Abstract
Background
Little is known of neutrophil-to-lymphocyte ratio (NLR) variations in septic shock. Hence, the predictive value of procalcitonin (PCT) and NLR variations for septic shock in bloodstream infection were explored.
Material/Methods
We analyzed 146 patients with bloodstream infection admitted to the Intensive Care Unit (ICU) of the First Affiliated Hospital of Anhui Medical University from October 2016 to May 2020. PCT and NLR were evaluated at 0 and 48 h after admission, and their variations (ΔPCT and ΔNLR) were calculated. The patients were divided into a shock group (n=80) and a non-shock group (n=66) and a gram-positive cocci group (n=69) and a gram-negative bacilli group (n=77). The predictive value of ΔPCT and ΔNLR was compared among groups.
Results
AUROC of NLR0h (0.756) higher than PCT0h (0.743).ΔPCT (0.561 vs 0.301) and ΔNLR (0.609 vs 0.361) were significantly higher in the shock group than in the non-shock group (P<0.05). No significant difference was seen in ΔPCT and ΔNLR in the gram-positive cocci infection group. However, the gram-negative bacilli infection group showed a significant difference in ΔPCT (0.606 vs 0.312) and ΔNLR (0.872 vs 0.508) between the shock and non-shock groups (P<0.05). ΔPCT+ΔNLR showed the best area under the curve (0.937), with a high sensitivity (78.80%) and specificity (90.80%), for predicting septic shock.
Conclusions
The prediction efficiency of initial NLR is higher than that of PCT. ΔPCT+ΔNLR best predicted septic shock in patients with bloodstream infections, with better accuracy for gram-negative infections.
Keywords: Intensive Care Units; Procalcitonin; Sepsis; Shock, Septic
Background
At present, sepsis is the leading cause of death from infections worldwide. The definition of sepsis is constantly updated, and as defined in the 2016 Surviving Sepsis Campaign guidelines (Sepsis-3), it is “a life-threatening organ dysfunction caused by a dysregulation of the host’s response to infection”. It is mainly caused by bloodstream, lung, urinary tract, and central nervous system infections. Septic shock is a kind of sepsis, defined as the disorder of circulation, cells, and metabolism, which are severe enough to increase mortality [1]. Sepsis 3.0 will help clinicians make a rough assessment of the patient’s condition, so as to identify and effectively treat critically ill patients early, protect organ function, and reduce mortality. However, early assessment of sepsis severity and prognosis remains inaccurate, and many studies are being carried out to solve this problem.
With the clinical application of broad-spectrum antibacterial drugs and immunosuppressive drugs and the development of minimally invasive treatment techniques, the incidence of BSI is increasing [2]. In particular, the incidence of septic shock due to bloodstream infections has continued to increase, and the mortality rate can be as high as 30–50%. Calcitoninogen is widely recognized as a diagnostic index for early bloodstream infections [3,4] and can be used to assess the severity of sepsis [5] and predict prognosis [6]. However, it is further influenced by several factors, such as multiple injuries, tumors, major surgery, poisoning, and other non-infectious diseases that can lead to changes in procalcitonin (PCT) levels. In recent years, the neutrophil-to-lymphocyte ratio (NLR) has been found to be a simple, inexpensive, and rapid indicator for detecting inflammation and can be used as a marker for early diagnosis and poor prognosis in bloodstream infections [7,8]. Various studies indicated that NLR and PCT have equal predictive value in patients with sepsis [9].There are several reports on PCT and PCT variations with sepsis [10–12]; however, few studies have assessed NLR variations in septic shock. Some studies suggested that the NLR level in patients with sepsis does not improve with treatment, which can predict poor prognosis [13].This study aimed to monitor NLR dynamically and compare it with an established sepsis-related biomarker (PCT), and observe the predictive value of PCT and NLR variations in bloodstream infections with septic shock.
Material and Methods
General Information
The clinical data of 146 patients with bloodstream infections between October 2016 and May 2020 in the Intensive Care Unit (ICU) of the First Affiliated Hospital of Anhui Medical University were retrospectively analyzed. The diagnostic criteria for bloodstream infections and septic shock were based on the International Guidelines for the Treatment of Sepsis and Septic Shock (2016 Surviving Sepsis Campaign guidelines) [1]. We categorized the 146 patients into shock (80 cases) and non-shock (66 cases) groups. Moreover, the 146 patients were divided into gram-positive bacteria (69 cases) and gram-negative bacteria (77 cases) infection groups based on the pathogenic bacteria causing the bloodstream infection. All enrolled patients were hospitalized for more than 48 h. The exclusion criteria were age <18 years; ICU stay <48 h; the onset time is more than 24 h; the presence of malignant tumors, hematologic diseases, AIDS, autoimmune system diseases; and the patients receiving immunosuppressive drugs that could affect hematologic and PCT parameters.
Research Methods
We retrospectively collected basic clinical data and related laboratory test results of the patients, including age, sex, underlying disease, white blood cell count, NLR, serum PCT, blood culture results, NLR0h and PCT0h at admission, and NLR48h and PCT48h after 48 h. Blood culture (bilateral and double sets) should be sent before the administration of antibiotics. The variations in PCT (ΔPCT) and NLR (ΔNLR) were analyzed [ΔPCT=|(PCT48h-PCT0h)|/PCT0h and ΔNLR=|(NLR48h-NLR0h)| /NLR0h].
Statistical Methods
In this study, all the statistical analyses were performed with IBM SPSS for Windows, Version 22.0 (IBM Corp., Armonk, N.Y., USA). Normally distributed data are presented as mean ± standard deviation and were compared with the t test. Non-normally distributed data are expressed as median and interquartile range and were compared with the rank-sum test. Categorical data are expressed as numbers or frequency (%) and were compared with the corrected χ2 test. The receiver operating characteristic curve (ROC) was drawn to analyze the predictive value of ΔPCT and ΔNLR for bloodstream infection complicated with septic shock. The area under the curve (AUC), sensitivity, and specificity were calculated. P<0.05 indicated the presence of a statistically significant difference.
Results
Analysis of Clinical Data of the 2 Groups of Patients
The mortality rate in the shock and non-shock groups was 61.25% and 24.24%, respectively. No significant difference was observed in sex, age, basic complications of diabetes, cardiovascular disease, respiratory disease, and kidney disease between the 2 groups. PCT0h, PCT48h, NLR0h, and NLR48h were significantly higher in the shock group than in the non-shock group (P<0.05; Table 1).
Table 1.
Comparison of baseline information between the 2 groups of patients.
| Baseline clinical characteristics | Shock group (n=80) | Non-shock group (n=66) | P value |
|---|---|---|---|
| Sex (Male/Female) | 47/33 | 39/27 | 0.081 |
| Age (years) | 57.64±17.71 | 62.75±18.16 | 0.153 |
| PCT0h (ng/mL) | 31.860 (6.355, 76.375) | 2.530 (0.320, 10.570) | <0.001 |
| PCT48h (ng/mL) | 22.860 (2.107, 40.305) | 0.879 (0.173, 5.320) | <0.001 |
| NLR0h | 21.367 (10.544, 37.838) | 10.019 (5.102, 15.093) | <0.001 |
| NLR48h | 10.232 (6.340, 20.067) | 6.467 (2.504, 10.136) | 0.026 |
| Complications of diabetes | 24 (30.00%) | 20 (30.30%) | 0.850 |
| Complications of cardiovascular disease | 16 (20.00%) | 12 (18.18%) | 0.274 |
| Complications of respiratory disease | 62 (77.50%) | 46 (69.69%) | 0.063 |
| Complications of kidney disease | 18 (22.50%) | 10 (15.15%) | 0.062 |
| Outcome [ICU mortality, n (%)] | 49 (61.25%) | 16 (24.24%) | <0.001 |
NLR – neutrophil-to-lymphocyte ratio; NLR0h – NLR at 0 hours; NLR48h – NLR at 48 hours; PCT – procalcitonin; PCT0h – PCT at 0 hours; PCT48h – PCT at 48 hours.
Comparison of ΔPCT and ΔNLR in Patients with Bloodstream Infection
Comparison of ΔPCT and ΔNLR Between the Shock and Non-Shock Groups
ΔPCT and ΔNLR were significantly higher in the shock group than in the non-shock group (P<0.05; Table 2).
Table 2.
Comparison of ΔPCT and ΔNLR.
| Index | Shock group (n=80) | Non-shock group (n=66) | P value |
|---|---|---|---|
| ΔPCT | 0.561 (0.246,2.730) | 0.301 (0.062,0.831) | 0.003 |
| ΔNLR | 0.609 (0.533,3.923) | 0.361 (0.126,0.920) | 0.000 |
ΔPCT=|(PCT48h-PCT0h)|/PCT0h; ΔNLR=|(NLR48h-NLR0h)|/NLR0h.
Comparison of ΔPCT and ΔNLR in Patients with Bloodstream Infection Caused by Different Pathogens
The gram-negative coccus infection group (n=77) comprised 45 and 32 cases in the shock and non-shock groups, respectively. ΔPCT and ΔNLR in the shock group were 0.606 (0.246, 5.038) and 0.872 (0.309, 7.061), respectively. In the non-shock group, ΔPCT and ΔNLR were 0.312 (0.172, 0.806) and 0.508 (0.314, 0.975), respectively. The differences between the shock and non-shock groups were significant (P<0.05).
The gram-positive coccus infection group (n=69) comprised 35 and 34 cases in the shock and non-shock groups, respectively. No significant difference was observed in ΔPCT [0.446 (0.194, 1.036) vs 0.346 (0.120, 0.732)] or ΔNLR [0.573 (0.213, 2.078) vs 0.417 (0.101, 0.895)] between the 2 groups (P>0.05). The gram-negative bacillus infection group (n=77) included 45 and 32 cases in the shock and non-shock groups, respectively. There was a significant difference in ΔPCT [(0.606 (0.246, 5.038) vs 0.312 (0.172, 0.806) and ΔNLR [0.872 (0.309, 7.061) vs 0.508 (0.314, 0.975)] between the 2 groups (P<0.05; Table 3).
Table 3.
ΔPCT and ΔNLR in patients with different bacterial bloodstream infections.
| Index | Gram-positive cocci | P value | Gram-negative bacilli | P value | ||
|---|---|---|---|---|---|---|
| Shock group (n=35) | Non-shockgroup (n=34) | Shock group (n=45) | Non-shock group (n=32) | |||
| ΔPCT | 0.446 (0.194, 1.036) | 0.346 (0.120, 0.732) | 0.067 | 0.606 (0.246, 5.038) | 0.312 (0.172, 0.806) | 0.001 |
| ΔNLR | 0.573 (0.213, 2.078) | 0.417 (0.101, 0.895) | 0.154 | 0.872 (0.309, 7.061) | 0.508 (0.314, 0.975) | 0.042 |
ΔPCT=|(PCT48h-PCT0h)|/PCT0h; ΔNLR=|(NLR48h-NLR0h)|/NLR0h.
Predictive Value of ΔPCT and ΔNLR for Septic Shock in Patients with Bloodstream Infection
ROC curves were drawn, with PCT0h, NLR0h, PCT48h, NLR48h, ΔPCT, ΔNLR, and ΔPCT+ΔNLR as independent variables and bloodstream infection complicated with septic shock as the outcome variable. The AUC of PCT0h, NLR0h, PCT48h, NLR48h, ΔPCT, ΔNLR, and ΔPCT+ΔNLR were 0.743, 0,756, 0.769, 0.682, 0.834, 0.852, and 0.937, respectively; the sensitivity was 70.50%, 38.36%, 32.23%, 41.09%, 80.98%,83.82%,and 80.02%, respectively; and the specificity was 78.00%, 92.13%, 94.08%, 81.25%, 75.03%,76.98%, and 92.80%, respectively (Table 4, Figure 1). ΔPCT+ΔNLR showed the highest predictive value for bloodstream infection complicated with septic shock, with high sensitivity and specificity.
Table 4.
Performance of variables in predicting septic shock in patients with bloodstream infection.
| Test variables | AUC (95%CI) | P value | Specificity (%) | Sensitivity (%) |
|---|---|---|---|---|
| PCT0h | 0.743 (0.661, 0.826) | <0.001 | 78.00 | 70.50 |
| NLR0h | 0.756 (0.686, 0.825) | <0.001 | 92.13 | 38.36 |
| PCT48h | 0.769 (0.702, 0.836) | <0.001 | 94.08 | 32.23 |
| NLR48h | 0.682 (0.606, 0.758) | <0.001 | 81.25 | 41.09 |
| ΔPCT | 0.834 (0.778, 0.891) | <0.001 | 75.03 | 80.98 |
| ΔNLR | 0.852 (0.797, 0.908) | <0.001 | 76.98 | 83.82 |
| ΔPCT+ΔNLR | 0.937 (0.905, 0.970) | <0.001 | 92.80 | 80.02 |
AUC – area under curve; NLR – neutrophil-to-lymphocyte ratio; PCT – procalcitonin; NLR0h – NLR at 0 hours; NLR48h – NLR at 48 hours; PCT0h – PCT at 0 hours; PCT48h – PCT at 48 hours.
Figure 1.
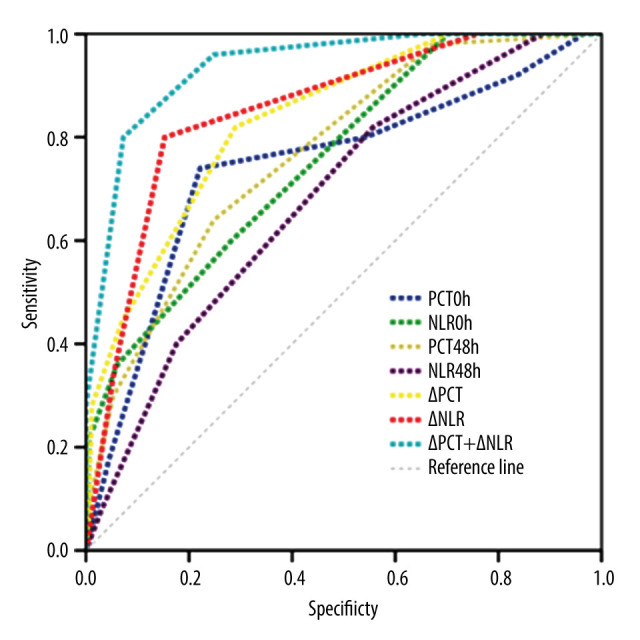
Performance of variables in predicting septic shock in patients with bloodstream infection. (SPSS version 22.0, IBM Corp., USA).
Discussion
Bloodstream infection is one of the common causes of sepsis, and septic shock caused by uncontrolled inflammatory response is a major cause of death in patients with bloodstream infection in the ICU [14,15], with fatality rates increasing yearly [16]. Early and accurate identification and effective initial treatment can reduce the probability of severe sepsis progressing to septic shock and reduce mortality. Serum PCT has been widely used in the diagnosis and treatment of sepsis [17–20] because of its high sensitivity, accuracy, and rapidity. However, the independent detection of serum PCT is affected by various factors, and combined detection and dynamic monitoring of variations can improve its diagnostic and prognostic value for bloodstream infection [21]. NLR is a new inflammatory indicator and can be used for early severity assessment, where higher NLR values indicate unfavorable prognoses in the sepsis patients [22,23]. Dynamic monitoring of changes is more beneficial for early disease severity assessment in patients with sepsis or septic shock [24].
Comparison Between PCT and NLR and Predictive Value for Septic Shock
In this study, we found that the mortality rate in the shock group of bloodstream infection was significantly higher than that in the non-shock group, which may be related to the multiple-organ failure caused by shock, further confirming that septic shock is a major lethal factor in bloodstream infection [25]. Excessive inflammatory response and immune dysfunction can lead to lymphocyte apoptosis and increased PCT, neutrophil count, and NLR [26], which have been reported to be correlated with higher 28-day mortality in patients with septic shock [27]. In this study, PCT0h (PCT at 0 h) and PCT48h (PCT at 48 h) in the septic shock group were significantly higher than those in the non-shock group, which could be used as predictors of septic shock. It further confirmed the role of PCT in the evaluation of sepsis and is consistent with earlier reports [12]. The normal reference range for NLR is 0.88–4.0, regardless of sex and age. Elevated NLR is related to physiological stress levels, especially in patients with septic shock. It reflects the severity of the disease, and NLR >10 can predict severe sepsis. In our study, NLR0h values were higher than normal in all patients, and the median NLR in patients with septic shock was 21.367, significantly higher than that in the non-septic shock group, which was 10.019, thereby indicating that NLR has some value in assessing the severity of sepsis. The NLR0h levels of septic shock patients obtained were similar to the findings of Liberski et al [28] but were higher than that of another study [29]. This may be related to sample size, the different sources of infection, and the different time-points of NLR assessment. The predictive value of NLR0h in septic shock patients is higher than that of PCT0h, which can be used as a biomarker of blood flow infection complicated with septic shock. A similar conclusion was put forward in a report on severe sepsis in children [30]. Therefore, in the early stage of severe sepsis or septic shock, NLR may be better than PCT in judging the severity of the disease.
Comparison Between ΔPCT and ΔNLR and Their Predictive Value for Septic Shock
This study found that ΔPCT had good predictive performance for bloodstream infections complicated by septic shock and its performance was better than that of PCT, in disagreement with a previous report [10]. The differences may be attributed to (1) sample size variation, (2) different sources of infection and age groups, (3) inclusion of factors affecting PCT, and (4) different detection time-points, which can cause PCT value errors. NLR variations have a better predictive value of bloodstream infections complicated with septic shock than any other index (such as PCT, NLR, and ΔPCT), but is worse than combined detection (ΔPCT+ΔNLR). For patients with sepsis without underlying autoimmune suppression, monitoring NLR changes may be preferred to assess disease severity because it is easier to perform and is less expensive, and, when combined with PCT variations, it can improve the predictive value.
Comparison of ΔPCT and ΔNLR in Patients with Bloodstream Infection Caused by Different Pathogens
Several studies have shown that the PCT level of patients with gram-negative bacterial bloodstream infections is significantly higher than that of patients with gram-positive bacterial bloodstream infections [31,32]. Because the cell wall of gram-negative bacteria is composed of lipopolysaccharide, which mainly produces endotoxin, it can directly induce and stimulate the production of high levels of PCT in vitro without cytokines [32]. The cell wall of gram-positive bacteria is composed of peptidoglycans, which mainly produce exotoxins and affect the production and release of PCT. Therefore, in patients with gram-negative bacterial infections, the release of PCT is increased significantly under the dual influence of endotoxins and inflammatory factors, resulting in a higher level of PCT than in those with gram-positive bacterial bloodstream infections. Studies have shown that the NLR of patients with gram-negative bacterial bloodstream infections is significantly higher than that of patients with gram-positive bacterial bloodstream infections. The specific explanation is unclear. Reports on the role of ΔPCT and ΔNLR in identifying the pathogens of bloodstream infection are lacking. In this study, we compared the ΔPCT and ΔNLR of patients with bloodstream infections due to different pathogens and found that ΔPCT and ΔNLR levels of patients with gram-negative bacterial infections with shock were significantly higher than those of non-shock patients, thereby indicating a higher predictive value for septic shock. However, ΔPCT and ΔNLR in patients with gram-positive bacteremia showed no statistically significant difference between shock and non-shock patients. This may be related to the slow decrease of PCT and NLR in patients with gram-positive bacterial bloodstream infections within 48 h after treatment, resulting in no significant difference in ΔPCT and ΔNLR between the 2 groups. In clinical work, we found that gram-positive bacterial anti-infective therapy was slow to respond, taking at least 3 days, which leads to a slow decline in early inflammatory indicators. Primary wound infection and insufficient drainage of abscesses also affect the rate of decline of infection indicators.
Study Limitations
As a single-center retrospective study, this study had the following limitations: the number of included subjects was small; there were few PCT, NLR, and other index data collection points; and detection and analysis at more time-points are required to reduce data bias. The validity and reliability of this study need to be verified by a large-sample and multi-center prospective study.
Conclusions
In conclusion, NLR can be used as a good inflammatory indicator to assist PCT in judging the severity of bloodstream infections, making the detection convenient and economical. Dynamic detection of changes can improve the accuracy of disease assessment, and the combined detection is more meaningful, especially for patients with gram-negative bacteremia complicated by septic shock. In clinical work, it can help us achieve early identification and prediction, thereby strengthening the clinical treatment management and improving the prognosis. Whether it can be widely used in the assessment and prognosis of patients with other sources of sepsis remains to be confirmed by further research.
Footnotes
Conflict of interest: None declared
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: The study was funded by the Anhui Province Key Clinical Specialties Construction Project, Anhui Province Clinical Medicine Application Project (Project No. 08B063)
References
- 1.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock. Crit Care Med. 2016;45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 2.Williams DW. Use of a policy-driven education program to reduce central line-associated bloodstream infection rates. J Infus Nurs. 2015;38:63–68. doi: 10.1097/NAN.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 3.Contenti J, Occelli C, Lemoel F, et al. Presepsin versus other biomarkers to predict sepsis and septic shock in patients with infection defined by Sepsis-3 criteria: The PREDI study of diagnostic accuracy. Emergencias. 2019;31:311–17. [PubMed] [Google Scholar]
- 4.Kargaltseva NM, Kotcherovets VI, Mironov AY, et al. Inflammation markers and bloodstream infection (review of literature) Klin Lab Diagn. 2019;64(7):435–42. doi: 10.18821/0869-2084-2019-64-7-435-442. [DOI] [PubMed] [Google Scholar]
- 5.Cabral L, Fernandes M, Marques S, et al. PCT kinetics in the first week postburn for sepsis diagnosis and death prognosis – an accuracy study. J Burn Care Res. 2021;42:545–54. doi: 10.1093/jbcr/iraa199. [DOI] [PubMed] [Google Scholar]
- 6.Ali WA, Bazan NS, Elberry AA, et al. A randomized trial to compare procalcitonin and C-reactive protein in assessing severity of sepsis and in guiding antibacterial therapy in Egyptian critically ill patients. Ir J Med Sci. 2021;190:1487–95. doi: 10.1007/s11845-020-02494-y. [DOI] [PubMed] [Google Scholar]
- 7.Ye W, Chen X, Huang Y, et al. The association between neutrophil-to-lymphocyte count ratio and mortality in septic patients: A retrospective analysis of the MIMIC-III database. J Thorac Dis. 2020;12:1843–55. doi: 10.21037/jtd-20-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martins EC, Silveira LDF, Viegas K, et al. Neutrophil-lymphocyte ratio in the early diagnosis of sepsis in an Intensive Care Unit: A case-control study. Rev Bras Ter Intensiva. 2019;31:64–70. doi: 10.5935/0103-507X.20190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ljungström L, Pernestig AK, Jacobsson G, et al. Diagnostic accuracy of procalcitonin, neutrophil-lymphocyte count ratio, C-reactive protein, and lactate in patients with suspected bacterial sepsis. PLoS One. 2017;12:e0181704. doi: 10.1371/journal.pone.0181704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui N, Zhang H, Chen Z, Yu Z. Prognostic significance of PCT and CRP evaluation for adult ICU patients with sepsis and septic shock: Retrospective analysis of 59 cases. J Int Med Res. 2019;47:1573–79. doi: 10.1177/0300060518822404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryoo SM, Han KS, Ahn S, et al. The usefulness of C-reactive protein and procalcitonin to predict prognosis in septic shock patients: A multicenter prospective registry-based observational study. Sci Rep. 2019;9:6579. doi: 10.1038/s41598-019-42972-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song J, Park DW, Moon S, et al. Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: A prospective controlled study according to the Sepsis-3 definitions. BMC Infect Dis. 2019;19:968. doi: 10.1186/s12879-019-4618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farkas JD. The complete blood count to diagnose septic shock. J Thorac Dis. 2020;12(Suppl 1):S16–S21. doi: 10.21037/jtd.2019.12.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moskowitz A, Omar Y, Chase M, et al. Reasons for death in patients with sepsis and septic shock. J Crit Care. 2017;38:284–88. doi: 10.1016/j.jcrc.2016.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikravan S, Song P, Bughrara N, Díaz-Gómez JL. Focused ultrasonography for septic shock resuscitation. Curr Opin Crit Care. 2020;26:296–302. doi: 10.1097/MCC.0000000000000730. [DOI] [PubMed] [Google Scholar]
- 16.Kontula KSK, Skogberg K, Ollgren J, et al. Population-based study of bloodstream infection incidence and mortality rates, Finland, 2004–2018. Emerg Infect Dis. 2021;27:2560–69. doi: 10.3201/eid2710.204826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang R, Wang J, Gao Y. [Advances of microfluidic technologies applied in diagnosis and treatment of sepsis]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31:789–92. doi: 10.3760/cma.j.issn.2095-4352.2019.06.026. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 18.Tosoni A, Paratore M, Piscitelli P, et al. Internal Medicine Sepsis Study Group. The use of procalcitonin for the management of sepsis in Internal Medicine wards: current evidence. Panminerva Med. 2020;62:54–62. doi: 10.23736/S0031-0808.19.03809-6. [DOI] [PubMed] [Google Scholar]
- 19.Pierrakos C, Velissaris D, Bisdorff M, et al. Biomarkers of sepsis: Time for a reappraisal. Crit Care. 2020;24:287. doi: 10.1186/s13054-020-02993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mustafić S, Brkić S, Prnjavorac B, et al. Diagnostic and prognostic value of procalcitonin in patients with sepsis. Med Glas (Zenica) 2018;15:93–100. doi: 10.17392/963-18. [DOI] [PubMed] [Google Scholar]
- 21.Hao C, Hu Q, Zhu L, et al. [Combined prognostic value of serum lactic acid, procalcitonin and severity score for short-term prognosis of septic shock patients]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021;33(3):281–85. doi: 10.3760/cma.j.cn121430-20201113-00715. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 22.Sarı R, Karakurt Z, Ay M, et al. Neutrophil to lymphocyte ratio as a predictor of treatment response and mortality in septic shock patients in the Intensive Care Unit. Turk J Med Sci. 2019;49:1336–49. doi: 10.3906/sag-1901-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Z, Fu Z, Huang W, et al. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am J Emerg Med. 2020;38:641–47. doi: 10.1016/j.ajem.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Terradas R, Grau S, Blanch J, et al. Eosinophil count and neutrophil-lymphocyte count ratio as prognostic markers in patients with bacteremia: A retrospective cohort study. PLoS One. 2012;7(8):e42860. doi: 10.1371/journal.pone.0042860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddiqui I, Jafri L, Abbas Q, et al. Relationship of serum procalcitonin, C-reactive protein, and lactic acid to organ failure and outcome in critically ill pediatric population. Indian J Crit Care Med. 2018;22:91–95. doi: 10.4103/ijccm.IJCCM_4_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mensa J, Barberán J, Ferrer R, et al. Recommendations for antibiotic selection for severe nosocomial infections. Rev Esp Quimioter. 2021;34:511–24. doi: 10.37201/req/126.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Xie J, Huang Y, et al. Leukocyte kinetics during the early stage acts as a prognostic marker in patients with septic shock in Intensive Care Unit. Medicine (Baltimore) 2021;100:e26288. doi: 10.1097/MD.0000000000026288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liberski PS, Szewczyk M, Krzych ŁJ. Haemogram-derived indices for screening and prognostication in critically ill septic shock patients: A case-control study. Diagnostics (Basel) 2020;10(9):638. doi: 10.3390/diagnostics10090638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drăgoescu AN, Pădureanu V, Stănculescu AD, et al. Neutrophil to lymphocyte ratio (NLR) – a useful tool for the prognosis of sepsis in the ICU. Biomedicines. 2021;10(1):75. doi: 10.3390/biomedicines10010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong X, Ma A, Zhang Z, et al. Neutrophil-to-lymphocyte ratio as a predictive marker for severe pediatric sepsis. Transl Pediatr. 2021;10(3):657–65. doi: 10.21037/tp-21-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bassetti M, Russo A, Righi E, et al. Role of procalcitonin in predicting etiology in bacteremic patients: report from a large single-center experience. J Infect Public Health. 2020;13(1):40–45. doi: 10.1016/j.jiph.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Gao Q, Li Z, Mo X, et al. Combined procalcitonin and hemogram parameters contribute to early differential diagnosis of Gram-negative/Gram-positive bloodstream infections. J Clin Lab Anal. 2021;35(9):e23927. doi: 10.1002/jcla.23927. [DOI] [PMC free article] [PubMed] [Google Scholar]


