 12.5-mg dosage level (approximately 0.25 mg/kg) and four- to fivefold higher at comparable dosages (J. Azuma, I. Yamamoto, M. Ogura, T. Mukai, H. Suematsu, H. Kageyama, K. Nakahara, K. Yoshida, and T. Takaya, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F146, 1998). Notwithstanding the different modes of drug administration, the plasma clearance of micafungin was approximately six- to sevenfold less in humans than that in rabbits. As a consequence of the different modes of administration (i.e., bolus versus 2-h infusion), direct scaling of therapeutically effective dosages from rabbits to humans would be best accomplished by formal pharmacodynamic models that link dosage, concentrations over time, and antifungal effects.
Despite the fact that tissue drug concentrations represent a mixture of drug concentrations in the intravascular, interstitial, and intracellular compartments (1), information on these concentrations is of potential utility in the selection of antifungal therapies (14). Cognizant of the fact that different equilibria may prevail during the dosing interval, assessment of tissue concentrations of micafungin after the administration of multiple once-daily doses for 8 days revealed potentially therapeutic drug concentrations in lung, liver, spleen, and kidneys near the completion of the initial distributive phase in plasma. Similar to amphotericin B (13), micafungin was undetectable in CSF and achieved relatively low levels in brain tissue compared to other sites. However, therapeutically effective levels of micafungin in brain tissue may be achieved in the state of tissue inflammation and/or necrosis, as evidenced by the effective clearance of Candida albicans from the central nervous system in our persistently neutropenic rabbit model of subacute disseminated candidiasis (V. Petraitis, R. Petraitiene, A. H. Groll, T. Sein, R. L. Schaufele, J. Bacher, and T. J. Walsh, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1684, 2000).
Micafungin achieved plasma and tissue drug concentrations that were severalfold in excess of the MICs at which 90% of the Candida and Aspergillus isolates tested are inhibited (21, 23, 25). In plasma, concentrations above these values were maintained in a dose-dependent manner for up to 18 h. Similar to cilofungin (26), caspofungin (7), and anidulafungin (10, 17, 22), micafungin exhibits predominantly concentration-dependent fungicidal activities against Candida spp. in vitro (Petraitis et al., 40th ICAAC). Concentration-dependent activity also was demonstrated in a Candida thigh infection model, where the ratio between tissue concentrations and MIC was found to be highly predictive for therapeutic efficacy of micafungin (S. Matsumoto, E. Warabe, Y. Wakai, Y. Koide, T. Ushitani, N. Teratani, K. Ohtomo, K. Hatano, F. Ikeda, T. Goto, F. Matsumoto, and S. Kuwahara, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1687, 2000). Pharmacokinetic and pharmacodynamic modeling of anidulafungin in our neutropenic rabbit model of disseminated candidiasis revealed that, apart from drug-specific threshold values for Cmax, AUC0–24, and tissue concentrations, maintenance of plasma drug concentrations above the minimum fungicidal concentration of the experimental isolate for ≥12 h was associated with 100% efficacy (14). These findings and the documentation of a concentration-dependent, prolonged postantifungal effect of up to 12 h and longer for caspofungin and anidulafungin (6) suggest that once-daily dosing regimens are also appropriate for micafungin. Nevertheless, pharmacodynamic studies comparing single- versus split-dose regimens are needed for a pharmacodynamically founded determination of the optimal dosing regimen.
In conclusion, micafungin displayed linear plasma pharmacokinetics that were best described by a two-compartment pharmacokinetic model. The drug achieved and maintained potentially therapeutic plasma drug concentrations exceeding the MICs of susceptible opportunistic fungi and distributed into tissues that are common sites of deeply invasive infections. The compound was well tolerated without evidence of clinical or laboratory toxicity. The characterization of the pharmacokinetics of micafungin in the rabbit will be of help for the design of pharmacodynamic animal models investigating the concentration-response relationships of this novel echinocandin-like lipopeptide. The findings from such studies are anticipated to support the determination of optimal dosing regimens in patients.
ACKNOWLEDGMENTS
We thank Azhar Kalim at MDS Harris, Lincoln, Nebr., for assistance with the analytical assay of micafungin in plasma and our colleagues Myrna Candelario and Aida Field-Ridley for expert technical support in conducting these experiments.
REFERENCES
- 1.Cars O. Pharmacokinetics of antibiotics in tissues and tissue fluids: a review. Scand J Infect Dis. 1991;74(Suppl.):23–33. [PubMed] [Google Scholar]
- 2.Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources; Commission on Life Sciences; National Research Council. Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- 3.D'Argenio D Z, Schumitzky A. ADAPT II user's guide. Biomedical Simulations Resource. Los Angeles: University of Southern California; 1997. [Google Scholar]
- 4.Debono M, Gordee R S. Antibiotics that inhibit fungal cell wall development. Annu Rev Microbiol. 1994;48:471–497. doi: 10.1146/annurev.mi.48.100194.002351. [DOI] [PubMed] [Google Scholar]
- 5.Denning D W. Echinocandins and pneumocandins–a new antifungal class with a novel mode of action. J Antimicrob Chemother. 1997;40:611–614. doi: 10.1093/jac/40.5.611. [DOI] [PubMed] [Google Scholar]
- 6.Ernst E J, Klepser M E, Pfaller M A. Postantifungal effects of echinocandin, azole, and polyene antifungal agents Candida albicans and Cryptococcus neoformans. Antimicrob Agents Chemother. 2000;44:1108–1111. doi: 10.1128/aac.44.4.1108-1111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst M E, Klepser M E, Ernst M E, Messer S A, Pfaller A. In vitro pharmacodynamic properties of MK-0991 determined by time-kill methods. Diagn Microbiol Infect Dis. 1999;33:75–80. doi: 10.1016/s0732-8893(98)00130-8. [DOI] [PubMed] [Google Scholar]
- 8.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker Inc.; 1982. pp. 455–459. [Google Scholar]
- 9.Graybill J R. The future of antifungal therapy. Clin Infect Dis. 1996;22(Suppl. 2):S166–S178. doi: 10.1093/clinids/22.supplement_2.s166. [DOI] [PubMed] [Google Scholar]
- 10.Green L J, Marder P, Mann L L, Chio L C, Current W. LY303366 exhibits rapid and potent fungicidal activity in flow cytometric assays of yeast viability. Antimicrob Agents Chemother. 1999;43:830–835. doi: 10.1128/aac.43.4.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groll A H, Gullick B M, Petraitiene R, Petraitis V, Candelario M, Piscitelli S C, Walsh T J. Compartmental pharmacokinetics of the antifungal echinocandin caspofungin (MK-0991) in rabbits. Antimicrob Agents Chemother. 2001;45:596–600. doi: 10.1128/AAC.45.2.596-600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groll A H, Walsh T J. FK-463. Curr Opin Anti-infect Investig Drugs. 2000;2:405–412. [Google Scholar]
- 13.Groll A H, Giri N, Petraitis V, Petraitiene R, Calendario M, Bacher J S, Piscitelli S C, Walsh T J. Comparative central nervous system distribution and antifungal activity of lipid formulations of amphotericin B in rabbits. J Infect Dis. 2000;182:274–282. doi: 10.1086/315643. [DOI] [PubMed] [Google Scholar]
- 14.Groll A H, Mickiene D, Petraitiene R, Petraitis V, Lyman C A, Bacher J S, Piscitelli S C, Walsh T J. Pharmacokinetic and pharmacodynamic modeling of anidulafungin ( LY303366): reappraisal of its efficacy in neutropenic animal models of opportunistic mycoses using optimal plasma sampling. Antimicrob Agents Chemother. 2001;45:2845–2855. doi: 10.1128/AAC.45.10.2845-2855.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hector R F. Compounds active against cell walls of medically important fungi. Clin Microbiol Rev. 1993;6:1–21. doi: 10.1128/cmr.6.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda F, Wakai Y, Matsumoto S, Maki K, Watabe E, Tawara S, Goto T, Watanabe Y, Matsumoto F, Kuwahara S. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis. Antimicrob Agents Chemother. 2000;44:614–618. doi: 10.1128/aac.44.3.614-618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klepser M E, Ernst E J, Ernst M E, Pfaller M A. Growth medium effect on the antifungal activity of LY 303366. Diagn Microbiol Infect Dis. 1997;29:227–231. doi: 10.1016/s0732-8893(97)00144-2. [DOI] [PubMed] [Google Scholar]
- 18.Kurtz M B, Douglas C M. Lipopeptide inhibitors of fungal glucan synthase. J Med Vet Mycol. 1997;35:79–86. doi: 10.1080/02681219780000961. [DOI] [PubMed] [Google Scholar]
- 19.Maesaki S, Hossain M A, Miyazaki Y, Tomono K, Tashiro T, Kohno S. Efficacy of FK463, a (1,3)-β-d-glucan synthase inhibitor, in disseminated azole-resistant Candida albicans infection in mice. Antimicrob Agents Chemother. 2000;44:1728–1730. doi: 10.1128/aac.44.6.1728-1730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto S, Wakai Y, Nakai T, Hatano K, Ushitani T, Ikeda F, Tawara S, Goto T, Matsumoto F, Kuwahara S. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of pulmonary aspergillosis. Antimicrob Agents Chemother. 2000;44:619–621. doi: 10.1128/aac.44.3.619-621.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikamo H, Sato Y, Tamaya T. In vitro antifungal activity of FK463, a new water-soluble echinocandin-like lipopeptide. J Antimicrob Chemother. 2000;46:485–487. doi: 10.1093/jac/46.3.485. [DOI] [PubMed] [Google Scholar]
- 22.Petraitiene R, Petraitis V, Groll A H, Candelario M, Sein T, Bell A, Peter J, Lyman C A, Schaufele R L, McMillian C L, Bacher J, Walsh T J. Antifungal efficacy, safety, and single-dose pharmacokinetics of LY30366, a novel echinocandin, in experimental disseminated candidiasis in persistently neutropenic rabbits. Antimicrob Agents Chemother. 1999;43:2148–2155. doi: 10.1128/aac.43.9.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tawara S, Ikeda F, Maki K, Morishita Y, Otomo K, Teratani N, Goto T, Tomishima M, Ohki H, Yamada A, Kawabata K, Takasugi H, Sakane K, Tanaka H, Matsumoto F, Kuwahara S. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob Agents Chemother. 2000;44:57–62. doi: 10.1128/aac.44.1.57-62.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomishima M, Ohki H, Yamada A, Takasugi H, Maki H, Tawara S, Tanaka A. FK463, a novel water-soluble echinocandin lipopeptide: synthesis and antifungal activity. J Antibiot. 1999;52:674–676. doi: 10.7164/antibiotics.52.674. [DOI] [PubMed] [Google Scholar]
- 25.Uchida K, Nishiyama Y, Yokota N, Yamaguchi H. In vitro antifungal activity of a novel lipopeptide antifungal agent, FK463, against various fungal pathogens. J Antibiot. 2000;53:1175–1181. doi: 10.7164/antibiotics.53.1175. [DOI] [PubMed] [Google Scholar]
- 26.Walsh T J, Lee J W, Kelly P, Bacher J, Lecciones J, Thomas V, Lyman C, Coleman D, Gordee R, Pizzo P A. Antifungal effects of the nonlinear pharmacokinetics of cilofungin, a 1,3-β-glucan synthetase inhibitor, during continuous and intermittent intravenous infusions in treatment of experimental disseminated candidiasis. Antimicrob Agents Chemother. 1991;35:1321–1328. doi: 10.1128/aac.35.7.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh T J, Bacher P, Pizzo P A. Chronic silastic central venous catheterization for induction, maintenance, and support of persistent granulocytopenia in rabbits. Lab Anim Med. 1988;38:467–470. [PubMed] [Google Scholar]
- 28.Yamaoka K, Nakagawa T, Uno T. Application of Akaike's information criterion in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]