Abstract
Spontaneous adrenal haemorrhage (SAH) is a rare condition. The incidence of adrenal haemorrhage in pregnancy is currently not known; however, an association with pregnancy has been reported.
An acute presentation with severe back or flank pain should raise suspicion of this condition. Diagnosis is based on imaging. An ultrasound scan is a basic and readily available investigation in pregnancy to rule out renal and suprarenal pathology while CT or MRI scan can help to confirm the diagnosis. A multidisciplinary team (MDT) approach, involving the obstetric, anaesthetic, medical and endocrine team, is essential in management of this condition.
We present a case of an SAH; managed conservatively, in an otherwise healthy and low-risk pregnant woman and describe the literature review on this rare condition, including pathophysiology and management.
Keywords: Adrenal disorders, Obstetrics and gynaecology, Pregnancy, Radiology
Background
Spontaneous adrenal haemorrhage (SAH) is defined as acute haemorrhage of the adrenal gland, without any prior trauma and history of anticoagulation and occurring without any predispositions.1 Adrenal haemorrhage in the general population is a rare condition.1 2 Many autopsy studies have stated that the incidence of adrenal haemorrhage ranges from 0.14% to 1.8%; with a higher incidence of up to 25% following trauma.3 4 The incidence of adrenal haemorrhage in pregnancy is currently not known; however, an association with pregnancy has been reported.1 2 5 6
Our case indicates the importance of SAH being kept as a differential diagnosis for flank pain as well as the importance of an MDT approach to its investigation and management. We hope that our case and the literature review will help other healthcare professionals be more aware of the condition and how to manage it in pregnancy.
Case presentation
A primiparous Caucasian woman was low risk with a body mass index of 24.8. Her previous medical history was unremarkable and her pregnancy was uneventful. She attended the obstetric emergency triage at 24+6 weeks of gestation following gradually worsening right-sided back pain over the last 48 hours radiating to the upper and lower right quadrants, with associated episodes of vomiting. There was no history of shortness of breath, fever, rigours or urinary symptoms. On examination, her blood pressure (BP) was 150/60 mm Hg (her booking BP at 10 weeks was 118/80 mm Hg), her heart rate was 92 bpm and her respiratory rate was 16 bpm. She had a normal temperature of 36.4 and was saturating 98% on air. Her chest had normal breathe sounds on examination and there was marked tenderness on the right renal angle. The urine sample showed +1 proteinuria.
Investigations
Her initial blood results showed serum lactate of 3.5, white cell count of 24.1 x109/L, C reactive protein of 30.0, normal electrolytes and renal function. She was started on the sepsis six care bundle in view of the high lactate and received intravenous antibiotics, intravenous fluids and analgesia. A stat dose of 200 mg labetalol was required for high BP, but no further treatment was required.
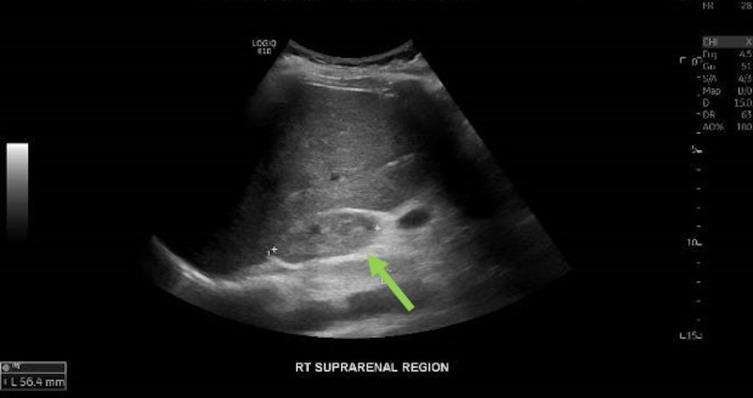
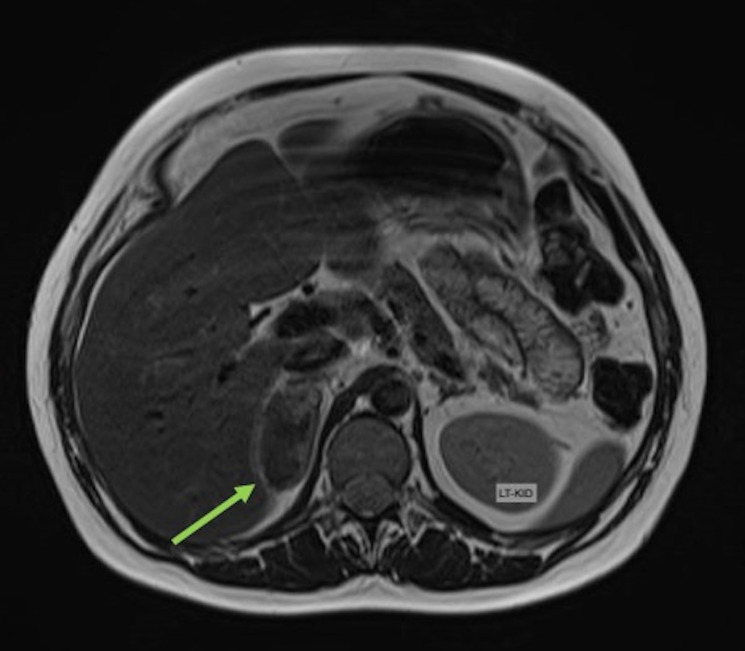
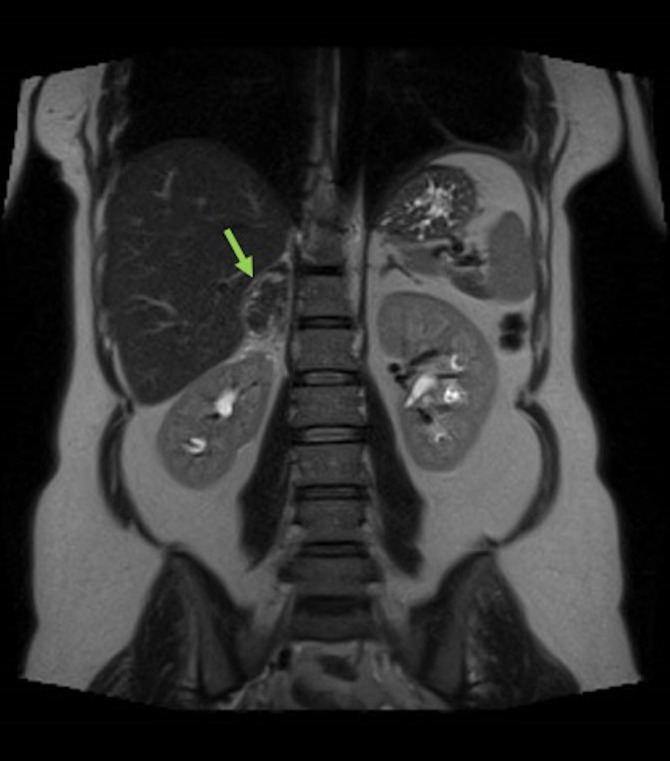
An urgent ultrasound scan of the renal tract system revealed a 5 cm mass in the right suprarenal region, with a high suspicion of it being of adrenal origin and a suspected diagnosis of SAH (figure 1). Subsequent MRI images (figures 2 and 3) confirmed an SAH (56×22 mm size haematoma).
Figure 1.
Ultrasound of the abdomen, showing a mildly hyperechoic avascular 56×20 mm mass in the right suprarenal area. It was noted to be separate from the upper pole of right kidney and appeared adrenal in origin.
Figure 2.
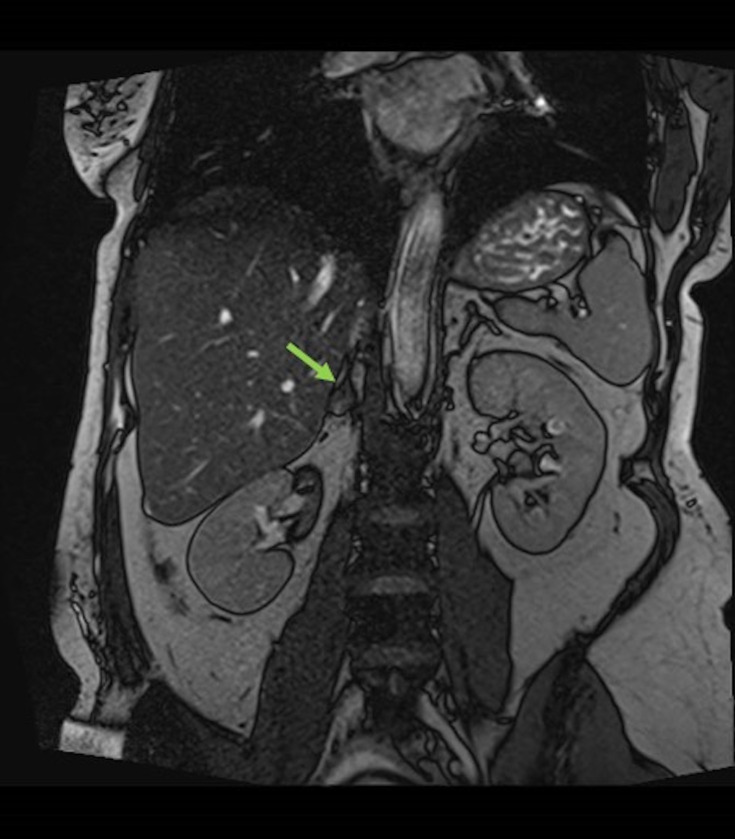
MRI, transverse plane, T2-weighted sequence demonstrating a 51×28 mm lesion centred in the right adrenal gland with heterogeneous T2 signal with small focal regions of hyperintensity. No loss of signal on chemical shift imaging to suggest intra-lipid content. Right adrenal lesion could be compatible with spontaneous acute/subacute adrenal haemorrhage.
Figure 3.
MRI, coronal plane, T2-weighted sequence (As in figure 2).
Differential diagnosis
Differential diagnoses included most likely pyelonephritis, but we also needed to rule out renal stones, renal hydronephrosis, pulmonary embolism, musculoskeletal pain and pre-eclampsia.
Pulmonary embolism was unlikely as there were no risk factors, including normal blood gases and saturations on air. Musculoskeletal pain was also unlikely considering there was no associated history.
Her placental growth factor (PlGF) ratio test was negative, which excluded any element of pre-eclampsia. Her initial urine PCR was 30, which subsequently normalised.
After the diagnosis of an SAH; her serum electrolytes, urine metnephrines, serum aldosterone and renin levels were measured and remained normal; ruling out the possibility of phaeochromocytoma at that stage.
Treatment
She was treated conservatively with analgesia and antibiotics for pyelonephritis and discharged home 6 days later with planned follow-up in obstetric, endocrinology and anaesthetic clinics.
At 33 weeks, she developed new-onset mild hypertension 140/90 mm Hg and treated as pregnancy-induced hypertension (PIH) on labetalol 200 mg two times per day.
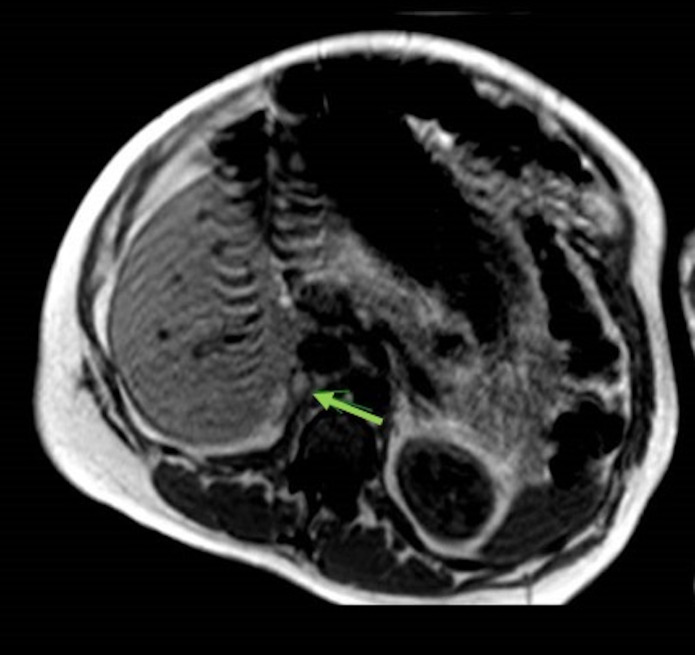
At 35+4 weeks, she presented with recurrent right-sided back pain, and a repeat MRI was arranged, which showed regression in the size of the haematoma to 26×14 mm (figures 4 and 5).
Figure 4.
MRI, transverse plane, T1-weighted sequence demonstrating a more homogeneous, decreased in size (22×14 mm) right adrenal lesion. A small hyperintense area within may represent a haemorrhagic cyst or residual haematoma.
Figure 5.
MRI, coronal plane, T1-weighted sequence (As in figure 4).
Following discussion with endocrinology team, the antihypertensive choice was changed to include an alpha blocker, doxazosin 4 mg two times per day; as there was a concern about a small possibility of phaeochromocytoma hiding in the haematoma in context of the new-onset hypertension. Repeat urine metnephrines remained negative.
Outcome and follow-up
She opted for a planned caesarean section for maternal request at 38+4 weeks and underwent an uneventful procedure with regional blockade and arterial line monitoring. A detailed obstetric and anaesthetic plan was generated in case she presented with an adrenal crisis.
Her BP remained normal postdelivery favouring the diagnosis of PIH. A repeat MRI postdelivery confirmed that the haematoma had further regressed in size to 4 mm and did not raise any concerns about phaeochromocytoma.
An MDT approach, involving the obstetric, anaesthetic, medical and endocrine team, was of essential importance throughout her case.
Discussion
Spontaneous or non-traumatic adrenal haemorrhage can be unilateral or bilateral. Bilateral adrenal haemorrhage, despite being extremely uncommon, has a greater potential to lead to acute adrenal insufficiency and if left untreated can be life threatening. The overall mortality rate for adrenal haemorrhage has been reported as 15%; but this may vary depending on the delay in diagnosis, prompt treatment and individual underlying illnesses.3–5
Pathophysiology
The pathophysiology of SAH is unclear; however, it has been noted that the gland’s rich arterial blood supply in combination with its slow venous drainage into a singular central adrenal vein can create a possible ‘vascular dam’. Therefore, it has been hypothesised that any increase in venous pressure or arterial pressure leading to vascular congestion in addition to the delicate architecture of the vessel walls could lead to a possible haemorrhage within the adrenal gland.1 3–5 It has also been thought that during times of stress, the increase in production of catecholamines by the adrenal gland results in platelet aggregation and vasoconstriction of the adrenal vein, which can further increase the venous pressure. This can subsequently lead to an adrenal haemorrhage.2 4
During pregnancy, there is noted to be physiological hypertrophy and hyperplasia of the adrenal cortex in addition to an increase in adrenocorticotropic hormone. This can subsequently increase vascular supply to the adrenal gland and possibly predispose the gland to bleeding.1 5 7 Furthermore, in pregnancies complicated by pre-eclampsia, the increase in BP may also cause rupture of the adrenal vessels. One-third of fatal cases of eclampsia have shown necrosis and haemorrhage of the adrenal gland.1 8 The hypercoagulable state in pregnancy in combination with the theory that the adrenal vein is prone to platelet thrombi can lead to thrombosis of the adrenal vein. Consequently, adrenal vein thrombosis has been thought to cause outflow obstruction and, as a result, can lead to adrenal haemorrhage.4 5
Aetiology
One of the most common causes of adrenal haemorrhage is trauma. However, in the context of SAH, certain predispositions have been studied; most notably stress, antiphospholipid syndrome and the use of anticoagulants. Some of the well-observed predisposing factors including in pregnancy, associated with adrenal haemorrhage, have been presented in table 1.
Table 1.
Predisposing factors for adrenal haemorrhage (Reprinted by permission from RightsLink:SpringerNature)4
| Trauma/mechanical injury | |
| Chronic venous congestion |
|
| Stress |
|
| Haemorrhagic tendency or coagulopathy |
|
| Underlying adrenal mass |
|
| Pregnancy-related conditions |
|
ACTH, adrenocorticotropic hormone.
Clinical presentation
The clinical presentation of an adrenal haemorrhage, both in the general population and in pregnant women, is very non-specific.3 8 The most common presenting symptom is that of acute abdominal or flank pain. Associated signs and symptoms include nausea, vomiting, a possible palpable abdominal mass, altered mental state, agitation and fever.1 3 4 8 Hypotension, though reported as a sign of adrenal haemorrhage, is often uncommon and seen in the presence of shock.3
Diagnosis
Blood tests are often the first investigations performed but can often be normal or non-specific. The most common laboratory findings include a reduction in haemoglobin and electrolyte disturbances, showing signs of adrenal insufficiency—hyponatraemia, hyperkalaemia and hypoglycaemia. These, however, are not diagnostic of an adrenal haemorrhage.1 3 4 8
For a definitive diagnosis of an adrenal haemorrhage, imaging is required. In pregnant patients, the first-line investigation is usually an ultrasound; however, the sonographic findings can be non-specific for an adrenal haemorrhage.6 8 9 Therefore, a CT or MRI scan is required for the confirmation of the diagnosis as well as to try to ascertain any underlying pathology or cause for the haemorrhage. Both modalities can be used in pregnancy on risk benefit analysis and for diagnostic purposes; however, MRI would be preferable than CT scan in pregnancy to gain further information and devise a management plan for this condition.10 11
In the recent literature, there appears to be no general consensus on whether CT or MRI is the most accurate in confirming the diagnosis of an adrenal haemorrhage. Despite this, both appear to be invaluable and each has characteristic findings on their images.1 3 4 6 8
A contrast CT scan showing an adrenal haemorrhage is most commonly described as round or oval echogenic masses encompassing the adrenal glands. Other findings may include an area of hypodense fluid with or without calcifications or periadrenal stranding, which results from the blood infiltrating through the retroperitoneal fat.3 4 6
On MRI, an adrenal haemorrhage appears as a heterogeneous mass of one or both of the adrenals.1 8 In addition to this, an adrenal haemorrhage can also be aged using MRI.9 In the acute phase (less than 7 days from onset), the haematoma appears either isointense or hypointense on T1-weighted images and hyperintense on T2-weighted images due to the presence of a high concentration of intracellular deoxyhaemoglobin. During the subacute phase (7 days to 7 weeks from onset), there is production of free methemoglobin from the oxidation of deoxyhaemoglobin; which, as a result, causes the haematoma to gradually become hyperintense on T1-weighted images, starting at the periphery and filling the centre over time. In the chronic phase (after 7 weeks from onset), a hypointense rim, attributed to haemosiderin deposits and a fibrous capsule, is present on T1-weighted and T2-weighted images. Gradient-echo imaging is beneficial in demonstrating the ‘blooming’ effect, caused by the magnetic susceptibility of haemosiderin deposition.1 8 12
Management
The definitive management of SAH is still undefined and depends on the likelihood of the patient developing life-threatening complications, such as haemorrhagic shock and adrenal insufficiency.2 7
Conservative, surgical and interventional treatments are the main management options for SAH in the general population as well as in pregnancy; with the recent trend in trauma literature moving towards non-operative management when possible.4 7 Conservative management, which includes intravenous fluids, pain control and serial haemoglobin assessments alongside as required blood transfusions, may be appropriate if the bleeding is less severe and the patient is haemodynamically stable.6 8 This was the case with our patient as well and she was managed conservatively with analgesia and intravenous fluids.
If the bleeding is massive, and the patient is unstable, surgery such as an adrenalectomy, may be required to control the bleeding. There is also evidence to show that persistent bleeding in haemodynamically unstable patients has responded well to angioembolisation. This method of radiological intervention is also beneficial for those patients who may be medically unfit for surgery and can allow for preservation of any functional adrenal parenchyma.1 4 6 8 Adrenalectomy can be safely performed in pregnancy. In the context of an adrenal tumour in pregnancy, adrenalectomy is preferably performed in the second trimester or deferred until after delivery if it is diagnosed in late third trimester. The procedure should be performed in a hospital where maternity services are available to facilitate delivery in case of an emergency or preterm labour. Depending on gestation, antenatal steroids may be required for fetal lung maturation, in case of a risk of preterm labour.13
In the acutely unwell patient exhibiting signs of shock even during labour, for example, hypotension, sweating, tachycardia, etc, steroid replacement with hydrocortisone may also be required and should not be delayed.4 Initially, intravenous or IM hydrocortisone 100 mg is given stat. Following this, 50 mg hydrocortisone intravenous or IM is given four times per day. When the patient is stable and able to eat and drink—this can be swapped to oral hydrocortisone, with the following regime—10 mg in the morning, 5 mg at lunch and 5 mg at 4 pm (teatime); in addition to a once daily dose of fludrocortisone 100 μg. Management of all these patients require an MDT review with both endocrinology and surgical teams.
Preterm delivery may be needed if the SAH is progressive or associated with severe pre-eclampsia or eclampsia, and, therefore, timely antenatal corticosteroids may be required.1 8
The choice of antihypertensives in a patient with suspected pheochromocytoma and an SAH area includes alpha and beta blockers, which can be safely used in pregnancy.
If conservative management is chosen as appropriate in pregnancy, the woman should be followed up throughout her pregnancy and in the postpartum period, as the stress of labour and delivery could result in a recurrence of the adrenal haemorrhage.1 8 There is no evidence that one mode of delivery is preferred than other. Woman should be referred to a tertiary hospital for delivery if there is a lack of local expertise in the management of pheochromocytoma, SAH or adrenal crises.
It is also important to ensure follow-up imaging in patients managed conservatively to confirm resolution of the haematoma as well as exclude any underlying lesion or neoplasm that may be the cause of the haemorrhage or may require surgical removal.1 4
Learning points.
Spontaneous adrenal haemorrhage is an important differential diagnosis for abdominal and/or flank pain in pregnancy.
Diagnosis may be confirmed using MRI or a CT scan.
Steroid treatment is an important part of management in adrenal shock.
Preterm delivery can be avoided if conservative management is possible.
There is no evidence that one mode of delivery is better than other in stable patients.
Footnotes
Contributors: RZ-E-H, EP and ED conceived the article. EP and ED researched and wrote the article. RZ-E-H reviewed, analysed and edited the article. All authors agreed on final version.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Gavrilova-Jordan L, Edmister WB, Farrell MA, et al. Spontaneous adrenal hemorrhage during pregnancy: a review of the literature and a case report of successful conservative management. Obstet Gynecol Surv 2005;60:191–5. 10.1097/01.ogx.0000157357.15401.c3 [DOI] [PubMed] [Google Scholar]
- 2.Ali A, Singh G, Balasubramanian SP. Acute non-traumatic adrenal haemorrhage-management, pathology and clinical outcomes. Gland Surg 2018;7:428–32. 10.21037/gs.2018.07.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Serafino M, Severino R, Coppola V, et al. Nontraumatic adrenal hemorrhage: the adrenal stress. Radiol Case Rep 2017;12:483–7. 10.1016/j.radcr.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon DR, Palese MA. Clinical update on the management of adrenal hemorrhage. Curr Urol Rep 2009;10:78–83. 10.1007/s11934-009-0014-y [DOI] [PubMed] [Google Scholar]
- 5.Anagnostopoulos A, Sharma S. Spontaneous adrenal haemorrhage in pregnancy. BMJ Case Reports 2011:1–3. 10.1136/bcr.07.2011.4496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A, Minhas R, Quant HS. Spontaneous adrenal hemorrhage in pregnancy: a case series. Case Rep Obstet Gynecol 2017;2017:1–3. 10.1155/2017/3167273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Zhu Y-C, Liu R-B. Spontaneous adrenal hematoma in pregnancy: a case report. Medicine 2018;97:1–3. 10.1097/MD.0000000000013329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keizer AL, Peters LW, de Vries C, et al. Spontaneous adrenal haemorrhage in early pregnancy. BMJ Case Rep 2013;2013:bcr2012008062–4. 10.1136/bcr-2012-008062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadhem S, Ebrahem R, Munguti C, et al. Spontaneous unilateral adrenal hemorrhage in pregnancy. Cureus 2017;9:1–4. 10.7759/cureus.977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American College of Obstetrician and Gynaecologist . Guidelines for diagnostic imaging during pregnancy and lactation, 2017. [Google Scholar]
- 11.Eskandar O, Eckford S, Watkinson T. Safety of diagnostic imaging in pregnancy. Part 1: X-ray, nuclear medicine investigations, computed tomography and contrast media. The Obstetrician and Gynaecologist 2010;12:71–8. 10.1576/toag.12.2.71.27571 [DOI] [Google Scholar]
- 12.Kawashima A, Sandler CM, Ernst RD, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics 1999;19:949–63. 10.1148/radiographics.19.4.g99jl13949 [DOI] [PubMed] [Google Scholar]
- 13.Diri H, Baryam F, Simsek Y. A pregnant woman who underwent laparoscopic adrenalectomy due to Cushing’s Syndrome. Case Rep Endocrinol 2014;283458. 10.1155/2014/283458 [DOI] [PMC free article] [PubMed] [Google Scholar]