Abstract
A nationwide multicenter susceptibility surveillance study which included 1,684 Streptococcus pneumoniae and 2,039 S. pyogenes isolates was carried out over 1 year in order to assess the current resistance patterns for the two most important gram-positive microorganisms responsible for community-acquired infections in Spain. Susceptibility testing was done by a broth microdilution method according to National Committee for Clinical Laboratory Standards M100-S10 interpretative criteria. For S. pneumoniae, the prevalences of highly resistant strains were 5% for amoxicillin and amoxicillin-clavulanic acid; 7% for cefotaxime; 22% for penicillin; 31% for cefuroxime; 35% for erythromycin, clarithromycin, and azithromycin; and 42% for cefaclor. For S. pyogenes, the prevalence of erythromycin resistance was 20%. Efflux was encountered in 90% of S. pyogenes and 5% of S. pneumoniae isolates that exhibited erythromycin resistance. Erythromycin resistance was associated with clarithromycin and azithromycin in both species, regardless of phenotype. Despite the different nature of the mechanisms of resistance, a positive correlation (r = 0.612) between the two species in the prevalence of erythromycin resistance was found in site-by-site comparisons, suggesting some kind of link with antibiotic consumption. Regarding ciprofloxacin, the MIC was ≥4 μg/ml for 7% of S. pneumoniae and 3.5% of S. pyogenes isolates. Ciprofloxacin resistance (MIC, ≥4 μg/ml) was significantly (P < 0.05) associated with macrolide resistance in both S. pyogenes and S. pneumoniae and with penicillin nonsusceptibility in S. pneumoniae.
Antibiotic agents can select resistant microorganisms (18), and clinically important bacteria involved in respiratory tract infections are rapidly developing resistance to common therapeutic options, as is the case for Streptococcus pneumoniae (13) and S. pyogenes (24). Decreased susceptibility to macrolides in S. pyogenes (2) and to β-lactams, including parenteral broad-spectrum cephalosporins, macrolides, and ciprofloxacin, in S. pneumoniae (3, 8) has already been described in Spain. These facts make good-quality data a necessity for effective interventions to counter the problem of antimicrobial resistance (18) and to help in designing prescription guidelines, since there is an established but complex relationship between antibiotic consumption and prevalence of resistance (18). This aspect is most important for community-acquired respiratory tract infections, since up to 85 to 90% of antibiotics are used in the community and 80% of this proportion is prescribed to treat this type of infection (14).
In addition to links between macrolide consumption and erythromycin resistance in S. pyogenes (11, 24) and consumption of β-lactams and macrolides and resistance to penicillin and erythromycin in S. pneumoniae (12), multiresistance and coselection of resistance (8, 10; C. García-Rey, L. Aguilar, J. García-de-Lomas, and the Spanish Surveillance Group for Respiratory Pathogens, Abstr. Proc. 3rd Eur. Congr. Chemother. Span. J. Chemother. 13(Suppl. 2):74, abstr. T161) may also be explained by antibiotic consumption. Although several authors have reported on variations in the prevalence of resistance in the absence of variations in antibiotic use (27, 28), the increase in resistance to a given antibiotic (e.g., erythromycin) often relates to the consumption of that antibiotic or of antibiotics belonging to the same group (11, 24). Because many pneumococcal strains display multiresistance, it is no wonder that the consumption of a given antibiotic leads to an increase in resistance to nonrelated antibiotics (8, 10; Garcia-Rey et al., Abstr. Proc. 3rd Eur. Congr. Chemother).
Our group has undertaken several studies in which we have described the pattern of resistance of S. pneumoniae and S. pyogenes to different antibiotics commonly used in Spain (2, 3) and reported the ecological relationship of the erythromycin resistance rates for both species (9). In this study, we report the current rates of resistance and further explore inter- and intraspecies ecological relationships, in light of new S. pneumoniae breakpoints.
Surveillance studies play a key role in tailoring antibiotic prescriptions in screened geographical areas. They are even more important when there is a need to provide awareness of recent changes in breakpoints based on pharmacokinetic and pharmacodynamic data for S. pneumoniae (19, 20) and how they affect its prevalence of resistance.
MATERIALS AND METHODS
Consecutive clinical isolates collected between November 1998 and October 1999 from patients with community-acquired respiratory infections (namely, acute pharyngitis caused by S. pyogenes and acute otitis media, acute exacerbations of chronic bronchitis, and pneumonia caused by S. pneumoniae) were collected by 17 university hospitals selected on the basis of population and geographical location in Spain. The demographic information submitted for each isolate included specimen collection date, specimen source, hospital ward, and unit of care.
At each center, isolates kept at −70°C were thawed once a month, seeded onto an enriched transport medium, incubated overnight at 35 to 37°C, and shipped to a central laboratory (Instituto Valenciano de Microbiología, Valencia, Spain), where compliance of the isolates with the criteria for inclusion in the study was checked. Confirmation of the identification of isolates was provided by positive bile solubility and inhibition by optochin for S. pneumoniae and serogroup A immunoagglutination (Streptex; Murex, Chantillon, France) for S. pyogenes. Serogrouping of all the pneumococcal isolates was performed at the reference central laboratory and the Centro Nacional de Microbiología, Instituto de Salud Carlos III, Majadahonda, Madrid, Spain, using the Quellung technique (Statens Seruminstitut, Copenhagen, Denmark) (17) and dot blot analysis (6). Isolates were kept frozen at −70°C in duplicate until antimicrobial susceptibility testing was performed.
Susceptibility testing was performed using double dilutions by a semiautomated microdilution method with custom-dried 96-well trays (Sensititre; Trek Diagnostics Inc., Westlake, Ohio) and Mueller-Hinton broth supplemented with 3% lysed horse blood according to the guidelines of the National Committee for Clinical Laboratory Standards (NCCLS) (20) and with a final inoculum of 5 × 105 CFU/ml. Cultures were incubated for 24 h at 35°C in ambient air with 11 antimicrobial agents (penicillin, amoxicillin, amoxicillin-clavulanate [2:1], cefaclor, cefuroxime, cefixime, cefotaxime, erythromycin, clarithromycin, azithromycin, and ciprofloxacin) selected on the basis of common empiric therapy options in Spain. S. pneumoniae ATCC 49619 and Escherichia coli ATCC 35218 were used as control strains. Breakpoints used were those recommended by NCCLS (NCCLS document M100-S10) (20). An arbitrary breakpoint for resistance to ciprofloxacin of ≥4 μg/ml was used (as it has not been established for S. pneumoniae by NCCLS) as a marker to indicate the development of quinolone resistance.
The mechanism of resistance to erythromycin was evaluated with a double-diffusion disk test as described elsewhere (26) with erythromycin (15 μg) and clindamycin (2 μg) disks placed 20 mm apart on 5% defibrinated horse blood agar and incubated overnight at 35°C in a 5% carbon dioxide atmosphere. After incubation, the presence or absence of blunting in the zone of inhibition of the clindamycin disk was recorded (22). If the clindamycin inhibition zone was blunted toward the erythromycin disk, the strain was interpreted as clindamycin inducible. Resultant phenotype patterns were clindamycin-sensitive strains (M phenotype) and clindamycin-resistant or -inducible strains (MLSB phenotype).
The Spearman nonparametric correlation coefficient for the prevalence of resistance to erythromycin in S. pneumoniae and S. pyogenes and combined erythromycin and penicillin resistance in S. pneumoniae was calculated for each center. Differences in the prevalence of resistance between different groups, together with calculation of the odds ratios (OR) and the 95% confidence intervals (CI), were assessed by the χ2 test with the Yates correction when necessary. Statistical analyses were carried out using SPSS version 8.0 for Windows and Epi-Info version 6.04.
RESULTS
Twelve percent (201 out of 1,684) of the S. pneumoniae isolates were from middle ear samples, whereas 88% (1,483 out of 1,684) were from patients suffering from lower respiratory tract (LRT) infections (1,169 [78.8%] from samples such as sputum, bronchial aspirate, or bronchoalveolar lavage fluid and 314 [21.2%] from blood). Eighty-three percent of the S. pneumoniae isolates were from the adult population. All 2,039 S. pyogenes isolates were from pharyngeal swabs; 87% were from the pediatric population.
Table 1 shows the in vitro susceptibility of S. pneumoniae and S. pyogenes isolates. Overall, a 50.1% prevalence of nonsusceptibility and a 21.7% prevalence of high-level resistance to penicillin were observed for S. pneumoniae isolates, with prevalences of resistance of 30 to 40% for macrolides and oral cephalosporins and of about 5 to 7% for amoxicillin (with or without clavulanate) and cefotaxime. The prevalence of intermediate resistance was below 9% for all antibiotics except penicillin (28.3%) and cefotaxime (16.7%). From the viewpoint of intrinsic activity, cefotaxime proved the most active agent (MIC at which 90% of the isolates tested were inhibited [MIC90], 1 μg/ml), followed by penicillin and amoxicillin (MIC90, 2 μg/ml). Considering both parameters, the prevalence of resistance and the MIC90 both cefotaxime and amoxicillin were the most active agents. Table 2 shows the percentages of isolates for which the MIC of every drug was increased.
TABLE 1.
In vitro activity of antimicrobial agents against S. pneumoniae and S. pyogenes isolates
| Organism | Antimicrobial agenta | MIC (μg/ml)
|
No. (%) of isolates that were:
|
|||
|---|---|---|---|---|---|---|
| 50% | 90% | Susceptible | Intermediate | Resistant | ||
| S. pneumoniae | Penicillin | 0.125 | 2 | 839 (49.8) | 479 (28.4) | 366 (21.7) |
| Amoxicillin | 0.06 | 2 | 1,519 (90.2) | 79 (4.7) | 86 (5.1) | |
| Amoxiclavb | 0.06 | 2 | 1,522 (90.4) | 75 (4.4) | 87 (5.1) | |
| Cefaclor | ≤1 | ≥64 | 893 (53.0) | 90 (5.3) | 701 (41.6) | |
| Cefuroxime | ≤0.25 | 8 | 1,008 (59.8) | 147 (8.7) | 529 (31.4) | |
| Cefiximec | 0.5 | ≥4 | ||||
| Cefotaxime | ≤0.25 | 1 | 1,287 (76.4) | 282 (16.7) | 115 (6.8) | |
| Erythromycin | ≤0.12 | ≥64 | 1,096 (65.1) | 0 (0) | 588 (34.9) | |
| Clarithromycin | ≤0.25 | ≥64 | 1,097 (65.1) | 3 (0.2) | 584 (34.7) | |
| Azithromycin | ≤0.12 | ≥64 | 1,062 (63.1) | 37 (2.2) | 585 (34.7) | |
| Ciprofloxacincd | 1 | 2 | 120 (7.1) | |||
| S. pyogenes | Penicilline | ≤0.015 | ≤0.015 | 2,039 (100) | 0 (0) | 0 (0) |
| Erythromycin | ≤0.12 | 8 | 1,621 (79.5) | 2 (0.1) | 416 (20.4) | |
| Clarithromycin | ≤0.25 | 8 | 1,622 (79.5) | 6 (0.3) | 411 (20.2) | |
| Azithromycin | ≤0.12 | 8 | 1,520 (74.5) | 103 (5.0) | 416 (20.4) | |
| Ciprofloxacincd | 1 | 1 | 70 (3.4) | |||
Breakpoints used for S. pneumoniae were ≤0.06 μg/ml (susceptible), 0.12 to 1 μg/ml (intermediate), and ≥2 μg/ml (resistant) for penicillin; ≤2 μg/ml (susceptible), 4 μg/ml (intermediate), and ≥8 μg/ml (resistant) for amoxicillin and amoxicillin-clavulanate; ≤1 μg/ml (susceptible), 2 μg/ml (intermediate), and ≥4 μg/ml (resistant) for cefaclor and cefuroxime axetil; ≤0.5 μg/ml (susceptible), 1 μg/ml (intermediate), and ≥2 μg/ml (resistant) for cefotaxime and azithromycin; and ≤0.25 μg/ml (susceptible), 0.5 μg/ml (intermediate), and ≥1 μg/ml (resistant) for erythromycin and clarithromycin. Breakpoints used for S. pyogenes were ≤0.12 μg/ml (susceptible), 0.25 to 2 μg/ml (intermediate), and ≥4 μg/ml (resistant) for penicillin; ≤0.5 μg/ml (susceptible), 1 μg/ml (intermediate), and ≥2 μg/ml (resistant) for azithromycin; and ≤0.25 μg/ml (susceptible), 0.5 μg/ml (intermediate), and ≥1 μg/ml (resistant) for erythromycin and clarithromycin.
Amoxiclav, amoxicillin-clavulanate (2:1). The concentrations listed are for amoxicillin.
No NCCLS breakpoints have been established.
Arbitrary ciprofloxacin breakpoint for resistance, ≥4 μg/ml.
In accordance with NCCLS, a streptococcal isolate that is susceptible to penicillin is considered susceptible to the remaining β-lactams.
TABLE 2.
MIC distribution for drugs tested against 1,684 pneumococcal isolates collected in Spain from 1998 through 1999
| Antimicrobial agent | No. (%) of inhibited isolates for which the MIC (μg/ml) was:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.015 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | ≥64 | |
| Penicillin | 710 (42.1) | 83 (4.9) | 46 (2.7) | 83 (4.9) | 102 (6.1) | 112 (6.6) | 182 (10.8) | 256 (15.2) | 110 (6.5) | 0 (0) | |||
| Amoxicillin | 369 (21.9) | 415 (24.6) | 88 (5.2) | 63 (3.7) | 86 (5.1) | 99 (5.9) | 159 (9.4) | 240 (14.2) | 79 (4.7) | 65 (3.9) | 21 (1.2) | ||
| Amoxiclav | 491 (29.1) | 313 (18.6) | 79 (4.7) | 58 (3.4) | 87 (5.2) | 89 (5.3) | 189 (11.2) | 217 (12.9) | 74 (4.4) | 69 (4.2) | 18 (1.1) | 0 (0) | |
| Cefaclor | 893 (53.0) | 90 (5.3) | 57 (3.4) | 79 (4.7) | 75 (4.5) | 180 (10.7) | 310 (18.5) | ||||||
| Cefuroxime | 879 (52.2) | 72 (4.3) | 57 (3.4) | 147 (8.7) | 246 (14.6) | 249 (14.8) | 34 (2.0) | 0 (0) | |||||
| Cefixime | 799 (47.4) | 82 (4.9) | 64 (3.8) | 77 (4.6) | 594 (35.3) | 68 (4.0) | |||||||
| Cefotaxime | 1,061 (63.0) | 226 (13.4) | 282 (16.7) | 109 (6.5) | 5 (0.3) | 1 (0.1) | 0 (0) | ||||||
| Erythromycin | 1,081 (64.2) | 15 (0.9) | 0 (0) | 4 (0.2) | 13 (0.8) | 9 (0.5) | 10 (0.6) | 122 (7.2) | 28 (1.7) | 402 (23.9) | |||
| Clarythromycin | 1,097 (65.1) | 3 (0.2) | 10 (0.6) | 7 (0.4) | 13 (0.8) | 4 (0.2) | 135 (8.0) | 8 (0.5) | 407 (24.2) | ||||
| Azithromycin | 844 (50.1) | 86 (5.1) | 132 (7.8) | 37 (2.2) | 6 (0.4) | 8 (0.5) | 8 (0.5) | 124 (7.4) | 9 (0.5) | 430 (25.5) | |||
| Ciprofloxacin | 289 (17.2) | 805 (47.8) | 470 (27.9) | 78 (4.6) | 13 (0.8) | 29 (1.7) | |||||||
When the prevalence of susceptibility to each of the other antibiotics of the S. pneumoniae isolates distributed according to their penicillin susceptibility categories (Table 3) is considered, it can be seen that the progressive increase in penicillin MICs has less influence on the activity of amoxicillin (97 and 60% prevalences of susceptibility for penicillin-intermediate and -resistant strains, respectively) than on those of cefotaxime (85 and 11% prevalences of susceptibility for penicillin-intermediate and -resistant isolates, respectively), macrolides (40% prevalence of susceptibility for both penicillin-intermediate and -resistant isolates), or oral cephalosporins (0% prevalence of susceptibility for penicillin-resistant isolates). Nonetheless, within the group of oral cephalosporins, differences between cefaclor and cefuroxime are evident for isolates exhibiting penicillin-intermediate resistance, since up to 70 and 35% of these isolates are resistant to the respective aforementioned antibiotics. All of the β-lactam nonsusceptibility and the majority of the macrolide nonsusceptibility happen to be clustered among the penicillin-nonsusceptible isolates. Notwithstanding, cefotaxime (and penicillin) is likely to be unfairly judged by considering raw NCCLS breakpoints rather than the proportion of isolates with progressively higher MICs. A simple glance at Table 2 shows that there are fewer isolates for which MICs of cefotaxime are high than for which amoxicillin MICs are high. Likewise, in terms of MICs, penicillin behaved in a manner very similar to that of amoxicillin.
TABLE 3.
In vitro activity of the antimicrobial agents against isolates of S. pneumoniae classified by penicillin susceptibility profile
| Penicillin susceptibility profile | Antimicrobial agenta | MIC90 | No. (%) of isolates that were:
|
||
|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | |||
| Susceptible (n = 839) | Amoxicillin | 0.03 | 839 (100) | 0 (0) | 0 (0) |
| Amoxiclavb | 0.03 | 839 (100) | 0 (0) | 0 (0) | |
| Cefaclorc | ≤1 | 805 (95.9) | 30 (3.6) | 4 (0.5) | |
| Cefuroxime axetilc | ≤0.25 | 838 (99.8) | 0 (0) | 1 (0.2) | |
| Cefiximecd | 0.5 | ||||
| Cefotaxime | ≤0.25 | 837 (99.8) | 2 (0.2) | 0 (0) | |
| Erythromycin | 8 | 747 (89.0) | 0 (0) | 92 (11.0) | |
| Clarithromycin | 4 | 748 (89.2) | 0 (0) | 91 (10.8) | |
| Azithromycin | 8 | 725 (86.4) | 22 (2.6) | 92 (10.9) | |
| Ciprofloxacind | 2 | 49 (5.8) | |||
| Intermediate (n = 479) | Amoxicillin | 2 | 464 (96.9) | 11 (2.3) | 4 (0.8) |
| Amoxiclavb | 2 | 463 (96.7) | 13 (2.7) | 3 (0.6) | |
| Cefaclor | 32 | 87 (18.2) | 58 (12.1) | 334 (69.7) | |
| Cefuroxime axetil | 4 | 170 (35.5) | 136 (28.4) | 173 (36.1) | |
| Cefiximed | 4 | ||||
| Cefotaxime | 1 | 408 (85.2) | 66 (13.8) | 5 (1.0) | |
| Erythromycin | ≥64 | 203 (42.4) | 0 (0) | 276 (57.6) | |
| Clarithromycin | ≥64 | 203 (42.4) | 3 (0.6) | 273 (57.0) | |
| Azithromycin | ≥64 | 191 (39.9) | 15 (3.1) | 273 (57.0) | |
| Ciprofloxacind | 2 | 39 (8.1) | |||
| Resistant (n = 366) | Amoxicillin | 8 | 216 (59.0) | 68 (18.6) | 82 (22.4) |
| Amoxiclavb | 8 | 220 (60.1) | 62 (16.9) | 84 (22.9) | |
| Cefaclor | ≥64 | 1 (0.3) | 2 (0.5) | 363 (99.2) | |
| Cefuroxime axetil | 8 | 0 (0) | 11 (3.0) | 355 (97.0) | |
| Cefiximed | ≥8 | ||||
| Cefotaxime | 2 | 42 (11.5) | 214 (58.5) | 110 (30.0) | |
| Erythromycin | ≥64 | 146 (39.9) | 0 (0) | 220 (60.1) | |
| Clarithromycin | ≥64 | 146 (39.9) | 0 (0) | 220 (60.1) | |
| Azithromycin | ≥64 | 146 (39.9) | 0 (0) | 220 (60.1) | |
| Ciprofloxacind | 2 | 32 (8.7) | |||
Significant differences were found regarding erythromycin nonsusceptibility in S. pneumoniae when isolates from patients with otitis were compared to those from patients with LTR infections (46.8 versus 33.2%; OR, 1.76; CI, 1.29 to 2.39; P < 0.01). Within the latter group, isolates from blood exhibited a lower erythromycin resistance rate than isolates from the remaining LRT samples (18.8 versus 37.2%; OR, 2.57; CI, 1.88 to 3.55; P < 0.01). As far as penicillin is concerned, middle ear samples had a significantly higher rate of nonsusceptibility than samples from patients with LRT infections (57.7 versus 49.2%; OR, 1.41; CI, 1.04 to 1.92; P = 0.02); blood samples had a nonsusceptibility rate of 34.1%, and the rate for samples from the respiratory tract was 53.2%. The rank order of penicillin resistance by sample, based on NCCLS breakpoints, was middle ear (27.4%) > respiratory tract (22.4%) > blood (16.5%) (P = 0.01). Likewise, clear differences in erythromycin resistance (48.5 versus 32.2%; OR, 1.98; CI, 1.51 to 2.58; P < 0.01) and penicillin resistance (28.4 versus 20.6%; OR, 1.53; CI, 1.14 to 2.07; P < 0.01) were evident between children and adults for S. pneumoniae.
Regarding S. pyogenes isolates, rates of macrolide resistance were about 20% (Table 1). No difference in erythromycin resistance was observed between children and adults (20.1 versus 23.3%), contrary to what was found with S. pneumoniae. As expected, all S. pyogenes isolates were 100% susceptible to the β-lactam drugs tested, with very low MIC50/MIC90 values.
The distribution of phenotypes of erythromycin resistance is shown in Table 4; 95% of the erythromycin-resistant S. pneumoniae isolates had constitutive MLSB phenotype, whereas the M phenotype was predominant (90%) among erythromycin-resistant S. pyogenes isolates.
TABLE 4.
Presumptive phenotypes of erythromycin resistance
| Organism | No. (%) of isolates with the following phenotype:
|
||
|---|---|---|---|
| Constitutive MLSB | Inducible MLSB | Efflux (M) | |
| S. pneumoniae(n = 588) | 551 (93.7) | 4 (0.6) | 33 (5.6) |
| S. pyogenes(n = 416) | 8 (2) | 36 (8.5) | 372 (89.5) |
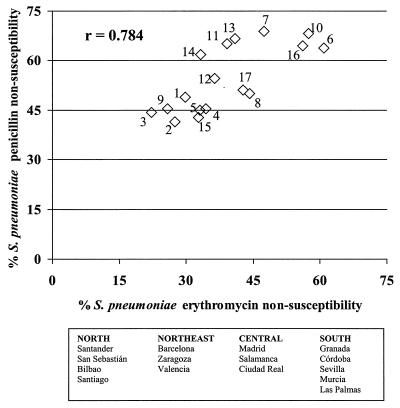
When the relationship between erythromycin nonsusceptibility and penicillin nonsusceptibility in S. pneumoniae was examined on a geographical basis (Fig. 1), a strong positive correlation (r = 0.784; P < 0.05) between both rates of resistance was observed. Therefore, centers with a high rate of erythromycin resistance also had a high rate of penicillin nonsusceptibility.
FIG. 1.
Relationship between erythromycin nonsusceptibility and penicillin nonsusceptibility in S. pneumoniae isolates by hospital. Hospitals (all in Spain) were as follows: 1, M. Valdecilla, Santander; 2, Donostia, San Sebastián; 3, Cruces, Bilbao; 4, General, Santiago Compostela; 5, Clinico, Barcelona; 6, San Juan de Dios, Barcelona; 7, Clínico, Zaragoza; 8, Peset, Valencia; 9, G. Marañón, Madrid; 10, La Paz, Madrid; 11, Clínico, Salamanca; 12, Alarcos, Ciudad Real; 13, V. Nieves, Granada; 14, R. Sofia, Córdoba; 15, V. Macarena, Sevilla; 16, V. Arrixaca, Murcia; and 17, Insular, Las Palmas. Distribution among the Geographical regions is shown below the graph.
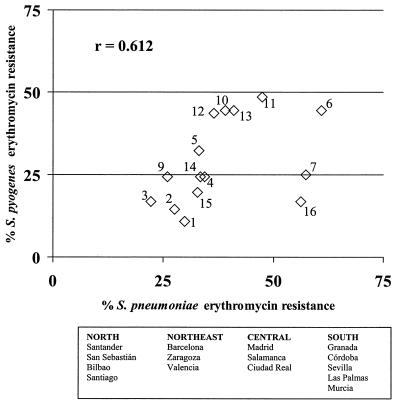
When the rates of erythromycin resistance for both species of the same Streptococcus genus were analyzed from a geographical perspective, it was found that centers with a high resistance rate for S. pneumoniae also had a high resistance rate for S. pyogenes and vice versa (Fig. 2). Hence, a fairly good correlation (r = 0.612; P < 0.05) between rates of erythromycin resistance for both species was shown on a geographical basis. Interestingly, centers in northern Spain (Santander, San Sebastián, and Bilbao) had the lowest rates of erythromycin resistance for S. pyogenes (10, 15, and 16%, respectively) and among the lowest rates for S. pneumoniae (27, 30, and 22%, respectively).
FIG. 2.
Relationship between erythromycin resistance in S. pneumoniae and S. pyogenes by hospital. Hospitals were as indicated in the legend to Fig. 1, with the following exceptions: 7, La Paz, Madrid; 10, Clínico, Salamanca; 11, Clínico, Zaragoza. Also, hospitals 8 and 17 did not provide S. pyogenes isolates.
The distribution by serogroup or serotype for the S. pneumoniae isolates was as follows: ST19 (14.0%), ST6 (10.8%), ST23 (10.1%), ST14 (9.0%), ST3 (8.5%), ST9 (6.4%), and ST15 (4.2%); these values accounted for up to 65% of all isolates.
For 86 pneumococcal isolates (5%), the amoxicillin MIC was ≥8 μg/ml, and among these, serotype 14 was by far the predominant one (45.9%), followed by serotypes 6 (17.6%), 9 and 23 (14% each), and 15 (5.8%). More than two-thirds of the isolates were isolated in northern centers, and only 6% were obtained from southern Spain. Penicillin MICs for these strains were two or three twofold dilutions lower than those of amoxicillin. The prevalence of nonsusceptibility for these 86 isolates was 100% for penicillin, amoxicillin-clavulanate, cefaclor, cefuroxime, and cefotaxime, whereas it was 65% for macrolides; the ciprofloxacin MIC was ≥4 μg/ml for 11.7%. For 21 of the isolates, the amoxicillin MIC was 16 μg/ml. With these isolates, there was again a clear geographical predominance, as more than 90% were from northern areas.
For up to 120 pneumococcal isolates (7.1%) and 70 S. pyogenes isolates (3.4%), the ciprofloxacin MIC was ≥4 μg/ml. Ciprofloxacin resistance was more prevalent among erythromycin-nonsusceptible pneumococcal isolates than among erythromycin-susceptible ones (9.67 versus 5.75%; OR, 1.76; CI, 1.19 to 2.60; P < 0.05). No statistically significant association between ciprofloxacin and penicillin resistance was found, although it was seen for penicillin-nonsusceptible isolates versus susceptible ones (8.4 versus 5.8%; OR, 1.48; CI, 1.01 to 2.20; P < 0.05). For S. pyogenes, clustering of ciprofloxacin resistance also was seen for erythromycin-resistant isolates versus susceptible ones (6.1 versus 2.7%; OR, = 2.29; CI, 1.34 to 3.88; P < 0.05). Ciprofloxacin resistance was also more prevalent among pneumococcal isolates from adults than among those from children (7.7 versus 4.2%; OR, 1.9; CI, 1.03 to 3.85; P < 0.05), but it was not significant (4.1 versus 3.3%; P > 0.05) among S. pyogenes isolates.
DISCUSSION
The prevalence of erythromycin resistance in S. pneumoniae currently remains at 35%, although it seems to have decreased to 20% for S. pyogenes compared to the results of a previous survey (2, 3) (33.7% for pneumococci and 26.7% for S. pyogenes). Participation in this study of three new centers in northern Spain (Santander, San Sebastián, and Bilbao) with very low rates of S. pyogenes erythromycin resistance (10 to 17%) is likely to have biased the mean rate.
With regard to high-level resistance to penicillin in S. pneumoniae, the current prevalence also has apparently decreased to 21.7%, but geographical imbalance in the proportions of resistant strains is very unlikely to have contributed significantly to this decrease.
Qualitative differences in erythromycin resistance were also observed for S. pneumoniae, since a higher rate of the M phenotype was found in Las Palmas (Canary Islands) relative to the entire study population (20 versus 5.6%). The Canary Islands are located approximately 10° south of the Spanish peninsula and close to the African coast. With this single exception, almost 95% of the erythromycin-resistant pneumococcal isolates displayed the MLSB phenotype.
From a quantitative viewpoint (i.e., prevalence of erythromycin resistance), a fairly good positive correlation (r = 0.612) was found between both species of the genus Streptococcus (Fig. 2), as already reported (9). This correlation indicates differential antibiotic consumption as a likely reason, as the species have different mechanisms of erythromycin resistance (constitutive for S. pneumoniae and efflux for S. pyogenes), different population sources (adults and children), and different pathologies (LRT infections and pharyngitis). Apart from the geographical factor, a temporal factor already has been reported in Spain, in which antibiotic consumption over time correlates well with the evolution of resistance in both S. pneumoniae and S. pyogenes (11, 12).
Nevertheless, the assumption that antibiotic consumption relates to resistance in a linear way needs to be made with care. Whether other factors, such as local clonal epidemiological differences (different prevalences of certain M protein types in S. pyogenes or serogroups in S. pneumoniae) or antibiotic pressure selection of strains carrying a more suitable gene trait and consequently “hitchhiking” their remaining genomes, are involved remains to be seen. Addressing such a question is of importance in order to either undertake societal infection control measures or control inappropriate antibiotic use. Erythromycin resistance of S. pyogenes in Spain has been related to the circulation of certain clones in a given area over time (21), which might account for the geographical differences in the prevalences of resistance. Certainly, antibiotic pressure somehow affects this turnover. When the prevalence of erythromycin resistance in S. pyogenes has been traced in a given location, it has varied widely over time (22). Oscillations ranging from 10 to 30% have been common in short follow-up periods. Another example of local oscillations in the prevalence of resistance is provided by the site in Córdoba, which showed a high rate of erythromycin resistance for S. pneumoniae and S. pyogenes (60% for both species) in a previous report (9), whereas in the current surveillance the rate was 30%. In addition to natural fluctuations in the spread of resistance (28), seasonal variations in either resistance rates (2, 3) or clonal turnover also may contribute to the global picture. All these factors may be important, since despite the fact that high resistance rates have been described for areas whose antibiotic consumption has increased (16), a given lower level of resistance has not been immediately seen after a decline in antibiotic use. Mathematical modeling shows that there is a lag until a decrease is perceived, and the declining rate is lower than the ascending rate (1). In addition, the new steady state is reached after a series of temporal oscillations whose intensity, duration, and stability depend on how long this biological lag takes.
In addition to coincident geographical and temporal selection of erythromycin resistance in both species, isolates that accumulate different resistance traits may also play a key role. In this study, amoxicillin nonsusceptibility clustered only in highly penicillin-resistant S. pneumoniae isolates, whereas cephalosporin nonsusceptibility and macrolide nonsusceptibility clustered in both penicillin-intermediate and penicillin-resistant isolates. Hence, it seems that penicillin nonsusceptibility is a better driver of nonsusceptibility for macrolides and cephalosporins than for amoxicillin. The selection of coresistance also occurs on a geographical basis, as shown in Fig. 1.
The fact that amoxicillin (and amoxicillin-clavulanate) now has higher breakpoints based on pharmacodynamic and pharmacokinetic considerations has resulted in a dramatic change in what was previously considered nonsusceptibility. As an example, a study conducted 2 years ago (3) with old NCCLS breakpoints (19) reported a rate of amoxicillin resistance of 23.7%, compared to the current 5.1%. If old breakpoints were now applied, the resultant rate would be the same (24%). These new breakpoints for amoxicillin may create the impression that amoxicillin and amoxicillin-clavulanate are more active in vitro than intravenous penicillin or cefotaxime, as breakpoints for these have not been changed. In any event, there is clinical evidence that amoxicillin and ceftriaxone are useful for the treatment of penicillin- and even cephalosporin-resistant pneumococcal pneumonia (23).
Among the most striking findings were the impressive rates of ciprofloxacin resistance found in S. pneumoniae (7.1%) and S. pyogenes (3.4%), which to our knowledge are the highest rates ever published. These figures were not so surprising for S. pneumoniae in Spain, since high rates of ciprofloxacin resistance have been reported (8, 15) in recent years. In addition, the prevalence of high ciprofloxacin MICs was mainly clustered in erythromycin-nonsusceptible (8, 15) and penicillin-nonsusceptible pneumococcal strains (4, 15). More interesting are the prevalence of ciprofloxacin resistance in S. pyogenes and its association with erythromycin resistance. These associations may explain the presence of high ciprofloxacin MICs in both species in children, who are not usually given fluoroquinolones. Ciprofloxacin resistance is becoming a matter of concern, since recent reports have communicated prevalence rates that might be a warning signal for a future increase in resistance (4, 8, 15). However, ciprofloxacin is not a good indicator for resistance to newer quinolones and does not imply the same findings for all drugs within this family (4, 7).
The existence of 86 strains for which the amoxicillin MIC was ≥8 μg/ml and the penicillin MICs were two or three twofold dilutions lower than those of amoxicillin is also a matter of concern. As a matter of fact, this phenomenon was recently reported in France for strains for which the amoxicillin MIC was ≥4 μg/ml (5), indicating that high-level resistance to amoxicillin emerged within preexisting penicillin-resistant clones. The geographical north-south gradient in Spain and the predominance of certain serogroups, such as 14, among our amoxicillin-resistant isolates both suggest a strong clonal relationship. A more detailed study with these isolates is in progress.
Country-based monitoring networks are of great importance in the study of local selection of resistance, coselection of resistance, selection of coresistance and resistance drivers in order to attain the appropriate level of local antibiotic consumption and/or evaluate natural clone fluctuations in the spread of resistance.
ACKNOWLEDGMENT
This study was supported by a grant from SmithKline Beecham S. A., Madrid, Spain.
Appendix
The members of the Spanish Surveillance Group for Respiratory Pathogens are J. Larruskain, Hospital Donostia, San Sebastián; E. Cercenado, Hospital Gregorio Marañón, Madrid; J. Barrón and L. López, Hospital de Cruces, Bilbao; T. Jiménez de Anta and F. Marco, Hospital Clinico Provincial, Barcelona; E. Perea and L. Martínez, Hospital Virgen de la Macarena, Sevilla; C. Latorre and A. Gené, Hospital Sant Joan de Déu, Barcelona; J. A. García-Rodríguez (Study Coordinator) and I. Trujillano, Hospital Clínico Universitario, Salamanca; A. García, S. García, and M. Güeni, Hospital La Paz, Madrid; J. Ruiz and E. Simarro, Hospital Virgen de la Arrixaca, Murcia; C. García-Riestra, B. Regueiro, A. Jato, and M. Prieto, Hospital Clínico Universitario, Santiago de Compostela; C. Rubio and C. García, Hospital Clínico Universitario, Zaragoza; M. de la Rosa, Hospital Virgen de las Nieves, Granada; A. M. Martín and F. Cañas, Hospital Insular, Las Palmas; D. Romero and M. González, Hospital Nuestra Señora de Alarcos, Ciudad Real; J. M. Nogueira, Hospital Dr. Peset, Valencia; M. Casal and A. Ibarra, Hospital Reina Sofia, Córdoba; A. Fenoll and J. Casal, Instituto Carlos III, Madrid; J. J. Granizo, Fundación Jiménez Diaz, Madrid; L. López and C. Gimeno, Instituto Valenciano de Microbiología, Valencia; and R. Dal-Ré, GlaxoSmithKline, Tres Cantos, Madrid.
REFERENCES
- 1.Austin D J, Kristinsson K G, Anderson R M. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc Natl Acad Sci USA. 1999;96:1152–1156. doi: 10.1073/pnas.96.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baquero F, García-Rodriguez J A, García-de-Lomas J, Aguilar L the Spanish Surveillance Group for Respiratory Pathogens. Antimicrobial resistance of 914 β-hemolytic streptococci isolated from pharyngeal swabs in Spain: results of a 1-year (1996–1997) multicenter surveillance study. Antimicrob Agents Chemother. 1999;43:178–180. doi: 10.1128/aac.43.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baquero F, García-Rodriguez J A, García-de-Lomas J, Aguilar L the Spanish Surveillance Group for Respiratory Pathogens. Antimicrobial resistance of 1,113 Streptococcus pneumoniae isolates from patients with respiratory tract infections in Spain: results of a 1-year (1996–1997) multicenter surveillance study. Antimicrob Agents Chemother. 1999;43:357–359. doi: 10.1128/aac.43.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D K, McGeer A, de Azavedo J C, Low D E. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. Canadian Bacterial Surveillance Network. N Engl J Med. 1999;341:233–239. doi: 10.1056/NEJM199907223410403. [DOI] [PubMed] [Google Scholar]
- 5.Doit C, Loukil C, Fitoussi F, Geslin P, Bingen E. Emergence in France of multiple clones of clinical Streptococcus pneumoniae isolates with high-level resistance to amoxicillin. Antimicrob Agents Chemother. 1999;43:1480–1483. doi: 10.1128/aac.43.6.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenoll A, Jado I, Vicioso D, Casal J. Dot blot assay for the serotyping of pneumococci. J Clin Microbiol. 1997;35:764–766. doi: 10.1128/jcm.35.3.764-766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuentes F, Giménez M J, Marco F, Alou L, Aguilar L, Prieto J. In vitro susceptibility to gemifloxacin and trovafloxacin of Streptococcus pneumoniae strains exhibiting decreased susceptibility to ciprofloxacin. Eur J Clin Microbiol Infect Dis. 2000;19:137–139. doi: 10.1007/s100960050446. [DOI] [PubMed] [Google Scholar]
- 8.García-Rey C, Aguilar L, Baquero F the Spanish Surveillance Group for Respiratory Pathogens. Influence of different factors on the ciprofloxacin resistance prevalence of Streptococcus pneumoniae in Spain. Antimicrob Agents Chemother. 2000;44:3481–3482. doi: 10.1128/aac.44.12.3481-3482.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gómez-Lus R, Granizo J J, Aguilar L, Bouza E, Gutiérrez A, García-de-Lomas J the Spanish Surveillance Group for Respiratory Pathogens. Is there an ecological relationship between rates of antibiotic resistance of species of the genus Streptococcus? J Clin Microbiol. 1999;37:3384–3386. doi: 10.1128/jcm.37.10.3384-3386.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goossens H, Sprenger M J W. Community acquired infections and bacterial resistance. Br Med J. 1998;317:654–657. doi: 10.1136/bmj.317.7159.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granizo J J, Aguilar L, Casal J, Baquero F, Dal-Ré R. Erythromycin resistance in Streptococcus pyogenes in relationship with macrolide consumption in Spain (1986–1997) J Antimicrob Chemother. 2000;46:959–964. doi: 10.1093/jac/46.6.959. [DOI] [PubMed] [Google Scholar]
- 12.Granizo J J, Aguilar L, Casal J, García-Rey C, Dal-Ré R, Baquero F. Streptococcus pneumoniae resistance to erythromycin and penicillin in relation to macrolide and β-lactam consumption in Spain (1979–1997) J Antimicrob Chemother. 2000;46:767–773. doi: 10.1093/jac/46.5.767. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann J, Cetron M S, Farley M M, et al. The prevalence of drug-resistant Streptococcus pneumoniae in Atlanta. N Engl J Med. 1995;333:481–486. doi: 10.1056/NEJM199508243330803. [DOI] [PubMed] [Google Scholar]
- 14.Huovinen P, Cars O. Control of antimicrobial resistance: time for action. Br Med J. 1998;317:613–614. doi: 10.1136/bmj.317.7159.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liñares J, de la Campa A G, Pallarés R. Fluoroquinolone resistance in Streptococcus pneumoniae. N Engl J Med. 1999;341:1546–1547. doi: 10.1056/nejm199911113412013. [DOI] [PubMed] [Google Scholar]
- 16.Liñares J, Pallarés R, Alonso T, Pérez J L, Ayats J, Gudiol F, Viladrich P F, Martín R. Trends in antimicrobial resistance of clinical isolates of Streptococcus pneumoniae in Bellvitge Hospital, Barcelona, Spain (1979–1990) Clin Infect Dis. 1992;15:99–105. doi: 10.1093/clinids/15.1.99. [DOI] [PubMed] [Google Scholar]
- 17.Lund E, Henrichsen J. Laboratory diagnosis, serology and epidemiology of Streptococcus pneumoniae. Methods Microbiol. 1978;12:241–262. [Google Scholar]
- 18.Monnet D L. European recommendations to respond to the threat of antimicrobial-resistant microorganisms. ASM News. 1999;65:390–391. [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard. NCCLS document M7–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard. NCCLS document M7–A5. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 21.Pérez-Trallero E, Marimón J M, Montes M, Orden B, de Pablos M. Clonal differences among erythromycin-resistant Streptococcus pyogenes in Spain. Emerg Infect Dis. 1999;5:235–240. doi: 10.3201/eid0502.990207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pérez-Trallero E, Urbieta M, Montes M, Ayestarán I, Marimón J M. Emergence of Streptococcus pyogenes strains resistant to erythromycin in Gipuzkoa, Spain. Eur J Clin Microbiol Infect Dis. 1998;17:25–31. doi: 10.1007/BF01584359. [DOI] [PubMed] [Google Scholar]
- 23.Roson B, Carratalá J, Tubau F, Dorca J, Liñares J, Pallarés R, Manresa F, Gudiol F. Usefulness of lactam therapy for community-acquired pneumonia in the era of drug-resistant Streptococcus pneumoniae: a randomized study of amoxicillin-clavulanate and ceftriaxone. Microb Drug Resist. 2001;7:85–96. doi: 10.1089/107662901750152864. [DOI] [PubMed] [Google Scholar]
- 24.Seppala H, Klaukka T, Lehtonen R, Nenonen E, Huovinen P. Outpatient use of erythromycin: link to increased erythromycin resistance in group A streptococci. Clin Infect Dis. 1995;21:1378–1385. doi: 10.1093/clinids/21.6.1378. [DOI] [PubMed] [Google Scholar]
- 25.Seppala H, Klaukka J, Vuopio-Varkila A, Muotiala A, Helenius H, Lager K, Huovinen P. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441–446. doi: 10.1056/NEJM199708143370701. [DOI] [PubMed] [Google Scholar]
- 26.Seppala H, Nissinen Q Y, Huovinen P. Three different phenotypes of erythromycin-resistant Streptococcus pyogenes in Finland. J Antimicrob Chemother. 1993;32:885–891. doi: 10.1093/jac/32.6.885. [DOI] [PubMed] [Google Scholar]
- 27.Stingemore N, Francis G R J, Toohey M, McGechie D B. The emergence of erythromycin resistance in Streptococcus pyogenes in Fremantle, Western Australia. Med J Aust. 1990;150:626–631. doi: 10.5694/j.1326-5377.1989.tb136725.x. [DOI] [PubMed] [Google Scholar]
- 28.Turnidge J. What can be done about resistance to antibiotics? Br Med J. 1998;317:645–647. doi: 10.1136/bmj.317.7159.645. [DOI] [PMC free article] [PubMed] [Google Scholar]