Abstract
A man in his 60s, with a medical history of gout, underwent total knee arthroplasty of his right knee followed by expeditious rehabilitation. Seven months after surgery, he was referred to the emergency ward with sudden onset of pain and swelling of his right knee accompanied with fever. Further inquiry revealed no trauma, infection or skin lesions besides a tongue bite several weeks earlier. An impaired range motion of the knee was seen on physical examination along with a tachycardia. Laboratory studies showed a C reactive protein of 345 mg/L, after which a debridement, antibiotics and implant retention procedure was performed. Intraoperatively obtained synovial fluid showed monosodium urate crystals consistent with crystalline arthropathy (ie, gout). However, unexpectedly, Streptococcus sanguinis was identified in all microbiological cultures too, confirming a coexistent periprosthetic joint infection. After comprehensive antibiotic treatment and gout flare therapy, this patient made a full recovery with retention of the implant.
Keywords: Bone and joint infections, Orthopaedics, Rheumatology, Orthopaedic and trauma surgery, Mouth
Background
Periprosthetic joint infection (PJI) is one of the most severe complications after total knee arthroplasty (TKA). PJI can result in severe subsequent complications such as sepsis and implant failure.1 2
Staphylococcus aureus, Streptococcus spp, coagulase-negative staphylococci and enterococci are known as the most common causative pathogens of haematogenous PJI.1 3 4 S. sanguinis as a causative pathogen of haematogenous PJI is rare.5 S. sanguinis, formerly known as S. sanguis, is known as a gram-positive bacterium which naturally originates within the oral cavity. Haematogenous PJI due to S. sanguinis is therefore associated with periodontal disease or injury.5–7
Differentially diagnostic non-infectious types of arthritis such as reactive arthritis or crystalline-induced arthritis (CIA) should always be ruled out, especially when there is a medical history of them.
CIA, which could be caused by gout after joint arthroplasty, is a rare but described phenomenon.8–10 CIA combined with a bacterial superinfection is even rarer with only four cases described in literature.8–10
Treatment of acute PJI should consist of a debridement, antibiotics and implant retention (DAIR) surgical procedure combined with extensive systemic intravenous antibiotic treatment. In the Netherlands, locally administered antibiotics during the surgical DAIR procedure is not a common practice. Only in rare cases a one-stage or two-stage revision is needed.1 2 4 Treatment of CIA in a prosthetic joint should consist of anti-inflammatory agents, with colchicine being the safest option until an infection is ruled out. Other options are non-steroidal anti-inflammatory drugs. Surgical treatment is not described as a viable option in cases of CIA.8–10
We present a rare case of CIA and PJI combined with additionally an uncommon pathogen, in a prosthetic knee joint.
This case demonstrates how a certain cause of arthritis does not rule out a second cause.
Case presentation
A healthy man, who is in his 60s, presented to the emergency ward with a painful right knee. Seven months earlier, he underwent TKA of his right knee with expeditious rehabilitation with knee flexion up to 130° with full extension. His medical history revealed hypertension, gout and a left total hip arthroplasty.
The patient reported pain in his knee, which started 4 days earlier and varied in intensity. Two days prior to presentation, he did a cycling tour and therefore attributed the pain due to overloading. However, he had also experienced transient fever with influenza symptoms prior to the cycling tour. Further inquiry revealed no trauma, skin lesions or infection.
On physical examination, a non-critically ill patient was seen. Vital signs showed a temperature of 36.2°C, and a tachycardia (120/min) with a normal blood pressure (128/88 mm Hg).
The right knee was swollen, slightly warmer compared with the left knee without erythema. Both medial and lateral parts of the joint were painful when palpated, knee flexion was limited to 100° due to pain and an extension deficit of 5° was observed.
Investigations
At the emergency department laboratory studies, blood cultures and conventional radiographs of the right knee were obtained. Laboratory results showed a C reactive protein (CRP) of 345 mg/L, leucocyte count of 8.93×109/L and an erythrocyte sedimentation rate (ESR) of 97 mm/hour. The radiographs showed proper positioning of the TKA, no post-traumatic defects, no signs of (early) loosening and no evident joint effusion as shown in figure 1.
Figure 1.
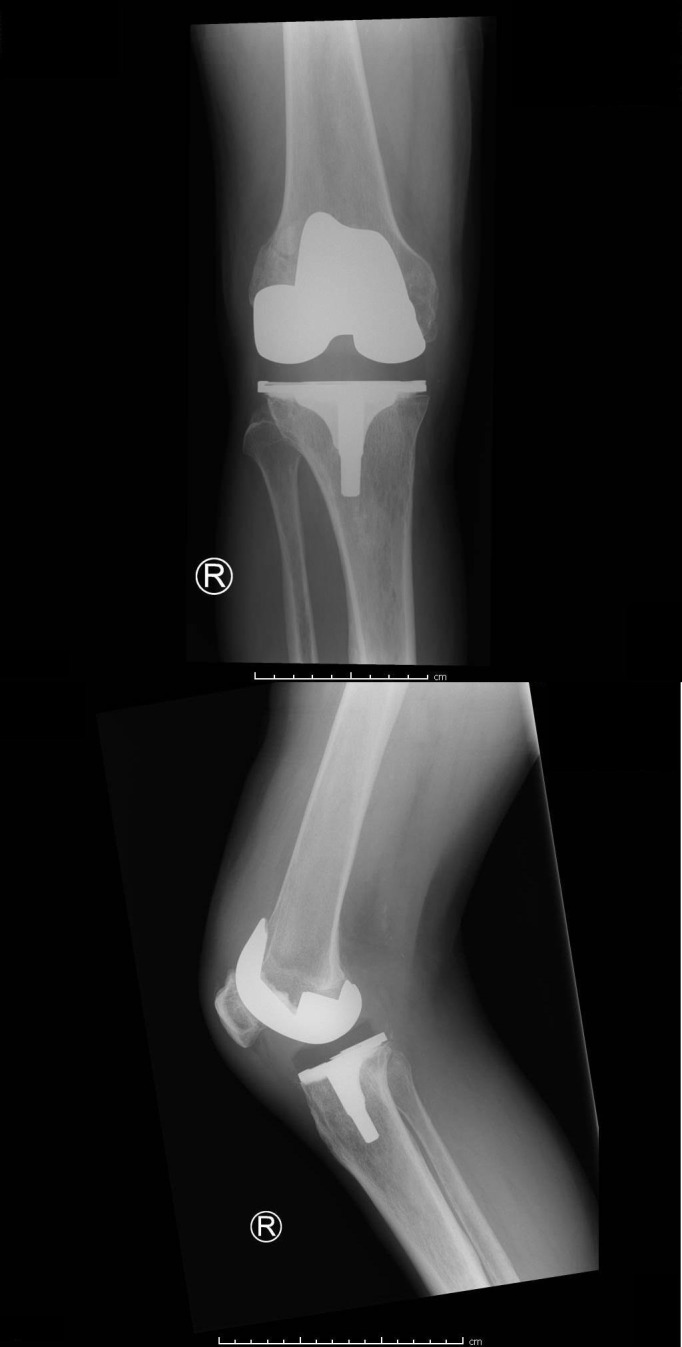
Anteroposterior and lateral conventional radiographs of the right knee.
Differential diagnosis
The clinical presentation in this case seemed very suggestive of PJI with transient fever, tachycardia on arrival and laboratory findings consistent with infection. Based on this initial presentation and work-up, the decision was made to perform a DAIR procedure. However, intraoperatively obtained synovial fluid showed birefringence needle-like crystals microscopically. This finding altered the diagnosis to CIA as the most probable cause of the arthritis. The day after surgery, however, S. sanguinis was cultivated from all six intraoperatively obtained microbiological tissue cultures, confirming a bacterial infection as secondary cause.
Treatment
A DAIR procedure of the right knee was performed with sampling of synovial fluid and six tissue samples for microbiological culturing. This procedure consisted of extensive surgical debridement, insert exchange and washout using pulse lavage while retaining the implant. In microscopic examination of the synovial fluid obtained during this procedure, a high leucocyte count and negative birefringence needle-like crystals were seen. Postoperatively, the patient started with cephazolin 3×1 g/24 hours intravenously with an 8-hour interval as a broad-spectrum antibiotic agent awaiting microbiological culture results.
Unexpectedly, in all six of the tissue cultures obtained during surgery, S. sanguinis was cultivated. With all microbiological cultures positive for S. sanguinis, antibiotic treatment was tailored towards penicillin 12 g/24 hours for a total of 2 weeks of intravenous antibiotic treatment. After 2 weeks, antibiotic treatment was continued with oral substitution towards clindamycin 3×600 mg/24 hours for a total of 3 months.
Gout flare therapy was started with colchicine 0.5 mg two times per day for 7 consecutive days by the rheumatologist.
The patient received in-hospital antibiotic treatment for 7 days. During his admission period, physiotherapy was started to mobilise the affected knee. A dietitian was consulted to optimise postoperative caloric intake. S. sanguinis is a pathogen found mainly in the oral region; therefore, the oral maxillofacial surgeon was consulted for evaluation of the dental status. No abnormalities were found except for a tongue bite 3 weeks earlier.
Outcome and follow-up
At the outpatient visit 3 months after surgery, antibiotic treatment was completed. The patient had already reintegrated at his work at 70% of his preadmission level. Clinically, reactive hydrops was persistent after excessive loading. On examination, primary wound healing was observed with a descent function of 125° of flexion with full extension. Completed antibiotic treatment resulted in a good biochemical response after 3 months with a CRP of 1 mg/L, leucocyte count of 6.7×109/L, ESR of 10 mm/hour and urate of 0.29 mmol/L.
Discussion
PJI, along with coexistent crystalline arthropathy after joint arthroplasty, is a rare combination with very few reported cases.8 Since the available literature is scarce, recommendations regarding diagnosis and treatment are difficult to generate.
We suggest obtaining synovial fluid to differentiate between PJI and CIA before performing surgery in all cases, since a surgical procedure could be prevented by microscopic examination and cultivating synovial fluid.9 When in doubt, however, such as in our case, a DAIR procedure is advised since delayed surgical debridement in case of PJI could impose complications in terms of implant retention. Treatment of PJI with coexistent CIA described in literature consists of a DAIR procedure, administering high doses of antibiotic agents and anti-inflammatory drugs, with a preference for colchicine.8
A recommendation for choice of which treatment should be performed, when the differentiation of both CIA and PJI is difficult, was not found in literature.
S. sanguinis is a known biofilm producing benign commensal of the oral cavity, which is relatively rare in correlation with septic arthritis.6 In the literature, all known cases of septic arthritis or PJI caused by S. sanguinis have been linked with severe bacterial periodontal disease with or without pocketing.5 As far as we know, this is the first reported case of S. sanguinis-induced PJI without the presence of periodontal disease with only a tongue bite as a probable cause.
Patient’s perspective.
The initial surgery of my right knee was followed by expeditious rehabilitation. I was satisfied with the range of motion without residual complaints. A few days prior to the hospital admission seven months after the initial surgery, I developed a fewer with flu symptoms which kept me in bed all weekend. I also experienced some knee complaints which I attributed to the flu. After this weekend, we left for a mini holiday with a cycling tour. During this mini holiday, walking became more and more difficult. When the fever recurred, we returned home and visited our general practitioner. After laboratory studies we were referred to the emergency ward.
Upon arrival at the emergency ward, we received the news that my fever might be caused by an infection of my prosthetic knee. First, I underestimated the gravity of the situation. My wife however, immediately recognized the danger of my condition. We experienced anxiousness and mental strain due to an uncertain prognosis prior to the surgery. After surgery, I made a fast recovery and was able to return home with intravenously antibiotic treatment along with gout therapy. The diagnosis, surgery and antibiotic treatment along with a hospital admission for 7 days made a great impact on me and my wife. The whole situation first negatively influenced my motivation for rehabilitation after surgery. Regardless of the cause of my hospital admission, I am grateful with the care provided.
Several months after the infection of my prosthetic knee, I still experience anxiousness for a new infection. Fortunately, these emotions are fading over time. I am content with the function of my knee and motivated to pursue further rehabilitation upon full recovery.
Learning points.
Periprosthetic joint infection can coexist with crystalline arthropathy after total knee arthroplasty.
Crystalline arthropathy can cause similar symptoms as a periprosthetic joint infection and should be considered as an alternative diagnosis when microbiological cultures are negative.
Streptococcus sanguinis is a rare causative pathogen for periprosthetic joint infection.
Haematogenous periprosthetic joint infection due to S. sanguinis is associated with periodontal disease.
Footnotes
Contributors: JBM—drafting the work. LMG/WCV—revising critically for important intellectual content. WCV—final approval of the version published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Izakovicova P, Borens O, Trampuz A. Periprosthetic joint infection: current concepts and outlook. EFORT Open Rev 2019;4:482–94. 10.1302/2058-5241.4.180092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the infectious diseases Society of America. Clin Infect Dis 2013;56:e1–25. 10.1093/cid/cis803 [DOI] [PubMed] [Google Scholar]
- 3.Rakow A, Perka C, Trampuz A, et al. Origin and characteristics of haematogenous periprosthetic joint infection. Clin Microbiol Infect 2019;25:845–50. 10.1016/j.cmi.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 4.Li C, Renz N, Trampuz A. Management of periprosthetic joint infection. Hip Pelvis 2018;30:138–46. 10.5371/hp.2018.30.3.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartzokas CA, Johnson R, Jane M, et al. Relation between mouth and haematogenous infection in total joint replacements. BMJ 1994;309:506–8. 10.1136/bmj.309.6953.506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papaioannides D, Boniatsi L, Korantzopoulos P, et al. Acute septic arthritis due to Streptococcus sanguis. Med Princ Pract 2006;15:77–9. 10.1159/000089391 [DOI] [PubMed] [Google Scholar]
- 7.Edson RS, Osmon DR, Berry DJ. Septic arthritis due to Streptococcus sanguis. Mayo Clin Proc 2002;77:709–10. 10.4065/77.7.709 [DOI] [PubMed] [Google Scholar]
- 8.Yahia SA, Zeller V, Desplaces N, et al. Crystal-Induced arthritis after arthroplasty: 7 cases. Joint Bone Spine 2016;83:559–62. 10.1016/j.jbspin.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 9.Buck M, Delaney M. Diagnosis and management of gout in total knee arthroplasty. Orthop Nurs 2014;33:37–40. 10.1097/NOR.0000000000000021 [DOI] [PubMed] [Google Scholar]
- 10.Chernoff DJ, Barker JP, Wingerter SA, et al. Gout after total knee arthroplasty. Arthroplast Today 2020;6:278–82. 10.1016/j.artd.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]


