Abstract
Chronic tinnitus and hyperacusis often develop with age-related hearing loss presumably due to aberrant neural activity in the central auditory system (CAS) induced by cochlear pathologies. However, the full spectrum of physiological changes that occur in the CAS as a result age-related hearing loss are still poorly understood. To address this issue, neurophysiological measures were obtained from the cochlea and the inferior colliculus (IC) of 2, 6 and 12 month old C57BL/6J mice, a mouse model for early age-related hearing loss. Thresholds of the compound action potentials (CAP) in 6 and 12-month-old mice were significantly higher than in 2-month-old mice. The sound driven and spontaneous firing rates of IC neurons, recorded with 16 channel electrodes, revealed mean IC thresholds of 22.8 ± 6.5 dB (n = 167) at 2 months, 37.9 ± 6.2 dB (n = 132) at 6 months and 47.1 ± 15.3 dB (n = 151) at 12 months of age consistent with the rise in CAP thresholds. The characteristic frequencies (CF) of IC neurons ranged from 3 to 32 kHz in 2-month-old mice; the upper CF ranged decreased to 26 kHz and 16 kHz in 6 and 12 month old mice respectively. The percentage of IC neurons with CFs between 8–12 kHz increased from 36.5% in 2-month-old mice, to 48.8% and 76.2% in 6 and 12-month-old mice, respectively, suggesting a downshift of IC CFs due to the high-frequency hearing loss. The average spontaneous firing rate (SFRs) of all recorded neurons in 2 month old mice was 3.2 ± 2.5 Hz (n = 167). For 6 and 12 month old mice, the SFRs of low CF neurons (<8 kHz) was maintained at 3–6 spikes/s; whereas SFRs of IC neurons with CFs > 8 kHz increased to 13.0 ± 15.4 (n = 68) Hz at 6 months of age and then declined to 4.8 ± 7.4 (n = 110) spikes/s at 12 months of age. In addition, sound-evoked activity at suprathreshold levels at 6 months of age was much higher than at 2 and 12 months of age. To evaluate the behavioral consequences of sound evoked hyperactivity in the IC, the amplitude of the acoustic startle reflex was measured at 4, 8 and 16 kHz using narrow band noise bursts. Acoustic startle reflex amplitudes in 6 and 12 month old mice (n = 4) were significantly larger than 2 month old mice (n = 4) at 4 and 8 kHz, but not 16 kHz. The enhanced reflex amplitudes suggest that high-intensity, low-frequency sounds are perceived as louder than normal in 6 and 12 month old mice compared to 2 month olds. The increased spontaneous activity, particularly at 6 months, may be related to tinnitus whereas the increase in sound-evoked activity and startle reflex amplitudes may be related to hyperacusis.
Keywords: Age-related hearing loss, Inferior colliculus, Hyperexcitability, Tinnitus, Hyperacusis
Introduction
In the past two decades, many animal studies have investigated the neural mechanisms underlying chronic tinnitus induced by intense noise exposures or ototoxic drugs (Chen et al., 2015; Eggermont, 2015; Roberts et al., 2010; Sun et al., 2009; Szczepaniak et al., 1996). Several studies found significant increases of spontaneous firing rates (SFRs) in the cochlear nucleus and inferior colliculus (IC) (Dong et al., 2010; Kaltenbach, 2000). Increases of SFRs, changes in neural synchrony and aberrant tonotopic map reorganization have also been observed at higher levels of the central auditory system (CAS); these changes have been considered as potential neural correlates of tinnitus (Eggermont, 2005).
The prevalence of tinnitus increases rapidly with age until 60–69 years (Chang et al., 2007; Shargorodsky et al., 2010). This increase in the prevalence of tinnitus could be due to aging alone or the progressive increase in age-related hearing loss. The severity of age-related tinnitus ranges widely from being a slight nuisance to severely affecting a person’s daily life. Although age-related hearing loss is the most common cause of sensorineural hearing loss, in adults, few studies have explored the neural correlates of tinnitus and hyperacusis related to age-related hearing loss.
The IC is an important binaural relay station, which routes information from key auditory nuclei in the brainstem to the auditory thalamus and auditory cortex (AC). Acute acoustic trauma, which decreases the neural output of the cochlea, paradoxically causes a significant increase in the amplitude of IC local field potentials indicative of enhanced central gain, but decreases the SFRs (Niu et al., 2013). Several weeks after acoustic overstimulation, the spontaneous activity of IC neurons increased significantly among neurons tuned to frequencies within the region of hearing loss (Robertson et al., 2013). This spontaneous hyperexcitability in the IC, which could result from reduced central inhibition, has been considered to be a neural correlate of central tinnitus (Dong et al., 2010). Willott et al. had reported functional changes in the CAS induced by age-related hearing loss (Willott et al., 1988a; Willott et al., 1988b). Most IC neurons of 6–12 month old C57 mice were well-driven by suprathreshold stimuli and the spontaneous activity increased with age in the central nucleus but not in other IC subnuclei (Willott et al., 1988b). Ison et al. found that low-frequency acoustic startle responses of C57 mice increased with age, behavior potentially related to hyperacusis (Ison et al., 2007). Studies of noise and drug-induced hearing loss suggest that spontaneous and sound evoked hyperexcitability in the CAS may be linked to tinnitus and hyperacusis; however, this association is not well established in cases of age-related hearing loss. To test this hypothesis, we measured the spontaneous activity and sound-induced excitatory responses in IC neurons from C57BL/6J mice at 2, 6 and 12 months of age. We predicted that SFRs in the IC of C57BL/6J mice, which develop early onset age-related hearing loss, would increase with advancing age. As hyperacusis is commonly associated with tinnitus, we hypothesized that sound driven activity would also increase with age-related hearing loss and that this would be associated with increases in the amplitude of the startle reflex.
Materials and Methods
Animals
Thirty-six C57BL/6J mice (Jackson Lab, Bar Harbor, ME) were used for physiological experiments; 18 mice were used for compound action potential (CAP) recordings and the other 18 were used for IC recordings. For CAP and IC recording, twelve mice at age of 1–2 months were used as the controls (G-2M, n = 6), twelve mice between 6–7 months and twelve mice between 12–14 months were used as the middle-age group (G-6M, n = 6) and the old-age group (G-12M, n = 6), respectively. Twelve additional mice were used for acoustic startle reflex measurements. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University at Buffalo and conform to the guidelines issued by the National Institutes of Health.
CAP recordings
Mice were anesthetized with a mixture of ketamine (100 mg/kg, i.p.) and xylazine (50 mg/kg, i.p.) and their heads were positioned with a custom head-clamp. The surgical procedure to record the CAP has been described in our recent paper (Wang et al., 2016). The bulla was opened and a silver-ball electrode was placed on the round window under a microscope. The cochlear responses were amplified using a DAM-50 amplifier (WPI, filter setting 300 Hz to 10 kHz). The external auditory meatus was opened at the end of the ear canal and the speaker was placed at the opening of the ear canal. Tone bursts (10 ms duration, rising/fall time 0.5 ms) centered at 4, 6, 8, 12, 16, 20, 24, 35 and 40 kHz were used to elicit the CAP. The threshold of CAP was defined as the minimal intensity of the minimal amplitude of CAP elicited.
IC surgery and electrophysiology
Mice were anesthetized with a mixture of ketamine (100 mg/kg, i.p.) and xylazine (50 mg/kg, i.p.). The pedal withdrawal reflex of the hind limbs was checked every 45 minutes to assess the anesthetic depth. Supplemental ketamine (20 mg/kg, i.p.) was given as needed to maintain the proper plane of anesthesia. The skin over the parietal and nasal bone was carefully removed to expose the skull. After removal of the tissue on the surface of the skull, the skull was treated with 3% hydrogen peroxide (H2O2) and a head-fixing pole was glued on the parietal bone with dental cement. The fixing pole attached to a magnetic stand was used to firmly hold the mouse’s head during the test. A ~3×3-mm region of cranial bone overlying the dorso-caudal aspect of the cerebellum was removed to expose left IC (Niu et al., 2013).
A 16-channel microelectrode (NeuroNexus, A1×16–5mm-100–177, Ann Arbor, MI) was used to record IC responses in anesthetized mice. The electrode was mounted on a hydraulic manipulator (FHC Inc., Bowdoinham, ME) and advanced into the IC at an angle of approximately 75–80° relative to the surface of the IC. The output of the electrode was connected to a 16-channel preamplifier (RA16PA, Tucker-Davis Technology, TDT). The output of the preamplifier was delivered to a digital signal processing module (RZ5, TDT) connected to a computer. A stainless-steel electrode inserted into the frontal lobe was used as the ground. Mouse body temperature was maintained at 37 °C using a thermally regulated heating pad system (Harvard Apparatus, Cambridge, MA). The multiunit recording typically finished in 2–3 hours. A small dose of supplemental ketamine and xylazine mixture (~0.1 ml) was added in every 30 minutes during the recording.
Recording were obtained from different regions of the central nucleus of the IC; this was accomplished by inserting the electrode into different locations starting from the lateral side and moving medially while avoiding major blood vessels. As the characteristic frequency (CF) of IC neurons increased with depth from the IC surface, the electrode was advanced to ~1600 μm in all the penetrations. Noise bursts (50 ms, 70–90 dB SPL) were presented three times per second as the electrode was advanced into the IC to search for neurons. The multiunit spike discharges were recorded using OpenEx (TDT) and custom software. Data were collected in the form of peristimulus time histograms (PSTH), frequency response area (FRA) maps and spike rate-level functions (Niu et al., 2013; Stolzberg et al., 2011). The CF from each recording site was defined as the frequency with the minimal threshold (MT). The sound-driven spike rate was calculated from a 50 ms window located near onset response of the averaged PSTHs.
Sound stimuli were generated with the TDT System-3 hardware and presented through a multi-field magnetic speaker (MF1, TDT). Tone bursts (50 ms duration, 1 ms rise/fall time, 1–42 kHz, 20 logarithmically spaced steps) were used to elicit responses. The sound intensity was varied from 10 to 90 dB SPL in 10 dB steps. Broadband noise burst rate-level functions (RLFs) were obtained from 0 to 100 dB SPL (50 repetitions). The sound intensity was calibrated using a sound level meter (824, Larson Davis, Depew, NY) with a 1/4-inch condenser microphone (Larson Davis).
Acoustical startle reflex test:
Twelve mice at 2, 6, or 12 month of age were used for acoustic startle reflex test following the procedure described previously (4 mice for each age group)(Sun et al., 2009). Animals were placed in a small, wire mesh cage mounted on a Plexiglas base that rested on a sensitive piezoelectric transducer. The wire mesh (0.5 cm × 0.5 cm) cage (4 cm W × 3.5 cm, H × L 7–8 cm) restricted the mouse’s movement within a calibrated sound field. The output of the piezo transducer was connected to an A/D converter on an RP2 Real-time Processor (TDT). The startle reflex was amplified and filtered (0–1000 Hz) using a low pass filter (LPF-300, World Precision Instruments, Sarasota, FL, USA). The root mean square of acoustic startle response (rms) was calculated using custom software. Sound stimuli were presented by a loud speaker (FT28D, Madisound Speaker Components Inc., Middleton, WI, USA) located approximately 28 cm above the mouse’s head. Sound signals were generated by an RP2 Real-time Processor (TDT) controlled by custom software. The startle eliciting stimuli consisted of narrow-band noise bursts centered at 4, 8 and 16 kHz (bandwidth 2 kHz, 20 ms) presented at intensities from 50 to 100 dB SPL (ten trails on each conditions). The inter-trial interval was randomly varied from 18 to 23 seconds.
Statistical data analysis
Graph-Pad Prism software (GraphPad Software, San Diego, CA) was used for plotting and statistical analysis unless otherwise noted. Results are presented as mean ± standard error (SE) of the mean. The alpha level was set to p<0.05 for all statistical tests.
Results
The CAP assessment of peripheral hearing loss in three groups
Age-related cochlear hearing loss was evaluated by measuring the CAP threshold at 2, 6 and 12 months of age. Mean CAP thresholds in 2-month-old C57BL/6 mice ranged from 25 dB at 4 kHz to 45 dB at 40 kHz with the lowest threshold of 19 dB at 8 kHz. CAP thresholds progressively deteriorated with age (Figure 1); the threshold increases were greater for high frequencies (~16 kHz) than low frequencies. The mean CAP thresholds of the middle-aged group (G-6M, n = 6, 6–7 months) and the oldest group (G-12M, n = 6, 12–14 months) were significantly higher than the youngest group (G-2M, n = six, 1–2 months) at all of the tested frequencies (two-way ANOVA, df = 2, F = 287.3, P < 0.0001). CAP thresholds at the four highest frequencies exceeded the limits of the acoustic system in the oldest group as indicated by the upward-pointing arrows. The age-related hearing loss is consistent with previous findings (Ison et al., 2007).
Figure 1.
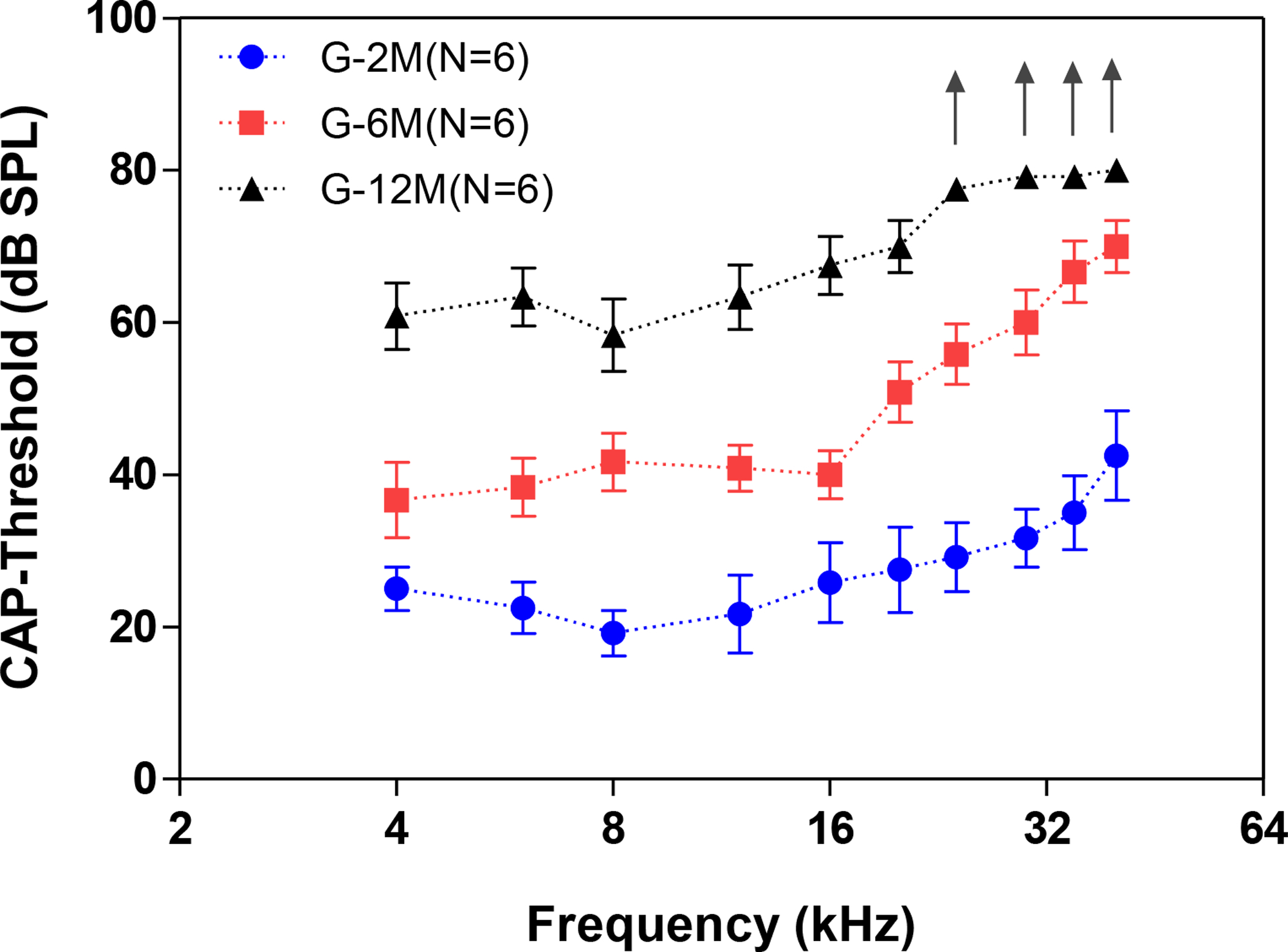
The thresholds of the compound action potentials (CAP) recorded from C57BL/6J mice showed a significant increase with age. The mean averaged CAP thresholds in the 6 month old group (G-6M, n=6) and 12 month old group (G-12M, n=6) were significantly higher than the 2 month old group (G-2M, n=6) (One-way ANOVA, df = 2, P<0.001. “↑” indicates there was no response at the maximum tested intensity at 80 dB SPL).
IC multiunit recordings
To determine whether the CF distribution of the IC was altered with the age-related hearing loss, MTs were plotted as a function of CF at different ages (Figure 2). In the young mice (G-2M), neurons with CFs ranged from 3 to 32 kHz and MTs varied from 10–45 dB SPL (n = 167, Figure 2A). With increasing age, fewer neurons were encountered with CFs above 16 kHz, and minimum MTs were above 25 dB SPL in the middle-aged mice (G-6M) (Figure 2B). In the oldest mice (G-12M), there was further restriction of CFs so that most neurons had CF < 12 kHz resulting in a preponderance of neurons around 8.5 kHz (Figure 2C). The percentages of recorded neurons in different CF bands were plotted for the three age groups (Figure 3A). In the 2 month group, the percentages of IC neurons were 22%, 36%, 20% and 22% for CF <8, 8–12, 12–16 and >16 kHz, respectively. For the 6 month age mice, the percentage of IC neurons in the 8–12 kHz band increased to 49% and the percentage >16 kHz dropped to 2%. For the 12-month age mice, the percentage of neurons in the 8–12 kHz band increased to 76% and no neurons were recorded with CFs above 12 kHz. The average MTs in the different frequency bands were determined for the three age groups (Figure 3B). There was a clear increase in mean MT in all frequency bands with increasing age consistent with the age-related increase in CAP threshold (Figure 3B, one-way ANOVA, df =2, *** P<0.0001, ** P<0.001).
Figure 2.
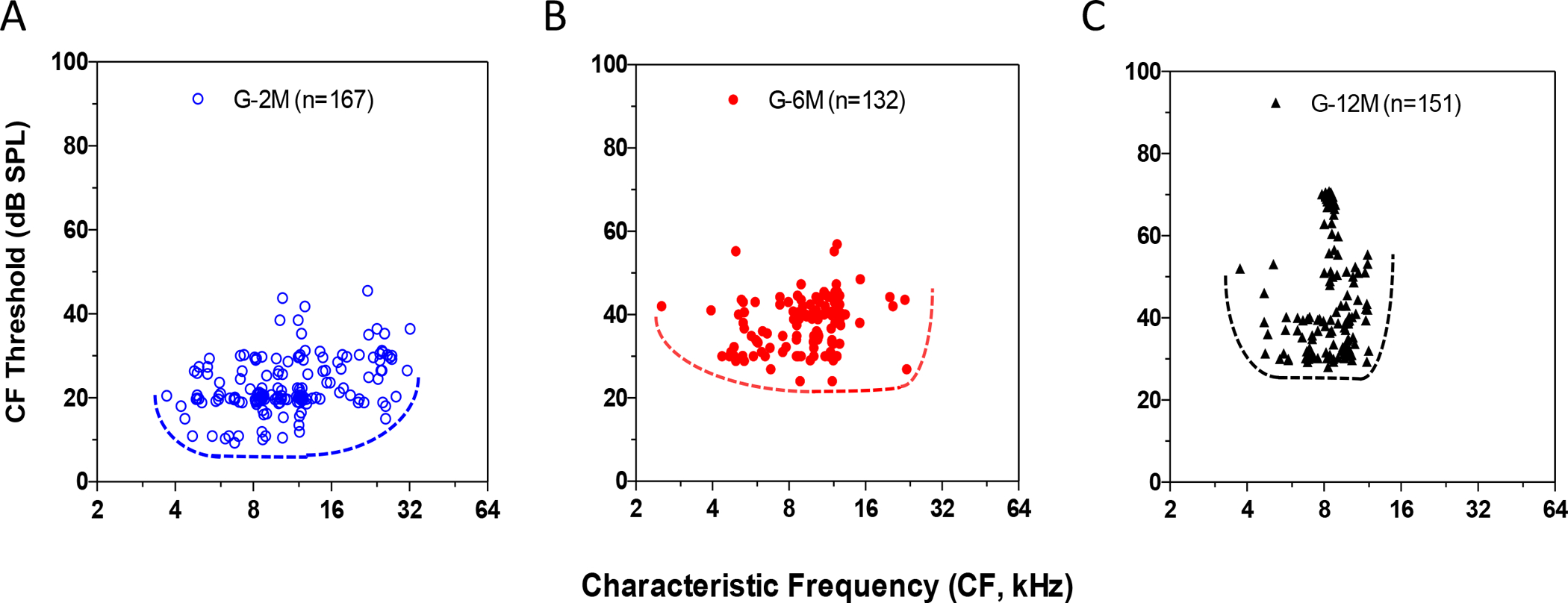
Distribution of the minimal thresholds (MT) and the characteristic frequencies (CF) of the inferior colliculus (IC) neurons recorded in 2, 6, and 12-month-old mice. (A) In the young mice (G-2M), the CFs was evenly distributed from 3 to 32 kHz with MTs from 10–45 dB SPL (n = 167). (B) In the 6 month old mice, animals displayed fewer high frequency neurons at above 16 kHz and the averaged MT increased about 20 dB compared to the 2 month old mice. (C) In the 12 month old mice (G-12M), all of the IC neurons showed a CFs less than 12 kHz and the preponderance of neurons concentrated in around the 8.5 kHz.
Figure 3.
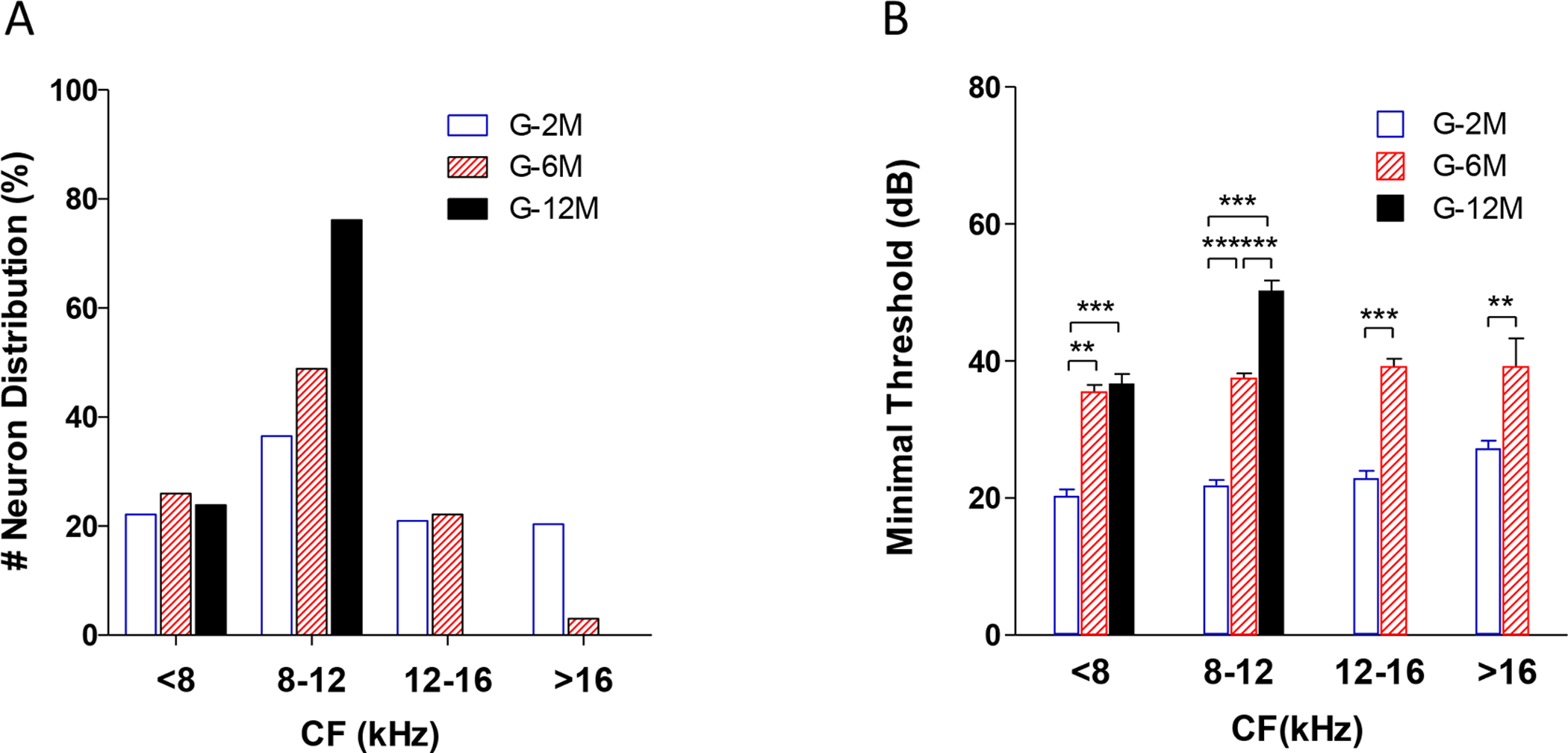
Changes of the distribution of the characteristic frequencies (CF) and minimal threshold changes with age. (A) The percentage of recorded neurons with different characteristic frequencies. There was a clear increment of the percentage of the 8–12 kHz neurons in the G-12M group (76.16%) and a reduction of the high frequency neurons at above 12 kHz in the G-6M and G-12M groups. (B) The averaged minimal threshold of IC neurons at different age groups. The mean minimal threshold increased with age (One-way ANOVA, df =2, *** P<0.0001, ** P<0.001).
Examples of the FRA responses recorded from IC neurons at 2, 6 and 12 months of age are shown in Figure 4. The tuning curves showed a steep slope above CF and a slightly shallower slope below CF in 2-month-old mice (Figure 4A–B). The tuning curves of IC neurons in the 6-month-old mice tended to be wider than those from the 2-month-old mice (Figure 4C–D). Moreover, the slopes above and below CF were shallower, particularly among neurons with higher CFs; this was particularly noticeable in the neuron with a CF around 20 kHz (Figure 4D), a region where thresholds started to increase with age. Tuning curves in the 12-month-old mice showed greatly elevated CF thresholds and very broad tuning at the tip of the tuning curve compared to the 2-month age group. To quantify these changes, the mean Q10 and mean Q30 values were computed for the different age groups (Figure 5A and 5B). There was a significant decrease of Q10 and Q30 at low frequencies IC neurons (<8 kHz) in the 12 month old mice compared to the 2 and 6 month old mice (One-way ANOVA, FQ10=16.46, df = 2, *** P<0.0001; FQ30=11.62, df = 2, *** P<0.0001, ** P<0.001).
Figure 4.
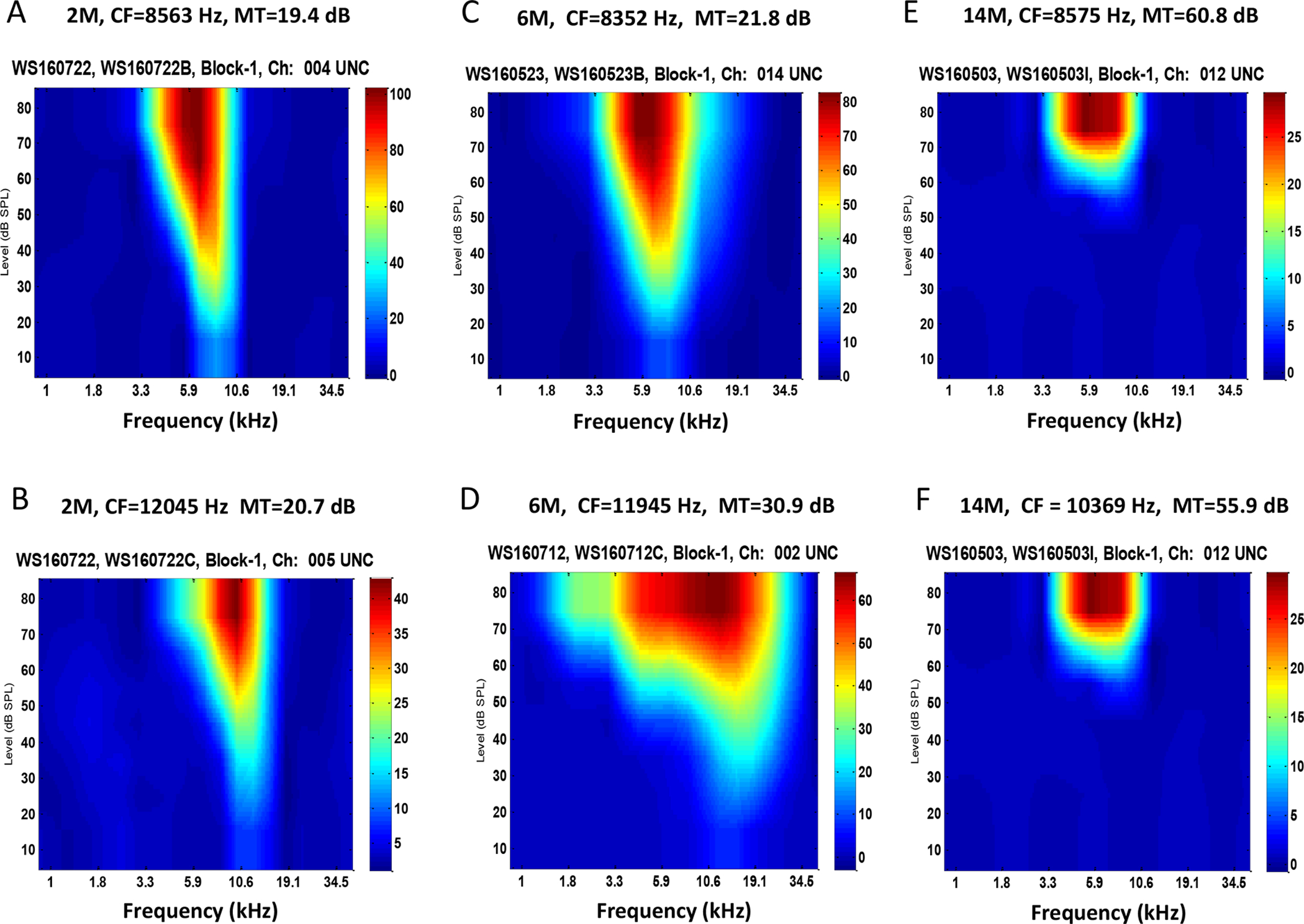
Tuning curves recorded from inferior colliculus neurons at 2, 6 and 12 month old mouse groups. (A-B) Examples of a low frequency neuron and a high frequency neuron in a 2-month old mouse. The tuning curve showed a steep slope on the frequency above the characteristic frequencies (CF) and a broad tuning on below the CF. (C-D) Examples of tuning curve recorded from a 6-month old mouse, which developed broad tunings on both side of the CF. (E-F) Examples of tuning curves recorded from a 12-month old mouse, which showed significant elevated thresholds and broad tunings compared the 2-month age group.
Figure 5.
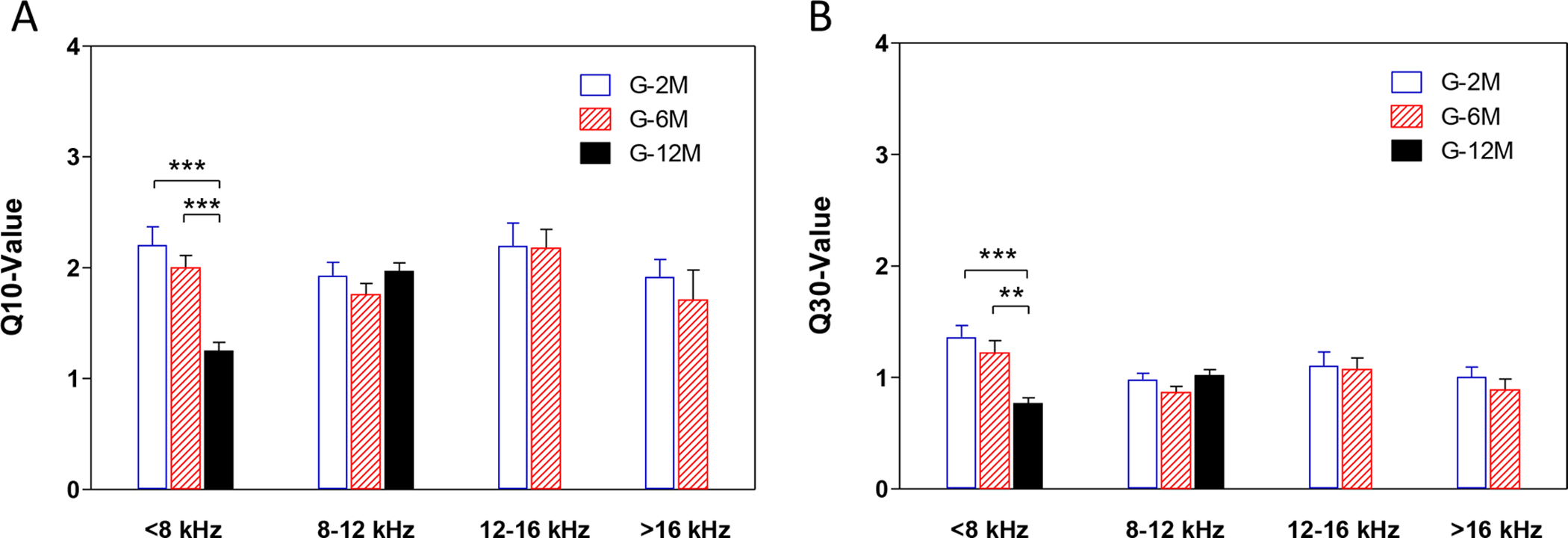
The Q10 and Q30 values of the tuning curve of the inferior colliculus neurons recorded at 2, 6 and 12 month old groups. There was a significant decrease of Q10 and Q30 at low frequencies IC neurons (<8 kHz) in the 12 month old mice compared to the 2 and 6 month old mice (One-way ANOVA, FQ10=16.46, df = 2, *** P<0.0001; FQ30=11.62, df = 2, *** P<0.0001, ** P<0.001). There was no significant difference of Q10 and Q30 in the IC neurons at other frequencies.
Spontaneous firing rate of the IC neurons
To determine if the spontaneous activity of IC neurons changed with age and age-related hearing loss, we compared the SFRs at different frequencies for the three age groups. Figure 6A shows the SFRs plotted as a function of CF in each age group. In 2 month old mice (blue circle), SFRs were lower than 10 spikes/s and the averaged SFRs was 3.16 ± 2.5 spikes/s for all the neurons with CFs from 3 to 32 kHz (n = 167). In contrast, the SFRs in 6-month-old mice (red dot) increased to 60 spikes/s for CFs near 12 kHz, much higher than in the 2-month-old mice. In 12-month-old mice, the SFRs (black triangle) decreased compared to the 6-month-old mice, but was still higher than in 2-month-old mice. There was a significant difference in the SFRs between these three groups (One-way ANOVA, F = 36.7. P<0.001). A Tukey post-hoc analysis test showed there were significate differences between the 6 month group compared to other two age groups (2 and 12-month) (P<0.001), but no difference between the 2 month group and 12 month group. To quantify these changes, we compared the mean SFRs for each age group in different frequency ranges (Figure 6B). The mean SFRs of IC neurons with CFs lower than 8 kHz were 3.53 ± 1.95 (n = 53), 6.17 ± 4.07 (n = 34) and 4.13 ± 4.66 (n = 41) spikes/s for the 2, 6 and 12 month groups, respectively. Statistical analysis showed a significant difference between the 6 month group compared to the 2 and 12 month groups (One-way ANOVA, F = 5.797, P<0.01; Bonferroni post-test, * P <0.05, *** P <0.001). In the 8–12 kHz band the mean SFRs was 13.01 ± 15.45 (n = 68) spikes/s in 6 month old mice, significantly higher than in the 2 month old and 12 month old mice (3.16 ± 2.52 spikes/s, n =65, and 4.80 ± 7.37 spikes/s, n= 110, respectively) (One-way ANOVA test, F = 21.08, P<0.0001. Bonferroni post-test, *** P <0.0001 between G-6M and G-2M, G-6M and G-12M). For neurons with CFs above 12 kHz, the SFRs in the 6 month old mice were much higher than the 2 month old mice (Student’s test, P<0.0001 ***). These data indicate that spontaneous activity increased between 2 and 6 months of age and then declined at 12 months of age.
Figure 6.
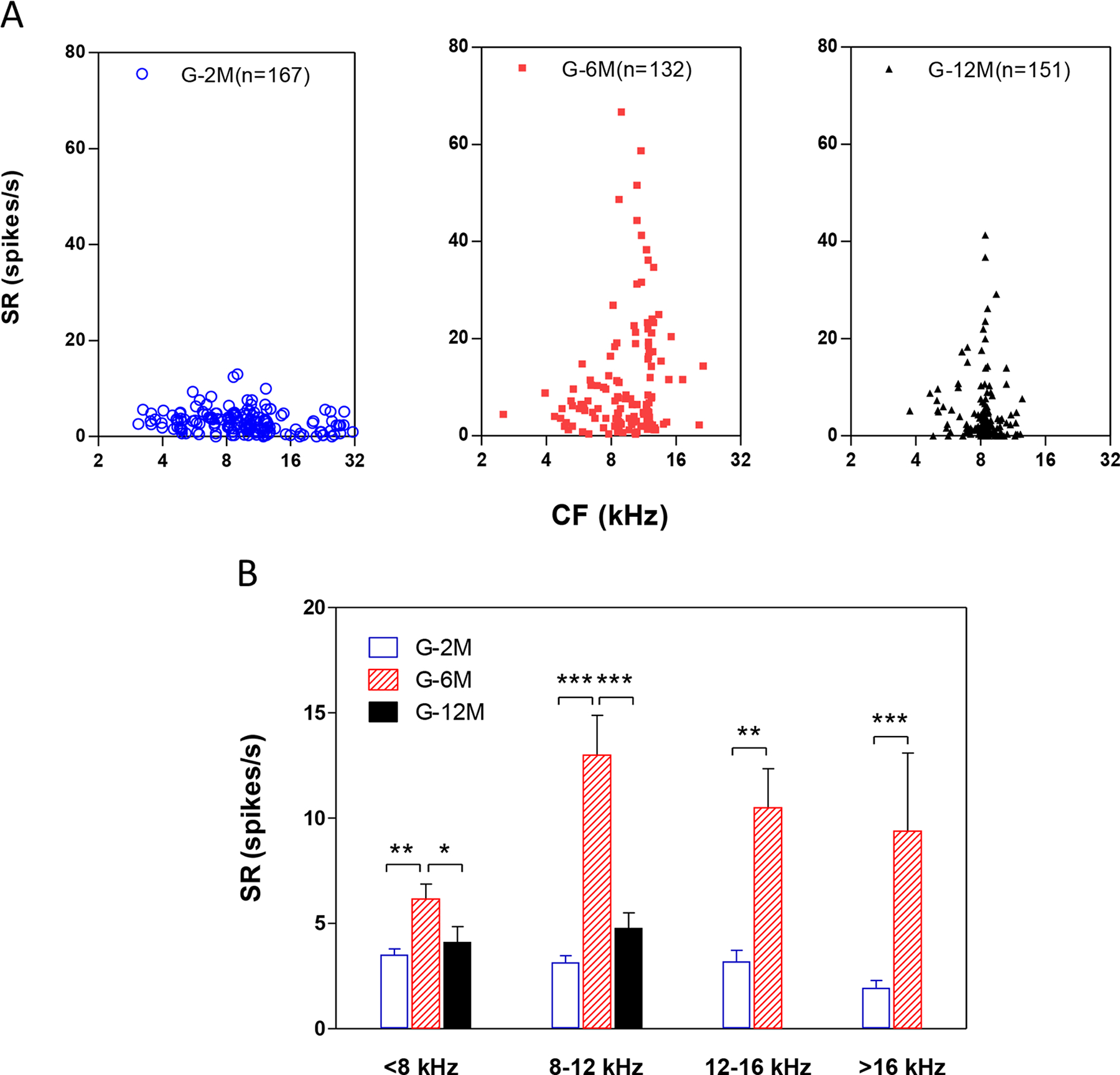
The spontaneous firing rates (SFRs) of the inferior colliculus neurons increased with age. (A) The SFRs changed with IC neurons with different characteristic frequencies in 2, 6 and 12 month old mice. In G-2M (blue circle), the SFRs were lower than 10 spikes/s for all the neurons with CFs at 3 to 32 kHz. In contrast, the SFRs in G-6M (red dot) increased to 60 spikes/s for CFs near 12 kHz, which were much higher than the 2-month-old mice. In G-12M, the SFRs (black triangle) decreased compared to the 6-month-old mice, but still higher than the 2-month-old mice. (B) The SFRs of below 8 kHz CF neurons were 3.53 ± 1.95 (n = 53), 6.17 ± 4.07 (n = 34) and 4.13 ± 4.66 (n = 41) spikes/s for the 2, 6 and 12 month groups, respectively (One-way ANOVA test, F = 5.797, P<0.01. Bonferroni post-test, *** P <0.001 between G-6M and G-2M, * P <0.05 between G-6M and G-12M). Between 8 – 12 kHz, the SFRs further increased to 13.01 ± 15.45 (n = 68) spikes/s in 6 month old mice, but did not change in the 2 month old mice and 12 month old mice (3.16 ± 2.52 spikes/s, n =65, and 4.80 ± 7.37 spikes/s, n= 110, respectively) (One-way ANOVA test, F = 21.08, P<0.0001. Bonferroni post-test, *** P <0.0001 between G-6M and G-2M, G-6M and G-12M). For high frequency neurons at above 12 kHz, the SFRs in the 6 month old mice were much higher than the 2 month old mice (Student’s test, *** P<0.0001).
Rate-level function of IC neurons evoked by broadband noise and tone bursts
To determine if age-related hearing loss had an effect on sound-driven activity, we measured the noise-burst evoked rate-level functions from 0 to 100 dB SPL (Figure 7). Figure 7 showed that the average firing rate increased monotonically from 20 to 100 dB SPL at 2 months of age (blue circles) reaching a maximum around 194 ± 15 spikes/s at 100 dB SPL. For the 6 month group, the mean firing rates began to increase just above 40 dB and then increased rapidly with level to a mean (G-6M; n = 132) of 334 ± 12 spikes/s at 100 dB SPL, a value 71% higher than the maximum firing rate in the G-2M group. The mean discharge rates in the G-12M group (n=151) began to increase around 80 dB SPL and rose steeply to a maximum firing rate of 218 ± 14 spikes/s at 100 dB SPL. The maximum firing rate in the 12-month-old group was slightly higher than the highest rate in the 2-month-old group. A two-way ANOVA was performed at intensity levels from 60 dB SPL to 100 dB SPL in three different age groups. There was a significant difference at 80, 90 and 100 dB SPL between the three groups (F= 232.9, P<0.0001, Bonferroni post-test showed a significant difference between the 6 month group and the 2 month group at 80 to 100 dB SPL. There was also a significant difference between the 12-month group and the 2-month group at 80 and 90 dB SPL, but not at 100 dB SPL. The rate-level functions were fitted with logarithm function (dashed lines). The slope of the logarithmic function was 0.04, 0.067 and 0.086, and the EC50 (the sound intensity that induced a half-maximum response) was 44, 60 and 95 dB SPL for the 2, 6 and 12 month groups, respectively.
Figure 7.
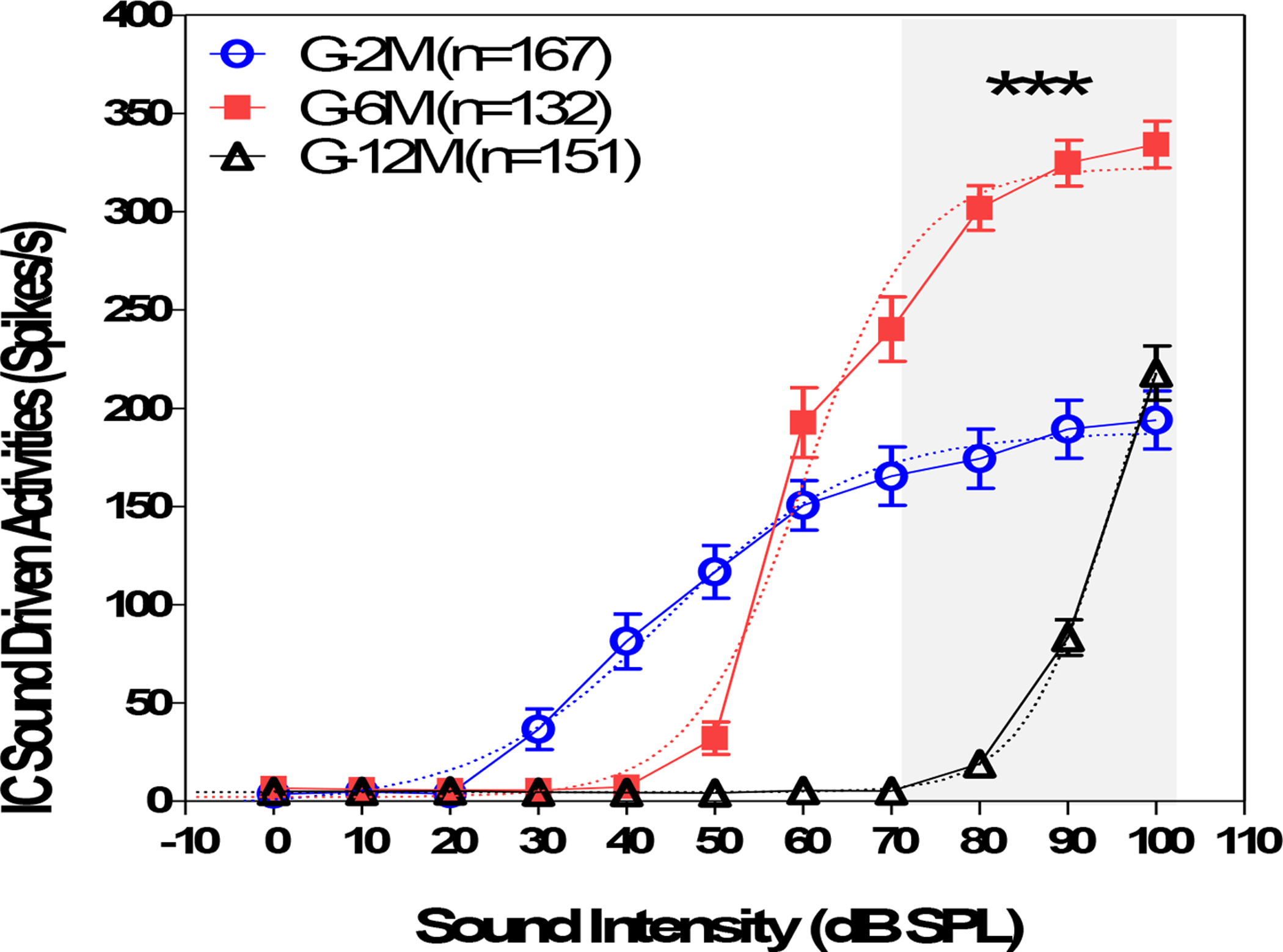
The firing-rate-level function of the inferior colliculus (IC) neurons elicited by noise-bursts (50 ms, 0 to 100 dB SPL) recorded from 2, 6, and 12-month-old mice. For the 2 month old group (blue circle), the averaged firing rates maintained at a very low level to sound stimuli between 0 and 20 dB SPL, increased almost linearly from 30 to 60 dB SPL and saturated at above 60 dB and reached to 194 ± 15 Hz at 100 dB SPL. For the 6 month group (red square), the firing rates was low at 0–40 dB SPL and showed a rapid increase at above 50 dB SPL and reached to 334 ± 12 Hz at 100 dB SPL (n = 132). The mean discharge rate of the G-12M group) was low at 60–90 dB SPL (black triangle, n=151) and was slightly higher than the 2 month old group at 100 dB SPL. The averaged firing rates were significant different at 70, 80, 90 and 100 dB SPL (grey shade area) between the three groups (two-way ANOVA, F= 225.8, df=11, 780, P<0.0001). The rate-level functions were fitted with logarithm curves (dashed lines). The slope of the logarithm function was 0.04, 0.067 and 0.086, and the EC50 (the sound intensity that induced halfway response between the baseline to the maximum response) was 44, 60 and 95 dB SPL for the G-2M, G-6M and G-12M groups, respectively.
Population PSTH responses elicited by tone-bursts (10 to 90 dB SPL, 4.8 to 23.3 kHz) were used to identify frequency specific changes of sound-driven activity (Chen et al., 2016). The mean PSTHs for each tested frequency-intensity combination was calculated from all of the recorded neurons at each age groups. Examples of PSTHs obtained at 4.8, 8.7, 15.7 and 23.3 kHz for 2 month (blue line), 6 month (red line) and 12 month groups (black line) are shown in Figure 8. For all of the tested frequencies, PSTH responses in the 12-month group were significantly lower than the 2 month and 6 month groups. For the 6 month group, the responses at supra-threshold levels (>60 dB SPL) were similar to the 2 month group at 4.8, 15.7 and 23.3 kHz. At 8.7 kHz, the PSTHs showed a clear increase at high intensities (> 50 dB SPL) in the 6-month group (red line) compared to the 2-month group (blue area). These increases were evident for both the onset and steady state components of the PSTH.
Figure 8.
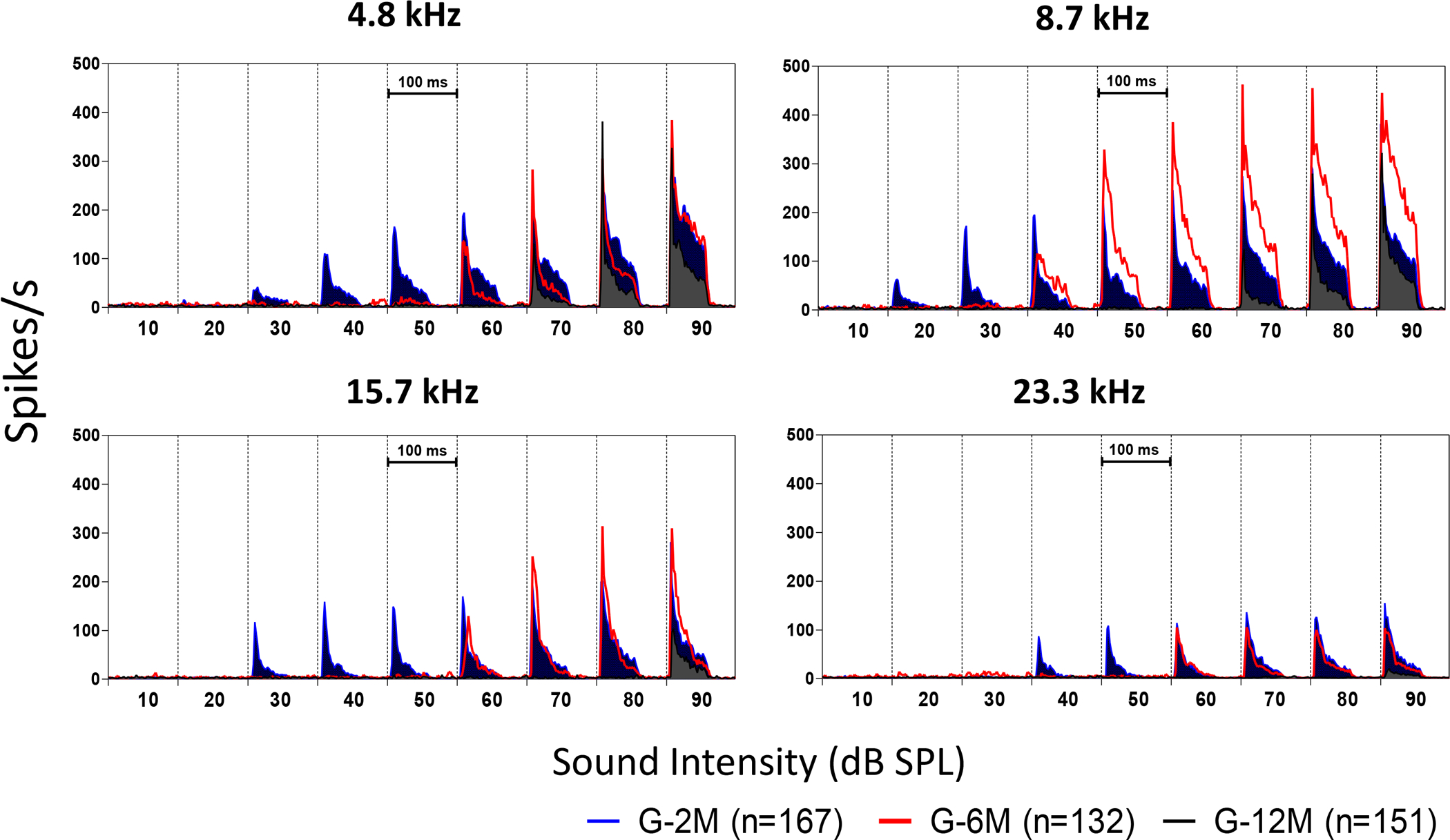
The peristimulus time histograms (PSTHs) recorded from the inferior colliculus (IC) neurons elicited by tone bursts (4.8, 8.7, 15.7 and 23.3 kHz at 80 dB SPL) of 2, 6, and 12 month old mice. Compared to the 2 month old group (blue), the PSTHs in the 6 month old group (red) were much higher at 8.7 kHz and 10.6 kHz (One-way ANOVA, df = 2, P<0.001). In the 12-month-old group, the PSTHs showed significant reduction at high frequencies (10.6 to 23.3 kHz) compared to the 2-month-old group. However, at low frequencies (4.8 to 8.7 kHz), the 12-month old group showed no difference or even slightly higher peaks compared to the 2-month-old group. These results suggest there may be a frequency sensitivity shift from high to low with age.
To quantify the frequency specific difference on the mean PSTH response at different age groups, the averaged PSTHs at 80 dB SPL (4.8 to 23.3 kHz) for all the neurons recorded in each of age group were calculated (Figure 9). A one-way ANOVA was performed on the mean PSTH in the 0 to 60 ms response window calculated at different tested frequencies. The mean PSTH in the 6 month group was significantly higher at 8.7 kHz and 10.6 kHz compared to the 2 and 12 month group, but not at other frequencies (one-way ANOVA, df =2, *** P<0.0001). In the 12 month group, the mean PSTH showed a significant reduction at high frequencies (10.6 to 19.1 kHz) compared to the 2-month group (one-way ANOVA, df =2, *** P<0.0001). However, at low frequencies (4.8 to 8.7 kHz), the average PSTH in the 12-month group was not significantly different from the 2-month group. These results suggest there was a frequency-dependent enhancement in the IC response at 8.7 and 10.6 kHz in the 6-month group, consistent with the rate-level function results (Figure 8).
Figure 9.
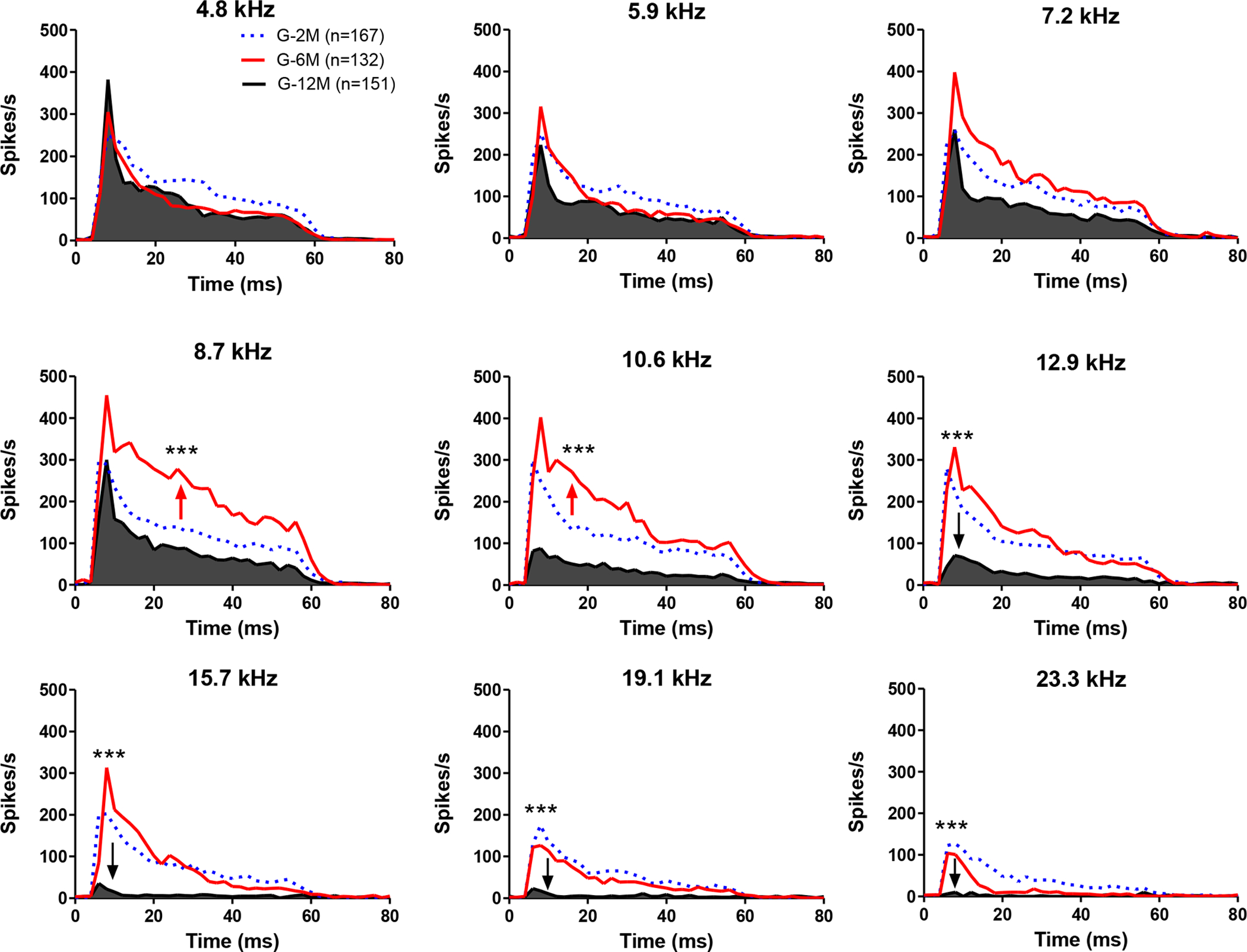
Examples of the peristimulus time histograms (PSTHs) recorded from 2, 6, and 12-month-old mice. PSTHs were elicited by tone bursts at different intensities (10 to 90 dB SPL) at 4.8 kHz (A), 8.7 kHz (B) and 15.7 kHz (C). (A) At 4.8 kHz, the PSTHs showed a significant reduction at low intensities (<50 dB SPL) and no obvious decrease at supra-threshold levels (>60 dB SPL) in the 6 month group (red, n = 132) and the 12 month group (black, n = 151) compared to 2 month group (blue, n=167). (B) At 8.7 kHz, the PSTHs showed a significant increase at high intensities (> 50 dB SPL) in the 6-month group (red) compared to the 2-month group (blue). The 12-month group showed a significant reduction at all tested intensities. (C) At 15.7 kHz, the peak amplitude of the PSTHs in the 6-month group showed a significant increase at high intensities (> 70 dB SPL) compared to the 2-month group (blue). In the 12-month group, there was no obvious response below 80 dB SPL.
Acoustic startle response
To determine the behavioral consequences of the high-frequency age-related hearing loss, the acoustic startle response was measured over three consecutive days in different age groups. The root-mean-square value of the startle amplitude (180 ms window after the onset of sound stimuli) was subtracted from baseline activity (Figure 10A). The averaged relative startle amplitudes as a function of intensity were generally much larger in the 6 month old mice compared to the 2 month and 12 month old groups at 4 and 8 kHz (two-way ANOVA, F=7.2, P<0.01 for 4 kHz and F=5.3, P<0.01 for 8 kHz, Figure 10B–C). At 16 kHz, the startle amplitudes in 2 month old mice was significantly greater than the 6 month and 12 month old mice at 100 dB SPL (two-way ANOVA, F=6.4, P<0.01, Bonferroni post-test, P<0.05, Figure 10D). These results indicate that the startle amplitude decreased at high frequencies, whereas the low frequency startle response increased significantly with age, consistent with the IC sound driven activity changes.
Figure 10.
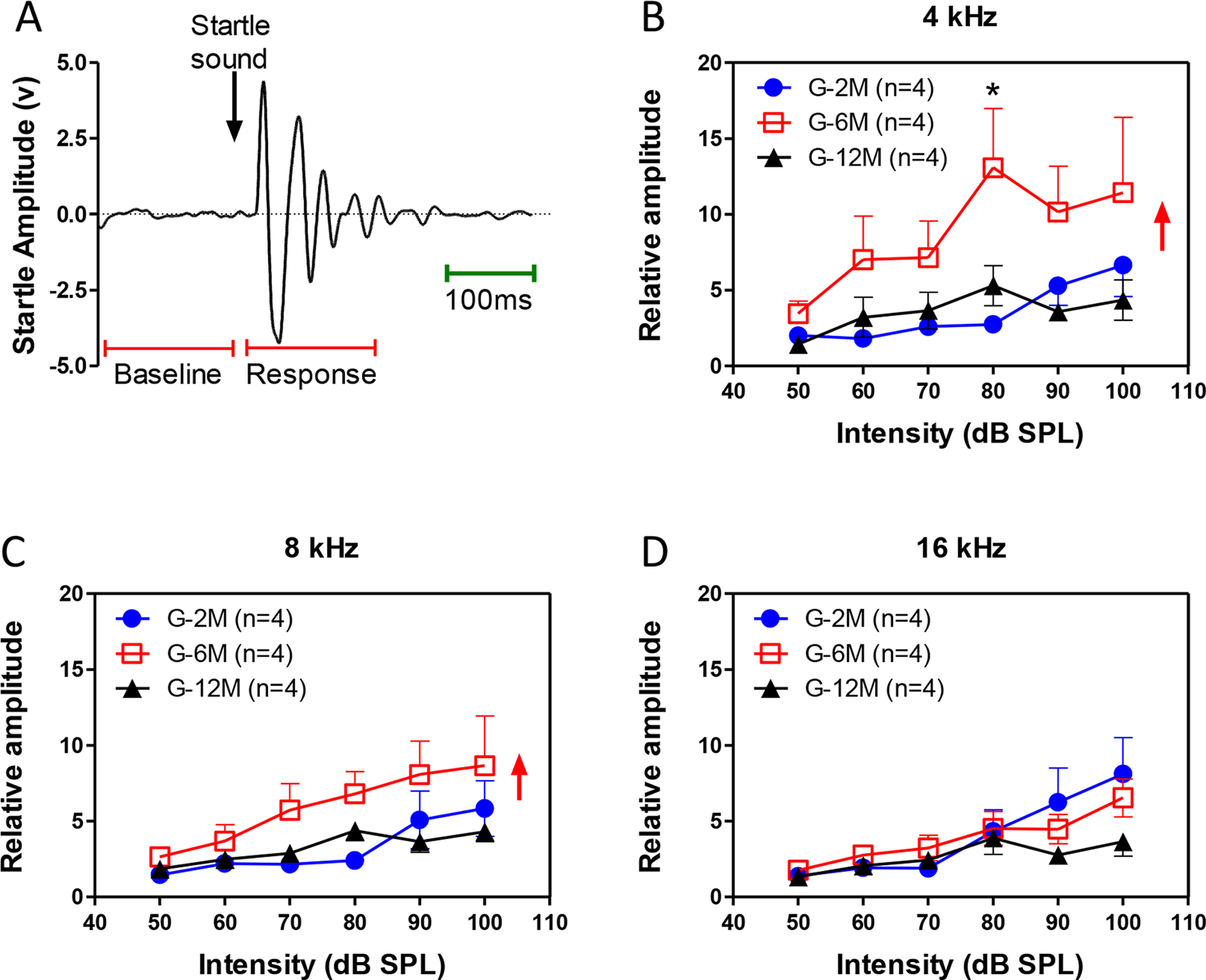
Response of acoustic startle reflex (ASR) of 2, 6 and 12 month old mice elicited by 4, 8, 16 kHz sound stimuli. (A) A raw startle response recorded from a mouse. (B) At 4 kHz, the mean amplitude of ASR in the 6-month-old mice was significantly higher than for 2 and 12 month old mice. (C) At 8 kHz, The ASR in the 6-month-old mice was also significant higher than the 2-month-old mice, but not to the 12-month-old mice. (D) At 16 kHz, the amplitude of ASR in the 6 and 12-month group was significantly lower than the 2-month-old mice.
Discussion
The goal of this study was to identify functional changes in the IC related to age- and age-related hearing loss. The three major findings were: (1) The distribution of CFs in the IC was truncated with age such that the proportion of high CFs neurons decreased while proportion of low CF neurons increased. The frequency shift, which has been shown previously by Willott (Willott, 1986), is reminiscent to the tonotopic map reorganization seen in the AC after noise induced hearing loss (Eggermont, 2005; Engineer et al., 2011). (2) The SFRs of IC neurons increased significantly in the 6 month and 12 month old mice compared to the 2 month old mice, this increase is similar that seen in chronic noise induced high-frequency hearing loss (Dong et al., 2010). Previous studies have suggested that the increase in SFR could be a neural correlate of tinnitus (Robertson et al., 2013). (3) Mice in the 6-month group had significantly higher sound driven activities in 8 to 10 kHz IC neurons compared to other age groups. This hyperactivity is intriguing because it coincides with the increased startle amplitudes to low frequency stimuli. The sound evoked increase in firing rate and the startle reflex could be the neural and behavioral correlates of hyperacusis associated with age-related hearing loss.
CF profiles and spontaneous activity changes with age-related hearing loss
The prevalence of tinnitus increases dramatically with age (Heller, 2003). Some of the common neurophysiological mechanisms for tinnitus include increased spontaneous rates in cortical and subcortical structures, increases in neural synchrony and the tonotopic map reorganization (Eggermont et al., 2004; Roberts et al., 2010). While we did not directly measure tonotopy in terms of CF vs. IC location, we did observe a dramatic change in the CF profile, i.e., the proportion of neurons with different CFs. In our study, a large region of IC neurons are tuned to 8–16 kHz, but very few IC neurons with CFs were above 16 kHz in the six-month old mice while no IC neurons were tuned above 12 kHz in 12 months-old mice. This change is not surprising given the progressive loss of high frequency hearing with advancing age in C57 mice. Our results suggest that the increased proportion of IC neurons responding to low frequency stimuli may be indicative of IC tonotopic reorganization. This change may be affected by the anatomical changes in inputs to the ventral IC, areas that represent low frequencies in the auditory brainstem nuclei sprout axon collaterals that then projected to non-tonotopic high frequency regions of the IC (Robinson et al., 2016; Wang et al., 2013; Willott et al., 2000).
SFRs of IC neurons increased with age (up to 6-month group); this increase was dominated by increases in IC neurons from 8–12 kHz, near the edge of the high-frequencies loss (~16 kHz). The largest increase occurred in the middle age group suggesting that the increase may be related with the onset of age-related hearing loss rather than the hearing loss severity, which increased with age. The increase in SFR in the middle age group may be due to homeostatic plasticity mechanisms attempting to compensate for the reduction in sound evoked activity. The decline in SFR between middle and old age group may be due to neural degeneration, loss of neurotrophic support or a decline in metabolic activity needed to main high SFR.
The rise and subsequent decline in SFR in our IC data may account for the age-related changes in tinnitus prevalence. In the 1999 – 2004 National Hearth Nutrition Examination Surveys, the prevalence of self-reported cases if frequent tinnitus increased with age until 60–69 years, after which it decreased with increasing age (Shargorodsky et al., 2010). Others have reported that tinnitus was more common in patients with presbycusis who have more severe degeneration of outer hair cells and stria vascularis (Terao et al., 2011). These results suggest that age could be an important factor contributing to the onset of tinnitus. Tinnitus is also strongly correlated occupational noise-induced hearing loss (Rosenhall et al., 1991). A recent Asian population study found that the prevalence of persistent tinnitus increased from 7% to 20.6% as average hearing loss increased from 25 to 55 dB HL (Chang et al., 2007). Taken together, these studies suggest that both hearing loss and aging contribute to the development of tinnitus. One possible approach to disentangle the contribution of age versus hearing loss and their relationship to SFR would be to measure SFR and hearing loss in hearing impaired mouse models that develop hearing loss at a much younger age than C57 mice.
Increased sound driven firing rates in age-related hearing loss
Loudness intolerance, known as hyperacusis, is a common symptom in tinnitus patients (Jastreboff et al., 2015). Although the cause of hyperacusis is still not clear, hearing loss is a risk factor that seems to be correlated with behavioral and neural hyperactivity. Hyperacusis-like augmentation of the acoustic startle response at high stimulus levels has been recorded in hamsters after noise exposure (Chen et al., 2013). Likewise, local field potentials in the central auditory system are enhanced after noise exposure (Salvi et al., 1990; Syka et al., 1994). Consistent with earlier reports, we observed increases in startle amplitude and sound evoked activity in C57 mice with age-related hearing loss. Our results are also consistent with Ison et al. (Ison et al., 2007); they observed large increase in startle responses elicited by intense 3 and 6 kHz stimuli between 6 and 10 months of age followed by a decline. In our study, we found that supra-threshold spike rates of IC neurons tuned to 8–10 kHz had increased significantly in the 6-month group compared to the young group. These results suggest that neural hyperactivity in the central auditory pathway, which compensates for the reduced neural output from the cochlea, may contribute to the enhancement of acoustic startle reflex amplitudes.
The hyperexcitability in the central auditory system caused by peripheral damage may be related with the reduction of inhibition in the central auditory system (Salvi et al., 2000; Sun et al., 2009). In the central auditory pathway, a neuron’s receptive field depends on the relative strength and overlap of excitatory and inhibitory inputs (Wehr et al., 2003; Wu et al., 2008). In normal hearing subjects, the excitatory and inhibitory inputs are maintained at a stable ratio, whereas in subjects with peripheral damage, this balance may be disrupted resulting in hyperactivity (Carcea et al., 2013). Our IC tuning curves became broader on the high frequency side and the rate-level functions become more monotonic in the middle-aged mice. These changes are consistent with the loss of side-band inhibition (Wang et al., 1996). This may explain the enhancement in spontaneous and sound driven activity near the frequencies showing a rapid increase in threshold (~16 kHz). In our 6-month age group, we found that IC spike rate-level functions increased significantly among neurons tuned to 8.7–10.6 kHz, but decreased in the 19.1–42 kHz. Together, these findings suggested that age-related hearing loss results in decreased inhibition in the CAS, which results in hyperexcitability in the IC neurons. Interestingly, reduced GABA-inhibition was observed in tinnitus patients, suggesting that reduced inhibition may contribute to tinnitus (Sedley et al., 2015).
4.4. Summary
Significant increases of SF and sound driven activity were observed in IC neurons in the C57 model of age-related heating loss. The changes were mainly seen at neurons with CFs at the border of the high frequency hearing loss. The sound evoked hyperactivity seen in the low frequency regions was associated with an increase in acoustic startle reflex amplitude in the same frequency region. Our results suggest that increased spontaneous firing rates and sound driven activities in the IC may be related with tinnitus and hyperacusis in aging population.
Highlights.
Inferior colliculus (ICs) neurons of 6 month old C57 mice showed a significant increase of spontaneous activity and suprathreshold sound driven activity, hyperexcitability linked to age-related cochlear hearing loss.
The characteristic frequency of the IC neurons showed a significant downshift from high to low frequency with progressive age-related cochlear hearing loss.
The age-related increase in spontaneous activity and suprathreshold sound driven activity may be linked to the upsurge in tinnitus and hyperacusis reported in elderly with age-related hearing loss.
Mice with age-related hearing loss showed a significant increase of acoustic startle response, consistent with the suprathreshold hyperactivity observed in low-CF IC neurons.
Acknowledgement
The study was supported by Chinese National Science Foundation (81528005) and NIH (R01DC014452)
References
- Carcea I, Froemke RC 2013. Cortical plasticity, excitatory-inhibitory balance, and sensory perception. Progress in brain research 207, 65–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HP, Chou P 2007. Presbycusis among older Chinese people in Taipei, Taiwan: a community-based study. International journal of audiology 46, 738–45. [DOI] [PubMed] [Google Scholar]
- Chen G, Lee C, Sandridge SA, Butler HM, Manzoor NF, Kaltenbach JA 2013. Behavioral Evidence for Possible Simultaneous Induction of Hyperacusis and Tinnitus Following Intense Sound Exposure. Journal of the Association for Research in Otolaryngology : JARO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Sheppard A, Salvi R 2016. Noise trauma induced plastic changes in brain regions outside the classical auditory pathway. Neuroscience 315, 228–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Li X, Liu L, Wang J, Lu CQ, Yang M, Jiao Y, Zang FC, Radziwon K, Chen GD, Sun W, Krishnan Muthaiah VP, Salvi R, Teng GJ 2015. Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. eLife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Mulders WH, Rodger J, Woo S, Robertson D 2010. Acoustic trauma evokes hyperactivity and changes in gene expression in guinea-pig auditory brainstem. The European journal of neuroscience 31, 1616–28. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ 2005. Tinnitus: neurobiological substrates. Drug discovery today 10, 1283–90. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ 2015. The auditory cortex and tinnitus - a review of animal and human studies. The European journal of neuroscience 41, 665–76. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE 2004. The neuroscience of tinnitus. Trends in neurosciences 27, 676–82. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP 2011. Reversing pathological neural activity using targeted plasticity. Nature 470, 101–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix RA 2nd, Portfors CV 2007. Excitatory, inhibitory and facilitatory frequency response areas in the inferior colliculus of hearing impaired mice. Hearing research 228, 212–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AJ 2003. Classification and epidemiology of tinnitus. Otolaryngologic clinics of North America 36, 239–48. [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen PD, O’Neill WE 2007. Age-Related Hearing Loss in C57BL/6J Mice has both Frequency-Specific and Non-Frequency-Specific Components that Produce a Hyperacusis-Like Exaggeration of the Acoustic Startle Reflex. Journal of the Association for Research in Otolaryngology : JARO 8, 539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff PJ, Jastreboff MM 2015. Decreased sound tolerance: hyperacusis, misophonia, diplacousis, and polyacousis. Handbook of clinical neurology 129, 375–87. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA 2000. Neurophysiologic mechanisms of tinnitus. Journal of the American Academy of Audiology 11, 125–37. [PubMed] [Google Scholar]
- Leong UC, Barsz K, Allen PD, Walton JP 2011. Neural correlates of age-related declines in frequency selectivity in the auditory midbrain. Neurobiology of aging 32, 168–78. [DOI] [PubMed] [Google Scholar]
- Niu Y, Kumaraguru A, Wang R, Sun W 2013. Hyperexcitability of inferior colliculus neurons caused by acute noise exposure. Journal of neuroscience research 91, 292–9. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA 2010. Ringing ears: the neuroscience of tinnitus. The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 14972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Bester C, Vogler D, Mulders WH 2013. Spontaneous hyperactivity in the auditory midbrain: relationship to afferent input. Hearing research 295, 124–9. [DOI] [PubMed] [Google Scholar]
- Robinson BL, Harper NS, McAlpine D 2016. Meta-adaptation in the auditory midbrain under cortical influence. Nature communications 7, 13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenhall U, Karlsson AK 1991. Tinnitus in old age. Scandinavian audiology 20, 165–71. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D 2000. Auditory plasticity and hyperactivity following cochlear damage. Hearing research 147, 261–74. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Saunders SS, Gratton MA, Arehole S, Powers N 1990. Enhanced evoked response amplitudes in the inferior colliculus of the chinchilla following acoustic trauma. Hearing research 50, 245–258. [DOI] [PubMed] [Google Scholar]
- Sedley W, Parikh J, Edden RA, Tait V, Blamire A, Griffiths TD 2015. Human Auditory Cortex Neurochemistry Reflects the Presence and Severity of Tinnitus. The Journal of neuroscience : the official journal of the Society for Neuroscience 35, 14822–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shargorodsky J, Curhan GC, Farwell WR 2010. Prevalence and characteristics of tinnitus among US adults. The American journal of medicine 123, 711–8. [DOI] [PubMed] [Google Scholar]
- Stolzberg D, Chen GD, Allman BL, Salvi RJ 2011. Salicylate-induced peripheral auditory changes and tonotopic reorganization of auditory cortex. Neuroscience 180, 157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Lu J, Stolzberg D, Gray L, Deng A, Lobarinas E, Salvi RJ 2009. Salicylate increases the gain of the central auditory system. Neuroscience 159, 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syka J, Rybalko N, Popelar J 1994. Enhancement of the auditory cortex evoked responses in awake guinea pigs after noise exposure. Hearing research 78, 158–168. [DOI] [PubMed] [Google Scholar]
- Szczepaniak WS, Moller AR 1996. Evidence of neuronal plasticity within the inferior colliculus after noise exposure: a study of evoked potentials in the rat. Electroencephalography & Clinical Neurophysiology 100, 158–64. [DOI] [PubMed] [Google Scholar]
- Terao K, Cureoglu S, Schachern PA, Morita N, Nomiya S, Deroee AF, Doi K, Mori K, Murata K, Paparella MM 2011. Cochlear changes in presbycusis with tinnitus. American journal of otolaryngology 32, 215–20. [DOI] [PubMed] [Google Scholar]
- Wang D, Xiong B, Xiong F, Chen GD, Hu BH, Sun W 2016. Apical hair cell degeneration causes the increase in the amplitude of summating potential. Acta oto-laryngologica, 1–6. [DOI] [PubMed] [Google Scholar]
- Wang F, Zuo L, Hong B, Han D, Range EM, Zhao L, Sui Y, Guo W, Liu L 2013. Tonotopic reorganization and spontaneous firing in inferior colliculus during both short and long recovery periods after noise overexposure. Journal of biomedical science 20, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Salvi RJ, Powers N 1996. Plasticity of response properties of inferior colliculus neurons following acute cochlear damage. Journal of neurophysiology 75, 171–83. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM 2003. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426, 442–6. [DOI] [PubMed] [Google Scholar]
- Willott JF 1986. Effects of aging, hearing loss, and anatomical location on thresholds of inferior colliculus neurons in C57BL/6 and CBA mice. Journal of neurophysiology 57, 391–408. [DOI] [PubMed] [Google Scholar]
- Willott JF, Turner JG 2000. Neural plasticity in the mouse inferior colliculus: relationship to hearing loss, augmented acoustic stimulation, and prepulse inhibition. Hearing research 147, 275–81. [DOI] [PubMed] [Google Scholar]
- Willott JF, Parham K, Hunter KP 1988a. Response properties of inferior colliculus neurons in young and very old CBA/J mice. Hearing research 37, 1–14. [DOI] [PubMed] [Google Scholar]
- Willott JF, Parham K, Hunter KP 1988b. Response properties of inferior colliculus neurons in middle-aged C57BL/6J mice with presbycusis. Hearing research 37, 15–27. [DOI] [PubMed] [Google Scholar]
- Wu FJ, Jen PH 2008. GABA-mediated modulation of the discharge pattern and rate-level function of two simultaneously recorded neurons in the inferior colliculus of the big brown bat, Eptesicus fuscus. The Chinese journal of physiology 51, 13–26. [PubMed] [Google Scholar]


