Abstract
The epidemiological association between exposure to particulate matter (PM10) and various respiratory and cardiovascular problems is well known, but the mechanisms driving these effects remain unclear. Neutrophils play an essential role in immune defense against foreign agents and also participate in the development of inflammatory responses. However, the role of these cells in the PM10 induced inflammatory response is not yet fully established. Thus, this study aims to evaluate the effect of PM10 on the neutrophil-mediated inflammatory response. For this, neutrophils from healthy adult human donors were in vitro exposed to different concentrations of PM10. The cell viability and cytotoxic activity were evaluated by MTT. LDH, propidium iodide and reactive oxygen species (ROS) were quantified by flow cytometry. Interleukin 8 (IL-8) expression, peptidyl arginine deiminase 4 (PAD4), myeloperoxidase (MPO), and neutrophil elastase (NE) expression were measured by RT-PCR. IL-8 was also quantified by ELISA. Fluorescence microscopy was used to evaluate neutrophil extracellular traps (NETs) release. The in vivo inflammatory responses were assessed in BALB/c mice exposed to PM10 by histopathology and RT-PCR. The analysis shows that PM10 exposure induced a cytotoxic effect on neutrophils, evidenced by necrosis and LDH release at high PM10 concentrations. ROS production, IL-8, MPO, NE expression, and NETs release were increased at all PM10 concentrations assessed. Neutrophil infiltration in bronchoalveolar lavage fluid (BALF), histopathological changes with inflammatory cell infiltration, and CXCL1 expression were observed in PM10-treated mice. The results suggest that lung inflammation in response to PM10 could be mediated by neutrophils activation. In this case, these cells migrate to the lungs and release pro-inflamatory mediators, including ROS, IL-8, and NETs. Thus, contributing to the exacerbation of respiratory pathologies, such as allergies, infectious and obstructive diseases.
Subject terms: Innate immune cells, Innate immunity, Environmental impact, Biological techniques
Introduction
Air pollution represents a major threat to human health1. According to the World Health Organization (WHO), exposure to air pollutants is the cause of 4.2 million premature deaths per year2. This mortality is related to adverse respiratory and cardiovascular health effects caused by exposure to particulate matter (PM)3,4.
Particulate pollutants are originated from the natural environment (volcanoes, forest fires and dust storms) and human activities (transportation, industry, power plants, combustion, and agriculture)5. According to their aerodynamic diameter, they are classified into particles including PM10 (less than 10 µm); PM 2.5 (less than 2.5 µm), and ultrafine particles (UFP, less than 0.1 µm)6,7. PM can penetrate the human respiratory tract and even reach circulation due to its small size, large surface area, penetration capacity, deposition, bioavailability, and long residence time in the air8. Exposure to PM can induce lung damage by oxidative stress and airway inflammation9, loss of immune functions against microorganisms10,11, thrombosis, coagulation, and vascular dysfunction that can lead to the development of different diseases12–14. Epidemiological and toxicological studies related to PM have shown positive correlations with the development of a wide range of diseases, including skin diseases15, asthma16, stroke, and ischemic heart disease17, chronic obstructive pulmonary disease (COPD)18 and lung cancer19.
Although the pathogenesis of diseases produced by PM exposure is not completely clear, several studies have demonstrated mechanisms potentially associated with innate and adaptive immune alterations. These processes include cytotoxicity, production of cytokines and pro-inflammatory molecules, mutagenicity, oxidative DNA damage, and genotoxicity20. Inflammatory responses activated by PM exposure may underlie asthma, chronic obstructive pulmonary disease, and lung cancer10,21.
Neutrophils constitute the most considerable fraction of leukocytes in the human body, with an essential role in lung immune response. These cells rapidly migrate to the lung, destroy the foreign agent and initiate an inflammatory response predominantly mediated by phagocytosis, ROS production, degranulation, and NETosis22. Neutrophil extracellular traps (NETs) are produced by the extracellular release of neutrophil nuclear material coated with antimicrobial peptides and enzymes, mainly activated by ROS production. NETosis activation is associated with exacerbated inflammatory response, tissue damage, and potentially, autoimmunity diseases23–25. Consequently, PM inhalation and deposition could induce ROS and oxidative stress molecules that induce neutrophil activation, NETs overproduction, and exacerbated inflammatory response.
Different in vivo studies have evidenced that PM exposure promotes neutrophil migration and lungs infiltration. Asthmatic BALB/c mice exposed to different concentrations of PM2.5 exhibited a significant increase in the frequency of eosinophils and neutrophils and high production of TNF-α in bronchoalveolar lavage fluid (BALF). Aberrant accumulation and altered functions of neutrophils in the lung are related to increased inflammation and tissue damage in people exposed to PM26. In this regard, it has been shown that neutrophils activated by PM exposure are accumulated in the pulmonary vasculature and induce an elevated release of inflammatory mediators such as myeloperoxidase (MPO)27 and leukotriene B4 (LTB4)28. Similarly, an in vitro study showed that neutrophil exposure to PM increased the release of (LTB4), leukotriene C4 (LTC4), and IL-829. These mediators may be responsible for oxidative and proteolytic tissue damage, leading to immune dysregulation and lung diseases.
Although different studies provide convincing evidence regarding PM exposure-related immune alterations, relatively few studies are available in the context of neutrophil-mediated inflammatory responses. Therefore, this study aimed to determine the in vivo and in vitro effect of PM10 on the neutrophil-mediated inflammatory response.
Materials and methods
Ethics statement
The study was performed according to the principles of the declaration of Helsinki and approved by the Ethical Committee of the Universidad Cooperativa de Colombia (certificate number 003/2018). The individuals enrolled provided signed informed consent forms.
Experiments in mice were approved by the corresponding Institutional Animal Care and Use Committees (CICUA), Universidad de Antioquia (certificate number 117), and performed following international guidelines and regulations.
The manuscript follows the recommendations in the ARRIVE guidelines.
Neutrophil isolation and culture
Neutrophilic polymorphonuclears (PMNs) were isolated from freshly drawn peripheral venous blood from healthy humans. Cells were separated by centrifugation at 3000 r.p.m for 40 min at room temperature in a double density gradient Ficoll/Histopaque 1119 and 1077 (Histopaque 1119 and 1077, Sigma—UK). The layer containing PMNs was then collected, washed twice with phosphate-buffered saline (PBS), and subsequently resuspended in RPMI medium (RPMI 1640, Gibco-USA) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich) and 1% penicillin–streptomycin (GIBCO Invitrogen, Breda, The Netherlands). Cell viability after isolation and before the start of each experiment was > 95%, as measured by the trypan exclusion test.
PM10 collection
PM10 was collected in Valle de Aburrá, Colombia, between January-June of 2018, due to the most intense air pollution episode of the year, and using the local environmental authority of Valle de Aburrá (Sistema de Alerta Temprana—SIATA). The PM10 sample was obtained from 100 quartz filters at ten BAM-1020 monitoring stations (TISCH Environmental, BM2000H). The PM10 filters were cut into small pieces; high purity sterile water was added, mixed by immersion, and sonicated for 2 h and 15 min at 37 Hz. The mixture was then filtered using six layers of sterile gauze. After distributing the collected solution into sterile vials and freeze-drying under a vacuum, the tube was weighed to determine the mass of the extracted particles and stored at 80 °C before biological testing. A vacuum lyophilizer (ALPHAI-2LDplus, Martin Christ, Osterode, Germany) was used to dry the PM10 to particulate powder.
Cell culture and treatment
PMNs (2.5 × 105 cells/well) were seeded in 96-well plates (Greiner-Bio-One, Solingen, Germany). Increasing concentrations (0.1, to 100 µg/mL) of PM10 were prepared in RPMI 1640 medium. Subsequently, the freshly prepared PM10 solutions were added to the cells at a maximum volume of 200 µL per well. After exposure at 37 °C and 5% CO2 for 5 h, the supernatant was removed and used for LDH analysis or stored at − 80 °C until further analysis (cytokine levels by ELISA). Cell pellets were used for total RNA extraction and MTT assay. As positive controls, we used cells stimulated with the following stimuli: MTT assays and LDH assay: 20% dimethyl sulfoxide (DMSO); RT-PCR and ELISA: 50 ng/mL lipopolysaccharide (LPS) (Invitrogen, San Diego, CA); flow cytometry: Zymosan; fluorescence microscopy: 50 nM Phorbol-12-myristate-13-acetate (PMA). In all assays, PM10 unexposed cells were used as a negative control. All reagents and materials used in the experiments were endotoxin-free.
Cytotoxicity assays
PM cytotoxic effect was evaluated using the colorimetric 3-(4,5-dimethylthiazol-2-yl) diphenyltetrazolium bromide (MTT) reduction, Lactate Dehydrogenase (LDH) release, and propidium iodide (PI) staining assays.
For MTT assay, PMNs were exposed to different concentrations of PM10 (0.1, 1, 10, 50, and 100 µg/mL) and 20% DMSO for 5 h. The supernatant was removed from each well, and the cells were washed with PBS. Then, the cells were incubated in a fresh medium containing 0.5 mg/mL MTT for 3 h at 37 °C. The formed crystalline formazan was dissolved in 100 µL DMSO. Finally, absorbance was measured at 570 nm using a microplate reader (Multiskan™ FC Microplate Photometer, Thermo Scientific). The data were normalized to untreated control cells.
LDH release in the PMNs culture medium was measured using the LDH toxicity assay kit (Roche, Germany) in supernatants of three different culture conditions: untreated PMNs (low control), treated with DMSO (high control) and exposed to different concentrations of PM10. After 5 h of culture, supernatants were transferred to a 96-well plate in triplicate and incubated for 30 min at room temperature with the reaction mixture. The final absorbance was measured at 490 nm using a microplate reader (Multiskan™ FC Microplate Photometer, Thermo Scientific). Cytotoxicity was calculated as: % cytotoxicity = [(LDH activity with stimulus (PM10)-(LDH activity low control)/(LDH activity high control-LDH activity low control)] × 100%.
For Propidium iodide (PI) staining assays (for necrosis), PMNs were cultured at a density of 1 × 106 on round coverslips and treated with different concentrations of PM10 (10, 50, and 100 µg/mL) for 30 min at 37 °C. The cells were washed, stained for 1 h, and observed with a fluorescent microscope.
RNA extraction and quantitative real-time PCR
IL-8, PAD4, NE, and MPO mRNA levels in PMNs exposed to different concentrations of PM10 (0.1, 1, 10, 50, and 100 µg/mL) and LPS (50 ng/ml) were determined by real-time PCR. Total RNA was isolated using the One-Step RNeasy Mini Kit extraction kit, according to the manufacturer's recommendations (Qiagen, Hilden, Germany). RNA concentration was quantified using a Nanodrop one spectrophotometer (Thermo Scientific). RNA was reverse transcribed into cDNA in a 20 μL reaction volume using the iScript High Capacity cDNA Reverse Transcription Kit, following the manufacturer's recommended protocol (Bio-Rad Laboratories, Hercules, CA). The qPCR was performed using SYBR Green Mastermix (Thermo Scientific, Waltham, MA) on a QuantStudio 3 real-time PCR detection system (Applied Biosystems). The list of primer sequences used to detect mRNAs is presented in Table 1. Relative gene expression levels obtained from reverse transcription qRT-PCR were calculated using the ΔΔCt method and normalized to phosphoglycerate kinase (PGK) gene expression.
Table 1.
Primers.
| Gen | Primers 5'-3' | Annealing temperature |
|---|---|---|
| IL-8 |
Fw: 5′-ACTGAGAGTGATTGAGAGTGGAC-3′ Rv: 5′-AACCCTCTGCACCCAGTTTTC -3′ |
60 °C |
| PAD4 |
FW: 5'-GGGGTGGTCGTGGATATTGC-3 ′ Rv: 5'-CCCGGTGAGGTAGAGTAGAGC-3 ′ |
64 °C |
| NE |
FW: 5′-GTGGCGAATGTAAACGTC-3 ′ Rv: 5′-CCGTTGAGCTGGAGAATC-3′ |
58 °C |
| MPO |
Fw: 5′-TGCTTCCTGGCAGGGGA-3′ Rv: 5′-CCACCTAG GGTTCAGGCTCT-3′ |
62 °C |
| PGK |
Fw: 5′-GTTGACCGAATCACCGACC -3′ Rv: 5′-CGACTCTCATAACGACCCGC -3′ |
60 °C |
Cytokine analysis
To investigate the effect of PM10 on IL-8 production, the concentration of this cytokine in cell culture supernatants was determined using a commercial ELISA assay (BioLegend, San Diego, CA) according to the manufacturer's instructions. All samples were performed in triplicate. Cytokine concentrations were calculated from a standard curve of the corresponding recombinant human cytokine.
NETs induction
Round coverslips were treated with ethanol and poly-L-lysine (Sigma) then placed in a 12-well plate with PBS. 5 × 105 PMNs were added per well with the following stimuli: (a) PMA as positive control; (b) 10 µg/ml of PM10, (c) 50 µg/ml of PM10, and (d) 100 µg/ml of PM10, PBS was used as a negative control. The cells were incubated without FBS for 3 h at 37 °C in the presence of 5% CO2, then fixed with 1 mL of 4% paraformaldehyde for 20 min at room temperature. Cell's DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI) and incubated at 4 °C overnight.
Afterward, cells were permeabilized with Triton X-100 and blocked with 1% BSA for 30 min at 37 °C. Then 1 mL of anti-neutrophil elastase (1:500, Abcam) and anti-myeloperoxidase (1:500, Abcam) primary antibodies were added to each well, and the plate was incubated for 1 h at 37 °C. 1 mL of anti-rabbit IgG (1:1000, Abcam) Alexa 488 and anti-rabbit IgG (1:500, Abcam) Alexa 647 secondary antibodies were then applied, the plate was incubated for 1 h at 37 °C. DNA, Neutrophil elastase (NE), and Myeloperoxidase (MPO) were identified by immunofluorescence. Images of random fields were taken through the 20 × and 40 × objectives. Each experiment was repeated at least three times.
Quantification of ROS production
Intracellular ROS production in PMNs exposed to PM10 was measured with the DHR-123 ROS dihydrorhodamine assay kit (Thermo Fisher Scientific, USA), according to the manufacturer's protocol. Briefly, PMNs (5 × 105 cells) were cultured in flow cytometry tubes (BD Falcon polystyrene) and exposed to different concentrations of PM10 (0.1, 1, 10, and 50 µg/mL) and zymosan (10 µg/mL) for 30 min. An unstimulated control tube was included in each assay. Cells were then washed with PBS, and 100 μL PBS supplemented with DHR-123 at a concentration of 0.07 μg/mL was added and incubated at 37 °C for one hour. Cells were then washed and resuspended in 200 μL of PBS. The analysis was performed on a BD LSR Fortessa™, and the collected data were analyzed in FlowJo v10.4 software (FlowJo, LLC). The neutrophil population was classified by forward scatter (FSC), and side scatter (SSC), where at least 10,000 events were recorded. Doublets were excluded by forward scatter height (FSC-H) and forward scatter area (FSC-A). FL-1 channel mean fluorescence (FITC) was examined for an increase in Rho123 fluorescence representing ROS production in activated versus unstimulated cells (Fig. S1). ROS production was calculated by subtracting the percentage of ROS production of stimulated PMNs from the percentage of ROS of control PMNs.
Mice
8-weeks-old male wild-type BALB/c mice weighing 18–20 g (Charles River, Portage, MI, USA) were bred and housed at 22 ± 1 °C under a 12 h light/dark cycle with food and water ad libitum and maintained under specific pathogen-free conditions at the animal facility of the Sede de Investigación Universitaria—Universidad de Antioquia (Medellín, Colombia). At the end of the experiments, euthanasia was performed using ketamine/xylazine 100/10 mg/kg intraperitoneally.
Mice PM10 model and experimental design
To establish a mouse model for PM-induced airway inflammation, mice were exposed to 100 µg PM10 (in 50 µl PBS) per day by intranasal instillation for six days. Meanwhile, control mice were treated with the same volume of PBS.
Lungs were perfused with 1 mL PBS sterile to obtain bronchoalveolar lavage fluid (BALF). The BALF cells were evaluated by Wright's stain, and neutrophil counts were performed. The mice's lung tissues were fixed with 4% buffered paraformaldehyde (Sigma-Aldrich, USA) for 48 h and processed using standard histological techniques. After embedding in paraffin, Sections (5-μm thick) were prepared and stained with HE (Sigma-Aldrich, USA) to evaluate neutrophils infiltration for histopathological analysis. A pathologist analyzed the histopathological sections. RT-PCR was performed as described above.
Statistical analysis
For data analysis, GraphPad® Prism 8.0.1 software. (San Diego, CA, USA) the software was used. Normality was determined using the Shapiro–Wilk test. Data were analyzed using the nonparametric Kruskal–Wallis test, followed by Dunn's multiple comparisons test. Values of p < 0.05 (*) were considered significant, and values of p < 0.01 (**) and p < 0.001 (***) were considered highly significant. Only statistically significant differences are stated with asterisks in each figure. All data are expressed as the mean ± standard error of the mean (SEM).
Results
PM10 did not reduce cell viability but increased plasma membrane damage and necrosis in PMNs
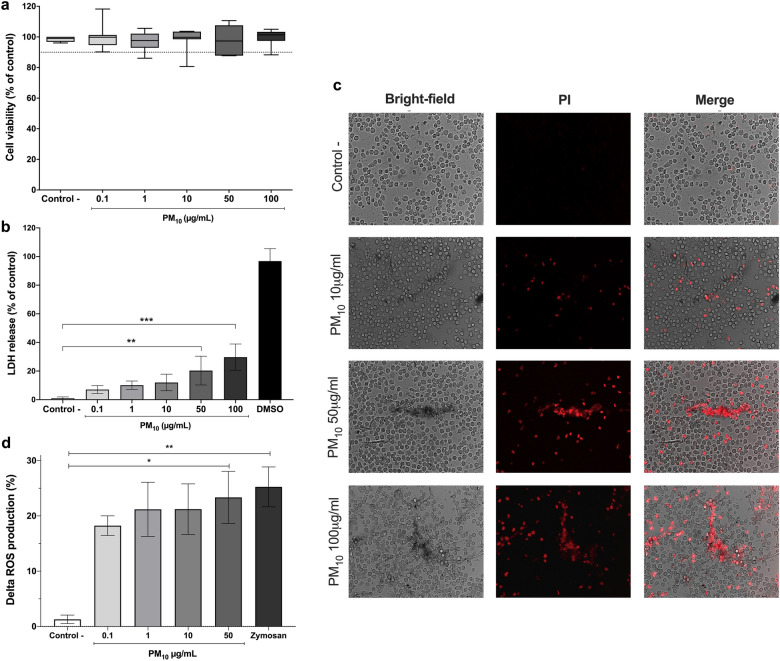
Cell viability was measured by MTT assay. PMNs exposed to 0.1, 1, 10, 50 and 100 µg/mL PM10 for 5 h did not show a significant concentration-dependent decrease in cell viability (Fig. 1a). However, 5 h after exposure of PMNs to the different PM10 concentrations, LDH release was increased in the two highest concentrations (50 µg/mL: 20.33 ± 2.77% and 100 µg/mL: 29.72 ± 3.18%) compared to control cells (p < 0.05) (Fig. 1b). As shown in Fig. 1c, stimulation of neutrophils with PM10 showed increased red fluorescence compared to control. Thus, PM10-induced necrosis is confirmed.
Figure 1.
Cytotoxic effects of PM10 and ROS production in PMNs. (a) Cell viability assay by MTT and (b) LDH release in PMNs exposed to different concentrations of PM10 (0.1, 1, 10, 10, 50 and 100 µg/mL) for 5 h. Negative control (culture medium); positive control (DMSO). (c) Necrotic effects in PMNs exposed to different concentrations of PM10 (10, 50, and 100 µg/mL) for 30 min by PI staining. Photographs of each slide were observed at 200X magnification. (d) Intracellular ROS production in PMNs exposed to different concentrations of PM10 (0.1, 1, 10, and 50 µg/mL) for 30 min, determined by flow cytometry. Negative control (culture medium); positive control (zymosan). ROS levels are presented as the mean fluorescent intensity of DHR of treated cells relative to control. All data are represented as mean ± SEM of 8–14 healthy donors; *p < 0.05; **p < 0.01; ***p < 0.001, by Kruskal–Wallis analysis with Dunn's post hoc test.
Exposure to PM10 leads to ROS formation in PMNs
ROS production in response to increasing doses of PM10 was determined by flow cytometry to assess their oxidative properties. An increase in ROS production was observed at all doses evaluated. However, there was a statistically significant increase from 50 µg/mL PM10 to the negative control (Fig. 1d).
Exposure to PM10 induces IL-8 expression in PMNs
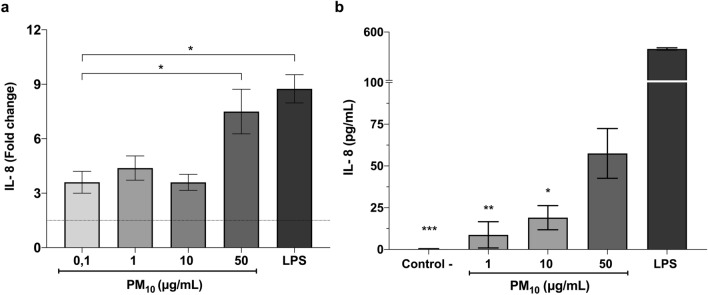
The IL-8 expression was quantified in PMNs after 5 h of PM10 exposure. IL-8 mRNA expression is significantly increased (7.49-fold) in PMNs after exposure to PM10 (50 μg/mL) (p < 0.05; vs. control group, Fig. 2a). Regarding IL-8 protein levels in PMNs supernatants, only a slight increase was observed at 50 μg/mL compared with the untreated control (Fig. 2b).
Figure 2.
PM10 exposure induces IL-8 in PMNs. (a) IL-8 mRNA expression and (b) IL-8 release in PMNs exposed to different concentrations of PM10 (0.1, 1, 10 and 50 µg/mL) for 5 h. Negative control (culture medium); positive control (LPS). The dotted line illustrates the 1.5-fold increase over the untreated negative control. All data are represented by the mean ± SEM of 8 healthy donors. * p < 0.05; **p < 0.01; ***p < 0.001, by Kruskal–Wallis analysis with post hoc Dunn's test. In (b), the asterisk represents differences compared with the LPS stimulated cells.
Exposure to PM10 induces the formation of NETs in PMNs
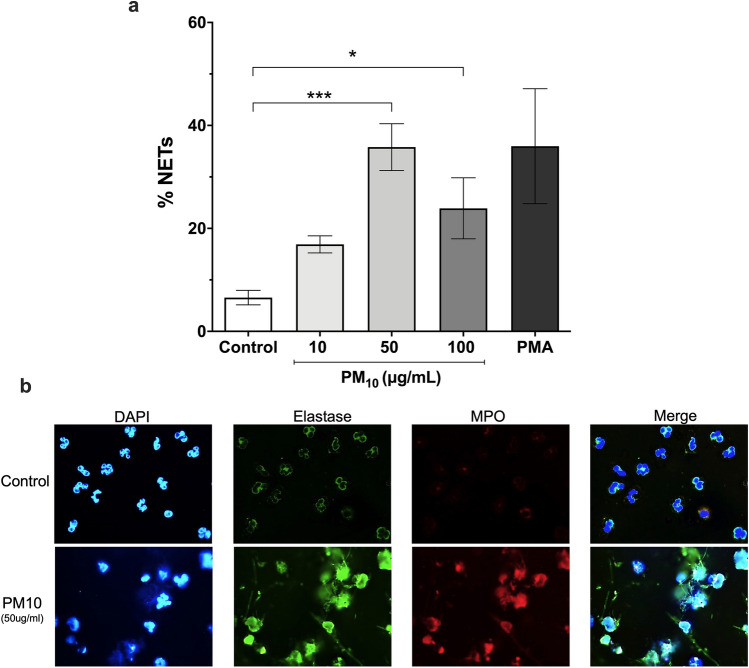
The formation of NETs in vitro in PMNs in response to PM10 was observed with 50 and 100 μg/mL of PM10 (35.76 and 23.90%, respectively) after 3 h, compared to unstimulated PMNs (p < 0.05; negative control) (Fig. 3a). Representative fluorescence photographs of NETs induction in PMNs, mediated by PM10, negative (unstimulated cells) and positive (PMA) controls are shown in Fig. 3b.
Figure 3.
NETs formation in PMNs exposed to PM10. PMNs were exposed to 10, 50, and 100 μg/mL PM10 for 3 h. Negative control (culture medium); positive control (PMA). (a) NETs release was analyzed microscopically, and MPO, NE, and DNA were quantified with ImageJ software. (b) Representative fluorescence microscopy images of NETs. Images show colocalization of MPO (red), NE (green) with DNA fibers released (blue) from released NETs. All data are represented as mean ± SEM of 9 healthy donors. *p < 0.05; ***p < 0.001 by Kruskal–Wallis analysis with Dunn's post hoc test.
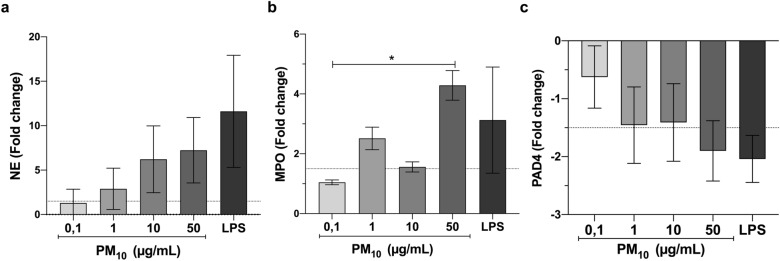
Neutrophil exposure to PM10 causes altered expression of NETs-associated genes
Gene expression of NE, MPO, and PAD4 was quantified in PMNs after PM10 exposure. MPO and NE mRNA expression increased in PMNs after PM10 exposure for 5 h (Fig. 4a,b). The PAD4 mRNA expression exhibited a negative regulation in PMNs after PM10 exposure (Fig. 4c).
Figure 4.
PM10 exposure alters NETS-associated gene expression in PMNs. mRNA expression of (a) NE (b) MPO and (c) PAD4 in PMNs exposed to different concentrations of PM10 (0.1, 1, 10 and 50 µg/mL) for 5 h. Negative control (culture medium); positive control (50 ng/ml LPS). The dotted line illustrates the value of 1.5-fold change compared with the untreated negative control. All data are represented as mean ± SEM of 15–18 healthy donors. *p < 0.05 by Kruskal–Wallis analysis with Dunn's post hoc test.
In vivo experiments with BALB/c Mice
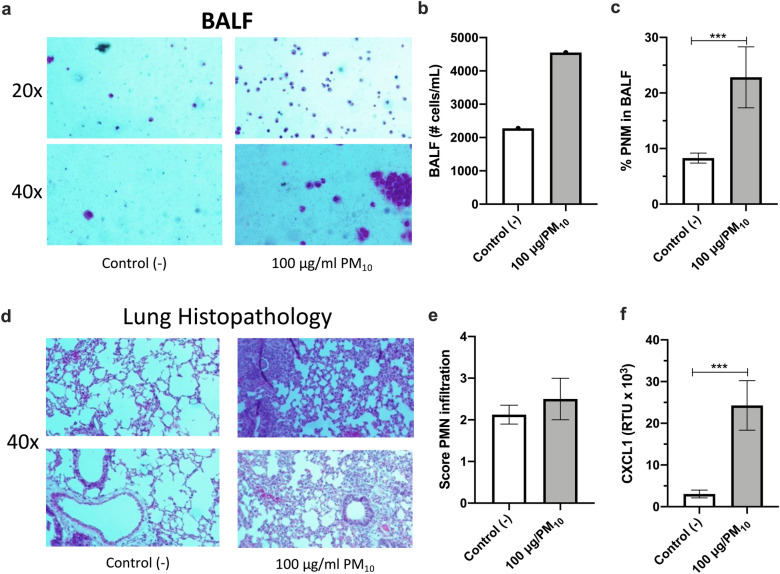
The cellular infiltrate in the BALF of PM10-exposed mice was analyzed to investigate the effects on lung inflammation induced by PM10 (Fig. 5a). PM10 induced an increase in the number of total inflammatory cells (Fig. 5b) and a statistically significant increase in the percentage of PMNs (Fig. 5c) in the BALF of exposed mice relative to controls. Histopathological changes in the lungs of BALB/c mice exposed to PM10 were also evaluated (Fig. 5d). We found pathological alterations with inflammatory cell infiltration and moderate PMNs infiltration score in the alveoli of PM10-exposed mice (Fig. 5e). In addition, we observed that PM10 increased mouse CXCL1 mRNA expression in the lung lysate of exposed mice compared to PBS controls (Fig. 5f).
Figure 5.
Intranasal treatment with PM10 increases cellularity and inflammatory infiltrate in the lung. BALB/c mice (n = 9 per group) were treated intranasally with 100 µg PM10 for five days. On day six post-treatment, the animals were euthanized, and bronchoalveolar lavage and lung tissue samples were collected. Wright's staining (a) quantification of total inflammatory cells (b) and quantification of PMNs in BALF (c) Representative images (d) and an inflammatory score of PMNs infiltrate (e) in hematoxylin–eosin stained lung sections, 1 = no infiltrate, 2 = mild infiltrate, 3 = moderate infiltrate, 4 = severe infiltrate. CXCL1 mRNA levels in lung lysate were measured by RT-PCR (f). All data are represented as mean ± SEM. ***p < 0.001 by Wilcoxon test.
Discussion
Inflammation caused by exposure to PM is involved in the pathogenesis of numerous diseases, such as COPD, asthma, lung cancer, cardiovascular and neurodegenerative diseases30. In a systematic review, we recently summarized different in vivo studies demonstrating that PM10 reaches the bronchi with a predominant infiltration of neutrophils31. Therefore, in this study, we evaluate the effect of PM10 on the in vitro and in vivo neutrophil-mediated inflammatory response.
In the in vitro model, no differences were observed in cell viability/metabolism when human neutrophils were exposed to increasing concentrations of PM10 up to 100 µg/mL (Fig. 1a). Different studies have shown that cellular exposure to PM10 decreased concentration- and time-dependent cell viability32,33. However, this is not consistent with the results of our investigation. On the other hand, two types of programmed cell death, pyroptosis, and necroptosis, are characterized by forming pores in the cell membrane and altering it, allowing the release of LDH34,35. In the present study, exposure to PM10 for 5 h increased LDH release at the highest concentrations (50 µg/mL: 20.33 ± 2.77% and 100 µg/mL: 29.72 ± 3.18%) in neutrophils (Fig. 1b). These results suggest that PM10 can alter the integrity of the neutrophil cell membrane, which can subsequently lead to cell death. Importantly, the assays used to analyze cytotoxicity in PMNs comprise respiratory chain activity (MTT) and membrane integrity (LDH). The latter is a much more sensitive assay, as it is based on the detection of LDH as an early event given by the loss of cell membrane integrity or damage. However, as mitochondrial metabolism is not yet affected, cytotoxicity is not detected by the MTT assay36.
On the other hand, necrosis, a passive and disordered cell death generated in response to physical damage or severe toxic stress, causes detrimental consequences such as induction of inflammation37. In our study, exposure of PMNs to PM10 induced necrosis, as demonstrated by increased red fluorescence of PI compared to control (Fig. 1c). Previous studies have described the necrotic effects of PM1038. In this study, PM10 exposure in the A549 cell line induced necrotic effects, probably due to the high metals content of PM. Therefore our data suggest that PM10 can generate different effects on PMNs, ultimately producing indirect toxicity.
Several factors are likely to contribute to the indirect toxic effects of PM, among which the most important may be the size and chemical composition39. However, there are no other studies on the cytotoxic effects of PM10 in neutrophils; some investigations have been carried out in vascular endothelial and epithelial cell lines of the respiratory tract, which corroborate the cytotoxic effects of PM1040. Likewise, in the BALF of murine models exposed to PM10, an increase in LDH concentrations has been demonstrated, evidencing epithelial injury41. Thus, exposure to these particles has been correlated with effects on human health31,42. Another study found that after a steel mill's closure, PM10 levels were decreased, and lower cases of respiratory admissions to hospitals were reported42. Interestingly, PM10 collected during this period produced less cytotoxicity and inflammatory response in human respiratory epithelial cells43. In addition, minor lung damage and neutrophilic inflammation in Sprague–Dawley rats were observed compared to PM10 collected while the steel mill was in operation44.
An additional factor that may contribute to the indirect toxic effects of PM10 is its ability to cause the generation of ROS, which in turn may initiate or exacerbate an inflammatory response. ROS are unstable molecules formed that play a significant role in the degradation of PM10 within the phagolysosome45. ROS species include hydroxyl radical (OH-), hypochlorous acid (HOCl), and hydrogen peroxide (H2O2)45,46. Their overabundance induces oxidative stress and reduces cell viability due to mitochondrial dysfunction, DNA damage, apoptosis, and genotoxicity47–49. In this study, we found that neutrophils exposed to PM10 presented an increase in ROS production (Fig. 1d). In this sense, our results agree with the study of Hitzfeld et al.50, who demonstrated that PM10 extracts collected in two German cities stimulated the production of ROS in human neutrophils. A recent report suggested that PM10 caused significant toxicity in the cardiovascular development of zebrafish larvae, as it led to an increase in the level of ROS and the expression of genes involved in endoplasmic reticulum stress and Nrf2 signaling pathway factors, which are essential in the regulation of cellular oxidative stress51.
In addition, to the indirect toxic effects of PM10 mediated by ROS, a recent study has shown that oxidative stress generated by PM exposure induces inflammatory responses by activating redox-sensitive transcription factors, which can lead to IL-8 expression in human bronchial epithelial cells52. Thus, scientific evidence has shown that IL-8 is a critical chemokine in airway inflammation induced by air pollutants53,54. In this study, we found up-regulation of IL-8 mRNA and IL-8 secretion in neutrophils exposed to PM10 (Fig. 2a,b). The secreted IL-8 mediates neutrophil recruitment into the lung and thus further amplifies inflammation in C57BL/6 mice exposed to cigarette smoke55. Oxidative stress and inflammation are essential factors in different respiratory and systemic diseases56. Dust particles can induce increased IL-8 transcriptional activity, leading to airway inflammation and exacerbation of asthma symptoms in human adults57. Concerning this, different studies have found that practices such as cooking with biomass are associated with increased IL-6, IL-8, and TNF-α, neutrophil infiltration, increased oxidative stress, hypertension, and tachycardia, increasing the risk of cardiovascular diseases58,59.
NETosis is a process that is highly dependent on ROS production60. In the present study, we observed that neutrophils produce NETs in response to PM10 than control (Fig. 3a,b). The beneficial role of NETs in infections is clear; however, new evidence has revealed a massive presence of NETs and neutrophils in the sputum of patients with cystic fibrosis and COPD61,62. Furthermore, NETs have recently been linked to severe clinical manifestations of COVID-19 and dysregulated immune-thrombosis in response to SARS-CoV-263, strongly suggesting the role of NETs in chronic airway diseases. Likewise our findings align with a recent work that found a significant increase in the percentage of human neutrophils forming NETs measured by flow cytometry after stimulation with diesel exhaust particles (another air pollutant)26. Similarly, has been found that neutrophils isolated from male BALB/c mice exposed to cigarette smoke produced more NETs in response to PMA compared to control mice64. To note the induction of NETs seems to be decreased in neutrophils exposed to 100 µg/mL PM10 compared to those exposed to 50 µg/mL (without statistical differences), this may be explained because PM10 particles at this concentration form aggregates and thus are less accessible to cells or as shown in Fig. 1c PM10 is inducing another type of cell death.
Our results suggest that exposure of human neutrophils to PM10 resulted in altered expression of NETs-associated genes because high expression of the NE gene mRNA, MPO, was observed (Fig. 4a,b). NETs are released by a programmed form of cell death characterized by chromatin decondensation, nuclear membrane disassembly, and cell membrane rupture65. NE and MPO play a central role in coordinating these processes66. Likewise, PAD4 is involved in NETosis through histone citrullination and chromatin decondensation65,66. However, our results show a negative regulation of the PAD4 gene in neutrophils stimulated with PM10 (Fig. 4c). In correlation to our results, scientific evidence demonstrates that various activating stimuli can mediate histone citrullination and PAD4 requirement in NETs formation67. Therefore, there is a large discrepancy in the role of PAD4 in NETs production, with several authors reporting that the involvement of PAD4 depends on the type of stimulus used68,69. In our investigation, PM was used to induce NETs. It is well known that PM10 from urban areas could contain lipopolysaccharide and activate NF-κB through the MyD88 pathway, dependent on the bound LPS70. Thus, NF-κB has been reported to regulate PAD4 in mouse and human cells differentially. For example, the human cell line HL-60 treated or not with TNF-α to activate the canonical NF-κB pathway showed a significant increase in IL-8 levels (a marker of NF-κB activation) and a significant decrease in PAD4 levels71.
Consistent with the in vitro results, our in vivo findings showed that five days after intranasal instillation with 100 µg/mL PM10, BALB/c mice exhibited airway inflammation and increased cellularity and PMNs in BALF (Fig. 5). In lung tissue, an increase in CXCL1 mRNA expression was evident and histological analysis showed pathological alterations and neutrophil recruitment with a moderate infiltration in the lung airspaces of PM10-exposed mice (Fig. 5). This inflammatory response could be partly due to the ability of PM10 to generate cellular cytotoxicity confirmed in vitro. Furthermore, although the composition of PM varies by region, our data are consistent with previous reports on neutrophilic inflammation. In the study by Yang et al.72, BALF and immunohistochemical staining of lung tissues in mice 12 h after PM10 exposure showed visible signs of inflammation neutrophil infiltration in the airways. Likewise, CXCL1 (human IL-8 homolog) expression has been demonstrated in the BALF of C57BL/6 mice exposed to diesel exhaust particulate matter (DEP), a significant component of particulate matter that induces neutrophil-dominant inflammatory responses. In general, acute neutrophil-dominated inflammation is a mechanism of the immune system to eliminate pathological agents, including PM. However, IL-8 secretion, infiltration, and overactivation of neutrophils at sites of inflammation provide a feedback loop leading to uncontrolled inflammation that could promote the development or progression of asthma, COPD, and other diseases73.
It is important to note that previous studies have suggested that PM10 exposure induces inflammatory cytokines production and immune cells recruitment, possibly due to their high content of transition metals74. On the other hand, recently published experimental studies evaluating the effects of air pollutant particles on inflammatory responses have focused mainly on PM2.5, which includes most of the particles derived from fossil fuel combustion75. While these particles pose a risk to human health, coarser particles such as PM10 should not go unnoticed because, as our data show, they are potentially cytotoxic and inflammatory.
In conclusion, our results suggest that neutrophils are critical players in PM10-induced inflammatory events. These molecules induce cytotoxicity, infiltration, and activation of neutrophils, leading to ROS production, NETs release, and MPO, NE, and IL-8 expression. Future studies should evaluate the effect of PM10 on other neutrophils responses, including their phagocytosis and tissue migration.
Supplementary Information
Acknowledgements
The authors would like to thank Sistema de Alerta Temprana (SIATA) and the Área Metropolitana del Valle de Aburrá (AMVA). We also acknowledge Carlos David Hoyos and Laura Herrera Mejia for their support in collecting the PM10 samples.
Authors contributions
Conceptualization: N.A.T., J.M.A.C., J.C.H. Writing—Original Draft: A.V., J.H.T.G. Writing—Review & Editing: J.F.Z.Z., J.M.A.C., P.O.H., D.M.G., N.A.T., J.C.H. Investigation: A.V., P.O.H., J.H.T.G. Methodology: D.M.G., J.M.A.C., J.C.H. Visualization: A.V., P.O.H., J.H.T.G. Formal analysis: J.M.A.C., J.C.H., N.A.T. Project administration: J.C.H.
Funding
This study was supported by Minciencias (Grant 141580763047), Corporación Universitaria Remington, Universidad Cooperativa de Colombia and Universidad Autónoma de Nayarit.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-11553-6.
References
- 1.Organización Mundial de la Salud. Las nuevas Directrices mundiales de la OMS sobre la calidad del aire tienen como objetivo evitar millones de muertes debidas a la contaminación del aire. https://www.who.int/news/item/22-09-2021-new-who-global-air-quality-guidelines-aim-to-save-millions-of-lives-from-air-pollution (2021).
- 2.World Health Organization. Ambient (outdoor) air pollution. https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health. pp 6–8 (2018).
- 3.Fasola S, et al. Effects of particulate matter on the incidence of respiratory diseases in the pisan longitudinal study. Int. J. Environ. Res. Public Health. 2020;17:2540. doi: 10.3390/ijerph17072540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang F, et al. Long-term exposure to fine particulate matter and cardiovascular disease in China. J. Am. Coll. Cardiol. 2020;75:707–717. doi: 10.1016/j.jacc.2019.12.031. [DOI] [PubMed] [Google Scholar]
- 5.Kelly FJ, Fussell JC. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ. 2012;60:504–526. doi: 10.1016/j.atmosenv.2012.06.039. [DOI] [Google Scholar]
- 6.United States Environmental Protection Agency (EPA). Particulate Matter (PM) Basics | US EPA. Particulate Matter (PM) Pollutionhttps://www.epa.gov/pm-pollution/particulate-matter-pm-basics (2020).
- 7.Traboulsi H, et al. Inhaled pollutants: The molecular scene behind respiratory and systemic diseases associated with ultrafine particulate matter. Int. J. Mol. Sci. 2017;18:243. doi: 10.3390/ijms18020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Billet S, et al. Ambient particulate matter (PM2.5): Physicochemical characterization and metabolic activation of the organic fraction in human lung epithelial cells (A549) Environ. Res. 2007;105:212–223. doi: 10.1016/j.envres.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Li N, et al. Use of a stratified oxidative stress model to study the biological effects of ambient concentrated and diesel exhaust particulate matter. Inhal. Toxicol. 2002;14:459–486. doi: 10.1080/089583701753678571. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Geng S, Prasad GL, Li L. Suppression of neutrophil antimicrobial functions by total particulate matter from cigarette smoke. Front. Immunol. 2018;9:2274. doi: 10.3389/fimmu.2018.02274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loaiza-Ceballos MC, Marin-Palma D, Zapata W, Hernandez JC. Viral respiratory infections and air pollutants. Air Qual. Atmos. Health. 2022;15:105–114. doi: 10.1007/s11869-021-01088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cano-Granda DV, Ramirez-Ramirez M, Gomez-Gallego DM, Hernandez JC, et al. Effects of particulate matter on endothelial, epithelial and immune system cells. Bionatura. 2022;7:1–7. doi: 10.21931/RB/2022.07.01.4. [DOI] [Google Scholar]
- 13.Baccarelli A, et al. Living near major traffic roads and risk of deep vein thrombosis. Circulation. 2009;119:3118–3124. doi: 10.1161/CIRCULATIONAHA.108.836163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim OJ, Lee SH, Kang SH, Kim SY. Incident cardiovascular disease and particulate matter air pollution in South Korea using a population-based and nationwide cohort of 02 million adults. Environ. Heal. Glob. A Access Sci. Sour. 2020;19:671. doi: 10.1186/s12940-020-00671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KE, Cho D, Park HJ. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016;152:126–134. doi: 10.1016/j.lfs.2016.03.039. [DOI] [PubMed] [Google Scholar]
- 16.Kiser D, et al. Particulate matter and emergency visits for asthma: A time-series study of their association in the presence and absence of wildfire smoke in Reno, Nevada, 2013–2018. Environ. Heal. Glob. A Access Sci. Source. 2020;19:2. doi: 10.1186/s12940-020-00646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nhung NTT, et al. Exposure to air pollution and risk of hospitalization for cardiovascular diseases amongst Vietnamese adults: Case-crossover study. Sci. Total Environ. 2020;703:134637. doi: 10.1016/j.scitotenv.2019.134637. [DOI] [PubMed] [Google Scholar]
- 18.Mbelambela EP, et al. Biomass energy, particulate matter (PM2.5), and the prevalence of chronic obstructive pulmonary disease (COPD) among Congolese women living near of a cement plant, in Kongo Central Province. Environ. Sci. Pollut. Res. 2020;27:40706–40714. doi: 10.1007/s11356-020-10099-2. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, et al. Association between particulate matter air pollution and lung cancer. Thorax. 2020;75:85–87. doi: 10.1136/thoraxjnl-2019-213722. [DOI] [PubMed] [Google Scholar]
- 20.Jaligama S, et al. Exposure to deepwater horizon crude oil burnoff particulate matter induces pulmonary inflammation and alters adaptive immune response. Environ. Sci. Technol. 2015;49:8769–8776. doi: 10.1021/acs.est.5b01439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra R, Krishnamoorthy P, Gangamma S, Raut AA, Kumar H. Particulate matter (PM10) enhances RNA virus infection through modulation of innate immune responses. Environ. Pollut. 2020;266:115148. doi: 10.1016/j.envpol.2020.115148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovalenko EI, et al. ROS production, intracellular HSP70 levels and their relationship in human neutrophils: Effects of age. Oncotarget. 2014;5:11800–11812. doi: 10.18632/oncotarget.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng IY, et al. Particulate matter increases the severity of bleomycin-induced pulmonary fibrosis through KC-mediated neutrophil chemotaxis. Int. J. Mol. Sci. 2020;21:227. doi: 10.3390/ijms21010227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bilyy R, Bila G, Vishchur O, Vovk V, Herrmann M. Neutrophils as main players of immune response towards nondegradable nanoparticles. Nanomaterials. 2020;10:1–14. doi: 10.3390/nano10071273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radermecker C, et al. Locally instructed CXCR4hi neutrophils trigger environment-driven allergic asthma through the release of neutrophil extracellular traps. Nat. Immunol. 2019;20:1444–1455. doi: 10.1038/s41590-019-0496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wooding DJ, et al. Acute air pollution exposure alters neutrophils in never-smokers and at-risk humans. Eur. Respir. J. 2020;55:1901495. doi: 10.1183/13993003.01495-2019. [DOI] [PubMed] [Google Scholar]
- 27.Nurkiewicz TR, et al. Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure. Environ. Health Perspect. 2006;114:412–419. doi: 10.1289/ehp.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee KH, et al. Effect of modifier structure on the activation of leukotriene A4 hydrolase aminopeptidase activity. J. Med. Chem. 2019;62:10605–10616. doi: 10.1021/acs.jmedchem.9b00663. [DOI] [PubMed] [Google Scholar]
- 29.Hitzfeld B, Friedrichs KH, Tomingas R, Behrendt H. Organic atmospheric dust extracts and their effects on functional parameters of human polymorphonuclear leukocytes (PMN) J. Aerosol Sci. 1992;23:531–534. doi: 10.1016/0021-8502(92)90466-9. [DOI] [Google Scholar]
- 30.Arias-Pérez RD, et al. Inflammatory effects of particulate matter air pollution. Environ. Sci. Pollut. Res. 2020;27:42390–42404. doi: 10.1007/s11356-020-10574-w. [DOI] [PubMed] [Google Scholar]
- 31.Valderrama A, Zapata MI, Hernandez JC, Cardona-Arias JA. Systematic review of preclinical studies on the neutrophil-mediated immune response to air pollutants, 1980–2020. Heliyon. 2022;8:e08778. doi: 10.1016/j.heliyon.2022.e08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steenhof M, et al. In vitro toxicity of particulate matter (PM) collected at different sites in the Netherlands is associated with PM composition, size fraction and oxidative potential–the RAPTES project. Part. Fibre Toxicol. 2011;8:26. doi: 10.1186/1743-8977-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xi M, Shen D, Dai P, Han G, Li C. TBHQ alleviates pyroptosis and necroptosis in chicken alveolar epithelial cells induced by fine particulate matter from broiler houses. Poult. Sci. 2022;101:101593. doi: 10.1016/j.psj.2021.101593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu W, Liu P, Li J. Necroptosis: An emerging form of programmed cell death. Crit. Rev. Oncol. Hematol. 2012;82:249–258. doi: 10.1016/j.critrevonc.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Wu X, et al. Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death Dis. 2018;9:2. doi: 10.1038/s41419-017-0013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Specian AFL, et al. LDH, proliferation curves and cell cycle analysis are the most suitable assays to identify and characterize new phytotherapeutic compounds. Cytotechnology. 2016;68:2729–2744. doi: 10.1007/s10616-016-9998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edinger AL, Thompson CB. Death by design: Apoptosis, necrosis and autophagy. Curr. Opin. Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Marchetti S, et al. In vitro lung toxicity of indoor PM10 from a stove fueled with different biomasses. Sci. Total Environ. 2019;649:1422–1433. doi: 10.1016/j.scitotenv.2018.08.249. [DOI] [PubMed] [Google Scholar]
- 39.Mandler WK, et al. In vitro toxicity assessment of respirable solid surface composite sawing particles. Toxicol. Ind. Health. 2020;36:250–262. doi: 10.1177/0748233720921683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hickey C, et al. Toxicity of particles emitted by fireworks. Part. Fibre Toxicol. 2020;17:4. doi: 10.1186/s12989-020-00360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li XY, Gilmour PS, Donaldson K, MacNee W. Free radical activity and pro-inflammatory effect of particulate air pollution (PM10) in vivo and in vitro. Thorax. 1996;51:1216–1222. doi: 10.1136/thx.51.12.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pope CA. Respiratory hospital admissions associated with PM10 pollution in Utah, Salt Lake, and Cache Valleys. Arch. Environ. Health. 1991;46:90–97. doi: 10.1080/00039896.1991.9937434. [DOI] [PubMed] [Google Scholar]
- 43.Frampton MW, et al. Effects of aqueous extracts of PM10 filters from the Utah Valley on human airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 1999;277:960–967. doi: 10.1152/ajplung.1999.277.5.L960. [DOI] [PubMed] [Google Scholar]
- 44.Dye JA, et al. Acute pulmonary toxicity of particulate matter filter extracts in rats: Coherence with epidemiologic studies in Utah valley residents. Environ. Health Perspect. 2001;109:395–403. doi: 10.1289/ehp.01109s3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curi R, et al. The critical role of cell metabolism for essential neutrophil functions. Cell. Physiol. Biochem. 2020;54:629–647. doi: 10.33594/000000245. [DOI] [PubMed] [Google Scholar]
- 46.Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: Implications for microbial killing. J. Biol. Chem. 2006;281:39860–39869. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- 47.Piao MJ, et al. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch. Toxicol. 2018;92:2077–2091. doi: 10.1007/s00204-018-2197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu R, et al. PM2.5 exposure elicits oxidative stress responses and mitochondrial apoptosis pathway activation in HaCaT keratinocytes. Chin. Med. J. 2017;130:2205–2214. doi: 10.4103/0366-6999.212942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hiura TS, Kaszubowski MP, Li N, Nel AE. Chemicals in diesel exhaust particles generate reactive oxygen radicals and induce apoptosis in macrophages. J. Immunol. 1999;163:5582–5591. [PubMed] [Google Scholar]
- 50.Hitzfeld B, Friedrichs KH, Ring J, Behrendt H. Airborne particulate matter modulates the production of reactive oxygen species in human polymorphonuclear granulocytes. Toxicology. 1997;120:185–195. doi: 10.1016/S0300-483X(97)03664-0. [DOI] [PubMed] [Google Scholar]
- 51.Cen J, et al. Particulate matter (PM10) induces cardiovascular developmental toxicity in zebrafish embryos and larvae via the ERS, Nrf2 and Wnt pathways. Chemosphere. 2020;250:126288. doi: 10.1016/j.chemosphere.2020.126288. [DOI] [PubMed] [Google Scholar]
- 52.Yoon JH, Jeong SH, Hong JH. The effect of therapeutic blockades of dust particles-induced Ca2+ signaling and proinflammatory cytokine IL-8 in human bronchial epithelial cells. Mediators Inflamm. 2015;2015:1–12. doi: 10.1155/2015/843024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berman R, Downey GP, Dakhama A, Day BJ, Chu HW. Afghanistan particulate matter enhances pro-inflammatory responses in IL-13-exposed human airway epithelium via TLR2 signaling. Toxicol. Sci. 2018;166:345–353. doi: 10.1093/toxsci/kfy217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holgate ST, et al. Health effects of acute exposure to air pollution. Part I: Healthy and asthmatic subjects exposed to diesel exhaust. Res. Rep. Health. Eff. Inst. 2003;2:1–67. [PubMed] [Google Scholar]
- 55.Lee KH, Lee CH, Jeong J, Jang AH, Yoo CG. Neutrophil elastase differentially regulates interleukin 8 (IL-8) and vascular endothelial growth factor (VEGF) production by cigarette smoke extract. J. Biol. Chem. 2015;290:28438–28445. doi: 10.1074/jbc.M115.663567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Eeden SF, Yeung A, Quinlam K, Hogg JC. Systemic response to ambient particulate matter: Relevance to chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2005;2:61–67. doi: 10.1513/pats.200406-035MS. [DOI] [PubMed] [Google Scholar]
- 57.Watanabe M, et al. Effects on asthma and induction of interleukin-8 caused by Asian dust particles collected in western Japan. J. Asthma. 2014;51:595–602. doi: 10.3109/02770903.2014.903965. [DOI] [PubMed] [Google Scholar]
- 58.Dutta A, Roychoudhury S, Chowdhury S, Ray MR. Changes in sputum cytology, airway inflammation and oxidative stress due to chronic inhalation of biomass smoke during cooking in premenopausal rural Indian women. Int. J. Hyg. Environ. Health. 2013;216:301–308. doi: 10.1016/j.ijheh.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Dutta A, Ray MR, Banerjee A. Systemic inflammatory changes and increased oxidative stress in rural Indian women cooking with biomass fuels. Toxicol. Appl. Pharmacol. 2012;261:255–262. doi: 10.1016/j.taap.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Vorobjeva NV, Chernyak BV. NETosis: Molecular mechanisms role in physiology and pathology. Biochem. Mosc. 2020;85:1178–1190. doi: 10.1134/S0006297920100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dwyer M, et al. Cystic fibrosis sputum DNA has NETosis characteristics and neutrophil extracellular trap release is regulated by macrophage migration-inhibitory factor. J. Innate Immun. 2014;6:765–779. doi: 10.1159/000363242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grabcanovic-Musija F, et al. Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with airflow limitation. Respir. Res. 2015;16:2. doi: 10.1186/s12931-015-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Middleton EA, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qiu SL, et al. Neutrophil extracellular traps induced by cigarette smoke activate plasmacytoid dendritic cells. Thorax. 2017;72:1084–1093. doi: 10.1136/thoraxjnl-2016-209887. [DOI] [PubMed] [Google Scholar]
- 65.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 66.Agraz-Cibrian JM, Giraldo DM, Urcuqui-Inchima S. 1,25-Dihydroxyvitamin D3 induces formation of neutrophil extracellular trap-like structures and modulates the transcription of genes whose products are neutrophil extracellular trap-associated proteins: A pilot study. Steroids. 2019;141:14–22. doi: 10.1016/j.steroids.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Guiducci E, et al. Candida albicans-induced NETosis is independent of peptidylarginine deiminase 4. Front. Immunol. 2018;9:1. doi: 10.3389/fimmu.2018.01573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holmes CL, et al. Insight into neutrophil extracellular traps through systematic evaluation of citrullination and peptidylarginine deiminases. J. Immunol. Res. 2019;2019:1–11. doi: 10.1155/2019/2160192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Claushuis TAM, et al. Role of peptidylarginine deiminase 4 in neutrophil extracellular trap formation and host defense during Klebsiella pneumoniae—induced pneumonia-derived sepsis. J. Immunol. 2018;201:1241–1252. doi: 10.4049/jimmunol.1800314. [DOI] [PubMed] [Google Scholar]
- 70.Song Y, et al. Lipopolysaccharide attached to urban particulate matter 10 suppresses immune responses in splenocytes while particulate matter itself activates NF-κB. Toxicol. Res. (Camb) 2016;5:1445–1452. doi: 10.1039/C6TX00216A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abbas AK, et al. Negative regulation of the peptidylarginine deiminase type IV promoter by NF-κB in human myeloid cells. Gene. 2014;533:123–131. doi: 10.1016/j.gene.2013.09.108. [DOI] [PubMed] [Google Scholar]
- 72.Yang C, Kwon DI, Kim M, Im SH, Lee YJ. Commensal microbiome expands Tγδ17 cells in the lung and promotes particulate matter-induced acute neutrophilia. Front. Immunol. 2021;12:645741. doi: 10.3389/fimmu.2021.645741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saygın M, et al. To investigate the effects of air pollution (PM10 and SO2) on the respiratory diseases asthma and chronic obstructive pulmonary disease. Turk. Thorac. J. 2017;18:33–39. doi: 10.5152/TurkThoracJ.2017.16016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu X, et al. Inflammatory response to fine particulate air pollution exposure: Neutrophil versus monocyte. PLoS ONE. 2013;8:71414. doi: 10.1371/journal.pone.0071414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeong S, et al. PM2.5 exposure in the respiratory system induces distinct inflammatory signaling in the lung and the liver of mice. J. Immunol. Res. 2019;2019:1–11. doi: 10.1155/2019/3486841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].