Abstract
Objective
End-stage chronic liver disease is associated with accelerated ageing and increased frailty. Frailty measures have provided clinical utility in identifying patients at increased risk of poor health outcomes, including those awaiting liver transplantation. However, there is limited data on the prevalence and severity of frailty in patients with non-cirrhotic non-alcoholic fatty liver disease (NAFLD). The aim of this study was to evaluate the prevalence of frailty and prefrailty in patients with non-cirrhotic NAFLD and correlate with severity of liver disease.
Design
A cross-sectional analysis of functional and laboratory frailty assessments, including the Fried frailty index (FFI), a self-reported frailty index (SRFI) and a lab-based frailty index (FI-LAB), was performed in a cohort of 109 patients with NAFLD, and results compared with fibrosis staging based on transient elastography.
Results
Patients with NAFLD had a high prevalence of prefrailty and frailty, with a median SRFI score of 0.18 (IQR: 0.18), FFI of 1 (IQR: 1) and FI-LAB of 0.18 (IQR: 0.12). Using the SRFI, 45% of F0/F1 patients were classified as prefrail and 20% were classified as frail, while in F2/F3 patients this increased to 36% and 41%, respectively. SRFI, 30 s sit-to-stand and FI-LAB scores increased with increasing liver fibrosis stages (p=0.001, 0.006 and <0.001, respectively). On multivariate linear regression, female gender was identified as a significant predictor of elevated frailty scores.
Conclusion
This study identifies a high prevalence of frailty in individuals with non-cirrhotic NAFLD. Addressing frailty through early rehabilitation interventions may reduce overall morbidity and mortality in this population.
Keywords: obesity, fatty liver, fibrosis, nonalcoholic steatohepatitis
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT?
Patients with cirrhosis due to non-alcoholic fatty liver disease (NAFLD) are known to be more frail than people without cirrhosis. It is unknown if patients with non-cirrhotic NAFLD are also frail.
WHAT ARE THE NEW FINDINGS?
Using a range of frailty assessment tools, our results demonstrate that in early-stage NAFLD, one-third of patients are frail, one-third are prefrail, and that frailty is more frequently found in female patients.
HOW MIGHT IT IMPACT ON CLINICAL PRACTICE IN THE FORESEEABLE FUTURE?
Frailty is important to recognise as it increases the risk of falls, disability and death. Incorporation of frailty assessment tools in clinical practice can allow earlier diagnosis and rehabilitation strategies to address physical frailty deficits, and consequently improve patient care.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a global health problem and is the leading cause of chronic liver disease (CLD) in North America and Europe, with an estimated global prevalence of 25%.1 This global impact is linked to the high prevalence of major risk factors for NAFLD, including obesity, type 2 diabetes mellitus (T2DM) and the metabolic syndrome.1–4 NAFLD is a spectrum of disease ranging from simple steatosis to non-alcoholic steatohepatitis (NASH) and cirrhosis, with approximately 20%–25% of patients with NAFLD at risk for developing NASH.1 3 Despite obesity, patients with advanced liver disease due to NAFLD often have concomitant reduced muscle mass, referred to as sarcopenic obesity and associated frailty,5–7 which contribute to poorer outcomes and mortality.8
Frailty is a concept widely used in medicine for the elderly and defined as a clinical state of decreased physiologic reserve and increased vulnerability to health stressors.9 Developed to identify older people at increased risk of poor health outcomes (falls, disability, dependency, hospitalisation and mortality), frailty is associated with the loss of functional, cognitive and physical capacity, leading to a vulnerable state.10 Physical frailty assessment includes determination of muscle mass, muscle strength, functional capacity and aerobic exercise capacity, and as such partially overlaps with sarcopenia.11 Sarcopenia, a term initially used to describe generalised loss of skeletal muscle mass in an ageing population, is now recognised as a syndrome requiring two of three diagnostic criteria: low muscle mass, and one of two additional criteria—low muscle strength or low physical performance.12
While frailty is a multidimensional construct, physical frailty is the component that has been most frequently described in CLD, especially in the setting of liver transplantation.13–18 The prevalence of frailty in patients with advanced liver disease ranges from 17% to 68%, with frailty being associated with the presence of ascites, hepatic encephalopathy and Child–Pugh class C.13–19 Consequently, frailty has emerged as a powerful predictor of clinical outcomes in patients with cirrhosis, and, in particular, patients requiring liver transplantation, with recently proposed guidelines recommending the incorporation of frailty assessments into routine clinical care.20 The aetiology of liver disease may also be important, with one study reporting the impact of frailty on mortality is more pronounced in cirrhotic patients with NAFLD compared with patients with alcoholic liver disease.21
Frail patients with CLD frequently exhibit sarcopenia, and sarcopenic cirrhotic patients are often frail, resulting in poorer outcomes, physical disability and poorer quality of life. The prevalence of sarcopenia in cirrhosis ranges from 30% to 70%, depending on the diagnostic tools used and the severity of the underlying liver disease.22 Recent data suggest that sarcopenia is frequently overlooked in patients with NAFLD, and that sarcopenia may be a risk factor for progression of disease in patients with NAFLD.23–26
Data on the prevalence of frailty in non-cirrhotic patients with NAFLD populations are lacking. In a study by Wang and colleagues,27 adults with non-cirrhotic CLD were less likely to be classed as ‘robust’ and more likely to be classed as ‘prefrail’ or ‘frail’ compared with adults without CLD, however, the authors did not analyse differences between liver disease aetiologies. A challenge in assessing frailty among patients with NAFLD is determining the optimal frailty assessment tools. A number of instruments to operationalise frailty have been established, including the Fried frailty index (FFI),28 the self-reported frailty index (SRFI)29 and the lab-based frailty index (FI-LAB).30 These instruments have been reported to be independent predictors of clinical outcomes such as mortality, hospitalisation and self-reported health scores in elderly populations. However, as the majority of these instruments were derived using cohorts of community-dwelling older adults without known liver disease, their application in patients with NAFLD is unknown.
This study aimed to address the lack of data on the prevalence of frailty across the full spectrum of NAFLD disease severity. Furthermore, we assessed frailty in patients with NAFLD, stratified on the basis of fibrosis, using a range of frailty assessment tools to assess their suitability in determining the presence of prefrailty and frailty in non-cirrhotic NAFLD disease.
Methods and analysis
Overview and study design
This study was a cross-sectional cohort study conducted in the Hepatology Department at St James’s Hospital, Dublin, Ireland to determine (1) the prevalence of frailty in non-cirrhotic and cirrhotic patients with NAFLD and (2) if frailty severity is correlated with severity of liver fibrosis in patients with NAFLD.
Patient cohort
Participants were recruited by convenience sampling from a tertiary referral University hospital outpatient hepatology clinic. A total of 109 individuals with NAFLD were consented to take part in the study. Before partaking in the assessment, patients had a medical screen to assess medical history. Inclusion criteria were: aged ≥18 years and vibration-controlled transient elastography (VCTE)-confirmed NAFLD. Exclusion criteria included: unwillingness to participate, alcohol consumption >30 g/day (men) or >20 g/day (women) and coexisting liver disease.
VCTE assessment
A VCTE device (FibroScan 502 touch, Echosens, France) was used to estimate liver stiffness measurement (LSM) as a validated marker for hepatic fibrosis, and controlled attenuation parameter (CAP) as a marker for hepatic steatosis. Participants were categorised into three groups based on LSM established cut-off values for NAFLD31: no/minimal fibrosis (F0/F1, <8.2 kPa); moderate/advanced fibrosis (F2/F3, 8.2–13.5 kPa) and cirrhosis (F4, ≥13.6 kPa), in order to assess between-group differences in outcomes.
Biochemistry and immunology blood tests
Routine clinical blood samples were collected to measure full blood counts, renal profiles, liver function tests, plasma glucose, glycated haemoglobin (HbA1c), insulin and lipid profiles.
Frailty assessments
Frailty was assessed using three established frailty assessment instruments developed in geriatric populations to operationalise frailty based on physical, functional or cognitive components. The frailty indices used in the current study included: the SRFI based on a cumulative deficits approach,29 the FFI28 and the FI-LAB.30 The SRFI is an investigator administered questionnaire, which assessed the presence of up to 44 health deficits (detailed in the online supplemental methods). A minimum completion of 70% was required for a valid SRFI. An SRFI of <0.10 indicates robustness; 0.10–0.249 indicates prefrailty and ≥0.25 indicates frailty.
bmjgast-2021-000861supp001.pdf (405.3KB, pdf)
The FFI assesses phenotypic frailty through a series of functional tests and self-report questions with categorical cut-offs. Five health deficits were assessed: (1) weakness; (2) slow gait; (3) self-reported exhaustion; (4) unintentional weight loss and (5) low physical activity.28 Weakness was assessed by a maximal grip strength test on participant’s dominant hand using a hand-grip dynamometer (Jamar Plus, JLW instruments, Chicago, Illinois) and scores were adjusted for gender and body mass index (BMI). Participant’s performed three maximum voluntary contractions and the highest score was recorded. Slow gait was measured through a walking test to assess average walking speed over 15 feet. Self-reported exhaustion was measured using two questions relating to experiences with fatigue in the previous week. Questions had four possible responses and were scored on a four-point Likert scale ranging from ‘not at all (0)’ to ‘a lot (3)’. A score of 2 or more in either question was considered positive for fatigue. Unintentional weight loss was assessed by asking participants if they had experienced unintentional weight loss of more than 10 pounds/4.5 kg in the past year. Low physical activity was defined as performing less than 150 min of moderate-to-vigorous physical activity per week in accordance with the WHO physical activity guidelines.32 An overall FFI score of 0 indicates robustness; 1–2 indicates prefrailty and ≥3 indicates frailty.
The FI-LAB encompasses multiple standardised blood-based laboratory tests and is based on a cumulative deficits approach using physiological measures.30 Each blood test was compared with local standardised reference ranges. Tests that were outside of the normal range were considered as a health deficit for the FI-LAB. The FI-LAB used in this study assessed 35 standardised blood tests (detailed in the onine supplemental methods). An index was then created by dividing the total amount of blood tests outside normal ranges by the total amount of blood tests investigated to give a score ranging from 0 to 1.
Functional assessments
In addition to the frailty assessments, two additional functional assessments were also conducted: the timed up and go (TUG) test33 and the 30 s sit-to-stand (30STST) test.34 The TUG test is used to assess functional mobility and balance and has been reported to be a sensitive and specific measure of frailty.35 Patients sat on a standardised chair with armrests in place and were prompted to rise out of the chair, walk around a cone 3 m away and sit back on the chair as quickly as possible. For the 30STST test, participants sat on a standardised chair and were asked to stand up from the chair and return to a seating position as many times as possible in thirty seconds.
Statistical analysis
Statistical analyses were performed using GraphPad Prism, V.9.1.1. Between-group differences were assessed using a one-way analysis of variance or the Kruskal-Wallis test for normal and non-normal continuous data, respectively. Tukey or Mann-Whitney post hoc tests were performed, where appropriate, for normal and non-normal continuous data, respectively. For between-group, independent categorical comparisons, Pearson’s χ2 test was used. Multivariate linear regression was performed to assess potential outcomes that were associated with the frailty assessment instruments. Statistical significance was set at p≤0.05 for all tests.
Results
Prefrailty and frailty are prevalent in patients with NAFLD
One hundred and nine patients with NAFLD completed the study assessments. Baseline participant characteristics for the cohort are detailed in table 1. The mean age of the cohort was 56±12 years, the median BMI was 32.3±9.4 kg/m2 and 50% were women. There was a high prevalence of hypertension (46%), T2DM (53%) and hypercholesteremia (61%) in the cohort.
Table 1.
Participant demographics grouped by LSM
| Variable | All (n=109) | F0–F1 (n=41) | F2–F3 (n=44) | F4 (n=24) | Between-group p value |
| Age, years † | 56 (12) | 53 (14) | 58 (11) | 57 (11) | 0.150 ‡ |
| Gender, n (%) | 0.187 § | ||||
| Female | 55 (50) | 18 (44) | 21 (48) | 16 (67) | |
| Male | 54 (50) | 23 (56) | 23 (52) | 8 (33) | |
| Smoking status, n (%) | 0.440 § | ||||
| Non-smoker | 44 (41) | 20 (49) | 16 (37) | 8 (35) | |
| Former-smoker | 51 (48) | 16 (39) | 21 (49) | 14 (61) | |
| Smoker | 12 (11) | 5 (12) | 6 (14) | 1 (4) | |
| T2DM, n (%n) | 58 (53) | 14 (34) | 26 (59) | 18 (75) | 0.004 **§ |
| Hypercholesteremia, n (%) | 66 (61) | 23 (56) | 28 (64) | 15 (63) | 0.758 § |
| Hypertension, n (%) | 50 (46) | 10 (24) | 25 (57) | 15 (63) | 0.002 **§ |
| BMI (kg/m2) ¶ | 32.3 (9.4) | 30.9 (4.2) | 35.0 (8.2) | 34.1 (14.3) | 0.002 **†† |
| AST (IU/L) ¶ | 28 (18) | 23 (12) | 28 (19) | 32 (14) | 0.004 **†† |
| ALT (IU/L) ¶ | 39 (30) | 36 (29) | 44 (33) | 41 (23) | 0.074 †† |
| ALP (IU/L) ¶ | 81 (43) | 75 (39) | 80 (43) | 93 (46) | 0.169 †† |
| GGT (IU/L) ¶ | 51 (63) | 41 (54) | 51 (64) | 74 (161) | 0.017 *†† |
| CRP (mg/L) ¶ | 2.4 (3.3) | 1.6 (2.3) | 2.6 (3.0) | 2.9 (7.1) | 0.047 *†† |
| NLR ¶ | 1.9 (1.1) | 1.9 (1.4) | 1.7 (1.0) | 2.3 (1.1) | 0.013 *†† |
| HbA1c (mmol/mol) ¶ | 43 (17) | 38 (10) | 44 (17) | 50 (20) | 0.001 **†† |
| Plasma glucose (mmol/L) ¶ | 6.0 (2.9) | 5.6 (1.4) | 6.5 (4.6) | 6.2 (3.5) | 0.050 †† |
| Hepatic CAP (dB/m) ¶ | 319 (64) | 302 (62) | 329 (61) | 340 (80) | 0.007 **†† |
| Hepatic stiffness (kPa) ¶ | 9.4 (6.3) | 5.9 (2.1) | 10.0 (2.3) | 15.9 (7.7) | ≤0.001 ***†† |
| FAST score ¶ | 0.36 (0.40) | 0.16 (0.28) | 0.44 (0.30) | 0.62 (0.21) | ≤0.001 ***†† |
Significant between-group difference (p≤0.05), **Significant between-group difference (p≤0.01), ***Significant between-group difference (p≤0.001).
†Normally distributed variable (mean (SD)).
‡One-way analysis of variance.
§Pearson’s χ2 test.
¶Non-normally distributed variable (median (IQR)).
††Kruskal Wallis test.
ALP, alkaline phosphatase; ALT, alanine transferase; AST, aspartate transferase; BMI, body mass index; CAP, controlled attenuation parameter; CRP, C reactive protein; GGT, gamma-glutamyl transferase; HbA1c, glycated haemoglobin; LSM, liver stiffness measurement; NLR, neutrophil to lymphocyte ratio.
Within the cohort, the median SRFI score was 0.18 (IQR=0.18; table 2) and the median FFI score was 1 (IQR=1; table 2). The FFI classified 59% of the study cohort as prefrail and 5% as frail. The frequency of prefrailty and frailty was higher when using the SRFI, with 38% of the cohort classified as frail and a further 39% classified as prefrail (table 2). Only 36% of patients were classified as robust using the FFI and only 23% of patients were classified as robust using the SRFI. The median FI-LAB score in the full cohort was 0.18 (IQR=0.12; table 2), the median TUG score was 7.0 (IQR=1.8; table 2), and the median 30STST score was 14 (IQR=7; table 2). Defined prefrail and frail cut-off values for the FI-LAB, TUG and 30STST scores were not available.
Table 2.
Frailty outcomes grouped by LSM
| Variable | All (n=109) | F0–F1 (n=41) | F2–F3 (n=44) | F4 (n=24) | Between-group p value |
| SRFI score † | 0.18 (0.18) | 0.15 (0.15) | 0.20 (0.19) | 0.27 (0.20) | 0.001 **‡ |
| SRFI categories | 0.007 **§ | ||||
| Robust, n (%) | 25 (23) | 14 (35) | 10 (23) | 1 (4) | |
| Pre-frail, n (%) | 42 (39) | 18 (45) | 16 (36) | 8 (33) | |
| Frail, n (%) | 41 (38) | 8 (20) | 18 (41) | 15 (63) | |
| FFI score † | 1 (1) | 1 (1) | 1 (2) | 1 (2) | 0.285 ‡ |
| FFI categories | 0.810 § | ||||
| Robust, n (%) | 29 (36) | 14 (41) | 11 (36) | 4 (25) | |
| Pre-frail, n (%) | 48 (59) | 18 (53) | 19 (61) | 11 (69) | |
| Frail, n (%) | 4 (5) | 2 (6) | 1 (3) | 1 (6) | |
| FI-LAB score † | 0.18 (0.12) | 0.14 (0.11) | 0.21 (0.09) | 0.24 (0.15) | ≤0.001 ***‡ |
| Timed-up and go (s) † | 7.0 (1.8) | 6.7 (1.8) | 6.8 (1.7) | 7.5 (1.9) | 0.110 ‡ |
| 30 s sit-to-stand † | 14 (7) | 16 (7) | 14 (6) | 11 (6) | 0.006 **‡ |
Post hoc for SRFI: F0/F1 significantly different from F4 (adjusted p=0.001). Post hoc for LBFI: F0/F1 significantly different from F2/F3 (adjusted p=0.001) and F4 (adjusted p≤0.001). Post hoc for 30 s sit-to-stand: F0/F1 significantly different from F4 (adjusted p=0.004).
**Significant between-group difference (p≤0.05), **Significant between-group difference (p≤0.01), ***Significant between-group difference (p≤0.001).
††Non-normally distributed variable (median (IQR))
‡‡Kruskal-Wallis test
§§Pearson’s χ2 test
FFI, Fried frailty index; FI-LAB, lab-based frailty index; SRFI, self-reported frailty index.
Frailty is increased in patients with NAFLD with advanced liver fibrosis and cirrhosis
To assess whether higher frailty scores were associated with stages of liver fibrosis and/or cirrhosis, the cohort was stratified on the basis of LSM using established cut-off values for NAFLD.31 These LSM groupings included 41 patients with no/minimal fibrosis (F0/F1,<8.2 kPa); 44 patients with moderate/advanced fibrosis (F2/F3, 8.2–13.5 kPa) and 24 patients with cirrhosis (F4, ≥13.6 kPa) (table 1). There was no significant difference in age or gender between these three groups but there were significant differences in the frequency of T2DM, hypertension, BMI, HbA1c and biochemical markers of liver damage (table 1), indicative of disease progression.
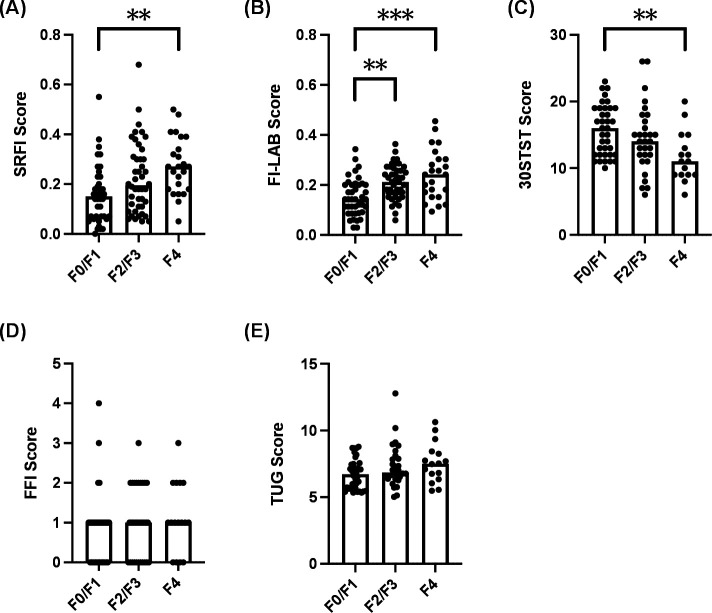
When comparing the frailty assessments between F0/F1, F2/F3 and F4 patients, a significant difference was observed in the SRFI scores (p=0.001; figure 1A and table 2), FI-LAB scores (p=≤0.001; figure 1B and table 2) and 30STST scores (p=0.006; figure 1C and table 2). No statistical differences were observed between the LSM groupings for either the FFI scores (p=0.285; figure 1D and table 2) or the TUG scores (p=0.110; figure 1E and table 2). Post hoc analysis identified a significant increase in SRFI scores between F0/F1 patients and F4 patients (median 0.15 vs 0.27; adjusted p=0.001). There was a significant decrease in 30STST scores between F0/F1 patients and F4 patients (median 16 vs 11; adjusted p=0.004). The post hoc analysis also identified a significant increase in FI-LAB scores between both F0/F1 patients and F4 patients (median 0.14 vs 0.24; adjusted p<0.001) as well as F0/F1 patients and F2/F3 patients (median 0.14 vs 0.21; adjusted p=0.001).
Figure 1.
The frequency of frailty increases in patients with non-alcoholic fatty liver disease (NAFLD) with higher stages of liver fibrosis. Participants were categorised into three groups based on liver stiffness measurement (LSM) cut-off values for NAFLD31: no/minimal fibrosis (F0/F1, <8.2 kPa); moderate/advanced fibrosis (F2/F3, 8.2–13.5 kPa) and cirrhosis (F4, ≥13.6 kPa). (A) Self-reported frailty index (SRFI) scores in patients classified as F0/F1, F2/F3 and F4. (B) Lab-based frailty index (FI-LAB) scores in patients classified as F0/F1, F2/F3 and F4. (C) 30 s sit-to-stand (30STST) scores in patients classified as F0/F1, F2/F3 and F4. (D) Fried frailty index (FFI) scores in patients classified as F0/F1, F2/F3 and F4. (E) Timed up and go (TUG) scores in patients classified as F0/F1, F2/F3 and F4. Between-group differences were assessed using a Kruskal-Wallis test and Mann-Whitney post-hoc analysis with Bonferroni correction. **p<0.01; ***p<0.001.
Female gender is associated with increased frailty scores in patients with NAFLD
In order to assess which clinical parameters were associated with elevated frailty scores within our cohort, we performed multivariate linear regression for the three instruments that demonstrated significant differences between F0/F1 and F4 groups (SRFI scores, FI-LAB scores and 30STST scores). Variables were selected on the basis of clinical relevance to disease progression and included CAP scores, LSM values, gender, smoking history, diabetes, hypercholesteremia, hypertension, age and BMI. CAP score, gender and hypercholesteremia were each identified as statistically significant independent predictors of SRFI score (online supplemental table 1). Gender was the only statistically significant independent predictors of FI-LAB score (online supplemental table 2), while gender and age were statistically significant independent predictors of 30STST score (online supplemental table 3).
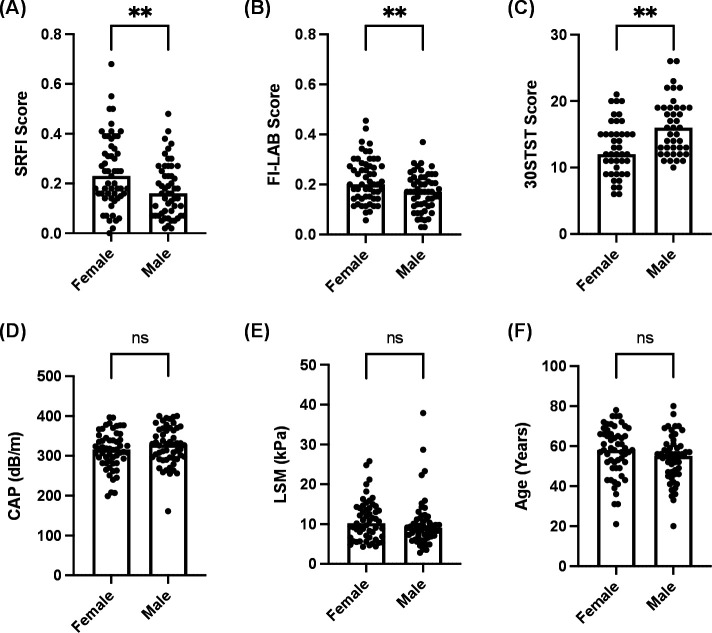
To further characterise this association with gender for the SRFI, the FI-LAB and the 30STST, we divided the full cohort on the basis of gender. Significant differences in SRFI scores (p<0.01; figure 2A), FI-LAB scores (p<0.01; figure 2B) and 30STST scores (p<0.01; figure 2C) were present when comparing male and female patients. No differences in CAP scores, LSM values or chronological age were observed between male and female patients (figure 2D–F). The SRFI scores and FI-LAB scores were both higher in women (figure 2A, B), while the 30STST score was lower in women (figure 2C), indicating a significantly higher prevalence of frailty in female patients with NAFLD.
Figure 2.
Female patients with non-alcoholic fatty liver disease (NAFLD) have higher frailty scores, despite no difference in liver fibrosis staging or age, compared with males. All participants were categorised into on the basis of gender, irrespective of the degree of liver disease. (A) Self-reported frailty index (SRFI) scores in female and male patients. (B) Lab-based frailty index (FI-LAB) scores in female and male patients. (C) 30 s sit-to-stand (30STST) scores in female and male patients. (D) Controlled attenuation parameter (CAP) measurements (dB/m) in female and male patients. (E) Liver stiffness measurement (LSM; in kPa) in female and male patients. (F) Age (in years) in female and male patients. Between-group differences were assessed using either an unpaired t test (F) or a Mann-Whitney test (A–E). ns, not significant; **p<0.01.
Discussion
This study highlights the high prevalence of prefrailty and frailty among patients with NAFLD, across the spectrum of fibrosis stages. By examining multiple different tools that operationalise frailty, we demonstrate that the SRFI, FI-LAB and 30STST instruments demonstrate increased frailty in patients with NAFLD as liver stiffness increases; in contrast, no significant changes in frailty were detected using TUG or FFI instruments between patients with different stages of fibrosis and those with cirrhosis. Furthermore, our study highlights the association of frailty and female gender.
Our data demonstrate a high prevalence of prefrailty and frailty among patients with NAFLD at non-cirrhotic disease stages. When employing the SRFI assessment instrument, 20% of patients with F0/F1, 41% of patients with F2/F3 compared with 63% of patients with F4 disease were classified as frail. This equated to a median SRFI score of 0.15, 0.20 and 0.27 in the F0/F1, F2/F3 and F4 groups, respectively. By comparison, data using the SRFI in community-based cohorts detected a mean SRFI score of approximately 0.10 in an Irish population.29 In this well-characterised Irish longitudinal cohort study, the overall frequency of frailty detected by the SRFI was 11% among 4961 individuals aged over 50 years of age. Similar data have been reported in a study of community-dwelling elders in Japan.36 The prevalence of frailty using the FFI was 5.6% in healthy community dwelling participants compared with a prevalence of 17.9% in non-cirrhotic patients and 37.0% in patients with cirrhosis.37 These data suggest that patients with non-cirrhotic CLD are more susceptible to frailty compared with the general population.
In our study, gender was an independent predictor of SRFI score, FI-LAB score and 30STST score, indicating a strong association between female gender and increased frailty. Female patients with NAFLD had significantly higher frailty scores compared with men. This finding aligns with a review of 4611 NHANES subjects, which assessed the association between NAFLD and sarcopenia. In this study, the independent predictors for the presence of sarcopenia among individuals with NAFLD included older age, female gender, non-Hispanic white ethnicity and lower physical activity.8 This gender difference has been identified in a number of population studies of frailty in the absence of CLD. Frailty researchers postulate multifactorial reasons for gender disparities in frailty, encompassing biological, sociological and environmental factors.38–40 Despite an increased preponderance for frailty, women tend to live longer, a phenomenon that is known as the frailty-mortality paradox. Future studies of frailty in patient with NAFLD will need to account for this gender-specific effect.
While gender was consistently associated with SRFI, FI-LAB and 30STST scores, other clinical parameters also showed significant associations with the different assessment instruments, including CAP score, age and hypercholesteremia. Due to the relatively small size of our cohort, we could not define the relative contributions of different clinical parameters on the likelihood of frailty. It is possible that the presence of comorbidities such as type 2 diabetes, elevated CRP and hypertension, which were all more common in patients with greater degrees of fibrosis, may also have influenced the risk of frailty among patients with NAFLD, independent of the presence of liver disease.
The SRFI, the FI-LAB and the 30STST each demonstrated a significant association with liver fibrosis; however, this was not evident with the FFI instrument and TUG test in our cohort. One of the components of the FFI is unintentional weight loss and consequently the FFI may under-represent frailty in obese patients as high BMI and weight gain can mask lean weight loss or sarcopenia.41 Similarly, the cut-off parameters for assessing grip strength based on BMI may not be sensitive for patients with non-cirrhotic NAFLD. Each component of the frailty index assesses different aspects of frailty and it is likely that some components of the frailty assessment may be more predictive of specific clinical outcomes compared with others. Our results highlight that FFI scores do not significantly increase in patients with NAFLD during the early stages of disease progression.
To address the unmet need for a frailty assessment tool specifically in patients with cirrhosis, Lai et al developed the Liver Frailty Index (LFI).16 This comprises three tests—grip strength, chair stands and balance testing. Lai and colleagues demonstrated that LFI performed better than MELD-Na in predicting waiting list mortality.16 19 However, as enrolment of patients in our study began, the LFI had been validated for use only in cirrhotic patient cohorts and was, therefore, not included in our study. Subsequent research has confirmed that the LFI has external validity in non-cirrhotic populations and is highly reproducible.27 Future studies should assess the LFI in non-cirrhotic patients with NAFLD to determine which frailty assessment instruments are best suited for this patient population.
We did not formally assess the presence of sarcopenia in our NAFLD study cohort. The European Working Group on Sarcopenia in Older People guideline recommends measurement of muscle mass, strength and function and requires documentation of low muscle mass with one of two further criteria; low muscle strength or low physical performance.12 Our study was not designed to formally assess muscle mass by CT, DEXA or US imaging, but we did assess muscle strength (as part of the FFI score) and physical performance (30STST and TUG). Physical performance as assessed by 30STST was significantly different across the spectrum of liver fibrosis, however, the TUG scores were not, possibly indicating differences in the sensitivity of these different measures within patients with NAFLD.
Identifying frailty in non-cirrhotic patients with NAFLD is an important first step in developing interventions to address frailty. A number of guidelines pertaining to dietary, lifestyle, exercise and pharmacotherapy interventions for individuals with frailty and/or sarcopenia highlight the importance of early identification and subsequent implementation of rehabilitation measures.5 42 Exercise interventions have already demonstrated significant improvements in liver fat content and fibrosis in patients with NAFLD, independent of overall weight loss,43 44 although patient adherence is a significant challenge that must be addressed. It will be important to assess the impact of exercise on frailty in non-cirrhotic NAFLD populations in future interventional studies.
This study has several limitations: (1) the small sample size (n=109) was due in part to the detailed assessment required for each patient and future prospective studies will need to prioritise the assessments of frailty, including the LFI as well as measurements of sarcopenia; (2) our cohort was selected from patients attending a tertiary referral service and as a result may have had more severe metabolic disease compared with patients with NAFLD in the community; (3) our study lacked a direct age-matched control group without NAFLD and while Irish data on the use of SRFI is available from community-based well-described cohorts from different age groups, these data are not directly matched for BMI or gender; (4) as a cross-sectional study, we were unable to assess the impact of frailty on clinical outcomes in non-cirrhotic NAFLD patients, or to report if changes in frailty measurements correlate with liver disease progression or regression. It is likely that frailty assessment tools will need to be assessed in a longitudinal cohort to determine the optimal frailty assessment tool for this patient population.
Our results highlight the complex and multifaceted impact of metabolic disease on overall health in patients with NAFLD, even in patients with early fibrosis stage. Frailty indices are important tools that aid early identification of patients with NAFLD who may be more vulnerable to adverse health outcomes. Incorporation of frailty assessment tools in clinical practice can facilitate more accurate prognostication, facilitate rehabilitation strategies to address physical frailty deficits and consequently improve patient care. Further validation studies of frailty assessment tools with long-term follow-up in patients with non-cirrhotic liver disease are required.
Acknowledgments
The authors would like to acknowledge all staff in the Department of Hepatology and Clinical Research Facility at St James’s Hospital, and staff in the Discipline of Physiotherapy at Trinity College Dublin.
Footnotes
Twitter: @philipogorman3
SN and PO contributed equally.
Contributors: SuN, JG, MR, DF, PO’G and SaN designed the study. PO’G, MM, EM, SaN and SuN collected the data. MR analysed the data. PO’G, SaN, MR and SuN drafted the original manuscript. All authors revised and edited the manuscript. All authors approved the final version of the manuscript. SuN is author acting as guarantor.
Funding: This work was supported by a grant held by SN from the Health Research Board, Ireland. MR was supported by the Health Research Board (EIA-2017–013).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Research Ethics Committee at St. James’s and Tallaght University Hospital, Dublin, Ireland. REC REF03:2018. Participants gave informed consent to participate in the study before taking part, and the study was conducted in line with the Declaration of Helsinki, 2013. The study assessments occurred between February 2018 and November 2019.
References
- 1.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11–20. 10.1038/nrgastro.2017.109 [DOI] [PubMed] [Google Scholar]
- 2.Jarvis H, Craig D, Barker R, et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of population-based observational studies. PLoS Med 2020;17:e1003100. 10.1371/journal.pmed.1003100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med 2017;377:2063–72. 10.1056/NEJMra1503519 [DOI] [PubMed] [Google Scholar]
- 4.Moore JB. From sugar to liver fat and public health: systems biology driven studies in understanding non-alcoholic fatty liver disease pathogenesis. Proc Nutr Soc 2019;78:290–304. 10.1017/S0029665119000570 [DOI] [PubMed] [Google Scholar]
- 5.El Sherif O, Dhaliwal A, Newsome PN, et al. Sarcopenia in nonalcoholic fatty liver disease: new challenges for clinical practice. Expert Rev Gastroenterol Hepatol 2020;14:197–205. 10.1080/17474124.2020.1731303 [DOI] [PubMed] [Google Scholar]
- 6.Nishikawa H, Fukunishi S, Asai A, et al. Sarcopenia and frailty in liver cirrhosis. Life 2021;11:399. 10.3390/life11050399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams FR, Milliken D, Lai JC, et al. Assessment of the frail patient with end-stage liver disease: a practical overview of sarcopenia, physical function, and disability. Hepatol Commun 2021;5:923–37. 10.1002/hep4.1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golabi P, Gerber L, Paik JM, et al. Contribution of sarcopenia and physical inactivity to mortality in people with non-alcoholic fatty liver disease. JHEP Rep 2020;2:100171. 10.1016/j.jhepr.2020.100171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013;381:752–62. 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–95. 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cesari M, Landi F, Vellas B, et al. Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci 2014;6:192. 10.3389/fnagi.2014.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working group on sarcopenia in older people. Age Ageing 2010;39:412–23. 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tandon P, Tangri N, Thomas L, et al. A rapid bedside screen to predict unplanned hospitalization and death in outpatients with cirrhosis: a prospective evaluation of the clinical frailty scale. Am J Gastroenterol 2016;111:1759–67. 10.1038/ajg.2016.303 [DOI] [PubMed] [Google Scholar]
- 14.Lai JC, Feng S, Terrault NA, et al. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant 2014;14:1870–9. 10.1111/ajt.12762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai JC, Rahimi RS, Verna EC, et al. Frailty associated with Waitlist mortality independent of ascites and hepatic encephalopathy in a multicenter study. Gastroenterology 2019;156:1675–82. 10.1053/j.gastro.2019.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017;66:564–74. 10.1002/hep.29219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhanji RA, Narayanan P, Moynagh MR, et al. Differing impact of sarcopenia and frailty in nonalcoholic steatohepatitis and alcoholic liver disease. Liver Transpl 2019;25:14–24. 10.1002/lt.25346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laube R, Wang H, Park L, et al. Frailty in advanced liver disease. Liver Int 2018;38:2117–28. 10.1111/liv.13917 [DOI] [PubMed] [Google Scholar]
- 19.Lai JC, Covinsky KE, McCulloch CE, et al. The liver frailty index improves mortality prediction of the subjective clinician assessment in patients with cirrhosis. Am J Gastroenterol 2018;113:235–42. 10.1038/ajg.2017.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai JC, Sonnenday CJ, Tapper EB, et al. Frailty in liver transplantation: an expert opinion statement from the American Society of transplantation liver and intestinal community of practice. Am J Transplant 2019;19:1896–906. 10.1111/ajt.15392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skladany L, Molcan P, Vnencakova J, et al. Frailty in nonalcoholic fatty liver cirrhosis: a comparison with alcoholic cirrhosis, risk patterns, and impact on prognosis. Can J Gastroenterol Hepatol 2021;2021:1–10. 10.1155/2021/5576531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tandon P, Low G, Mourtzakis M, et al. A model to identify sarcopenia in patients with cirrhosis. Clin Gastroenterol Hepatol 2016;14:1473–80. 10.1016/j.cgh.2016.04.040 [DOI] [PubMed] [Google Scholar]
- 23.Hong HC, Hwang SY, Choi HY, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean sarcopenic obesity study. Hepatology 2014;59:1772–8. 10.1002/hep.26716 [DOI] [PubMed] [Google Scholar]
- 24.Bhanji RA, Narayanan P, Allen AM, et al. Sarcopenia in hiding: the risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology 2017;66:2055–65. 10.1002/hep.29420 [DOI] [PubMed] [Google Scholar]
- 25.Fernández-Mincone T, Contreras-Briceño F, Espinosa-Ramírez M, et al. Nonalcoholic fatty liver disease and sarcopenia: pathophysiological connections and therapeutic implications. Expert Rev Gastroenterol Hepatol 2020;14:1141–57. 10.1080/17474124.2020.1810563 [DOI] [PubMed] [Google Scholar]
- 26.Koo BK, Kim D, Joo SK, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol 2017;66:123–31. 10.1016/j.jhep.2016.08.019 [DOI] [PubMed] [Google Scholar]
- 27.Wang CW, Lebsack A, Chau S, et al. The range and reproducibility of the liver frailty index. Liver Transpl 2019;25:841–7. 10.1002/lt.25449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–57. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 29.Theou O, O'Connell MDL, King-Kallimanis BL, et al. Measuring frailty using self-report and test-based health measures. Age Ageing 2015;44:471–7. 10.1093/ageing/afv010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howlett SE, Rockwood MRH, Mitnitski A, et al. Standard laboratory tests to identify older adults at increased risk of death. BMC Med 2014;12:171. 10.1186/s12916-014-0171-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1717–30. 10.1053/j.gastro.2019.01.042 [DOI] [PubMed] [Google Scholar]
- 32.Bull FC, Al-Ansari SS, Biddle S, et al. World Health organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54:1451–62. 10.1136/bjsports-2020-102955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 34.Jones CJ, Rikli RE, Beam WC. A 30-S chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport 1999;70:113–9. 10.1080/02701367.1999.10608028 [DOI] [PubMed] [Google Scholar]
- 35.Savva GM, Donoghue OA, Horgan F, et al. Using timed up-and-go to identify frail members of the older population. J Gerontol A Biol Sci Med Sci 2013;68:441–6. 10.1093/gerona/gls190 [DOI] [PubMed] [Google Scholar]
- 36.Yoshimura N, Muraki S, Oka H, et al. Do sarcopenia and/or osteoporosis increase the risk of frailty? A 4-year observation of the second and third road study surveys. Osteoporos Int 2018;29:2181–90. 10.1007/s00198-018-4596-4 [DOI] [PubMed] [Google Scholar]
- 37.Saeki C, Kanai T, Nakano M, et al. Relationship between Osteosarcopenia and frailty in patients with chronic liver disease. J Clin Med 2020;9:2381. 10.3390/jcm9082381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon EH, Peel NM, Samanta M, et al. Sex differences in frailty: a systematic review and meta-analysis. Exp Gerontol 2017;89:30–40. 10.1016/j.exger.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 39.Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012;60:1487–92. 10.1111/j.1532-5415.2012.04054.x [DOI] [PubMed] [Google Scholar]
- 40.Hubbard RE. Sex differences in frailty. Interdiscip Top Gerontol Geriatr 2015;41:41–53. 10.1159/000381161 [DOI] [PubMed] [Google Scholar]
- 41.Gallagher D, Ruts E, Visser M, et al. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab 2000;279:E366–75. 10.1152/ajpendo.2000.279.2.E366 [DOI] [PubMed] [Google Scholar]
- 42.Dent E, Morley JE, Cruz-Jentoft AJ, et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Health Aging 2019;23:771–87. 10.1007/s12603-019-1273-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Gorman P, Norris S. Exercising in the COVID-19 era: implications in non-alcoholic fatty liver disease (NAFLD). BMJ Open Gastroenterol 2021;8:e000568. 10.1136/bmjgast-2020-000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Gorman P, Naimimohasses S, Monaghan A, et al. Improvement in histological endpoints of MAFLD following a 12-week aerobic exercise intervention. Aliment Pharmacol Ther 2020;52:1387–98. 10.1111/apt.15989 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2021-000861supp001.pdf (405.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request.