Abstract
Introduction
Heart failure (HF) is a growing global public health burden. However, due to the very limited regenerative capacity of mature cardiomyocytes in the adult mammalian heart, conventional treatments can only improve the symptoms of HF but fail to restore cardiac function. Heart transplantation is limited by a severe shortage of donors. Cell-based transplantation for the treatment of HF has become a promising strategy. Human-induced-pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) have been tested in animal models to assess safety and efficacy. This study aims at evaluating the safety and efficacy of epicardial injection of hiPSC-CMs in patients with advanced HF during coronary artery bypass grafting (CABG) surgery.
Methods
This study is a dose-escalation, placebo-controlled, single-centre phase I/IIa clinical trial. Dose escalation will be guided by a modified 3+3 design for three doses (1×108, 2×108 and 4×108 cells, sequentially). Patients with advanced heart failure will be enrolled and randomly allocated to receive epicardial injection of hiPSC-CMs during CABG surgery or CABG surgery alone, followed by a 12-month follow-up investigation. The primary endpoint is to assess the safety of hiPSC-CMs transplantation, including haemodynamic compromised sustained ventricular arrhythmias and newly formed tumours during 6 months postoperatively. The secondary endpoint is to evaluate the efficacy of epicardial injection of hiPSC-CMs and CABG surgery combination by comparison with CABG surgery alone.
Ethics and dissemination
The study protocol has been approved by the Institutional Ethical Committee of Nanjing Drum Tower Hospital (No. SC202000102) and approved by National Health Commission of the PRC (MR-32-21-014649). Findings will be disseminated to the academic community through peer-reviewed publications and presentation at national and international meetings.
Trial registration number
Keywords: Heart failure, Coronary heart disease, SURGERY
Strengths and limitations of this study.
This study is the first dose-finding and placebo-controlled trial of induced pluripotent stem cell-based cardiac regenerative therapy in patients with advanced heart failure.
This dose-finding study will assess both the safety and efficacy of epicardial injection of human-induced-pluripotent stem cell-derived cardiomyocytes during coronary artery bypass grafting surgery for treating advanced heart failure.
This study will be limited to a Chinese population with a target sample size.
Introduction
Heart failure (HF) is a growing global public health concern with an estimated prevalence of over 37 million individuals worldwide.1 HF is caused by several causes of cardiovascular diseases, resulting in poor quality of life, high morbidity and mortality.1 2 Ischaemic heart disease (IHD) is a major cause of heart failure,2 3 causing over 8.9 million or 16% deaths in the year of 2019 globally.4 Although the treatments for HF, including medications and interventional devices have continuously improved in the past few decades, currently there is no treatment to restore cardiac function by addressing the underlying mechanism of IHD, loss of massive contractile cardiomyocytes.5–7 Adult mammalian heart has limited capability to regenerate after cardiac injury.8–10 Hence, it is reasonable to hypothesise that transplantation of exogenous cardiomyocytes as a promising therapeutic to repair cardiac function by remuscularisation of the otherwise irreversibly impaired human myocardium.11–13
Human pluripotent stem cells, including embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs), can differentiate into cardiomyocytes with high purity in vitro,14–16 providing an ideal source to regenerate the impaired cardiac function. Ethical controversy has long been a key concern of clinical application of hESCs.17 In contrast, hiPSCs are derived from adult somatic cells (peripheral blood mononuclear cells, skin fibroblasts, etc) through reprogramming, which overcomes supply limits and avoids ethical issues.18 19 In 2015, the First-In-Human (FIH) study involving transplantation of hESCs-derived cardiac progenitor cells was completed by Dr Menasché et al in patients with severe ischaemic left ventricular dysfunction.20 A subsequent clinical report from the same team further suggested that transplantation of these cells was safe and potentially promoted some functional recovery in the transplanted myocardial areas.21 Because of the mentioned limitations of hESCs, hiPSCs-derived cardiomyocytes have been investigated in both small and large animals, including non-human primates,22–24 demonstrating promising results to remuscularise and restore cardiac function.
Previously, our group has performed an FIH clinical study of hiPSC-CMs transplantation during open-chest surgery,25 ‘Treating Heart Failure with hPSC-CMs (HEAL-CHF)’, and observed no serious adverse event, such as mortality or tumourigenicity, which related to the epicardial injection exogenous hiPSC-CMs during a 24-month follow-up. Here, we design a dose-escalation, placebo-controlled, phase I/IIa clinical trial to evaluate the safety and efficacy of epicardial injection of allogeneic hiPSC-CMs in patients with advanced HF during CABG surgery by comparison with CABG surgery alone.
Methods and analysis
Study design
This study is a dose-escalation, placebo-controlled, single-centre phase I/IIa clinical trial. An overview of the modified 3+3 dose-escalation trial is presented in figure 1. This study protocol follows the Standard Protocol Items: Recommendations for Interventional Trials guidelines, developed to provide a standardised guidance for recommended items to be included in a clinical trial protocol.26 The primary endpoint is to assess the safety of the epicardial injection of allogeneic hiPSC-CMs in the treatment of patients with advanced IHF during CABG surgery. The secondary endpoint is to evaluate the efficacy of epicardial injection of hiPSC-CMs and CABG surgery combination by comparison with the CABG surgery alone.
Figure 1.
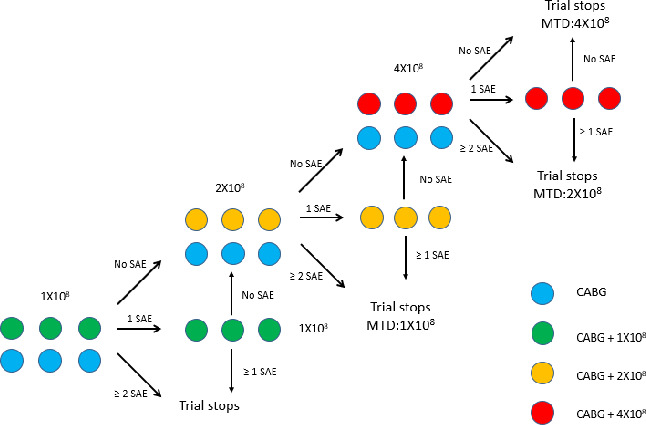
The modified 3+3 dose-escalation study design. CABG, coronary artery bypass graft; MTD, maximum tolerated dose; SAE, serious adverse event, grade 4 or above cell implant-related adverse event.
Study population
Patients with advanced chronic HF secondary to IHD fulfilling all inclusion/exclusion criteria will be enrolled at Nanjing Drum Tower Hospital, the affiliated hospital of Nanjing University Medical School, China. The study will be conducted in compliance with the requirements of governmental regulatory bodies and ethics committees.
Inclusion criteria
Patients aged 35–75 years (including 35 and 75).
Have signed the Informed Consent Form (ICF).
Patients have chronic left ventricular dysfunction.
Patients have New York Heart Association (NYHA) Functional Classification III-IV despite receiving guideline-directed medical therapy.
Patients have indications for coronary artery bypass grafting.
20%≤ left ventricular ejection fraction (LVEF) ≤ 45% as determined by echocardiographic assessment (data collected up to 6 months prior to inclusion evaluation are valid; data collected within 1 month since a myocardial infarction are invalid).
Weakening or the absence of segmental regional wall motion as determined by standard imaging.
Exclusion criteria
Panel reactive antibody (PRA)≥20% or donor specific antibody (DSA) positive.
Patients with valvular heart disease or received heart valvular disease.
Patients with acute myocardial infarction or percutaneous transluminal coronary intervention treatment within 1 month.
Patients requiring atrial fibrillation radiofrequency ablation.
Patients having previously suffered from sustained ventricular tachycardia.
Baseline glomerular filtration rate <30 mL/min/1.73 m2.
Liver dysfunction, as evidenced by enzymes (AST and ALT) greater than three times the upper limit of normal.
Haematological abnormality: a haematocrit<25% as determined by Hematocrit, white blood cell <2500/µL or platelet values<100 000/µL.
Serious radiographic contrast allergy, penicillin allergy, streptomycin allergy.
Coagulopathy (INR>1.3) not due to a reversible cause.
Contraindication to performance of MRI scan and positron emission tomography/emission-computed tomography (PET/ECT) scan.
Recipients of organ transplant.
Clinical history of malignancy within 5 years (patients with prior malignancy must be disease-free for 5 years).
Non-cardiac condition that limits lifespan<1 year.
On chronic therapy with immunosuppressant medication, such as glucocorticoid and TNFα antagonist.
Contra-indication to take immunosuppressant medication.
Serum positive for HIV, hepatitis B virus (HBV), hepatitis C virus (HCV) or treponema pallidum (TP).
Currently enrolled in another investigational therapeutic or device study.
Patients who are pregnant or breast feeding.
Other conditions that researchers consider not suitable to participate in this study.
Randomisation and groups
Six patients will be enrolled and randomly allocated to CABG+1×108 cells group or CABG group. Randomisation will be similarly applied for the 2×108 cells and 4×108 cells patient groups (figure 1).
Intervention
Screening and baseline phase
See table 1 for the schedule and assessments to be performed during this phase I/IIa clinical trial. Subjects fulfilling all inclusion/exclusion criteria and who have signed the ICF will be enrolled. Baseline information and data required should be collected from all enrolled subjects within 4 weeks before the operation. Key information and data to be collected include subject demographics, vital signs, laboratory tests, cardiac function evaluation and immunological evaluation (HLA typing, determination of PRA and DSA).
Table 1.
Schedule of events and assessments
| Visit time assessments |
Baseline screening | Inpatient visit | Outpatient monitoring visits | ||||||
| Day 0 | Days 1–7 | Day 14 | Day 21 | Month 1±7d | Month 3±7d | Month 6±7d | Month 12±7d | ||
| Informed consent form | X | ||||||||
| Medical history | X | X | X | X | X | ||||
| Physical examination | X | X | X | X | X | X | X | X | X |
| 12-lead ECG | X | X | X | X | X | X | X | X | |
| Concomitant medications | X | X | X | X | X | X | X | X | X |
| CAG (SYNTAX score) | X | ||||||||
| iPSC-CM administration | X | ||||||||
| Echocardiography | X | X | X | X | X | ||||
| Cardiac MRI | X | X | X | X | X | ||||
| PET/CT | X | X | X | ||||||
| CT (brain/chest/pelvic) | X | X | X | X | X | ||||
| 6-min walk test (m) | X | X | X | X | X | ||||
| NYHA classification | X | X | X | X | X | ||||
| MLHFQ | X | X | X | X | X | ||||
| 24 hours Holter | X | X | X | X | X | X | X | X | |
| Cardiac enzymes and troponins | X | X | X | X | X | X | X | X | |
| NTproBNP | X | X | X | X | X | X | X | X | |
| Blood routine and PCT | X | X | X | X | X | X | X | X | |
| Blood type | X | ||||||||
| Biochemistry | X | X | X | X | X | X | X | X | |
| Routine urine and stool test | X | X | X | X | X | ||||
| Thyroid function test | X | X | X | X | X | ||||
| Tumour marker | X | X | X | X | X | ||||
| Immunoassay (C3, C4, IgA, IgG, IgM) | X | X | X | X | X | X | |||
| Infectious test | X | X | X | X | X | ||||
| Coagulation function | X | X | X | X | X | X | X | ||
| HLA typing | X | ||||||||
| Plasma renin activity | X | X | X | X | X | X | |||
| Donor-specific antibody | X | X | X | X | X | X | |||
| Cytokines (IFNγ, TNFα, IL-2, IL-4, IL-6, IL-10) | X | Days 1/3/7 | X | ||||||
| Adverse events | X | X | X | X | X | X | X | X | |
CAG, coronary artery bypass grafting; HLA, human lymphocyte antigen; iPSC-CM, induced Pluripotent Stem Cell-derived CardioMyocyte; MLHFQ, The Minnesota Living with Heart Failure Questionnaire; NTproBNP, N-terminal (NT)-pro hormone BNP; NYHA, New York Heart Association; PCT, procalcitonin; PET/CT, positron emission tomography/CT.
Preparation of hiPSC-CMs
Donors were screened and tested for relevant communicable disease agents and diseases, including HIV-1 (antigen and nucleic acid), HIV-2, HBV (nucleic acid and surface and core antigen), HCV (antigen and nucleic acid) and TP (syphilis), according to ‘Guidance for Industry: Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps)’ by FDA. In order to prevent promotion of delayed carcinogenesis, donors were also screened and tested by exome sequencing for target genes presumably responsible for primary somatic cell mutation in cancer, according to COSMIC, an existing cancer genome mutation database. A health, 28 years old, Chinese female, who met the criteria of donor eligibility tests was selected. Her peripheral mononucleate cells were collected and reprogrammed to induced pluripotent stem cells under current good manufacturing practice (cGMP) condition.
The allogeneic hiPSC-CMs were manufactured at Help Therapeutics under cGMP condition and cryopreserved after quality control analysis.22 The hiPSC-CMs will be thawed in a 37°C water bath and resuspended in 5% human serum albumin solution before epicardial injection.
Dose and treatment method
Six patients will be enrolled and randomly allocated to CABG +1×108 group or CABG group (n=3 for each arm). For patients allocated to the CABG +1×108 group, hiPSC-CMs will be injected at 10 sites (0.25–0.30 mL of cell suspension at each site). Details regarding injection site location and volume of cell suspension injected at each site will be carefully recorded. Patients in CABG groups will receive standard CABG surgery alone. All patients will be transferred to the intensive care unit for 1 week after surgery. If no grade 4 or above cell implant-related adverse event occurs within 1 month postoperatively in the CABG +1×108 group, dose escalation will proceed to 2×108 cells. If one grade 4 or above cell implant-related adverse event occurs within 1 month postoperatively, three more patients will be enrolled and injected with 1×108 cells during CABG surgery. If no grade 4 or above cell implant-related adverse event occurs in the second three-patient cohort, dose escalation will then proceed to 2×108 cells. Otherwise, the trial will be stopped. The dose-escalation design is depicted in figure 1.
Concomitant medications
Immunosuppressive drugs
Subjects in the cell treatment groups will receive immunosuppressive treatment as described below:
2.5 g of immunoglobulin will be injected intravenously 1 day preoperatively, on the day of surgery and 3 days postoperatively, respectively
20 mg of simulect (basiliximab for injection) will be injected intravenously on the day of surgery and 4 days postoperatively, respectively.
1 g of mycophenolate mofetil (oral) will be given 1 day preoperatively and subsequently at the dose of 1.5 g for 28 days postoperatively.
Tacrolimus tablets will be given from 3 days preoperatively to 28 days postoperatively, dose will be adjusted according to a target blood concentration of 3–5 ng/mL.
Antiarrhythmic drugs
The following antiarrhythmic medications will be provided for subjects who developed accelerated idioventricular rhythm over 100 bpm:
450 mg amiodarone hydrochloride, intravenous injection.
200 mg amiodarone tablet, three times per day.
5 mg ivabradine tablet, two times per day.
Day 1–21 postoperatively
See table 1 for different timepoints of assessments to be performed during this period. Key assessments to be performed include vital sign evaluation, ECG-based heart rhythm monitoring and laboratory tests including biochemistry, cardiac injury markers (NT-Pro-BNP, cardiac troponin, cardiac enzymes, etc), cytokines (IFNγ, TNFα, IL-2, IL-6 and IL-10), PRA and DSA.
Months 1–12 visit
See table 1 for the schedule and assessments to be performed during this period. Outpatient visits should be completed as close to the scheduled visit dates as possible. The visit window is ±7 days from the intended date of the visit (1, 3, 6 and 12 months postoperatively). Key assessments to be performed include vital sign evaluation, ECG, echocardiogram-based and MRI-based cardiac function evaluation, NYHA classification, 6-min walk test, chest and abdominal PET scan, and laboratory tests including biochemistry, cardiac injury markers (NT-Pro-BNP, cardiac troponin, cardiac enzymes, etc), cytokines (IFNγ, TNFα, IL-2, IL-6 and IL-10), PRA, DSA and tumour markers. Subjects will also fill the Minnesota Living with Heart Failure Questionnaire (MLHFQ).
Endpoints
Primary endpoints
Dose limiting toxicity, the adverse event occurs within 30 days post-CABG surgery and is considered to be related to hiPSC-CMs transplantation, including the following:
Grade 4 cardiac arrhythmia based on Common Terminology Criteria for Adverse Events V.5.0.
Graft versus host disease disregard the continuous prophylaxis immunosuppressant treatment.
Death.
Incidence of newly formed tumour, chest and abdominal CT at 1, 3, 6 months and PET/CT at 6 months postoperatively.
Haemodynamic compromised sustained ventricular tachycardia, from 1 to 6 months postoperatively.
Secondary endpoints
-
Changes in left ventricle function evaluation by cardiac MRI-based evaluation of left ventricular function at baseline, 1, 3, 6 and 12 months postoperatively, including the following:
Infarct size.
LVEF, %.
Left ventricular fractional shortening (%).
Left ventricular end-diastolic volume (mL).
Left ventricular end-systolic volume (mL).
Left ventricular thickness at sites of injection.
Changes in left ventricle function evaluation by echocardiogram-based evaluation of left ventricular function at baseline, 1, 3, 6 and 12 months postoperatively.
PET/ECT-based evaluation of myocardial perfusion at baseline, 6 and 12 months postoperatively.
Functional status by 6min walk test at baseline, 1, 3, 6 and 12 months postoperatively.
Functional status by NYHA Classification at baseline, 1, 3, 6 and 12 months postoperatively.
Functional status by MLHFQ at baseline, 1, 3, 6 and 12 months postoperatively.
Incidence of major adverse cardiac events during months 1–12 visit postoperatively, including death, non-lethal myocardial infraction and hospitalisation for worsening HF.
Changes in PRA, DSA and NT-pro BNP at baseline, 1, 3, 6 and 12 months postoperatively.
Statistical considerations
This is a phase I/IIa dose-escalation clinical trial. The sample size is estimated based on a modified 3+3 design to achieve the primary endpoint. Sample size will be ranged from 6 to 27.
Descriptive statistical analysis will be used for the primary and secondary endpoints. The 95% CIs of the frequency of developing ventricular tachycardia sustained for >30 s and tumourigenesis due to allogeneic hiPSC-CMs will be determined with the use of Miettinen’s method.
Descriptive statistical analysis will be used for secondary endpoint. Depending on the variables, different statistical methods will be used to compare the outcomes. For measurement data, mean and SD, median, maximum, minimum and range will be calculated and presented. For enumeration data and rating data, frequency (composition ratio), rate and CI will be calculated and presented. Student’s t-test will be employed to determine the 95% CIs of enumeration data and rating data, while Miettinen’s method will be employed to determine the 95% CIs of measurement data. Where appropriate, differences between low dose and high dose groups will be calculated and significance tests will be performed. A bilateral p value less than or equal to 0.05 is considered significant.
Data collection, management and monitoring
The schedule of data collection is shown in table 1. An electronic data capture (EDC) system will be established for this study. A database manager (DM) will be appointed, who will be responsible for the design of the EDC system. Data will be collected from medical notes and hospital records in Nanjing Drum Tower Hospital. Before freezing the database, the DM will compose the data validation report based on the study plan, data validation standards and database contents. The Sponsor, Principal Investigator, Statistician and DM should engage in a meeting to validate the data and come to a resolution regarding database freezing. Once approved, the DM will be responsible for the freezing of the database and the Statistician will conduct statistical analysis afterwards.
Data monitoring and validation will be regularly conducted throughout the study. The frequency of monitoring will be once a year by the Medical Ethics Committee of Affiliated Nanjing Drum Tower Hospital, Nanjing University Medical College, starting from the beginning of this study.
Quality control
The clinical trial investigators will implement a quality assurance and quality control system based on the standard operating procedures prescribed by the investigators. Implementation of clinical trial, data creation, recording, monitoring and reporting will be conducted in compliance with ‘Administrative Measures for Clinical Studies of Stem Cell-based Therapeutics’. The study will be monitored by a third-party Data and Safety Monitoring Board.
Patient and public involvement
Neither patients nor the public were involved in the development of the research question, choice of outcome measures, design of the trial, recruitment of participants or conduct of the trial. Results of the trial will be disseminated to study participants through direct consultation with a trial clinician at completion of the trial, as well as through the publication of results.
Discussion
Loss of cardiomyocytes in the myocardium contributes to severe impairment of cardiac function and may lead to heart failure. The implantation of cardiomyocytes presents an alternative treatment to heart transplantation.11–13 After a roll-in experience as part of the ‘Treating Heart Failure With hPSC-CMs (HEAL-CHF)’ study, we now initiate a dose-escalation phase I/IIa trial to evaluate the safety and efficacy of epicardial injection of hiPSC-CMs during CABG surgery in patients with advanced heart failure. This study will be undertaken with sufficient safety considerations and based on the implementation plan and relevant laws. The allogenic approach can bring down the cost for iPSC-based cell therapy compared with the autologous approach and will also obviate the need for approval of individual patient-derived products by regulatory authorities.27 This clinical trial will shed the light on the hiPSC-CMs cell therapy for the unmet clinical needs for advanced heart failure patients.
Ethics and dissemination
The study protocol has been approved by the Medical Ethics Committee of Affiliated Nanjing Drum Tower Hospital, Nanjing University Medical College (No. SC202000102) in May 2020. This study has then been registered and approved by National Health Commission of People’s Republic of China (MR-32-21-014649). Participants and their guardians (where applicable) have the right to withdraw at any time and if they do withdraw, will be treated according to hospital standard procedures. Participants who choose to withdraw from the trial will be asked if we can continue to use any data already collected and whether they are willing to participate in the trial follow-up. We will present the trial findings at international meetings and in peer-reviewed publications. We will inform the public through patient organisations and a newsletter to participants.
Supplementary Material
Acknowledgments
The authors would like to thank Dr Ph Menasché for his help during preparation of this manuscript.
Footnotes
Contributors: D-JW and J-W designed the whole protocol, reviewed and approved the paper. HZ and YX wrote the paper and prepared the figure and table. TP, XZ, HC, CX, FF, HC, BZ, JP, QZ and GY reviewed the paper and provided valuable suggestions.
Funding: The dose-escalation study is fully sponsored by HELP Therapeutics.
Competing interests: JW is a full-time employee of HELP Therapeutics. All other authors declare no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol 2016;13:368–78. 10.1038/nrcardio.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng H, Abdel-Latif A. Cellular therapy for ischemic heart disease: an update. Adv Exp Med Biol 2019;1201:195–213. 10.1007/978-3-030-31206-0_10 [DOI] [PubMed] [Google Scholar]
- 3.Elgendy IY, Mahtta D, Pepine CJ. Medical therapy for heart failure caused by ischemic heart disease. Circ Res 2019;124:1520–35. 10.1161/CIRCRESAHA.118.313568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rana JS, Khan SS, Lloyd-Jones DM, et al. Changes in mortality in top 10 causes of death from 2011 to 2018. J Gen Intern Med 2021;36:2517–8. 10.1007/s11606-020-06070-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossignol P, Hernandez AF, Solomon SD, et al. Heart failure drug treatment. Lancet 2019;393:1034–44. 10.1016/S0140-6736(18)31808-7 [DOI] [PubMed] [Google Scholar]
- 6.Normand C, Kaye DM, Povsic TJ, et al. Beyond pharmacological treatment: an insight into therapies that target specific aspects of heart failure pathophysiology. Lancet 2019;393:1045–55. 10.1016/S0140-6736(18)32216-5 [DOI] [PubMed] [Google Scholar]
- 7.Willerson JT. The medical and device-related treatment of heart failure. Circ Res 2019;124:1519. 10.1161/CIRCRESAHA.119.315268 [DOI] [PubMed] [Google Scholar]
- 8.González A, Schelbert EB, Díez J, et al. Myocardial Interstitial Fibrosis in Heart Failure: Biological and Translational Perspectives. J Am Coll Cardiol 2018;71:1696–706. 10.1016/j.jacc.2018.02.021 [DOI] [PubMed] [Google Scholar]
- 9.Uygur A, Lee RT. Mechanisms of cardiac regeneration. Dev Cell 2016;36:362–74. 10.1016/j.devcel.2016.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponnusamy M, Liu F, Zhang Y-H, et al. Long noncoding RNA CPR (cardiomyocyte proliferation regulator) regulates cardiomyocyte proliferation and cardiac repair. Circulation 2019;139:2668–84. 10.1161/CIRCULATIONAHA.118.035832 [DOI] [PubMed] [Google Scholar]
- 11.Bertero A, Murry CE. Hallmarks of cardiac regeneration. Nat Rev Cardiol 2018;15:579–80. 10.1038/s41569-018-0079-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura K, Murry CE. Function Follows Form - A Review of Cardiac Cell Therapy. Circ J 2019;83:2399–412. 10.1253/circj.CJ-19-0567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murry CE, MacLellan WR. Stem cells and the heart-the road ahead. Science 2020;367:854–5. 10.1126/science.aaz3650 [DOI] [PubMed] [Google Scholar]
- 14.Burridge PW, Matsa E, Shukla P, et al. Chemically defined generation of human cardiomyocytes. Nat Methods 2014;11:855–60. 10.1038/nmeth.2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lian X, Bao X, Zilberter M, et al. Chemically defined, albumin-free human cardiomyocyte generation. Nat Methods 2015;12:595–6. 10.1038/nmeth.3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y-W, Chen B, Yang X, et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol 2018;36:597–605. 10.1038/nbt.4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilic D, Ogilvie C. Concise review: human embryonic stem Cells-What have we done? what are we doing? where are we going? Stem Cells 2017;35:17–25. 10.1002/stem.2450 [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–72. 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318:1917–20. 10.1126/science.1151526 [DOI] [PubMed] [Google Scholar]
- 20.Menasché P, Vanneaux V, Hagège A, et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J 2015;36:2011–7. 10.1093/eurheartj/ehv189 [DOI] [PubMed] [Google Scholar]
- 21.Menasché P, Vanneaux V, Hagège A, et al. Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction. J Am Coll Cardiol 2018;71:429–38. 10.1016/j.jacc.2017.11.047 [DOI] [PubMed] [Google Scholar]
- 22.Guan X, Xu W, Zhang H, et al. Transplantation of human induced pluripotent stem cell-derived cardiomyocytes improves myocardial function and reverses ventricular remodeling in infarcted rat hearts. Stem Cell Res Ther 2020;11:73. 10.1186/s13287-020-01602-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawamura M, Miyagawa S, Miki K, et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 2012;126:S29–37. 10.1161/CIRCULATIONAHA.111.084343 [DOI] [PubMed] [Google Scholar]
- 24.Shiba Y, Gomibuchi T, Seto T, et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016;538:388–91. 10.1038/nature19815 [DOI] [PubMed] [Google Scholar]
- 25.Mallapaty S. Revealed: two men in China were first to receive pioneering stem-cell treatment for heart disease. Nature 2020;581:249–50. 10.1038/d41586-020-01285-w [DOI] [PubMed] [Google Scholar]
- 26.Chan A-W, Tetzlaff JM, Altman DG, et al. Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eschenhagen T, Weinberger F. Heart repair with myocytes. Circ Res 2019;124:843–5. 10.1161/CIRCRESAHA.118.314336 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


