Abstract
Introduction
Pneumonia is a leading cause of mortality and a common indication for antibiotic in elderly patients. However, its diagnosis is often inaccurate. We aim to compare the diagnostic accuracy, the clinical and cost outcomes and the use of antibiotics associated with three imaging strategies in patients >65 years old with suspected pneumonia in the emergency room (ER): chest X-ray (CXR, standard of care), low-dose CT scan (LDCT) or lung ultrasonography (LUS).
Methods and analysis
This is a multicentre randomised superiority clinical trial with three parallel arms. Patients will be allocated in the ER to a diagnostic strategy based on either CXR, LDCT or LUS. All three imaging modalities will be performed but the results of two of them will be masked during 5 days to the patients, the physicians in charge of the patients and the investigators according to random allocation. The primary objective is to compare the accuracy of LDCT versus CXR-based strategies. As secondary objectives, antibiotics prescription, clinical and cost outcomes will be compared, and the same analyses repeated to compare the LUS and CXR strategies. The reference diagnosis will be established a posteriori by a panel of experts. Based on a previous study, we expect an improvement of 16% of the accuracy of pneumonia diagnosis using LDCT instead of CXR. Under this assumption, and accounting for 10% of drop-out, the enrolment of 495 patients is needed to prove the superiority of LDCT over CRX (alpha error=0.05, beta error=0.10).
Ethics and dissemination
Ethical approval: CER Geneva 2019-01288.
Trial registration number
Keywords: Thoracic medicine, Respiratory infections, GERIATRIC MEDICINE
Strengths and limitations of this study.
Direct comparison of chest X-ray, low-dose CT scanner-based and ultrasound-based strategies for the diagnosis of pneumonia in a randomised trial.
All three imaging modalities obtained in all patients irrespective of randomisation arm, thus minimising information bias.
Standardisation of ultrasound conduct and interpretation.
The primary outcome (difference in accuracy) is not a clinical outcome.
Introduction
By 2050, one in four people in Europa and Northern America will be aged 65 years or over according to demographic projections.1 Pneumonia principally affects older people, with two thirds of patients hospitalised for pneumonia aged more than 70 years.2 Accordingly, the burden of pneumonia on health and economic outcomes is expected to increase. Pneumonia is also the most frequent cause of antimicrobial therapy prescription in this population.3 4 However, studies specifically investigating the elderly are scarce.
Diagnosis of pneumonia
According to international guidelines, the diagnosis of pneumonia is based on suggestive clinical signs and symptoms and the presence of a new infiltrate on chest X-ray (CXR).5 6 However, signs and symptoms of pneumonia have poor sensitivity and specificity in older patients who often present with unspecific complaints. There is a significant overlap in the clinical presentation between pneumonia and other respiratory or infectious conditions frequently present in the elderly like acute heart failure, chronic obstructive pulmonary disease exacerbation and non-respiratory sepsis. As for the radiologic demonstration of an acute lung infiltrate, the elderly have a higher prevalence of pre-existing cardiac, pulmonary and thoracic wall diseases complicating the interpretation of radiologic studies. Moreover, several diseases can simultaneously affect the same individual.7 8 Obtaining good-quality radiographs can be challenging in this population and CXR also lacks sensitivity to detect pneumonia, which exposes patients to the risk of late initiation of appropriate antibiotic treatment.9 All these issues may lead to a high prevalence of diagnostic errors, delays in the correct management of patients’ conditions, overprescription of antimicrobial drugs and finally poor patients’ outcome.10–18 This translates in a positive predictive value of only 60%–75% for an initial diagnosis of community-acquired pneumonia (CAP) in hospitalised patients when compared with the final diagnosis.19 20 Misdiagnosis of pneumonia may translate in harmful delays in the correct management of the real cause of patients’ symptoms, an understudied issue. Alternative imaging strategies have been proposed to surpass the acknowledged drawbacks of the current diagnostic workup of pneumonia.
Thoracic CT scan
The use of CT scan is sometimes recommended when standard imaging is inconclusive.9 21 In a cohort of 319 adult patients with suspected pneumonia, Claessens et al reported that early CT scan changed the diagnostic classification of pneumonia in 59%.22 Modification of pneumonia probability (mostly, but not only, downgrading of the probability of pneumonia) was concordant with the final classification of an adjudication committee in 80%. The absolute Net Reclassification Improvement was 60/319 (18%). The authors also demonstrated the feasibility of rapidly obtaining a CT scan in the emergency room (ER) settings for patients suspected of pneumonia.
Similar results were obtained for elderly patients in the monocentric PneumO-LD-CT cohort, which included patients aged 65 years and older with suspected pneumonia treated with antibiotics.23 All of them had a CXR followed by a low-dose CT scan (LDCT). The clinician in charge of the patients assessed the probability of pneumonia before and after the LDCT. The main outcome was the difference of probability of pneumonia according to the clinician in charge before and after LDCT. Among 200 patients (median age 84 years, IQR: 79–90), performing an LDCT immediately after admission led to Net Reclassification Improvement in 16/200 patients (8%) (reference diagnosis adjudicated a posteriori by a panel of experts, blinded for the results of LDCT). Correct reclassification was mainly observed in patients not having pneumonia, suggesting that LDCTwould mainly reduce the overdiagnosis of pneumonia in this population.24
The advantages of a CT scan-based strategy could be greater in elderly patients as it can be challenging to obtain good quality CXR and, as mentioned above, pneumonia can be difficult to distinguish from other frequent conditions. Native LDCT is appropriate to study the lung fields, is free of risks associated with contrast medium injection and the irradiation burden is low.
Lung ultrasonography
Lung ultrasonography (LUS) is another imaging modality under investigation for the diagnosis of pneumonia. LUS has significant advantages, being increasingly available, non-irradiating, easy to perform directly at the bedside, and is increasingly done by trained emergency physicians. LUS realisation can be taught quickly.25 Its main drawback is the operator-dependent accuracy. Diagnostic studies evaluating LUS have reported a sensitivity of 80%–90% and a specificity of 70%–90% in pneumonia, using various reference standards.26–29 In studies using CT scan as the reference standard, LUS showed a higher sensitivity than CXR, with similar specificity.29 30 However, LUS has never been compared with CXR and LDCT for the diagnosis of pneumonia in a randomised controlled study, and data on its performance in elderly patients are scarce.31 32 In a non-randomised monocentric study including patients hospitalised for a pneumonia in an acute geriatric ward, Ticinesi and colleagues showed that LUS was more accurate than CXR (Area under the ROC Curve (AUC) of 0.90, 95% CI 0.83 o 0.96 vs 0.67, 95% CI 0.60 to 0.74, p<0.001), particularly in frail patients.32 A recent review emphasised the possible advantages of LUS in geriatrics, including the fact that it is little affected by age-related changes of lower respiratory tract and mobility limitations. The authors further emphasised the urgent need to perform studies focused on elderly patients.33
Rationale for the study
On these premises, an LDCT or LUS-based work-up for suspected pneumonia may have significant advantages over a standard CXR-based strategy. Superior diagnostic accuracy of either modality may lead to better short-term outcomes through early appropriate management of the disease causing the symptoms; to more appropriate antibiotic prescription; and to less additional diagnostic tests ordered. However, the impacts of reduced false positive and false negative diagnoses on costs, prognosis and quality of life should be assessed, along with costs associated with the three imaging modalities. Only a randomised trial comparing each diagnostic strategy head to head can allow for a reliable comparison of strategies’ performance.
Based on the results of our previous PneumO-LD-CT study, we hypothesise that an LDCT-based diagnostic strategy will have better accuracy than the standard of care CXR-based strategy for the diagnosis of pneumonia in elderly patients admitted to the ER.23 This could translate in better clinical and cost outcomes and less inappropriate use of antibiotics. The same could be true if a LUS-based strategy is more accurate than the CXR-based strategy.
Methods and analysis
Setting
This study will be conducted in three academic hospitals and one tertiary care hospital in Switzerland: Geneva University Hospitals, Geneva; Inselspital, Bern; Regional Hospital Lugano, Lugano; and Riviera Chablais Hospital, Rennaz.
Study design
We used the Standard Protocol Items: Recommendations for Interventional Trials checklist when writing our protocol.34
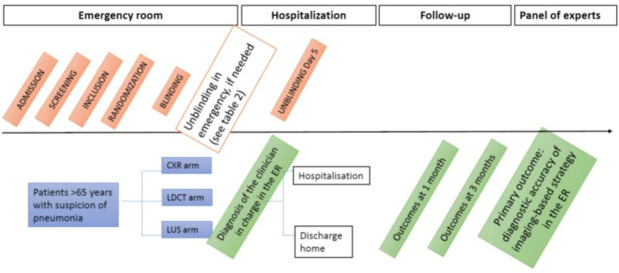
This is a multicentre randomised superiority clinical trial with three parallel arms aiming to compare the accuracy of imaging-based strategies for diagnosis of pneumonia in elderly patients admitted to the ER (figure 1).
Figure 1.
Study design. CXR, chest X-ray; ER, emergency room; LDCT, low-dose CT scan; LUS, lung ultrasonography.
Each patient will be randomly allocated in the ER to one of the three imaging examination (CXR, LDCT or LUS), which will be immediately performed, interpreted by one of two independent radiologists (one for CXR and one for LDCT) or by an independent emergency physician trained in ultrasonography (LUS) and reported in a standardised form. The physician in charge of the patient will have access to the imaging examination and the corresponding report, in addition to usual clinical and biological data obtained in the diagnostic workup of suspected pneumonia; he/she will be asked to assess the probability of pneumonia before the patient is discharged from the ER.
For each patient, the two other imaging examinations will also be performed and interpreted as described above, but the physician in charge of the patient will be blinded to these results. The results of all three imaging examinations and the interpretation will however be available to the panel of experts, whose final diagnosis of pneumonia will be the reference diagnosis for the study.
This study is carried out in an emergency setting. Pneumonia is a major cause of morbidity and mortality, and diagnosing and the decision of treating the patient consulting the ER must be performed within the first hours. Indeed international guidelines recommend treating the patient for a suspicion of pneumonia while he/she is still in the ER.
All enrolled patients will be followed up by study staff during hospitalisation and by phone at month 1 and 3.
Sample size
The sample size calculation was based on the accuracy of diagnostic strategies for pneumonia assessed in the PneumO-LD-CT cohort (unpublished results, online supplemental file 1). In this study, the accuracy of the clinician’s diagnosis was 68% when based on CXR and 84% when based on LDCT.
bmjopen-2021-055869supp001.pdf (116.9KB, pdf)
With an expected improvement of 16% of the accuracy using LDCT instead of CXR, 150 patients will be required in each arm to demonstrate the superiority of LDCT over CXR with a two-sided alpha error of 0.05 and a power of 90%. Allowing for a 10% drop-out after randomisation, the final recruitment objective is 165 patients in each arm, for a total of 495 patients.
Patient population
Patients aged >65 years consulting in the ER with suspected CAP or nursing-home acquired pneumonia. Any patients admitted to the ER will be included in the study if eligible according prespecified criteria. They may be referred by a doctor, an ambulance, a relative or come on their own initiative.
Eligibility criteria
Eligible patients will be patients aged >65 years consulting in the ER with suspected pneumonia. At least one respiratory symptom and one sign or laboratory finding compatible with infection are required (details in box 1). In the oldest old (patients aged 80 years or older), the presence of acute delirium or unexplained acute fall can substitute for the presence of either the respiratory or the infectious symptoms.
Box 1. Eligibility criteria.
Inclusion criteria
Age >65 years.
Suspected community-acquired pneumonia or nursing-home acquired pneumonia with:
At least one respiratory symptom (new or increasing cough or dyspnoea, purulent sputum, pleuritic chest pain, respiratory rate >20/min, focal auscultatory findings or oxygen saturation <90% on room air).
AND at least one sign or laboratory finding compatible with an infection (temperature >37.8°C or <36.0°C, C reactive protein >10 mg/L, procalcitonin (PCT)>0.25 µg/L, leucocyte count >10 G/L with >85% neutrophils or band forms).
In the oldest old signed informed consent (patients aged 80 years or older), the presence of acute delirium or unexplained acute fall can substitute for the presence of either the respiratory or the infectious symptom
Exclusion criteria
Immediate admission to the intensive care unit.
Pneumonia in the past 3 months.
Positive PCR for SARS-CoV-2 or antigenic test within 3three past weeks and at the arrival in the ER.
Transfer from another hospital with a diagnosis of pneumonia.
Thoracic CXR or CT scan or US during the present episode.
Immediate contrast-enhanced thoracic CT scan needed.
Advanced care planning limiting therapy to comfort care only.
Prisoners.
Patients with known uncontrolled psychiatric disorders.
Previous enrolment into the current study.
Inclusion and exclusion criteria are listed in details in box 1.
Patients with suspicion of bacterial, viral or aspiration pneumonia will be included on a non-preferential basis. Patients with a current diagnosis of pneumonia or a chest imaging obtained during the recent episode will be excluded, as well as those with a recent diagnosis of COVID-19 to minimise the overrepresentation of COVID-19 cases in the cohort. Patients with hospital-acquired pneumonia will be excluded.
Recruitment
At the ER of each recruiting site, dedicated research staff will screen admissions and ask suitable patients for participation in the study. In addition, triage nurses and physicians working at the ER will be asked to call research staff when identifying any potential participant.
Due to the high complexity of the inclusion process and of the simultaneous realisation of three imaging modalities, the patients will only be included during working hours.
The inclusions began on June 2021 and in the study is planned to last until August 2023. However, recruitment may be extended if necessary, in particular in view of the COVID-19 pandemic interfering with inclusions in times of high demands on emergency departments.
Randomisation
Patients will be randomised using Research Electronic Data Capture (REDCap) tool, a secured web-based application designed to support data capture and randomisation for research studies. Randomisation will be done immediately after inclusion, stratified by centre and using permuted block sizes.
Interventions
The three images can be performed in random order and order will depend on the availability of the clinician practicing LUS. However, images will be obtained in the shortest time possible to avoid any significant impact on patient’s care.
Standard of care: CXR will be done preferentially standing and with two incidences, which is the recommended and most commonly used diagnostic imaging modality for pneumonia in guidelines.5 6
LDCT scan will be obtained without administration of intravenous contrast. Its performance lasts 10 min. Mean radiation exposure is 1.5±0.47 mSv, to be compared with a mean exposure of 0.05±0.03 mSv for conventional CXR, 7 mSv for a full-dose CT scan, and to Switzerland’s natural background radiation level of 4 mSv/year.35 LDCT and CXR will be interpreted by two independent radiologists who will not be allowed to communicate.22
LUS will be performed at bedside by a trained physician not involved in management of the patient, using the device available in the corresponding ER (models and commercial brand may differ between different ER). All physicians performing the LUS (therafter: sonographists) will be board certified in the realisation of point-of-care ultrasonography (POCUS). To enhance homogeneity of LUS reporting, physicians performing LUS will be trained to use the standardised report form before the beginning of inclusions, using a common protocol agreed on by al site investigators. All examiners will have participated in a joint POCUS workshop to standardise the use of the study protocol and practice and their years of practice will be recorded.
Blinding/unblinding procedure
The two masked radiological examinations will be concealed to the physicians caring for the patients during 5 days.
As soon as the randomisation has been conducted, research staff will know which tests to blind. Those will be sent to a research PACS instead of the electronic health records with the help of the local IT team. Furthermore, the radiologists or sonographist who perform/interpret the tests will be asked not to communicate with each other. Research staff will be present throughout the process to ensure a smooth conduct of the study.
Emergency unblinding will be allowed in case of identification of an immediately life-threatening finding (box 2). In this occurrence, the radiologist or ultrasonographist will make an emergency call to the investigator before communicating the results to the clinician.
Box 2. Reasons for emergency unblinding.
Pneumothorax.
Haemothorax.
Indirect signs of aortic dissection.
Indirect signs of aneurysmal rupture (haemomediastinum).
Massive pericardial effusion.
Tracheal foreign body.
Pneumoperitoneum.
Pneumomediastinum.
Malignant airway obstruction.
Suspected acute tuberculosis.
The clinician in charge of the patient will be allowed to prescribe any new imaging deemed necessary in case of later clinical deterioration (eg, full dose chest CT scan with intravenous contrast for suspected pulmonary embolism).
At day 5, research staff will unmask all radiological examinations along with the standardised reports in the patient’s medical record.
Reference diagnosis
A panel of experts composed of senior clinicians and board-certified specialists, including internists, geriatricians, infectious diseases specialists and radiologists, blinded to the allocation arm and the probability of pneumonia estimated by the clinician in charge, will rate prospectively and a posteriori the probability of pneumonia. They will be trained before the adjudication process and asked to follow international guidelines for the diagnosis of pneumonia. They will have access to all available but deidentified patient data present in the medical records, including clinical, biological, microbiological data—as results of biomarkers and polymerase chain reaction (PCR) viral detection on naso-pharyngeal swabs—and images of CXR, LDCT, LUS and corresponding reports, hospital notes and the final medical report. Each patient’s diagnosis of pneumonia will be analysed using a Delphi method as follows: each expert will give an individual opinion on the probability of pneumonia on a 3-point Likert scale (low, intermediate, high). Next, each expert will re-examine the cases where there was a disagreement between expert ratings, in full knowledge of the other experts’ first decisions. Finally, the adjudication committee will make consensus decisions in a plenary session and in the presence of a radiologist. The adjudication committee’s final decision will be considered as the reference diagnosis.
Data safety monitoring board
A safety analysis will be performed after 200 patients have completed the 1-month follow-up. Safety outcomes will be: unplanned transfer to the intensive care unit (ICU) and 1-month mortality. Based on this analysis, the data safety monitoring board can recommend to discontinue one arm or all arms of the trial. No interim analysis for futility or superiority is planned.
Objectives
Primary objective
Comparison between CXR-based and LDCT-based diagnostic strategies for diagnostic performance (including accuracy, sensitivity, specificity, positive and negative predictive value and likelihood ratio)
Secondary objectives
Treatment and management (antibiotic prescription and additional imaging).
Clinical outcomes (including length of stay, mortality, quality of life).
Cost per patient.
Association between biomarkers and imaging-based diagnosis.
Other secondary objectives
Comparison between CXR-based and LUS-based diagnostic strategies (secondary objectives) for the same outcomes as above.
Comparison between LDCT- and LUS based diagnostic strategies (secondary objectives) for the same outcomes as above.
Calibration of physician confidence with their actual diagnostic accuracy (CIRCUS substudy for Calibration of reasoning confidence in uncertain situation) and factors associated with their confidence.
Other secondary objectives : Comparison between CXR-based and LUS-based diagnostic strategies (secondary objectives) for the same outcomes as above.
Outcomes
Primary outcome: diagnostic accuracy of imaging-based strategies, CXR, LDCT and LUS, at the end of the ER evaluation, using the expert panel as the reference standard.
Secondary outcomes are summarised in table 1.
Table 1.
Secondary outcomes
| Diagnostic outcomes parameters and measurement | |
| Sensitivity and specificity of imaging-based strategies (CXR, LDCT and LUS) | Using panel of experts as reference |
| Unmasked imaging modalities in emergency | Number of unmasked imaging results (reasons shown in box 2) |
| Alternative diagnoses | Standardised report at the ER |
| Diagnosis of aspiration pneumonia | Diagnosis of panel of experts |
| Diagnosis of viral pneumonia Diagnosis of bacterial pneumonia |
Diagnosis of panel of experts |
| Additional imaging studies ordered | Number of additional CXR, thoracic CT scan and US prescribed by the clinician during the acute setting |
| The association between biological markers and the presence of an infiltrate | C reactive protein, procalcitonin at admission |
| Treatment outcomes parameters and measurement | |
| Antibiotic free days at day 30 (for any indication) | By phone or patient record |
| Clinical outcomes parameters and measurement | |
| Quality of life | European Quality of Life 5 Dimensions 3 Level Version (EQ-cx5D-3L) questionnaire and CAP score questionnaire40 (pneumonia-specific quality of life questionnaire) |
| Length of hospital stay | Patient record |
| Transfer to rehabilitation or long-term care facility | Patient record |
| Transfer to the intensive care unit | Patient record |
| All cause mortality All cause readmission |
Patient record, follow-up |
| Cost outcomes parameters and measurement | |
| Costs | Hospital financial database using the Swiss standard called REKOLE |
CAP, community-acquired pneumonia; CXR, chest X-ray; ER, emergency room; LDCT, low-dose CT; LUS, lung ultrasonography; US, ultrasonography.
Outcome assessment
Primary outcome
The primary outcome is the accuracy of the clinician’s diagnosis using the experts’ diagnosis as reference. The probability of pneumonia will be rated by the clinician, before the patient is discharged from the ER, on a three-level Likert scale (‘low’, ‘intermediate’ or ‘high’). The probability of pneumonia will be rated by the panel of experts a posteriori on the same scale. For the primary outcome, a diagnosis of pneumonia will be positive if the probability is rated ‘intermediate’ or ‘high’ and negative if the probability is rated ‘low’. The accuracy will be the proportion of patients with a clinician’s diagnosis (either negative or positive) matching with the panel of experts’ diagnosis which is considered as the reference. Grouping the levels ‘intermediate’ or ‘high’ makes sense from a medical decision making perspective since a patient rated ‘intermediate’ will be treated with antibiotics in the same way as a patient rated ‘high’. In a secondary analysis, the diagnosis of pneumonia will be considered positive only if the probability is rated ‘high’.
Secondary outcomes
Cost outcomes are defined as costs within the hospital calculated using a Swiss standard called REKOLE (https://rekole.hplus.ch/fr/produkt/rekole-comptabilite-analytique-a-lhopital/).
The main costs components are: nursing care, physician, imaging, laboratory, treatment (including antibiotic therapy) and others per patient during hospitalization, health related quality of life at 3 months; unit of work consumption per hospital (number of minutes of care, physician, laboratory and imaging points) up to 3 months.
Safety outcomes
The research staff will report any transfer to the ICU. All-cause mortality will be determined at 1 month (from the civil registry if necessary) (table 1).
For the study schedule, see table 2.
Table 2.
Timeline of patient enrolment/allocation, interventions and assessments
| Study periods | Screening | Randomisation | Discharge from ER | Discharge from the acute setting | Day 30 | Day 90 | Reference diagnosis |
| Visit | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Time (hour, day) | hr0 | hr2* | hr6* | dx | d30 | d90 | |
| Demographics | x | ||||||
| Medical history | x | ||||||
| Inclusion/exclusion criteria | x | ||||||
| Physical examination | x | ||||||
| Vital signs | x | ||||||
| Laboratory tests | x | x | |||||
| CXR, LDCT, LUS | x | ||||||
| Main diagnosis before ER discharge | x | x | |||||
| Other diagnosis outcomes | x | x | x | ||||
| Number of antibiotic free days | x | ||||||
| Clinical, safety and cost outcomes | x | x | |||||
| Readmission and mortality | x | x | |||||
| QoL questionnaire | x | x | x | ||||
| Panel of experts | x |
*Approximately.
CXR, chest x-ray; d, day; ER, emergency room; hr, hour; LDCT, low-dose CT; LUS, lung ultrasonography; QoL, quality of life.
Statistical methods
We hypothesise that the diagnostic accuracy will be higher with the LDCT-based strategy than with CXR-based strategy. The null hypothesis that will be tested in the primary analysis is the equality of accuracy with these two strategies.
Population analysis
The analysis will follow the intention-to-treat principle. A sensitivity analysis will be conducted on the per protocol population (ie, excluding patients for whom an imaging or its standardised report which should have been masked has been available to the clinician who assesses the probability of pneumonia at the ER).
Primary analysis
The proportions of correctly classified patients in the LDCT and CXR arms will be calculated with 95% Clopper-Pearson confidence intervals (CIs), and will be compared with a logistic regression model adjusted for sites to account for the stratified randomisation. The statistical test will be two-sided and the significance threshold will be 0.05.
Secondary analyses
-
Analyses related to diagnostic outcomes:
The primary analysis will be repeated to compare CXR-based and LUS-based strategies.
Sensitivity, specificity, predictive values and likelihood ratios will be assessed by arm. Sensitivity (respectively, specificity) will be compared between strategies (logistic regression model).
The proportions of imaging results needing unmasking and of additional radiological studies (and invasive procedures) during the hospitalisation will be compared between strategies (logistic regression model).
The proportion of patients with an alternative diagnosis consistent between clinicians and experts will be compared between strategies (logistic regression model).
The association between biological markers (C reactive protein, procalcitonin) and infiltrates will be assessed with multiple linear regression models. Transformations on markers will be applied if needed. Similar methods will be used to investigate the association between markers and the probability of pneumonia rated on the three-level scale. The analyses will be conducted for each arm.
-
Analyses related to the treatment outcome:
The cumulative incidence of antibiotic free patients will be investigated over 30 days by using a non-parametric competing risk model with death before antibiotic intake as a competing event and will be compared between arms (CXR-based vs LDCT-based strategies and CXR-based vs LUS-based strategies).
-
Analyses related to clinical outcomes:
Quality of life: it will be compared between the strategies by using a linear regression model.
Length of stay: if appropriate the length of hospital stay will be compared between the strategies by using a linear regression.
Transfer: the proportion of patients with an unplanned transfer to the ICU will be compared between arms (logistic regression model).
Mortality and readmission: all-cause mortality during the 3 months follow-up will be investigated with Kaplan-Meier’s survival curves, and compared between the arms using a log-rank test stratified on sites. Readmissions during the 3-month follow-up will be investigated using survival models with competing risk. Death before readmission will be the competing event.
Cost outcomes: Mean cost per patient will be assessed and compared between arms using multiple linear regression models. Reweighted estimators will be used to account for censoring.
All relevant proportions (sensitivity, specificity, predictive values…) will be reported with the exact 95% CIs assess by Clopper-Pearson method. All regression models will be adjusted for sites and tests for comparison of survival or cumulative incidence will be stratified on sites to account for the stratified randomisation. All statistical tests will two-sided with a level of 5%.
Sensitivity analyses
As a sensitivity analysis, the primary analysis and the secondary analyses 1a and 1b will be repeated, whereby only the level ‘high’ on the Likert scale is regarded as positive diagnosis (clinician’s diagnoses and reference diagnoses).
Interim and safety analyses
There will be no comparative analysis for efficacy or futility. An initial safety analysis will be performed after 200 patients have reached the 1-month follow-up. The study’s data manager will ensure that the data will be exported with ‘scrambled’ allocation labels and that study investigators do not have access to the data. The results will be transmitted to the principal investigators and the safety monitoring board. The latter will be free to lift the blind if deemed necessary, and will be asked to make the final decision on the continuation of the study without modification, or terminating the study.
Subgroup analyses
The primary analysis and the secondary analyses related to the assessment of diagnostic performances (secondary analyses 1) to 4)) will be conducted in subgroups of patients: (1) patients aged less than 80 years vs patients aged over 80 years, (2) patients with proven viral pneumonia vs bacterial pneumonia vs pneumonia with no identified pathogen, 3) patients with aspiration pneumonia vs patients with other causes of pneumonia.
Handling of missing data and drop-outs
Some patients will be discharged from the ED, but they won’t be considered as dropout patients as the diagnosis of pneumonia will be made before the discharge, and follow-up will be done by phone. All available data from all included patients will be included in the intention-to-treat analysis whereas patients with missing data will be excluded from the complete case analysis. In addition, multiple imputation will be performed if more than 10% of data of the outcome are missing. R (R Foundation for Statistical Computing, Vienna, Austria) will be used for statistical analyses.
Data management
Data will be collected by using electronic case record forms in REDCap software, and will be securely stored for 10 years.
Patient and public involvement
No patients or public have been included in the study design but it was presented at a patient–partner meeting.
Ethics and dissemination
OCTOPLUS will be carried out in accordance to the research plan and the Declaration of Helsinki, the Swiss Law and Swiss regulatory authority’s requirements as applicable.36 37 The application has been approved by the lead committee, that is, the Ethic Committee of Geneva (CEC, number 2019-01288).
Vulnerable participants will be included. Clinical studies in elderly patients are scarce, in part because informed consent is difficult to obtain due to delirium or permanent cognitive impairment in this group. If the patient is able to consent in the ER, he/she will be invited to participate to the study and will be asked to read and sign the consent. If not, a physician independent of the study will safeguard the patient’s interests and possibly give consent for the patient to participate in the study and will sign the dedicated written confirmation according to Swiss law on Human research (HRA, Art. 7, 16, and 18, 42; ClinO, Art. 7-9). When the patient recovers his capacity to consent during hospitalisation, he will sign the standard consent of the participant. Otherwise it will be signed by the legal representative (who can be a relative in Switzerland). If the latter finally deny consent, all data collected until then will be kept and used in the analysis as written in the letter of information and consent. Consents and written confirmation of the independent physician are provided in online supplemental files 2–4.
bmjopen-2021-055869supp002.pdf (8.6MB, pdf)
bmjopen-2021-055869supp003.pdf (9.8MB, pdf)
bmjopen-2021-055869supp004.pdf (736.6KB, pdf)
Data storage will be handled according to international standards. The CEC will receive annual safety and interim reports and be informed about study stop/end in agreement with local requirements. Risk-based monitoring will be performed by the clinical trial unit on each site.
The results of the study will be disseminated through conference presentations in national and international conferences and peer-reviewed manuscripts published in open-access journals. Trial findings will be integrated into national and international guidelines. As described in the data management plan, data will be shared according to FAIR data principles and thus in a FAIR-compliant data repository.
Discussion and implications
To our knowledge, no randomised trial has ever compared the performance of CXR, CT scan and LUS in patients with suspected pneumonia. The OCTOPLUS trial will allow a direct comparison of CXR-based, LDCT-based and LUS-based strategies for the diagnosis of pneumonia in a randomised trial.
The results will have an important impact for emergency medicine, internal medicine and general practice, because the question of the superiority of LDCT over CXR in diagnosing pneumonia in the elderly will be addressed. Confirmed superiority could profoundly affect recommendations for the diagnosis of pneumonia in elderly patients, and LDCT could become the preferred diagnostic option over CXR. This could also apply to the superiority of LUS over CXR, with the added benefit of being more readily available and non-irradiating compared with LDCT. Other strengths of the study will be the random blinding of two of the three imaging modalities to the patients and clinicians, and the LUS standardisation among sonographists.
We will use an adjudication expert committee for the reference diagnosis using a Delphi method to obtain consensus.38 Experts will have access to all three imaging modalities obtained for all patients irrespective of randomisation arm in order to minimise information bias. However, this might induce an incorporation bias as the tests under evaluation will be known when interpreting the reference standard.39 This might be considered as a limitation of the study but such a methodology is recommended in the absence of a gold standard, which is the case in the diagnosis of pneumonia in elderly patients in whom it seems not ethical to perform a bronchoalveolar lavage. Another limitation is that the primary outcome (difference in accuracy) is not a clinical outcome but we have included clinical outcomes as secondary ones.
Supplementary Material
Acknowledgments
We thank all members of OCTOPLUS study group, the ER and IT teams, the research nurses, the coordinators and monitors, and the Clinical Research Centre of Hôpitaux Universitaires de Genève (HUG).
Footnotes
Collaborators: Other members of the OCTOPLUS study group: Nicolas Garin, Christophe Combescure and in alphabetical order: Gianluca Argentieri, Christine Baumgartner, Cristina Boehm-Bosmani, Tanja Birrenbach, Clémence Cuvelier, Christophe Fehlmann, Pauline Gosselin, Olivier Grosgurin, François Herrmann, Alessandro Jessula, Laurent Kaiser, Aileen Kharat, Véronique Lachat, Cornelia Lambrigger, Beat Lehmann, Antonio Leidi, Elisa Marchi, Christophe Marti, Mihaela Martinvalet, Lara Morosoli, Daniel Ott, Thibault Parent, Pierre-Alexandre Poletti, Jean-Luc Reny, Xavier Roux, Frédéric Rouyer, Thomas Ruder, Max Scheffler, Guillaume Soret, Jérôme Tessieras, Catherine Vindret, Dina Zekry, Enrico Zucconi.
Contributors: VP has the primary responsability for the final content. Study concept and design : VP, WH, NG, JS, TS, AP, CC and EB. Draft of manuscript and statistical analysis: VP, WH, NG, TS, JS and CC. Revision of manuscript: all authors read and approved the manuscript for final publication.
Funding: This study is funded by the Swiss National Science Foundation (grant number 32 003B_197398) and by the Ligue Pulmonaire Genevoise (no grant number).
Competing interests: WH has received research funding from the European Union, the Swiss National Science foundation, Zoll foundation, Dräger Medical Germany, Mundipharma Research UK, MDI International Australia, Roche Diagnostics Germany, all outside the submitted work. WH has provided paid consultancies to AO foundation Switzerland and MDI International Australia, all outside the submitted work. WH has received financial support for a congress he chaired from EBSCO Germany, Isabel Healthcare UK, Mundipharma Medical Switzerland, VisualDx USA, all outside the submitted work.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
The OCTOPLUS study group:
Nicolas Garin, Christophe Combescure, Gianluca Argentieri, Christine Baumgartner, Cristina Boehm-Bosmani, Tanja Birrenbach, Clémence Cuvelier, Christophe Fehlmann, Pauline Gosselin, Olivier Grosgurin, François Herrmann, Alessandro Jessula, Laurent Kaiser, Aileen Kharat, Véronique Lachat, Cornelia Lambrigger, Beat Lehmann, Antonio Leidi, Elisa Marchi, Christophe Marti, Mihaela Martinvalet, Lara Morosoli, Daniel Ott, Thibault Parent, Pierre-Alexandre Poletti, Jean-Luc Reny, Xavier Roux, Frédéric Rouyer, Thomas Ruder, Max Scheffler, Guillaume Soret, Jérôme Tessieras, Catherine Vindret, Dina Zekry, and Enrico Zucconi
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. OFSP . Vieillissement actif, 2008. Available: www.bfsadminch/bfs/fr/home.html
- 2. Ewig S, Klapdor B, Pletz MW, et al. Nursing-home-acquired pneumonia in Germany: an 8-year prospective multicentre study. Thorax 2012;67:132–8. 10.1136/thoraxjnl-2011-200630 [DOI] [PubMed] [Google Scholar]
- 3. Chami K, Gavazzi G, Carrat F, et al. Burden of infections among 44,869 elderly in nursing homes: a cross-sectional cluster nationwide survey. J Hosp Infect 2011;79:254–9. 10.1016/j.jhin.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 4. Wroe PC, Finkelstein JA, Ray GT, et al. Aging population and future burden of pneumococcal pneumonia in the United States. J Infect Dis 2012;205:1589–92. 10.1093/infdis/jis240 [DOI] [PubMed] [Google Scholar]
- 5. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious diseases Society of America/American thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27–72. 10.1086/511159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections--full version. Clin Microbiol Infect 2011;17 Suppl 6:E1–59. 10.1111/j.1469-0691.2011.03672.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aliberti S, Brambilla AM, Chalmers JD, et al. Phenotyping community-acquired pneumonia according to the presence of acute respiratory failure and severe sepsis. Respir Res 2014;15:27. 10.1186/1465-9921-15-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Black AD. Non-infectious mimics of community-acquired pneumonia. Pneumonia 2016;8:2. 10.1186/s41479-016-0002-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Syrjälä H, Broas M, Suramo I, et al. High-resolution computed tomography for the diagnosis of community-acquired pneumonia. Clin Infect Dis 1998;27:358–63. 10.1086/514675 [DOI] [PubMed] [Google Scholar]
- 10. Albaum MN, Hill LC, Murphy M, et al. Interobserver reliability of the chest radiograph in community-acquired pneumonia. Port Investigators. Chest 1996;110:343–50. 10.1378/chest.110.2.343 [DOI] [PubMed] [Google Scholar]
- 11. Faverio P, Aliberti S, Bellelli G, et al. The management of community-acquired pneumonia in the elderly. Eur J Intern Med 2014;25:312–9. 10.1016/j.ejim.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernández-Sabé N, Carratalà J, Rosón B, et al. Community-acquired pneumonia in very elderly patients: causative organisms, clinical characteristics, and outcomes. Medicine 2003;82:159–69. 10.1097/01.md.0000076005.64510.87 [DOI] [PubMed] [Google Scholar]
- 13. Haga T, Fukuoka M, Morita M, et al. Computed tomography for the diagnosis and evaluation of the severity of community-acquired pneumonia in the elderly. Intern Med 2016;55:437–41. 10.2169/internalmedicine.55.5556 [DOI] [PubMed] [Google Scholar]
- 14. Loeb M, Carusone SC, Goeree R, et al. Effect of a clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial. JAMA 2006;295:2503–10. 10.1001/jama.295.21.2503 [DOI] [PubMed] [Google Scholar]
- 15. Metlay JP, Schulz R, Li YH, et al. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch Intern Med 1997;157:1453–9. 10.1001/archinte.1997.00440340089009 [DOI] [PubMed] [Google Scholar]
- 16. Sauter TC, Capaldo G, Hoffmann M, et al. Non-Specific complaints at emergency department presentation result in unclear diagnoses and lengthened hospitalization: a prospective observational study. Scand J Trauma Resusc Emerg Med 2018;26:60. 10.1186/s13049-018-0526-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hautz WE, Kämmer JE, Hautz SC, et al. Diagnostic error increases mortality and length of hospital stay in patients presenting through the emergency room. Scand J Trauma Resusc Emerg Med 2019;27:54. 10.1186/s13049-019-0629-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mattsson B, Ertman D, Exadaktylos AK, et al. Now you see me: a pragmatic cohort study comparing first and final radiological diagnoses in the emergency department. BMJ Open 2018;8:e020230. 10.1136/bmjopen-2017-020230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chandra A, Nicks B, Maniago E, et al. A multicenter analysis of the ED diagnosis of pneumonia. Am J Emerg Med 2010;28:862–5. 10.1016/j.ajem.2009.04.014 [DOI] [PubMed] [Google Scholar]
- 20. Kanwar M, Brar N, Khatib R, et al. Misdiagnosis of community-acquired pneumonia and inappropriate utilization of antibiotics: side effects of the 4-h antibiotic administration rule. Chest 2007;131:1865–9. 10.1378/chest.07-0164 [DOI] [PubMed] [Google Scholar]
- 21. Waterer GW. The diagnosis of community-acquired pneumonia. do we need to take a big step backward? Am J Respir Crit Care Med 2015;192:912–3. 10.1164/rccm.201507-1460ED [DOI] [PubMed] [Google Scholar]
- 22. Claessens Y-E, Debray M-P, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med 2015;192:974–82. 10.1164/rccm.201501-0017OC [DOI] [PubMed] [Google Scholar]
- 23. Prendki V, Scheffler M, Huttner B, et al. Low-dose computed tomography for the diagnosis of pneumonia in elderly patients: a prospective, interventional cohort study. Eur Respir J 2018;51:1702375. 10.1183/13993003.02375-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garin N, Marti C, Scheffler M, et al. Computed tomography scan contribution to the diagnosis of community-acquired pneumonia. Curr Opin Pulm Med 2019;25:242–8. 10.1097/MCP.0000000000000567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soldati G, Smargiassi A, Inchingolo R. Is there a role for lung ultrasound during the COVID-19 pandemic? J Med Ultrasound 2020. 10.1002/jum.15284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Llamas-Álvarez AM, Tenza-Lozano EM, Latour-Pérez J. Accuracy of lung ultrasonography in the diagnosis of pneumonia in adults: systematic review and meta-analysis. Chest 2017;151:374–82. 10.1016/j.chest.2016.10.039 [DOI] [PubMed] [Google Scholar]
- 27. Xia Y, Ying Y, Wang S, et al. Effectiveness of lung ultrasonography for diagnosis of pneumonia in adults: a systematic review and meta-analysis. J Thorac Dis 2016;8:2822–31. 10.21037/jtd.2016.09.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu X-lei, Lian R, Tao Y-kang, Tao YK, et al. Lung ultrasonography: an effective way to diagnose community-acquired pneumonia. Emerg Med J 2015;32:433–8. 10.1136/emermed-2013-203039 [DOI] [PubMed] [Google Scholar]
- 29. Nazerian P, Volpicelli G, Vanni S, et al. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am J Emerg Med 2015;33:620–5. 10.1016/j.ajem.2015.01.035 [DOI] [PubMed] [Google Scholar]
- 30. Amatya Y, Rupp J, Russell FM, et al. Diagnostic use of lung ultrasound compared to chest radiograph for suspected pneumonia in a resource-limited setting. Int J Emerg Med 2018;11:8. 10.1186/s12245-018-0170-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Staub LJ, Mazzali Biscaro RR, Kaszubowski E, et al. Lung ultrasound for the emergency diagnosis of pneumonia, acute heart failure, and exacerbations of chronic obstructive pulmonary Disease/Asthma in adults: a systematic review and meta-analysis. J Emerg Med 2019;56:53–69. 10.1016/j.jemermed.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 32. Ticinesi A, Lauretani F, Nouvenne A, et al. Lung ultrasound and chest X-ray for detecting pneumonia in an acute geriatric ward. Medicine 2016;95:e4153. 10.1097/MD.0000000000004153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ticinesi A, Scarlata S, Nouvenne A, et al. The geriatric patient: the ideal one for chest ultrasonography? A review from the chest ultrasound in the elderly Study Group (GRETA) of the Italian Society of gerontology and geriatrics (SIGG). J Am Med Dir Assoc 2020;21:447-454.e6. 10.1016/j.jamda.2019.06.018 [DOI] [PubMed] [Google Scholar]
- 34. Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. Spirit 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Larke FJ, Kruger RL, Cagnon CH, et al. Estimated radiation dose associated with low-dose chest CT of average-size participants in the National lung screening trial. AJR Am J Roentgenol 2011;197:1165–9. 10.2214/AJR.11.6533 [DOI] [PubMed] [Google Scholar]
- 36. Declaration of Helsinki, 2013. Available: http://www.wma.net/en/30publications/10policies/b3/index.html
- 37. Humanforschungsgesetz, HFG Bundesgesetz über die Forschung am Menschen (Bundesgesetz über die Forschung am Menschen, HFG) vom 30. September 2011/ Loi fédérale relative la recherche sur l’être humain (loi relative la recherche sur l’être humain, LRH) du 30 septembre 2011. Available: https://www.bag.admin.ch/bag/fr/home/medizin-und-forschung/forschung-am-menschen.html
- 38. Niederberger M, Spranger J. Delphi technique in health sciences: a MAP. Front Public Health 2020;8:457–57. 10.3389/fpubh.2020.00457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reitsma JB, Rutjes AWS, Khan KS, et al. A review of solutions for diagnostic accuracy studies with an imperfect or missing reference standard. J Clin Epidemiol 2009;62:797–806. 10.1016/j.jclinepi.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 40. El Moussaoui R, Opmeer BC, Bossuyt PMM, et al. Development and validation of a short questionnaire in community acquired pneumonia. Thorax 2004;59:591–5. 10.1136/thx.2003.015107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-055869supp001.pdf (116.9KB, pdf)
bmjopen-2021-055869supp002.pdf (8.6MB, pdf)
bmjopen-2021-055869supp003.pdf (9.8MB, pdf)
bmjopen-2021-055869supp004.pdf (736.6KB, pdf)