Abstract
Immune responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in pediatric patients with malignant disease may be affected by tumor therapy. Here, we report the case of a child with rhabdomyosarcoma and recurrent SARS-CoV-2 infection. Immunologic responses, analyzed by T-cell activity and anti-viral IgG levels, were impaired and not durable as a result of intensive radiochemotherapy.
Keywords: pediatric oncology, immune response
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has caused substantial morbidity and mortality. The impact of SARS-CoV-2 infection in children is an ongoing matter of discussion, as children appear to be infected less commonly and with a milder course of the disease.1 However, the rate of infection is likely to be underestimated because infections in children are often asymptomatic and are therefore tested less frequently. Throughout the pandemic, severe courses of the primary disease as well as pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 have been observed in children. Increased symptomatic infections were observed as the coronavirus evolved, including variants B1.1.72 and B1.617.2.3
Although children with underlying malignant disease are usually at a higher risk for infections in general, only a few coronavirus disease 2019 cases have been reported during the first wave of the pandemic.4 Nevertheless, these children are generally classified as high risk. Apart from SARS-CoV-2 associated complications, they may also be affected by a delay in tumor therapy and increased exposure to pathogens within hospital facilities.5 Furthermore, the ability of these children to develop an adequate immune response is not entirely understood.
MATERIALS AND METHODS
To assess T-cell responses against SARS-CoV-2, patient’s peripheral blood mononuclear cells were incubated with SARS-CoV-2-specific peptide mixes (SI and SII, containing human leukocyte antigen class I and II SARS-CoV-2-exclusive T-cell epitopes) and cross-reactive peptide mixes (CI and CII, containing human leukocyte antigen class I and II T-cell epitopes derived from conserved sequences of coronavirus antigens).6 After in vitro peptide stimulation, intracellular cytokine staining was performed to detect antigen-specific T cells as described previously.6 For the gating strategy for the detection of specific T-cell responses, see Figure 1 (Supplemental Digital Content, http://links.lww.com/INF/E696).
RESULTS
We report the case of an 11-year-old boy with rhabdomyosarcoma and subsequent SARS-CoV-2 reinfection. The patient was diagnosed with disseminated stage IV alveolar rhabdomyosarcoma in November 2019 (day 1). Histology was confirmed by paratesticular biopsy of the suspected primary site. Subsequent radiochemotherapy was initiated according to the Cooperative Weichteilsarkom Study Group guidance. Orchidectomy was performed after neoadjuvant therapy. Following the fifth cycle of polychemotherapy, including ifosfamide, vincristine, actinomycin, carboplatin, epirubicin and etoposide, the patient presented with a globus sensation and subfebrile temperature. There were no additional signs suggestive of SARS-CoV-2 infection, such as respiratory symptoms or an altered sense of smell and taste. SARS-CoV-2-RNA was detected in a pharyngeal swab by real-time polymerase chain reaction (RT-PCR; Ct value of E-gene PCR 32) in April 2020 (day 135) during the first pandemic wave in Germany. Laboratory results showed neutropenia (40/µL), lymphopenia (131/µL) and elevated C-reactive protein (CRP) value (4.82 mg/dL). Lactate dehydrogenase and D-dimer levels were not significantly altered.
The follow-up pharyngeal swab, 1 week after the first SARS-CoV-2 detection, was negative for SARS-CoV-2-RNA, as were consecutive oropharyngeal swabs from days 146 to 261. Three weeks after the first negative RT-PCR and after hematopoietic recovery, oncological therapy was continued with polychemotherapy and radiation, targeting retroperitoneal, mediastinal, left-side supraclavicular and hilar lymph nodes; bone metastasis and central nervous system. Subsequently, oral maintenance chemotherapy was started.
Blood samples of our patient were retrospectively analyzed for the immune response towards SARS-CoV-2. At the time of the first SARS-CoV-2 infection, the patient received chemotherapy, resulting in significantly decreased lymphocyte counts (Fig. 1A).
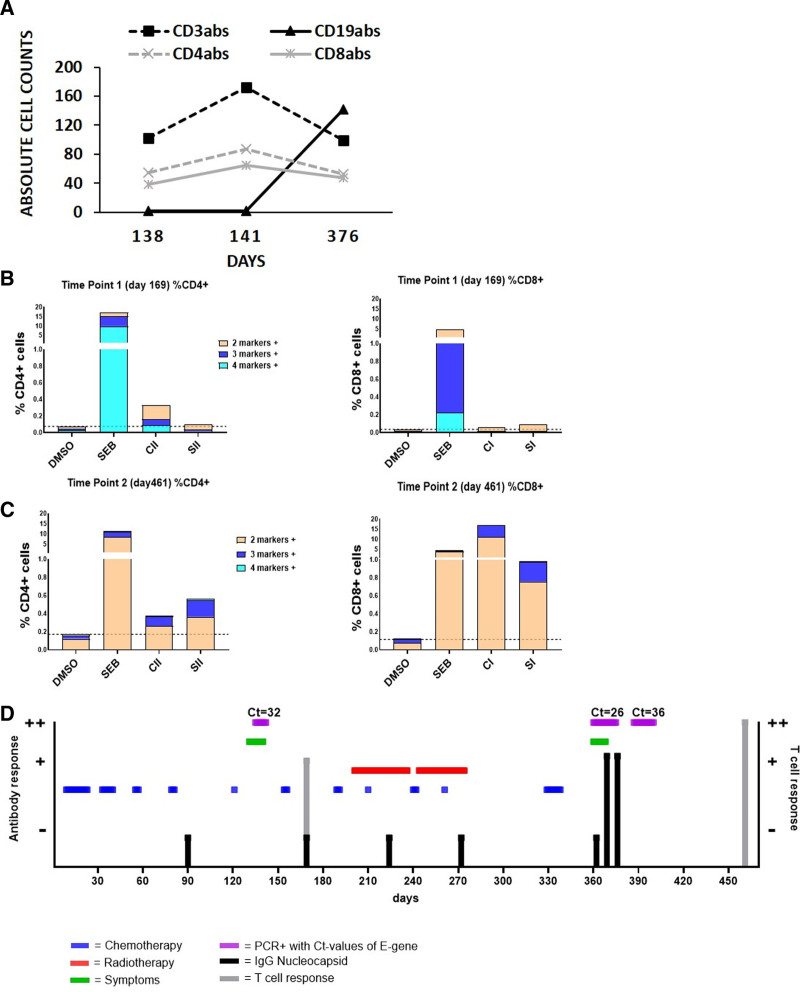
FIGURE 1.
Immunologic findings and time course of infection, radiochemotherapy and laboratory parameters. A: Distribution of absolute cell counts CD4+, CD8+, CD19+, and CD3+ cells at days 138, 141 and 376. B-cell counts increase from day 376. B: CD4+/CD8+ T-cell activity measured using intracellular cytokine staining (ICS). Multiple positive cytokine markers were detected on day 169. Positive control: staphylococcal enterotoxin B (SEB), negative control: dimethyl sulfoxide (DMSO). Specific peptide mix HLA class I and II (SI and SII), cross-reactive peptide mix HLA class I and II (CI and CII). A clear CD4+ T-cell response against CII but only a very weak CD8+ response to the specific SI mix could be observed. C: CD4+/CD8+ T-cell activity measured by ICS on day 461 shows a clear specific and cross-reactive CD4+ and CD8+ T-cell response following the second SARS-CoV-2 infection. D: Time course starting on the day of diagnosis of sarcoma by biopsy (day 1: November 2019). Chemotherapy: 10 cycles and oral maintenance according to the Cooperative Weichteilsarkom Study (CWS) guidance (blue). Radiotherapy: cycle 1 infradiaphragmal; cycle 2, supradiaphragmal (red). COVID-19-related symptoms: during first/second infection (green). Positive SARS-CoV-2 RNA detection (purple). Antibody responses were measured at days 90, 169, 224, 272, 362, 369 and 376. No SARS-CoV-2 antibodies were detected before or after the first infection. Detection of IgG nucleocapsid antibodies during and after second infection. T-cell response: weak positive response after the first infection and explicit response after the second infection. No sufficient immune response was observed during chemotherapy or radiotherapy.
At time point 1 (day 169), three weeks after clearing the first SARS-CoV-2 infection (Fig. 1D), only a borderline in vitro CD8+ T-cell response and no CD4+ response were observed against SARS-CoV-2-specific peptides (peptide mixes SI/SII). In contrast, a clear response of CD4+ T cells against cross-reactive peptides (peptide mix CII) was observed (Fig. 1B). During subsequent intense radiochemotherapy, lymphocyte counts remained low (maximum 580/µL between March and November). However, stimulation of T cells with staphylococcal enterotoxin B, serving as a positive control, resulted in cytokine secretion, indicating a remaining T-cell function (Fig. 1B). No antibody response was detected under chemotherapy.
In November 2020 (360 days after the initial diagnosis of sarcoma), the patient developed dry cough and headaches. Subsequent testing for SARS-CoV-2 revealed RNA in the pharyngeal swab on days 360 and 369. Next-generation sequencing (Illumina) detected the SARS-CoV-2 lineage B.1.1.70 (Pangolin tool v3.1.11, https://pangolin.cog-uk.io/ and https://pangolin.cog-uk.io/), a strain circulating in Germany that time (GISAID Accession Number EPI_ISL_4528405). Infection likely occurred after exposure to SARS-CoV-2 positive close family members. In addition to neutropenia and lymphopenia, the patient did not develop fever or significantly altered laboratory results. However, routine magnetic resonance imaging performed to evaluate remission status revealed lung infiltration, possibly attributable to coronavirus disease 2019. In a second swab obtained 1 week later, SARS-CoV-2 RNA was still detectable (Ct value E-gene = 26). Following 2 consecutive negative SARS-CoV-2 swabs (days 376 and 379), subsequent swabs demonstrated faintly positive results (Ct value E-gene >35) on days 387 and 394. All further follow-up swabs (sampled at 16 time points from days 401 to 524) remained negative for SARS-CoV-2 RNA, indicating that SARS-CoV-2 reinfection was cleared. Stool specimens were not analyzed for viral shedding. SARS-CoV-2 antibody ELISA revealed seroconversion with the detection of anti-nucleocapsid IgG. The antibody response occurred with recovery of B-cell counts after completion of intense radiochemotherapy (Fig. 1A).
At time point 2, 9 weeks after the last positive PCR test, T-cell responses were re-evaluated. Strong CD3+CD4+ and CD3+CD8+ T-cell responses against specific SARS-CoV-2 peptide mixes SI and SII, as well as against cross-reactive peptide mixes CI and CII, were evident (Fig. 1C).
Despite salvage radiation and intrathecal chemotherapy, the patient died as a result of the underlying oncological disease after experiencing intracranial relapse.
DISCUSSION
Prolonged virus shedding for more than 100 days,7 recurrent detection of SARS-CoV-2 RNA,5 and de novo reinfections8 have been reported for SARS-CoV-2, especially in immunocompromised patients. Differentiating between persistent shedding and reinfection is a challenging task. In the present case, it is likely that the patient had experienced 2 subsequent SARS-CoV-2 infections. Although sampling at a low viral load during the first episode was insufficient for sequencing, the SARS-CoV-2 lineage was successfully determined for the second episode. Since the detected strain was predominantly circulating during the second wave in Germany (November 2020), a second de novo reinfection is most likely. Additionally, the relatively long interval between April and November, the known infection source (exposure from SARS-CoV-2 positive family members), and typical clinical symptoms including pulmonary infiltration during the second infection9 argue in favor of a reinfection.
It is likely that the T-cell response at time point 1 reflects preexisting cross-reactive CD4+ T cells originating from previous infections with endemic coronaviruses related to SARS-CoV-2. These T cells represent preformed memory cells that are reactive to peptide sequences included in the cross-reactive peptide mix. The peptide mixtures CI and CII consist of sequences conserved across coronaviruses, SARS-CoV-2 and other endemic coronaviruses. Therefore, a large proportion of the healthy population has memory T cells against these antigens.6 The cross-reactive cells in the present patient might have been acquired before the first SARS-CoV-2 infection but could also have developed as a result of it. Kundu et al10 recently showed that such cross-reactive memory T cells are associated with protection against SARS-CoV-2 infection.
Thus, the detected cross-reactive CD4+ and the borderline specific CD8+ response might have contributed to the clearance of the patient’s first infection. Antitumor therapy before and after the first infection seemed to hamper the production of specific antibodies, which were not detected by ELISA. The absence of seroconversion is associated with profound therapy-related lymphopenia and B-cell deficiency. Moreover, insufficient T-cell priming and cytokine support may negatively influence seroconversion, antibody production and release.
In contrast, the second infection resulted in strong and persistent CD4+ and CD8+ responses against both cross-reactive and specific peptides, as well as clear seroconversion. At that time point, the intense antitumor therapy was terminated and did not severely interfere with the cellular immune response.
Reinfections with SARS-CoV-2 have also been reported in patients without preexisting oncological conditions, but are still very rare, considering the high number of infections worldwide.8 The duration of protective immune responses is not clear, since long-term follow-up studies are limited to date, and immunity following the first SARS-CoV-2 infection might be insufficient against emerging virus variants. Nevertheless, the patient experienced a second infection in close temporal proximity to the first infection, which did not result in a sufficient antibody response. Even though the marginal T-cell response appears to be sufficient to clear the virus initially, long-lasting protection was impaired by subsequent therapy. Thus, radiochemotherapy can inhibit a durable immune response and increase the risk of reinfections.
Conversely, cancer patients are not only endangered by the virus but also by a delay in treatment protocols, and mild SARS-CoV-2 symptoms should not necessarily lead to a long-lasting interruption.
Regular screening can help detect reinfections at an early stage and initiate appropriate measures. Since family members are a common source of infection, immunization of caretakers and siblings with priority, according to national approval and guidelines, should be encouraged.
In summary, a limited and transient immune response can occur in children diagnosed with cancer undergoing radiochemotherapy, and previously acquired immunity might be impaired by these therapies.
ACKNOWLEDGMENTS
We thank Silke Peter from the Institute of Medical Microbiology (University Tuebingen) for typing of the SARS-CoV-2 lineage by high-throughput sequencing. The authors acknowledge the financial support by the German ministry of education and research (BMBF, FKZ:01KI20130) by which the virus specific peptides were identified. We would like to thank Editage (www.editage.com) for English language editing.
Supplementary Material
Footnotes
This work was supported by grants from the excellence cluster iFIT (EXC 2180) Gefördert durch die Deutsche Forschungsgemeinschaft (DFG) im Rahmen der Exzellenzstrategie des Bundes und der Länder—WXC 2180—390900677 to P.L. and A.R., from the Dieter Schwarz Stiftung Neckarsulm, from the Reinhold-Beitlich Stiftung Tuebingen, from the Foerderverein and from the Stiftung fuer krebskranke Kinder Tuebingen e.V. to P.L.
J.W. is listed as inventor for patents on peptides described in this manuscript. The other authors have no conflicts of interest to disclose.
Thomas Eichholz and Anne-Marie Arendt contributed equally to the writing of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Ursula Holzer, Email: Ursula.Holzer@med.uni-tuebingen.de.
Christian Seitz, Email: Christian.Seitz@med.uni-tuebingen.de.
Reinhild Klein, Email: Reinhild.Klein@med.uni-tuebingen.de.
Juliane Walz, Email: Juliane.Walz@med.uni-tuebingen.de.
REFERENCES
- 1.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loenenbach A, Markus I, Lehfeld AS, et al. SARS-CoV-2 variant B.1.1.7 susceptibility and infectiousness of children and adults deduced from investigations of childcare centre outbreaks, Germany, 2021. Euro Surveill. 2021;26:2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheikh A, McMenamin J, Taylor B, et al. ; Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balduzzi. Management of the COVID-19 outbreak in a pediatric hemato-oncology department Early experience in protecting immunocompromised patients in Lombardia, Italy. https://www.piernetwork.org/uploads/4/7/8/1/47810883/covid_19_comments_letter.pdf.pdf. Accessed March 18, 2020.
- 5.Ferrari A, Trevenzoli M, Sasset L, et al. Prolonged SARS-CoV-2-RNA detection from nasopharyngeal Swabs in an oncologic patient: what impact on cancer treatment? Curr Oncol. 2021;28:847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelde A, Bilich T, Heitmann JS, et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat Immunol. 2021;22:74–85. [DOI] [PubMed] [Google Scholar]
- 7.Avanzato VA, Matson MJ, Seifert SN, et al. Case Study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183:1901–1912.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tillett RL, Sevinsky JR, Hartley PD, et al. Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect Dis. 2021;21:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falahi S, Kenarkoohi A. COVID-19 reinfection: prolonged shedding or true reinfection? New Microbes New Infect. 2020;38:100812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kundu R, Narean JS, Wang L, et al. Cross-reactive memory T cells associate with protection against SARS-CoV-2 infection in COVID-19 contacts. Nat Commun. 2022;13:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.