Abstract
Objectives
Investigating end-of-life use of anticancer drugs and of palliative care services.
Design
Population based cohort linked to mortality registry and administrative databases.
Setting
Emilia-Romagna Region (Northern Italy).
Participants
55 625 residents who died of cancer between 2017 and 2020.
Primary and secondary outcome measures
Multivariate analyses were carried out to assess the relationship between cancer drug therapy and palliative care services, and their association with factors related to tumour severity.
Results
In the last month of life, 15.3% of study population received anticancer drugs (from 12.5% to 16.9% across the eight Local Health Authorities—LHA) and 40.2% received palliative care services (from 36.2% to 43.7%). Drug therapy was inversely associated with receiving palliative care services within the last 30 days (OR 0.92, 95% CI 0.87 to 0.97), surgery within the last 6 months (OR 0.59, 95% CI 0.52 to 0.67), aggressive tumours (OR 0.88, 95% CI 0.84 to 0.93) and increasing age (OR 0.95, 95% CI 0.95 to 0.95). Drug therapy was more likely among those with haematologic tumours (OR 2.15, 95% CI 2.00 to 2.30) and in case of hospital admissions within the last 6 months (OR 1.63, 95% CI 1.55 to 1.72). Palliative care was less likely among those with haematologic compared with other tumours (OR 0.52, 95% CI 0.49 to 0.56), in case of surgery (OR 0.44, 95% CI 0.39 to 0.49) or hospital admissions (OR 0.70, 95% CI 0.67 to 0.72) within the last 6 months, if receiving anticancer drugs during the last 30 days (OR 0.90, 95% CI 0.85 to 0.94) and for each year of increasing age (OR 0.99, 95% CI 0.99 to 0.99). Palliative care was more likely in the presence of aggressive tumours (OR 1.12, 95% CI 1.08 to 1.16).
Conclusion
Use of anticancer drugs and palliative care in the last month of life were inversely associated, showing variability across different LHAs. While administrative data have limits, our findings are in line with conclusions of other studies.
Keywords: CHEMOTHERAPY, ONCOLOGY, PALLIATIVE CARE
Strengths and limitations of this study.
Inclusion of all people deceased from cancer in a region with 4.4 million residents, linking information on the use of anticancer drugs and palliative care services with tumour characteristics and severity, are major strengths of this study.
Caution should be taken since administrative data could not capture all the elements that may contribute to clinical decision-making.
Moreover, although multivariate analyses provide adjustment for factors associated with tumour severity, residual confounding may be present.
Introduction
The appropriate use of anticancer drugs in end-of-life care is increasingly debated, both for clinical and economic reasons.1 2 Aggressive treatments, facilitated by the availability of newer anticancer agents that have fewer side effects,3 often do not alleviate patients’ condition or provide hope for extending significantly life of decent quality. Focus on clinically irrelevant treatments may lead to the underuse of palliative care,4–6 defined by WHO as ‘an approach that improves the quality of life (QoL) of patients and their families facing the problem associated with life-threatening illness, through … assessment and treatment of pain and other problems, physical, psychosocial and spiritual’.7 Palliative care is generally provided in dedicated hospices or as home care services by a specially trained team of doctors, nurses and other specialists who work together with a patient’s other doctors to provide an extra layer of support.8 9 Expectations of patients’ and parents on one side,10 and difficulties in predicting and communicating patients’ prognosis on the other,11 12 are among the main determinants of overuse of anticancer drugs (box 1). Some patients may perceive continued active treatment as the only acceptable option.10 For example, in a prospective cohort of terminally ill patients with cancer (n = 386), 31% preferred life-extending care rather than comfort care and as many as 77% preferred to receive drug treatment even if it would extend their life by only 1 week.12 Communication between the care team, patient and family seem to be a central element that can influence this phenomenon.13
Box 1. Main determinants of potential overuse of anticancer drugs.
Expectations of patients’ and parents (and ‘never give up’ attitude).
Difficulties in predicting patients’ prognosis.
Difficulties in communicating patients’ prognosis.
Physician’s perception of potential harm by early withdrawal.
Therapy seen as a form of palliative care.
From the clinicians’ point of view, withdrawal of drugs during the final, but not exactly predictable, stages of life is challenging:14 early withdrawal can cause potential harm, whereas late withdrawal would involve unnecessary treatment and stress (box 1). Research findings suggest that culture may impact the utilisation of aggressive treatment in patients with advanced cancer. For example, a study from Japan stated that only 3.7% of patients receive chemotherapy in their last 2 weeks of life.15
However, anticancer therapy itself is frequently considered a form of palliative care, aimed at reducing tumour-related symptoms, so that boundaries between curative and palliative intent are sometimes difficult to establish (box 1).16–18 According to the American Society of Clinical Oncology (ASCO), anticancer drugs can potentially improve QoL in late stages of life even if they do not impact survival length.19 In this regard, their use promotes a simultaneous care approach, using palliative care alongside usual oncology care as the standard of care for any patient with advanced cancer.20
Several studies have analysed the use of anticancer drugs in the last weeks of life with results that, although variable, show a tendency to prolong treatment beyond realistic expectations of a favourable benefit–risk ratio.16 21–26 Analysis of data available in administrative and clinical databases can inform about prescribing patterns and the utilisation of healthcare services in the end of life, in order to provide useful basis for discussion helping clinicians and healthcare managers identify areas of improvement, enhance the appropriateness and value of cancer care and make judicious use of available resources. In keeping with these targets, this study aims at providing insights on the use of anticancer drugs, hospital, hospice and home care services in the last month of life in a region of Northern Italy with more than 4 million residents, also to assess whether palliative care services are inversely associated with overuse of antineoplastic therapy.
Methods
A cohort of residents in the Emilia-Romagna Region who had cancer as the underlying cause of death between 2017 and 2020 (ICD-X classification: C00-C97, D00-D09, D37-D48) were selected from the regional mortality registry. This cohort was linked with the routinely available administrative databases, specifically: (1) hospital discharge records (including inpatient use of anticancer drugs, type of tumour, patients’ age, surgery and hospital admissions); (2) ambulatory services (specifying use of anticancer drugs); (3) outpatient pharmacological prescriptions (use of drugs within ATC classes L01 and L02); (4) hospice and (5) domiciliary care databases (also collectively considered as palliative care services). These databases do not include any personal details (eg, name or fiscal code) that can allow direct identification of included subjects: anonymity is warranted since each resident is associated to a unique identification number, allowing record linkage procedures. A list of codes used to select hospital discharge information and ambulatory care is available in the online supplemental appendix.
bmjopen-2021-057437supp001.pdf (54.3KB, pdf)
Analyses were specifically aimed at describing frequency of anticancer drug use, palliative care services or both received within the last 30 days of life among eight Local Health Authorities (LHA). Logistic multivariate two-level analyses27 were carried out to assess whether (1) anticancer drug use, (2) palliative care services (3) or both within the last 30 days of life could be associated with each other as well as with type of tumour (solid versus haematological, or aggressive tumours—see list in the online supplemental appendix), patients’ age, any surgery and hospital admissions within the last 6 months, considering LHA clustering as the second level (random intercept) to eliminate the effect of a possible correlation of results of residents in the same province. One-level models28 adding each LHA as covariates (each compared with a reference LHA) were subsequently used to assess whether the use of anticancer drugs and of palliative care could present variability among LHA. OR with 95% CIs were calculated. SAS V.8.2 (SAS Institute, Cary, NC, USA) and STATA/SE V.16.1 (STATACorp, College Station) were used for statistical analyses.
Patient and public involvement
No patient involved.
Results
In Emilia-Romagna, 55 625 people died from cancer between 1 January 2017 and 31 December 2020. Table 1 quantifies the main cancer diagnosis associated with death. Online supplemental extra table 1 also provides specific data on each LHA: no substantial differences are shown among them. Table 2 shows the use of anticancer drugs and of palliative care services within the last 30 days of life by main cancer diagnosis in the whole cohort. The highest use of anticancer drugs was in people with breast, prostate and haematologic tumours (in more than 20% of patients), whereas the lowest use was in people with nervous system and urinary tumours (in less than 10% of patients). Use of palliative care services appears relatively uniform across tumour types, except for a lower observed use in genital tumours in men and in haematologic tumours. Overall, 15.3% of patients received anticancer drugs within the last 30 days of life, with an increasing trend from 2017 (14.6%) to 2020 (16.2%). About palliative care services, 40.2% of patients received them (from 39.7% in 2017 to 40.8% in 2020). 4.1% received surgery within the last 30 days.
Table 1.
Study population of patients dying for cancer in Emilia-Romagna between 2017 and 2020, by tumour site: number (white background) and percentage (grey background)
| Head and neck | 1439 |
| 2.6 | |
| Digestive | 17 575 |
| 31.6 | |
| Respiratory | 10 098 |
| 18.2 | |
| Muscoloskeletal | 409 |
| 0.7 | |
| Skin | 1472 |
| 2.7 | |
| Nervous system | 1892 |
| 3.4 | |
| Breast | 3583 |
| 6.4 | |
| Genital (women) | 2548 |
| 4.6 | |
| Genital (men) | 215 |
| 0.4 | |
| Urinary | 4043 |
| 7.3 | |
| Prostate | 2253 |
| 4.1 | |
| Haematologic | 5874 |
| 10.6 | |
| Other/metastatic | 4224 |
| 7.6 | |
| Region | 55 625 |
| 100.0 |
Table 2.
Percentage of the use of anticancer drugs and palliative care (home or hospice care) during the last 30 days of life, by cancer type
| Cancer type | Treatment during the last 30 day of life | |||
| % anticancer drugs | % home care | % hospice care | % overall palliative | |
| Head and neck | 13.0 | 19.3 | 28.7 | 41.3 |
| Digestive | 10.3 | 23.1 | 27.7 | 44.8 |
| Respiratory | 19.3 | 21.5 | 28.0 | 43.3 |
| Musculoskeletal | 10.4 | 20.5 | 21.8 | 38.1 |
| Skin | 16.5 | 25.1 | 30.9 | 47.8 |
| Nervous system | 6.1 | 20.3 | 31.9 | 45.2 |
| Breast | 27.1 | 20.8 | 25.5 | 40.4 |
| Genital (women) | 16.4 | 20.8 | 29.6 | 44.6 |
| Genital (men) | 13.0 | 15.8 | 11.2 | 24.2 |
| Urinary | 8.7 | 19.2 | 22.9 | 37.4 |
| Prostate | 26.1 | 18.4 | 23.2 | 37.1 |
| Haematologic | 24.7 | 15.3 | 13.2 | 26.2 |
| Other/metastatic | 8.4 | 18.2 | 15.8 | 30.8 |
| Region | 15.3 | 20.7 | 24.9 | 40.2 |
bmjopen-2021-057437supp002.pdf (59.6KB, pdf)
Among the eight LHA, there was variability in the use of anticancer drugs (from 12.5% to 16.9%—figure 1) and of palliative care (from 36.2% to 43.7%—figure 2) in the last 30 days of life; 39.1% of patients died in hospital, with wide variability among the LHAs (range: from 29.4% to 44.0%).
Figure 1.
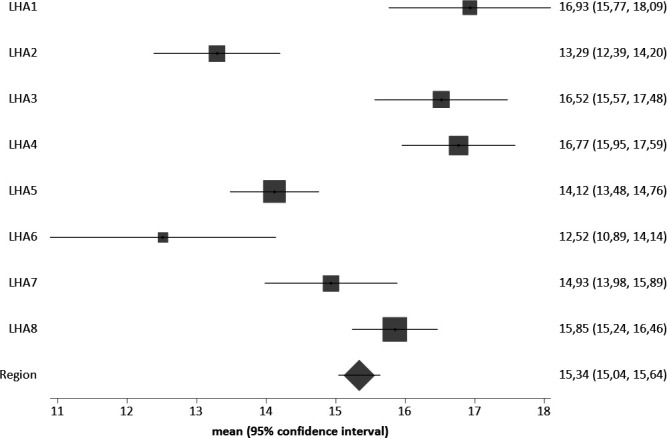
Percentage of the use of anticancer drugs during the last 30 days of life, by LHA of residence (all tumours). LHA, Local Health Authorities.
Figure 2.
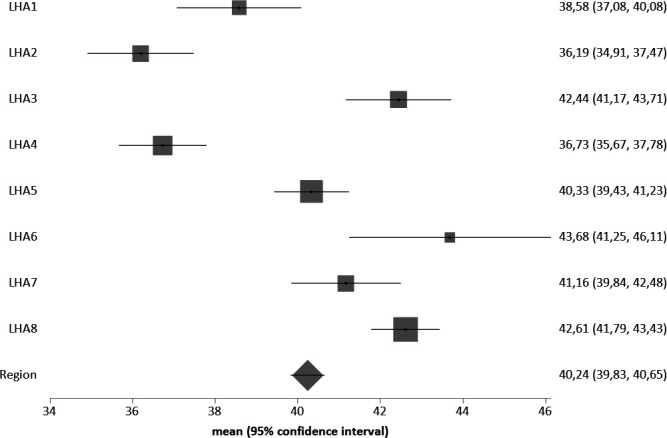
Percentage of home care services use during the last 30 days of life, by LHA of residence (all tumours). LHA, Local Health Authorities.
The likelihood to receive anticancer drugs during the last 30 days of life mostly increased in case of haematologic compared with other tumours; it also increased in case of hospital admissions within the last 6 months. It was reduced in case of surgery within the last 6 months and (less) in case of aggressive compared with other tumours, receiving home care or hospice services during the last 30 days and for every year of increasing age. Detailed data are available in table 3. The intracluster correlation coefficient (ICC) is 0.3%, showing low intra-LHA correlation.
Table 3.
ORs of receiving anticancer drugs, palliative care or both given each covariate (two-level multivariate model considering LHA clustering)
| Factor | Anticancer drugs | Palliative care | Anticancer drugs+palliative care |
| OR (CI 95%) | OR (CI 95%) | OR (CI 95%) | |
| Anticancer drugs within the last 30 days | – | 0.90* (0.85 to 0.94) | – |
| Palliative care within the last 30 days | 0.92* (0.87 to 0.97) | – | – |
| Haematologic tumour (ref. solid/metastatic) | 2.15* (2.00 to 2.30) | 0.52* (0.49 to 0.56) | 1.03 (0.92 to 1.16) |
| Age (continuous, in year) | 0.95* (0.95 to 0.95) | 0.99* (0.99 to 0.99) | 0.96* (0.96 to 0.96) |
| Hospital admission within the last 6 months | 1.63* (1.55 to 1.72) | 0.70* (0.67 to 0.72) | 1.05 (0.98 to 1.14) |
| Surgery within the last 6 months | 0.59* (0.52 to 0.67) | 0.44* (0.39 to 0.49) | 0.42*(0.33 to 0.54) |
| Aggressive tumour | 0.88* (0.84 to 0.93) | 1.12* (1.08 to 1.16) | 0.84*(0.78 to 0.90) |
*Significance at p<0.05.
LHA, Local Health Authorities.
The likelihood to receive palliative care during the last 30 days of life shows a limited increase in the presence of aggressive compared with other tumours. It was reduced in case of haematologic compared with other tumours, hospital admissions within the last 6 months, surgery within the last 6 months and (less) in case of receiving anticancer drugs during the last 30 days and for every year of increasing age (table 3). Also in this case, the ICC (0.3%) shows no intra-LHA correlation.
The likelihood to receive concurrent anticancer drugs and palliative care during the last 30 days of life was reduced in case of surgery within the last 6 months and (less) in case of aggressive compared with other tumours, in keeping with the result of the first model, suggesting that clinicians in such cases tend not to insist on drug therapy (table 3). Also in this case, the ICC (0.4%) shows no intra-LHA correlation.
Since no effect of clustering of subjects in the eight LHAs was shown, we replicated the latter models without LHA clustering and including LHA as covariates, in order to assess variability among LHAs (online supplemental extra table 2). Covariate coefficients are the same as in the cluster models, confirming no effect of LHA clustering on the outcome. As raw data suggested in figures 1 and 2, place of residence may also be associated with the likelihood to receive end-of-life drug therapies and palliative care after adjusting for the other covariates.
bmjopen-2021-057437supp003.pdf (81.6KB, pdf)
Discussion
This study shows that the use of anticancer drugs and of palliative care services in the last month of life are inversely associated rather than complementary. A variable use of anticancer drugs and of palliative care services in different LHAs and across different tumours in the last month of life is also shown. Compared with solid cancers, haematologic tumours tend to be treated more frequently with anticancer drugs and to be provided less frequently with palliative care. This circumstance could be related to the more frequent availability of effective in-hospital therapies leading to longer survival,29 to perceiving a more favourable benefit–risk ratio of ‘not giving up’ and to the often rapid pace of decline near death. This has also been observed in other studies.30–33 An opposite pattern is associated with aggressive tumours, treated more frequently with palliative care and less frequently with anticancer drugs.
Variability among different LHAs may depend either on a different epidemiological distribution of the tumours and of their severity, or on different prescribing attitudes and availability of palliative services in the areas of residence. Main cancer diagnosis associated with death appears similar across different LHAs. In addition, multivariate analyses provide adjustment for factors associated with tumour severity (age, haematologic tumour, previous surgery and hospital admission) and, although residual confounding can be reasonably present, we consider unlikely that it could provide the main explanation for the observed variability. Therefore, despite limits in our data and taking the possibility of unobserved factors (residual confounding) into account, we consider that this variability may be explained to a higher degree by different prescribing and management attitudes rather than by local epidemiology/case mix. As for the availability of palliative services in the areas of residence, the Emilia-Romagna Region has been quite active in implementing a national law issued in 201034 to guarantee such availability as well as adequate access to these services.35 Further qualitative research could analyse whether attitudes and level of endorsement in different LHAs may in part explain differential use/access, aside from their availability which is relatively homogeneous across the region.
Inclusion of all people deceased from cancer in a region with 4.4 million residents, linking information on the use of anticancer drugs and palliative care services with tumour characteristics and severity, are major strengths of this study. However, our results should be taken with caution since administrative data are grossly descriptive and have obvious limits in capturing all the elements that may contribute to clinical decision making. As for quality and completeness of available data, they are collected during the patient’s care for the purpose of reimbursements to healthcare rather than for research. No scientific validation of the unique patient identification number is available.
Nonetheless, our findings are in line with conclusions of several other studies. There may be a potential to reduce the use of end-of-life anticancer therapies increasing at the same time the provision of palliative care services. In general, shifting resources from aggressive pharmacological treatments to comprehensive approaches to palliative care services should be a priority in cancer care, and palliative care may be one of the determinants ‘protecting’ against the overuse of anticancer drugs. While the high variability observed among LHA in the use of these services is worrying, it also suggests that a huge potential exists to better organise end-of-life care for cancer patients.
Clinical and administrative data can help promote discussion among oncologists, specialists in palliative care, nurses, general practitioners, pharmacists, healthcare managers and (ideally) patients’ representatives to maximise quality of end-of-life care, especially in blood malignancies, in light of available resources. Local multidisciplinary groups can/should use data to analyse possible determinants of inappropriate care and propose strategies to offer patients and their families the best possible support. This especially in light of the increasing availability and accelerated approval of new therapies36 that often have a limited added value but a wide range of indications, targeting resistant cases and/or administered by oral route. These circumstances may favour an increase in the use of anticancer drugs, sometimes (or often) without a real clinical benefit, and may hinder or delay access to palliative care services.
Data on pharmaco-utilisation can also help local multidisciplinary groups to discuss to what extent anticancer drugs are used with a palliative intent, and to foster the design of research protocols aimed at evaluating the impact of drug utilisation on patients’ QoL. Record linkage studies generally cannot provide such specific information, since QoL information is generally unavailable in administrative databases, and this is also one of the limits of our study. A few randomised controlled trials and systematic reviews addressing different types of tumours have shown some effect of different anticancer therapies on reducing pain and improving patients’ QoL.37–42 However, this issue is largely debated as evidence is controversial or lacking, so that the guideline from the European Society for Medical Oncology (ESMO) explicitly contraindicates the use of anticancer drugs in the last weeks of life.43
In any case, the availability of adequate prognostic tools is key to improve the appropriateness of end-of-life care. In theory, ECOG performance status can be used as such to guide clinicians and palliative care specialists to make choices for appropriate healthcare,44 although it is subjectively assessed and may lead to optimistic assessments.45 A palliative prognostic score integrating subjective judgements with a series of more objective parameters has been validated and extensively discussed, showing a good balance between accuracy and applicability in clinical practice.46 47 48 49 Physicians should be also prepared to address patients’ and relatives’ concerns and expectations by refining their communication skills. Interventions that include communication about advanced care planning and care preferences with goals-of-care conversations,50 have been found to improve concordance between care preferences and actual care delivered.51 Nurses play a pivotal role too in accompanying patients and their families through their cancer journey, being in an ideal position52 53 to provide cancer patients and their families with emotional and social support, together with adequate communication about the diagnosis, prognosis and treatment alternatives.52 54
Conclusion
By showing, through administrative data, that the use of anticancer drugs and of palliative care services in the last month of life may be inversely associated rather than complementary, this study suggests the need to further explore the hypothesis that palliative care services may have a role in preventing inappropriate use of anticancer drugs. Administrative data may help highlight macro issues that should be addressed with a multidisciplinary approach involving clinicians, nurses, specialists in palliative care, pharmacists, healthcare managers and members of the public, eventually helping the promotion of palliative care and limiting the use of aggressive treatments that may not be beneficial.
Supplementary Material
Acknowledgments
The authors want to thank Dr Alberto Bagnulo for his comments on a preliminary version. All authors approved the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Correction notice: This article has been corrected since it was published. The institution in both the affiliations has been updated to 'Azienda USL – IRCCS di Reggio Emilia'. The funding statement has also been updated.
Contributors: GF: conception and design, interpretation of data, drafting the article. MM: conception and design, analysis and interpretation of data. MG: interpretation of data, revising the article critically for important intellectual content. RGG: conception and design, interpretation of data, revising the article critically for important intellectual content. GF acts as guarantor.
Funding: This study was partially supported by Italian Ministry of Health – Ricerca Corrente Annual Program 2023.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by an Ethics Committee. Name of the Ethics Committee: Comitato Etico dell'Area Vasta Emilia Nord, Reggio Emilia (Italy).
References
- 1.Specchia ML, La Torre G, Calabrò GE, et al. Disinvestment in cancer care: a survey investigating European countries' opinions and views. Eur J Public Health 2018;28:987–92. 10.1093/eurpub/cky033 [DOI] [PubMed] [Google Scholar]
- 2.Blanke CD, Fromme EK. Chemotherapy Near the End of Life: First--and Third and Fourth (Line)--Do No Harm. JAMA Oncol 2015;1:785–6. 10.1001/jamaoncol.2015.2379 [DOI] [PubMed] [Google Scholar]
- 3.Chan W-lok, Lam K-on, Siu W-kwan, et al. Chemotherapy at end-of-life: an integration of oncology and palliative team. Support Care Cancer 2016;24:1421–7. 10.1007/s00520-015-3031-z [DOI] [PubMed] [Google Scholar]
- 4.Wu C-C, Hsu T-W, Chang C-M, et al. Palliative chemotherapy affects aggressiveness of end-of-life care. Oncologist 2016;21:771. 10.1634/theoncologist.2015-0445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huynh L, Moore J. Palliative and end-of-life care for the older adult with cancer. Curr Opin Support Palliat Care 2021;15:23–8. 10.1097/SPC.0000000000000541 [DOI] [PubMed] [Google Scholar]
- 6.El-Jawahri A, Nelson AM, Gray TF, et al. Palliative and end-of-life care for patients with hematologic malignancies. J Clin Oncol 2020;38:944–53. 10.1200/JCO.18.02386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Care P, Organization WH. Available: https://www.who.int/cancer/palliative/definition/en/ [Accessed 19 Jan 2022].
- 8.Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of clinical oncology clinical practice guideline update. J Clin Oncol 2017;35:96–112. 10.1200/JCO.2016.70.1474 [DOI] [PubMed] [Google Scholar]
- 9.Page JS, Lederman L, Kelly J, et al. Teams and teamwork in cancer care delivery: shared mental models to improve planning for discharge and coordination of follow-up care. J Oncol Pract 2016;12:1053–8. 10.1200/JOP.2016.013888 [DOI] [PubMed] [Google Scholar]
- 10.Brom L, Onwuteaka-Philipsen BD, Widdershoven GAM, et al. Mechanisms that contribute to the tendency to continue chemotherapy in patients with advanced cancer. qualitative observations in the clinical setting. Support Care Cancer 2016;24:1317–25. 10.1007/s00520-015-2910-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui D. Prognostication of survival in patients with advanced cancer: predicting the unpredictable? Cancer Control 2015;22:489–97. 10.1177/107327481502200415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorne SE, Bultz BD, Baile WF, et al. Is there a cost to poor communication in cancer care?: a critical review of the literature. Psychooncology 2005;14:875–84. 10.1002/pon.947 [DOI] [PubMed] [Google Scholar]
- 13.Skov Benthien K, Adsersen M, Petersen MA, et al. Is specialized palliative cancer care associated with use of antineoplastic treatment at the end of life? a population-based cohort study. Palliat Med 2018;32:1509–17. 10.1177/0269216318786393 [DOI] [PubMed] [Google Scholar]
- 14.Clarke G, Johnston S, Corrie P, et al. Withdrawal of anticancer therapy in advanced disease: a systematic literature review. BMC Cancer 2015;15:892. 10.1186/s12885-015-1862-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sano M, Fushimi K. Association of palliative care consultation with reducing inpatient chemotherapy use in elderly patients with cancer in Japan: analysis using a nationwide administrative database. Am J Hosp Palliat Care 2017;34:685–91. 10.1177/1049909116650238 [DOI] [PubMed] [Google Scholar]
- 16.Harrington SE, Smith TJ. The role of chemotherapy at the end of life: "when is enough, enough?". JAMA 2008;299:2667–78. 10.1001/jama.299.22.2667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright AA, Zhang B, Keating NL, et al. Associations between palliative chemotherapy and adult cancer patients' end of life care and place of death: prospective cohort study. BMJ 2014;348:g1219. 10.1136/bmj.g1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neugut AI, Prigerson HG, Curative PHG. Curative, life-extending, and palliative chemotherapy: new outcomes need new names. Oncologist 2017;22:883–5. 10.1634/theoncologist.2017-0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pacetti P, Paganini G, Orlandi M, et al. Chemotherapy in the last 30 days of life of advanced cancer patients. Support Care Cancer 2015;23:3277–80. 10.1007/s00520-015-2733-6 [DOI] [PubMed] [Google Scholar]
- 20.Smith CB, Phillips T, Smith TJ. Using the new ASCO clinical practice guideline for palliative care concurrent with oncology care using the team approach. Am Soc Clin Oncol Educ Book 2017;37:714–23. 10.1200/EDBK_175474 [DOI] [PubMed] [Google Scholar]
- 21.Näppä U, Lindqvist O, Rasmussen BH, et al. Palliative chemotherapy during the last month of life. Ann Oncol 2011;22:2375–80. 10.1093/annonc/mdq778 [DOI] [PubMed] [Google Scholar]
- 22.Cardona-Morrell M, Kim J, Turner RM, et al. Non-Beneficial treatments in hospital at the end of life: a systematic review on extent of the problem. Int J Qual Health Care 2016;28:456–69. 10.1093/intqhc/mzw060 [DOI] [PubMed] [Google Scholar]
- 23.Rochigneux P, Raoul JL, Beaussant Y, et al. Use of chemotherapy near the end of life: what factors matter? Ann Oncol 2017;28:809–17. 10.1093/annonc/mdw654 [DOI] [PubMed] [Google Scholar]
- 24.Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol 2015;1:778–84. 10.1001/jamaoncol.2015.2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massa I, Nanni O, Foca F, et al. Chemotherapy and palliative care near end-of life: examining the appropriateness at a cancer Institute for colorectal cancer patients. BMC Palliat Care 2018;17:86. 10.1186/s12904-018-0339-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pellizzari M, Rolfini M, Ferroni E, et al. Intensity of integrated cancer palliative care plans and end-of-life acute medical hospitalisation among cancer patient in Northern Italy. Eur J Cancer Care 2018;27:e12742. 10.1111/ecc.12742 [DOI] [PubMed] [Google Scholar]
- 27.InGoldstein H. Multilevel statistical models. 3rd. London UK: Arnold, 2003. [Google Scholar]
- 28.Hosmer DW, Lemeshow S. Applied logistic regression. New York, NY: John Wiley & Sons, Inc, 2000. [Google Scholar]
- 29.Pulte D, Jansen L, Brenner H. Changes in long term survival after diagnosis with common hematologic malignancies in the early 21st century. Blood Cancer J 2020;10:56. Article number. 10.1038/s41408-020-0323-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Metre Baum L, Rosemblum R, Scarborough B. Evaluating end-of-life chemotherapy for solid tumor and hematologic malignancy patients. Prog Palliat Care 2021. 10.1080/09699260.2021.1872138 [DOI] [Google Scholar]
- 31.Hui D, Didwaniya N, Vidal M, et al. Quality of end-of-life care in patients with hematologic malignancies: a retrospective cohort study. Cancer 2014;120:1572–8. 10.1002/cncr.28614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Odejide OO, Salas Coronado DY, Watts CD, et al. End-Of-Life care for blood cancers: a series of focus groups with hematologic oncologists. J Oncol Pract 2014;10:e396–403. 10.1200/JOP.2014.001537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez MA, DeJesus AY, Cheng L. Use of chemotherapy within the last 14 days of life in patients treated at a comprehensive cancer center. JAMA Intern Med 2014;174:989–91. 10.1001/jamainternmed.2014.1001 [DOI] [PubMed] [Google Scholar]
- 34.Legge 15 marzo 2010, n.38. G.U.Serie Generale, n.65 del 19 marzo 2010.
- 35.Emilia-Romagna R. Assessorato politiche per La salute. Indagine sullo stato di attuazione degli hospice nella rete di cure palliative dell'Emilia-Romagna anno, 2013. Available: http://salute.regione.emilia-romagna.it/siseps/sanita/sdhs/files/AO_1_3_All_PG_91336_2015.pdf/view [Accessed 15 Sep 2021].
- 36.Gyawali B, Hey SP, Kesselheim AS. Assessment of the clinical benefit of cancer drugs receiving accelerated approval. JAMA Intern Med 2019;179:906–13. 10.1001/jamainternmed.2019.0462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. colorectal cancer Collaborative group. BMJ 2000;321:531–5. 10.1136/bmj.321.7260.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bezjak A, Tu D, Seymour L, et al. Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National cancer Institute of Canada clinical Trials Group study BR.21. J Clin Oncol 2006;24:3831–7. 10.1200/JCO.2006.05.8073 [DOI] [PubMed] [Google Scholar]
- 39.Oostendorp LJM, Stalmeier PFM, Donders ART, et al. Efficacy and safety of palliative chemotherapy for patients with advanced breast cancer pretreated with anthracyclines and taxanes: a systematic review. Lancet Oncol 2011;12:1053–61. 10.1016/S1470-2045(11)70045-6 [DOI] [PubMed] [Google Scholar]
- 40.Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of Folfirinox compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol 2013;31:23–9. 10.1200/JCO.2012.44.4869 [DOI] [PubMed] [Google Scholar]
- 41.Janmaat VT, Steyerberg EW, van der Gaast A, et al. Palliative chemotherapy and targeted therapies for esophageal and gastroesophageal junction cancer. Cochrane Database Syst Rev 2017;11:CD004063. 10.1002/14651858.CD004063.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Kleef JJ, Ter Veer E, van den Boorn HG, et al. Quality of life during palliative systemic therapy for esophagogastric cancer: systematic review and meta-analysis. J Natl Cancer Inst 2020;112:12–29. 10.1093/jnci/djz133 [DOI] [PubMed] [Google Scholar]
- 43.Crawford GB, Dzierżanowski T, Hauser K, et al. Care of the adult cancer patient at the end of life: ESMO clinical practice guidelines. ESMO Open 2021;6:100225. 10.1016/j.esmoop.2021.100225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jang RW, Caraiscos VB, Swami N, et al. Simple prognostic model for patients with advanced cancer based on performance status. J Oncol Pract 2014;10:e335–41. 10.1200/JOP.2014.001457 [DOI] [PubMed] [Google Scholar]
- 45.Johansen J, Boisen MK, Mellemgaard A, et al. Prognostic value of ECoG performance status in lung cancer assessed by patients and physicians. J Clin Oncol 2013;31:8103. 10.1200/jco.2013.31.15_suppl.8103 [DOI] [Google Scholar]
- 46.Maltoni M, Nanni O, Pirovano M, et al. Successful validation of the palliative prognostic score in terminally ill cancer patients. J Pain Symptom Manage 1999;17:240–7. 10.1016/S0885-3924(98)00146-8 [DOI] [PubMed] [Google Scholar]
- 47.Tarumi Y, Watanabe SM, Lau F. Evaluation of the palliative prognostic score (PAP) and routinely collected clinical data in prognostication ofSurvival for PatientsReferred to a palliative care consultation service in an acute care hospital. J Pain Symptom Manage 2011;42:419:e431. 10.1016/j.jpainsymman.2010.12.013 [DOI] [PubMed] [Google Scholar]
- 48.Glare PA, Eychmueller S, McMahon P. Diagnostic accuracy of the palliative prognostic score in hospitalized patients with advanced cancer. J Clin Oncol 2004;22:4823–8. 10.1200/JCO.2004.12.056 [DOI] [PubMed] [Google Scholar]
- 49.Hui D, Park M, Liu D, et al. Clinician prediction of survival versus the palliative prognostic score: which approach is more accurate? Eur J Cancer 2016;64:89–95. 10.1016/j.ejca.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. IOM. Dying in America: improving quality and honoring individual preferences near the end of life. Washington (DC: National Academies Press, 2015. 10.17226/18748 [DOI] [PubMed] [Google Scholar]
- 51.Starr LT, Ulrich CM, Corey KL, et al. Associations among end-of-life discussions, health-care utilization, and costs in persons with advanced cancer: a systematic review. Am J Hosp Palliat Care 2019;36:913–26. vol.. 10.1177/1049909119848148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banerjee SC, Manna R, Coyle N, et al. Oncology nurses' communication challenges with patients and families: a qualitative study. Nurse Educ Pract 2016;16:193–201. 10.1016/j.nepr.2015.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baer L, Weinstein E. Improving oncology nurses' communication skills for difficult conversations. Clin J Oncol Nurs 2013;17:E45–51. 10.1188/13.CJON.E45-E51 [DOI] [PubMed] [Google Scholar]
- 54.Hack TF, Ruether JD, Pickles T, et al. Behind closed doors II: systematic analysis of prostate cancer patients' primary treatment consultations with radiation oncologists and predictors of satisfaction with communication. Psychooncology 2012;21:809–17. 10.1002/pon.1984 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-057437supp001.pdf (54.3KB, pdf)
bmjopen-2021-057437supp002.pdf (59.6KB, pdf)
bmjopen-2021-057437supp003.pdf (81.6KB, pdf)
Data Availability Statement
Data are available upon reasonable request.


