Abstract
In the present study we assessed the use of a new in vitro testing method and graphical representation of the results to investigate the potential effectiveness of combinations of amoxicillin (AMZ) plus ceftriaxone (CRO) and of CRO plus vancomycin (VAN) against strains of Streptococcus pneumoniae highly resistant to penicillin and cephalosporins (PRP strains). We used the fractional maximal effect (FME) method of time-kill curves to calculate adequate concentrations of the drugs to be tested rather than relying on arbitrary choices. The concentrations obtained, each of which corresponded to a fraction of the maximal effect, were tested alone and in combination with the bacterial strains in a broth medium. Synergy was defined as a ratio of observed effect/theoretical effect, called FME, of greater than 1, additivity was defined as an FME equal to 1, and antagonism was defined as an observed effect lower than the best effect of one of the antibiotics used alone. The area between antagonism and additivity is the indifference zone. The well-known synergy between amoxicillin and gentamicin against a reference strain of Enterococcus faecalis was confirmed, with a best FME equal to 1.07. Two strains of PRP, strains PRP-1 and PRP-2, were studied. The MICs for PRP-1 and PRP-2 were as follows: penicillin, 4 and 16 μg/ml, respectively; AMZ, 2 and 8 μg/ml, respectively, CRO, 1 and 4 μg/ml, respectively; and VAN, 0.5 and 0.25 μg/ml, respectively. For PRP-1 the best FME for the combination AMZ-CRO was 1.22 with drug concentrations of 1.68 mg/liter for AMZ and 0.17 mg/liter for CRO; the best FME for the combination VAN-CRO was 1.75 with VAN at 0.57 mg/liter and CRO at 0.17 mg/liter. For PRP-2 the best FME obtained for the combination AMZ-CRO was 1.05 with drug concentrations of 11.28 mg/liter for AMZ and 0.64 mg/liter for CRO; the best FME obtained for the combination VAN-CRO was 1.35 with VAN at 0.25 mg/liter and CRO at 1.49 mg/liter. These results demonstrated the synergy of both combinations, AMZ-CRO and VAN-CRO, against PRP strains at drug concentrations achievable in humans. Consequently, either of the combinations can be proposed for use for the treatment of PRP infections.
The emergence of strains of Streptococcus pneumoniae highly resistant to penicillin and cephalosporins (PRP strains) (5, 10) and, often, to other classes of antimicrobial agents (33) complicates empirical treatment of pneumococcal infections. Therefore, all potential modalities for the treatment of these infections must be considered (6). Ceftriaxone or vancomycin is often one of the drugs recommended for the treatment of severe infections due to PRP such as meningitis. However, a combination of amoxicillin and ceftriaxone could also be a candidate for the treatment of such infections.
In this context, our purpose was, first, to investigate the in vitro interaction between amoxicillin and ceftriaxone and between vancomycin and ceftriaxone against PRP and, second, to identify the range of concentrations that are of clinical interest.
The traditional methods used to test the in vitro interactions between drugs are not very satisfactory, with difficulties in the interpretation of the results (16). All these methods come from two original models: the Loewe additivity model (24), illustrated by isobolograms, and the Bliss independence model (4), illustrated by dose-effect curves. The checkerboard method, with fractional inhibitory concentration indices and isobolograms, and the killing-curve method are used. Both methods have their advantages and drawbacks (7, 8, 32, 36, 37).
There is also a great disparity in the definitions used to characterize the interactions between drugs. For example, the limit value of fractional inhibitory concentration indices used to define antagonism varies from 1 to 8 according to previous studies. This disparity also appears in the duration of the times of killing, which can vary from 4 to 24 h and even longer (2).
The lack of standard definitions causes major problems, as it is impossible to compare the results of different studies.
Several investigators have looked further into the interpretation of the interactions between drugs (3) or the graphical representation of bacterial killing (34) or have proposed the use of the area under the curve (AUC) of bacterial killing as a criterion (1). Thus, additivity, synergy, antagonism, and autonomy or indifference have been defined (17, 21, 35).
Methods based on mathematical models have been used as a general approach to study the interactions between drugs (15). By these methods, the characterization of the dose-effect curve of each agent alone is critical; for this, the better-adapted structural model is the Hill model applied to a Michaelis-Menten curve.
According to these principles, the use of the AUC obtained from killing curves seems to be one of the better ways to evaluate in vitro the interactions of drugs used together in comparison with the theoretical AUC (26).
A further study has been carried out by using the fractional maximal effect (FME) method (23). The main characteristic of this method is that it tests calculated and not arbitrarily chosen concentrations of drugs. As a result, a maximal effect (Emax) can be determined for each drug.
The theoretical effect of the combination is calculated by adding the effects of each antibiotic used alone at the concentration tested in the combination. This theoretical effect is then compared with the effect obtained during the experiment.
The different interaction areas are defined as follows: additivity is an observed effect equal to the theoretical effect, and the ratio between them is equal to 1; synergy is an observed effect higher than the theoretical effect, and the ratio between them is more than 1; antagonism is an observed effect lower than the theoretical effect, and the ratio between them is less than 1.
For the graphical representation, Li et al. chose the isobologram method (23).
In the present study, we used the isobologram method with two modifications: first, as proposed previously (17, 35), we introduced indifference as a fourth zone of interaction; second, the graphical representation of the concentration-effect curve in two dimensions was preferred, as it allows a global illustration of the effects of each drug alone, drug-drug interactions, and theoretical addition.
MATERIALS AND METHODS
Drugs.
Ceftriaxone was obtained from Roche (Nutley, N.J.), vancomycin was obtained from Lilly Laboratories (Indianapolis, Ind.), and amoxicillin was obtained from SmithKline Beecham (Brentford, United Kingdom). The drugs were reconstituted as recommended by the manufacturers.
Strains.
Two strains of PRP (strains PRP-1 and PRP-2) isolated from a clinical specimen were used. The penicillin MICs for strains PRP-1 and PRP-2 were 4 and 16 mg/liter, respectively. Strains were identified by common tests and were stored at −70°C in brain heart infusion (BHI) with 15% glycerol. Enterococcus faecalis CIP 76117 was used as a reference strain and was provided by the Institut Pasteur (Paris, France).
In vitro testing. (i) MIC.
MICs were determined by the agar dilution method described by the National Committee for Clinical Laboratory Standards (31).
(ii) Killing curves.
The organisms were grown in BHI for 4 h at 37°C and were then adjusted by dilution in BHI to obtain a final inoculum of 5.5 × 106 organisms per ml. The antibiotics were diluted in BHI to obtain the different concentrations tested. To obtain a 1.9-ml final volume in 5-ml sterile hemolysis tubes, 0.1 ml of the bacterial inoculum was finally added to the tubes. They were then placed in an incubator at 37°C. A 100-μl volume was then removed; and cultures were performed after serial dilutions at 0, 3, 6, and 12 h for PRP strains and 24 h for E. faecalis on Mueller-Hinton agar plates supplemented with sheep blood at 37°C and in 5% CO2 for S. pneumoniae. These counts were then expressed as log number of CFU per milliliter and were used to calculate the AUC by using the trapezoidal method.
This protocol was used with the concentrations of antibiotics in each tube required to achieve bactericidal activity, as well as with the antibiotics alone and in combination, to create the killing curves.
Criterion to achieve concentrations tested and calculations.
We derived a method from the FME method of time-kill curves in which the concentrations of the drugs to be tested are calculated from the bactericidal activity curves and are chosen rather arbitrarily. The AUC was the criterion chosen to construct the bactericidal activity curves in triplicate for each antibiotic; the effect (E) is equal to the ratio 1/AUC. A nonlinear regression (the formula used was E = Emaxn × Cn/EC50n + Cn), performed with SPSS software (SPSS Inc., Chicago, Ill.), was applied to each curve (for each concentration C) to determine the following constants: Emax (maximal effect), EC50 (the concentration that produces one-half of the Emax), and n (Hill's coefficient).
The FME is defined by the ratio observed effect/theoretical effect.
Next, the two antibiotics (antibiotics A and B) are combined so that the sum of FME is always equal to 1. The following pairs were tested: 0.1FMEA + 0.9FMEB, 0.3FMEA + 0.7FMEB, 0.5FMEA + 0.5FMEB, 0.7FMEA + 0.3FMEB, and 0.9FMEA + 0.1FMEB.
The corresponding concentrations (C) to be tested alone and in combination were calculated by using the appropriate formula: C = (FME × EC50)/(1 − FME).
Because the relation between the two antibiotics is not linear, the theoretical effect of the combination had to be calculated by using the following formula (M. Katzper, personal communication):
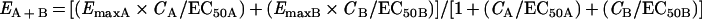 |
These results (the FME for each test) were then plotted versus the concentrations (antibiotics alone and in combination). In this way, different areas of interaction were defined as follows: additivity was defined as an effect equal to the theoretical sum of the effects of each antibiotic tested alone and was expressed as an FME equal to 1; synergy was defined as an effect superior to additivity and was expressed as an FME of greater than 1; antagonism was defined as an observed effect lower than the best effect of an antibiotic used alone and was expressed as an FME lower than the best FME of the antibiotics tested alone; and the area between antagonism and additivity was the indifference zone and was expressed as an FME for the antibiotic combination between the FME values for antagonism and additivity.
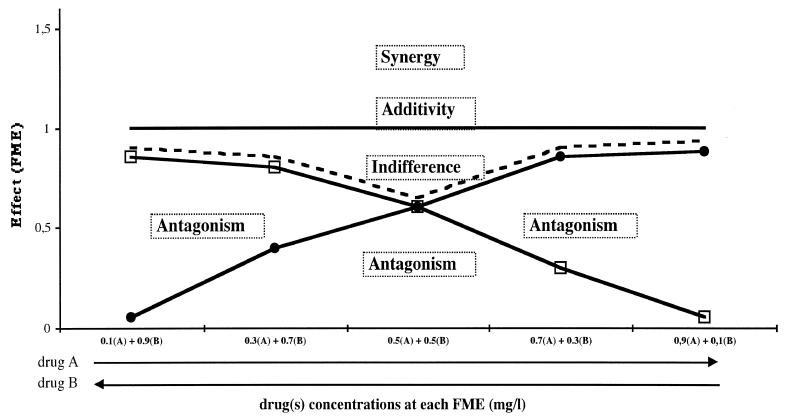
These theoretical interactions are represented graphically in Fig. 1.
FIG. 1.
Theoretical graphical representation of the different interactions between two antibiotics, antibiotics A and B. The y axis represents the FME of each antibiotic alone (A, B) and the antibiotic pair (A + B). The x axis represents the concentration of either antibiotic A (increasing concentrations from the left) or antibiotic B (increasing concentrations from the right). The concentrations tested are those corresponding to each fraction of maximal effect. □, effect of antibiotic A; this effect is expressed as the FME of antibiotic A; ●, effect of antibiotic B; this effect is expressed as the FME of antibiotic B; - - - , the best effect of either antibiotic A or B (“best alone” line); additivity line, theoretical addition of the effects of A and B; this sum is always equal to 1; Synergy, area of synergy above the additivity line; Indifferent, indifference zone between the additivity line and the best alone line; antagonism, area of antagonism below the best alone line.
RESULTS
MIC.
The MICs of the antibiotics tested are presented in Table 1. Both pneumococcal strains were resistant to penicillin, while PRP-2 was also resistant to ceftriaxone.
TABLE 1.
MICs for the strains tested
| Strain | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| Amoxicillin | Ceftriaxone | Vancomycin | Gentamicin | |
| E. faecalis | 0.38 | NDa | ND | 12.00 |
| PRP-1 | 2.00 | 1.00 | 0.50 | ND |
| PRP-2 | 8.00 | 4.00 | 0.25 | ND |
ND, not determined.
E. faecalis.
Killing curve studies were done over 24 h in triplicate at multiples of the MICs of amoxicillin and gentamicin alone. The AUC was calculated for each concentration tested, and the effect was defined as the inverse of the AUC. The criteria for the nonlinear regression obtained from the killing curve studies of each antibiotic are shown in Table 2. Figure 2A illustrates the FME for each antibiotic concentration tested and the FMEs for the antibiotic combinations tested. Synergy was observed when the concentration of amoxicillin was above 0.3 mg/liter. Below this concentration, the effect of the combination was indifferent.
TABLE 2.
Emax concentrations that produce EC50 and Hill's coefficients deduced from in vitro killing curvesa
| Strain | Antibiotic | Emax | EC50 (multiple of MIC) | Hill's coefficient | r2 |
|---|---|---|---|---|---|
| E. faecalis | Amoxicillin | 0.018 | 0.76 | 0.96 | 0.87 |
| Gentamicin | 0.032 | 4.5 | 0.88 | 0.97 | |
| PRP-1 | Amoxicillin | 0.030 | 0.36 | 1.29 | 0.49 |
| Ceftriaxone | 0.027 | 0.39 | 0.61 | 0.53 | |
| Vancomycin | 0.038 | 0.49 | 1.83 | 0.74 | |
| PRP-2 | Amoxicillin | 0.060 | 1.41 | 1.11 | 0.7 |
| Ceftriaxone | 0.026 | 0.16 | 0.57 | 0.58 | |
| Vancomycin | 0.072 | 2.33 | 0.63 | 0.74 |
See Materials and Methods.
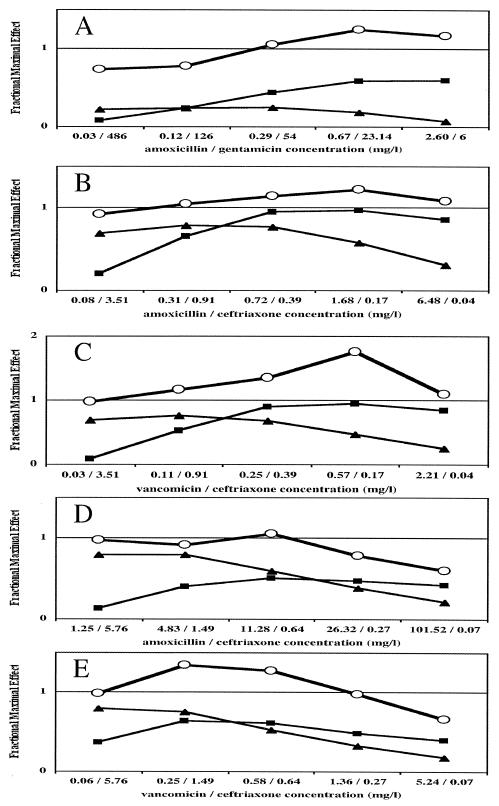
FIG. 2.
In vitro FMEs of amoxicillin, gentamicin, ceftriaxone, and vancomycin against a reference strain of E. faecalis and two penicillin-resistant pneumococcal strains (strains PRP-1 and PRP-2). (A) FME of the combination (open circle), amoxicillin (increasing concentrations from the left; ▪), or gentamicin (increasing concentration from the right; ▴) against E. faecalis. (B) FME of the combination (open circle), amoxicillin (increasing concentrations from the left; ▪), or ceftriaxone (increasing concentration from the right; ▴) against strain PRP-1. (C) FME of the combination (open circle), vancomycin (increasing concentrations from the left; ▪), or ceftriaxone (increasing concentration from the right; ▴) against strain PRP-1. (D) FME of the combination (open circle), amoxicillin (increasing concentrations from the left; ▪), or ceftriaxone (increasing concentration from the right; ▴) against strain PRP-2. (E) FME of the combination (open circle), vancomycin (increasing concentrations from the left; ▪), or ceftriaxone (increasing concentration from the right; ▴) against strain PRP-2.
S. pneumoniae.
For both PRP strains, the criteria for the nonlinear regression obtained from the killing curve studies with the range of antibiotic concentrations tested are shown in Table 2. The FMEs of each antibiotic tested alone and the FMEs of the different combinations are shown in Fig. 2B to E.
For strain PRP-1 and the combination amoxicillin-ceftriaxone, synergy was observed with a concentration of amoxicillin of 0.3 mg/liter and relatively low concentrations of ceftriaxone (Fig. 2B). For this strain and the combination of vancomycin and ceftriaxone, synergy was obtained with any concentration of each antibiotic (Fig. 2C).
For strain PRP-2, the combination amoxicillin-ceftriaxone was found to be synergistic for only one pair of concentrations. With the other pair of concentrations, the results were in the indifference area (Fig. 2D). A synergistic effect was obtained for the vancomycin-ceftriaxone combination, but it did not exist for the lowest concentration of ceftriaxone tested (Fig. 2E).
DISCUSSION
By the method used in the present study, we used the logarithmic transformation of CFU to construct AUCs. Previous experiments were performed with the metric results of CFU (data not shown), which allows one to cope with very large variations in bacterial contents that range from 0 to 1011 but with no advantages for the graphical representation. Therefore, the method used to count the bacterial concentration in the present work is commonly used and is easier to perform. This logarithmic transformation of CFU probably minimizes the contrasts by smoothing the graphical representation and so leads to a more cautious interpretation.
Our findings demonstrated synergy for amoxicillin and gentamicin against E. faecalis (FME > 1) and thus confirmed conclusions drawn from previous studies performed by different methods (18, 19, 27, 30). The concentrations tested were very high for gentamicin because the MIC was also very high; therefore, high concentrations were needed to obtain the Emax. Despite this, synergy was observed at concentrations achievable in humans. More precisely, synergy appears when the amoxicillin concentration is at least at 0.29 mg/liter, which is about the MIC of this antibiotic for this strain. This is in accordance with previous data (25), in which synergy was achieved if the penicillin concentration was at least equal to the MIC for the strain, even for very resistant bacteria. Taken together, these findings are in accordance with the known mechanism of the interaction between beta-lactams and aminoglycosides (29).
For penicillin-resistant (PRP-1) and broad-spectrum cephalosporin-resistant pneumococci (PRP-2), the effect obtained with both combinations (amoxicillin-ceftriaxone and vancomycin-ceftriaxone) was not constant with the concentrations tested because this method was very dynamic. However, in all cases there was always a section of the curve that was in the area for synergism; the worst values were always in the indifference zone, and antagonism was never found.
A closer analysis reveals that the vancomycin-ceftriaxone combination seems to be slightly more effective than the amoxicillin-ceftriaxone combination, as it has higher FME values (the highest FMEs, 1.73 versus 1.21, respectively) and more extensive areas of synergy (the part of the curve above the additivity line). For both combinations, the synergy seems to be greater for strain PRP-1, which is the less resistant strain, than for strain PRP-2, which is also reflected by higher FME values (the highest FMEs for the two strains were 1.73 and 1.34, respectively) and more extensive areas of synergy.
However, the results that we obtained with the amoxicillin-ceftriaxone combination are in accordance with those previously obtained with the same strains (9). In the previous study, we demonstrated by two different in vitro methods (the checkerboard method and classical time-kill curve studies) that this antibiotic combination has an improved antibacterial effect, but there were interpretation difficulties; this improvement was also observed in an in vivo model of pneumococcal infection. The method described here allows a simple interpretation of results; notably, it provides a clearly defined distinction between the different interaction areas and the range of concentrations of interest.
Our results are also concordant with those of other studies that used different in vivo or in vitro methods. When studying the interactions between a broad-spectrum cephalosporin and amoxicillin against 25 PRP strains, Johnson and Jones (20) have always observed favorable interactions: synergy, partial synergy, additivity, or indifference. Friedland et al. (13, 14) have shown at least an additive or a synergistic interaction between ceftriaxone and vancomycin against four PRP strains in vivo and in vitro. Marton and Major (28) have also demonstrated the superiority of the vancomycin–broad-spectrum cephalosporin combination over cephalosporins alone against two PRP strains. Other investigators (22) have studied the bacterial activities of drug combinations against cephalosporin-resistant S. pneumoniae in the cerebrospinal fluid (CSF) of children with acute bacterial meningitis. Their results showed that a combination of ceftriaxone and vancomycin or a combination of ceftriaxone and rifampin had higher levels of antibacterial activity in CSF than ceftriaxone alone.
Similarly, a French group (11) has shown that the cefotaxime-vancomycin combination was at least additive against a broad-spectrum cephalosporin-intermediate pneumococcal strain by measuring bactericidal activity in the CSF of children with acute bacterial meningitis. They also reported the results of in vitro studies of different drug combinations against PRP strains that had various levels of susceptibility to broad-spectrum cephalosporins. The activities of trovafloxacin-vancomycin and beta-lactam–vancomycin combinations were found to be additive or indifferent even against broad-spectrum cephalosporin-resistant strains (12).
Conclusion.
The new method for the testing of drug-drug interactions described here permits a simple graphical representation of the interaction and then an approach that uses the range of concentrations of interest.
Our results confirm the results of previous in vitro and in vivo studies in which amoxicillin-ceftriaxone and vancomycin-ceftriaxone combinations appeared to be synergistic at some concentrations against penicillin- and/or broad-spectrum cephalosporin-resistant pneumococcal strains. Moreover, this synergy is present at drug concentrations achievable in humans. Therefore, the clinical interest in these combinations for the treatment of PRP infections seems to be confirmed.
REFERENCES
- 1.Barriere S L, Ely E, Kapusnik J E, Gambertoglio J G. Analysis of a new method for assessing activity of combinations of antimicrobials: area under the bactericidal activity curve. J Antimicrob Chemother. 1985;16:49–59. doi: 10.1093/jac/16.1.49. [DOI] [PubMed] [Google Scholar]
- 2.Berenbaum M C. Minor synergy and antagonism may be clinically important. J Antimicrob Chemother. 1987;19:271–273. doi: 10.1093/jac/19.2.271. [DOI] [PubMed] [Google Scholar]
- 3.Blaser J. Interactions of antimicrobial combinations in vitro: the relativity of synergism. Scand J Infect Dis Suppl. 1991;74:71–79. [PubMed] [Google Scholar]
- 4.Bliss C I. The toxicity of poisons applied jointly. Ann Appl Biol. 1939;26:585–615. [Google Scholar]
- 5.Bulletin Epidémiologique Annuel Numéro 2. Epidémiologie des maladies infectieuses en France. Situation en 1997 et tendances évolutives récentes. 1999. Réseau National de Santé Publique, Paris, France. [Google Scholar]
- 6.Butler J C, Dowell S F, Breiman R F. Epidemiology of emerging pneumococcal drug resistance: implications for treatment and prevention. Vaccine. 1998;16:1693–1697. doi: 10.1016/s0264-410x(98)00132-7. [DOI] [PubMed] [Google Scholar]
- 7.Cappelletty D M, Rybak M J. Comparison of methodologies for synergism testing of drug combinations against strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1996;40:677–683. doi: 10.1128/aac.40.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan E L, Zabransky R J. Determination of synergy by two methods with eight antimicrobial combinations against tobramycin-susceptible and tobramycin-resistant strains of Pseudomonas. Diagn Microbiol Infect Dis. 1987;6:157–164. doi: 10.1016/0732-8893(87)90101-5. [DOI] [PubMed] [Google Scholar]
- 9.Chavanet P, Dalle F, Delisle P, Duong M, Pechinot A, Buisson M, D'Athis P, Portier H. Experimental efficacy of combined ceftriaxone and amoxicillin on penicillin-resistant and broad-spectrum cephalosporin-resistant Streptococcus pneumoniae infection. J Antimicrob Chemother. 1998;41:237–246. doi: 10.1093/jac/41.2.237. [DOI] [PubMed] [Google Scholar]
- 10.Doern G, Heilmann K, Huynh H, Rhomberg P, Coffman S, Brueggemann A. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999–2000, including a comparison of resistance rates since 1994–1995. Antimicrob Agents Chemother. 2001;46:1721–1729. doi: 10.1128/AAC.45.6.1721-1729.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doit C, Barre J, Cohen R, Bonacorsi S, Bourrillon A, Bingen E. Bactericidal activity against intermediately cephalosporin-resistant Streptococcus pneumoniae in cerebrospinal fluid of children with bacterial meningitis treated with high doses of cefotaxime and vancomycin. Antimicrob Agents Chemother. 1997;41:2050–2052. doi: 10.1128/aac.41.9.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitoussi F, Doit C, Geslin P, Bingen E. Killing activities of trovafloxacin alone and in combination with β-lactam agents, rifampin, or vancomycin against Streptococcus pneumoniae isolates with various susceptibilities to extended-spectrum cephalosporins at concentrations achievable in cerebrospinal fluid. Antimicrob Agents Chemother. 1999;43:2372–2375. doi: 10.1128/aac.43.10.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedland I, Paris M, Ehrett S, Hickey S, Olsen K, MacCracken G. Evaluation of antimicrobial regimens for treatment of experimental penicillin and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1993;37:1630–1636. doi: 10.1128/aac.37.8.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedland I R, Paris M, Shelton S, MacCracken G H. Time-kill studies of antibiotic combinations against penicillin-resistant and -susceptible Streptococcus pneumoniae. J Antimicrob Chemother. 1994;34:231–237. doi: 10.1093/jac/34.2.231. [DOI] [PubMed] [Google Scholar]
- 15.Greco W, Unkelbach H D, Pöch G, Sühnel J, Kundi M, Bödeker W. Consensus on concepts and terminology for combined-action assessment: the Saariselkä agreement. Arch Complex Environ Studies. 1992;4:65–69. [Google Scholar]
- 16.Greco W R, Bravo G, Parsons J C. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47:331–385. [PubMed] [Google Scholar]
- 17.Hamilton-Miller J M T. Rationalization of terminology and methodology in the study of antibiotic interaction. J Antimicrob Chemother. 1985;15:655–658. doi: 10.1093/jac/15.6.655. [DOI] [PubMed] [Google Scholar]
- 18.Hook E W, Roberts R B, Sande M A. Antimicrobial therapy of experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1975;8:564–570. doi: 10.1128/aac.8.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jawetz E, Gunnison J B, Coleman V R. The combined action of penicillin with streptomycin or chloromycetin on enterococci in vitro. Science. 1950;111:254. doi: 10.1126/science.111.2880.254. [DOI] [PubMed] [Google Scholar]
- 20.Johnson D M, Jones R N. In vitro activity of a combination of two oral β-lactams (cefpodoxime and amoxicillin) against Streptococcus pneumoniae isolates with reduced susceptibilities to penicillin. J Antimicrob Chemother. 1998;42:555–557. doi: 10.1093/jac/42.4.555. [DOI] [PubMed] [Google Scholar]
- 21.King T C, Schlessinger D, Krogstad D J. The assesment of antimicrobial combinations. Rev Infect Dis. 1981;3:627–633. doi: 10.1093/clinids/3.3.627. [DOI] [PubMed] [Google Scholar]
- 22.Klugman K P, Friedland I R, Bradley J S. Bacterial activity against cephalosporin-resistant Streptococcus pneumoniae in cerebrospinal fluid of children with acute bacterial meningitis Antimicrob. Agents Chemother. 1995;39:1988–1992. doi: 10.1128/aac.39.9.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li R C, Schentag J J, Nix D E. The fractional maximal effect method: a new way to characterize the effect of antibiotic combinations and other nonlinear pharmacodynamic interactions. Antimicrob Agents Chemother. 1993;37:523–531. doi: 10.1128/aac.37.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loewe S. The problem of synergism and antagonism of combined drugs. Arzneim-Forsch. 1953;3:285–290. [PubMed] [Google Scholar]
- 25.Lopardo H A, Venuta M E, Rubeglio E A. Penicillin resistance and aminoglycoside-penicillin synergy in enterococci. Chemotherapy (Basel) 1995;41:165–171. doi: 10.1159/000239339. [DOI] [PubMed] [Google Scholar]
- 26.MacGowan A P, Wooton M, Hedges A J, Bowker K E, Holt H A, Reeves D S. A new time-kill method of assessing the relative efficacy of antimicrobial agents alone and in combination developed using a representative β-lactam, aminoglycoside and fluoroquinilone. J Antimicrob Chemother. 1996;38:193–203. doi: 10.1093/jac/38.2.193. [DOI] [PubMed] [Google Scholar]
- 27.Mandell G L, Kaye D, Levison M E, Hook E W. Enterococcal endocarditis: an analysis of 38 patients observed at the New York Hospital-Cornell Medical Center. Arch Intern Med. 1970;125:258–264. doi: 10.1001/archinte.125.2.258. [DOI] [PubMed] [Google Scholar]
- 28.Marton A, Major P. In vitro susceptibility of Streptococcus pneumoniae strains to nine β-lactam antibiotics and the killing kinetics of cephalosporins alone and in combination with vancomycin or gentamicin. Microb Drug Resist. 1996;2:361–369. doi: 10.1089/mdr.1996.2.361. [DOI] [PubMed] [Google Scholar]
- 29.Moellering R C, Jr, Eliopoulos G M, Allan J D. Beta-lactam/aminoglycoside combinations: interactions and their mechanisms. Am J Med. 1986;80(Suppl. 5C):30–34. [PubMed] [Google Scholar]
- 30.Moellering R C, Jr, Wennersten C, Weinberg A N. Studies on antibiotic synergism against enterococci. I. Bacteriological studies. J Lab Clin Med. 1971;77:821–828. [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards. Performance for antimicrobial susceptibility testing. Standard M100-S5. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1994. [Google Scholar]
- 32.Norden C W, Wentzel H, Keleti E. Comparison of techniques for measurement of in vitro antibiotic synergism J. Infect Dis. 1979;140:629–633. doi: 10.1093/infdis/140.4.629. [DOI] [PubMed] [Google Scholar]
- 33.Novak R, Henriques B, Charpentier E, Normark S, Tuomanen E. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature. 1999;399:590–593. doi: 10.1038/21202. [DOI] [PubMed] [Google Scholar]
- 34.Prichard M N, Prichard L E, Shipman C J. Strategic design and three-dimensional analysis of antiviral drug combinations. Antimicrob Agents Chemother. 1993;37:540–545. doi: 10.1128/aac.37.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahal J J. Antibiotic combinations: the clinical relevance of synergy and antagonism. Medicine. 1978;57:179–195. [PubMed] [Google Scholar]
- 36.Ryan R W, Kwasnik I, Tilton R C. Methodological variation in antibiotic synergy tests against enterococci. J Clin Microbiology. 1981;13:73–75. doi: 10.1128/jcm.13.1.73-75.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanders C C, Sanders W E, Jr, Moland E S. Decimal assay for additivity of drugs permits delineation of synergy and antagonism. Antimicrob Agents Chemother. 1993;37:260–264. doi: 10.1128/aac.37.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]