Abstract
Background
Currently nanomedicines are the focus of attention from researchers and clinicians because of the successes of lipid-nanoparticles-based COVID-19 vaccines. Nanoparticles improve existing treatments by providing a number of advantages including protection of cargo molecules from external stresses, delivery of drugs to target tissues, and sustained drug release. To prevent premature release-related side effects, stable drug loading in nanoformulations is required, but the increased stability of the formulation could also lead to a poor drug-release profile at the target sites. Thus, researchers have exploited differences in a range of properties (e.g., enzyme levels, pH, levels of reduced glutathione, and reactive oxygen species) between non-target and target sites for site-specific release of drugs. Among these environmental stimuli, pH gradients have been widely used to design novel, responsive nanoparticles.
Area covered
In this review, we assess drug delivery based on pH-responsive nanoparticles at the levels of tissues (tumor microenvironment, pH ~ 6.5) and of intracellular compartments (endosome and lysosome, pH 4.5–6.5). Upon exposure to these pH stimuli, pH-responsive nanoparticles respond with physicochemical changes to their material structure and surface characteristics. These changes include swelling, dissociation, or surface charge switching, in a manner that favors drug release at the target site (the tumor microenvironment region and the cytosol followed by endosomal escape) rather than the surrounding tissues.
Expert opinion
Lastly, we consider the challenges involved in the development of pH-responsive nanomedicines.
Keywords: Nanomedicines, pH-responsiveness, Tumor microenvironment, Endosomal escape
Introduction
Recently nanomedicines have gained significant attention from researchers and clinicians because of the successes of lipid-nanoparticles-based COVID-19 vaccines (Reichmuth et al. 2016; Chaudhary et al. 2021; Damase et al. 2021; Gao et al. 2021). Nanoparticles improve existing treatments by providing a number of advantages, including protection of cargo molecules from external stresses, delivery of drugs to target tissues, and sustained drug release (Lee et al. 2021). Nanoparticles are intended to release their cargo at the target sites without premature release, which can cause side effects in other tissues (Lee et al. 2021). To prevent premature release, stable drug loading in nanoformulations is required, but the increased stability of the formulation can also lead to a poor drug-release profile at the target sites (Lee et al. 2021). Thus, researchers have exploited differences in properties (e.g., enzyme levels, pH, levels of reduced glutathione (GSH), and reactive oxygen species) between non-target and target sites for site-specific release of drugs (Mura et al. 2013). Researchers have also explored the use of environmentally responsive nanoparticles that can, when exposed to external stimuli (e.g., temperature, electric field, magnetic field, or ultrasound), produce physicochemical changes that favor drug release at the target site (Mura et al. 2013). Liposomes, polymeric micelles, lipoplexes, and polyplexes have been developed to use these physical and chemical cues to modify drug release properties (Gao et al. 2010; Wu et al. 2018; Deirram et al. 2019; Zhuo et al. 2020).
Among environmental stimuli, pH gradients have been widely used to design novel, pH-responsive nanoparticles (Gao et al. 2010; Wu et al. 2018; Deirram et al. 2019; Zhuo et al. 2020). The success of lipid nanoparticles having pH-responsive properties, which enable the loaded RNA drugs to escape from endosome by changing protonation status (Gao et al. 2021), especially emphasizes the use of pH differences between endo/lysosomal (pH 4.5–6.5) (Hu et al. 2015) and extracellular (pH ~ 7.4) environments. Because the tumor microenvironment (TME) is mildly acidic (pH ~ 6.5) compared to normal tissues (pH ~ 7.4) (He et al. 2020; Thomas et al. 2020), pH-targeting nanomedicines have also been widely developed for the TME (He et al. 2020; Thomas et al. 2020). This review assesses drug delivery based on pH-responsive nanoparticles at the levels of tissues and of intracellular compartments. In particular, specific examples of targeting the TME and of intracellular delivery will be demonstrated to highlight the designs of conceptually interesting pH-responsive nanoparticles. At the tissue level, many researchers have designed nanoparticle formulations to exploit the more acidic pH of TMEs, compared to the neutral pH of normal tissue, to achieve high local drug concentrations. At the intracellular level, a variety of pH-responsive nanoparticles have been designed for cytosolic delivery of endolysosomal-liable therapeutics, such as small molecule drugs, peptides, proteins, and nucleic acid drugs (mRNA, siRNA), from the acidic endolysosomal compartments to the cytosol. As endosomal escape has a crucial role in the cytosolic delivery of therapeutics by pH-responsive nanoparticles, endosomal escape mechanisms have been highlighted. Finally, we discuss the future research directions and challenges in the development of pH-responsive nanomedicines.
Nanoparticle responsiveness to tissue pH: tumor targeting
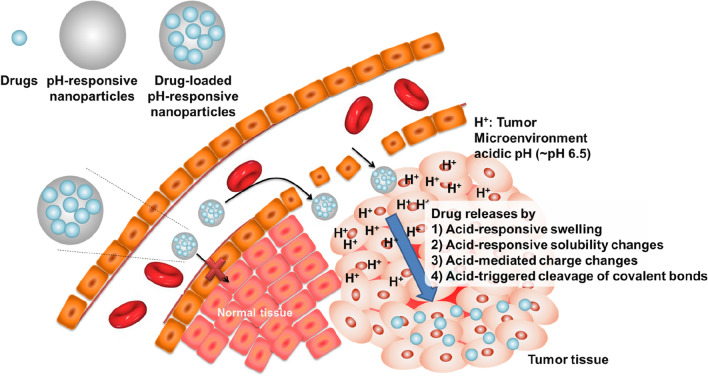
Whereas normal cells acquire energy via oxidative phosphorylation, tumor cells utilize the energy of oxygen-independent glycolysis to adapt to the abnormal conditions of insufficient oxygen and energy sources (e.g., glucose) due to the heterogeneously distributed tumor vasculatures (Kim and Dang 2006). The oncogenic metabolism results in the accumulation of a large amount of lactic acid along with excess protons and carbon dioxide (termed the Warburg effect) (Christofk et al. 2008). This leads to enhanced acidification of the extracellular TME with pH often in the range of 6.5–6.8 (He et al. 2020; Thomas et al. 2020), which can cause increased tumor metastasis and treatment resistance (Thews and Riemann 2019; Ward et al. 2020). In addition, insufficient blood supply and poor lymphatic drainage, characteristics of most tumors, also contribute to the acidity of the TME (Thews and Riemann 2019; He et al. 2020; Thomas et al. 2020; Ward et al. 2020). Thus, the acidity of the TME is a general characteristic for most types of solid tumors (Fig. 1).
Fig. 1.
Nanoparticles that are responsive to the pH of acidic tissue: tumor targeting. pH-responsive nanoparticles can be accumulated in tumor tissue by enhanced permeability and retention effects that mediate both passive and active targeting. The acidic pH (~ 6.5 to 6.8) of the tumor microenvironment (TME) induces physicochemical changes in the nanoparticles (swelling, solubility changes, charge changes, and cleavage of covalent bonds), resulting in efficient drug release in the TME
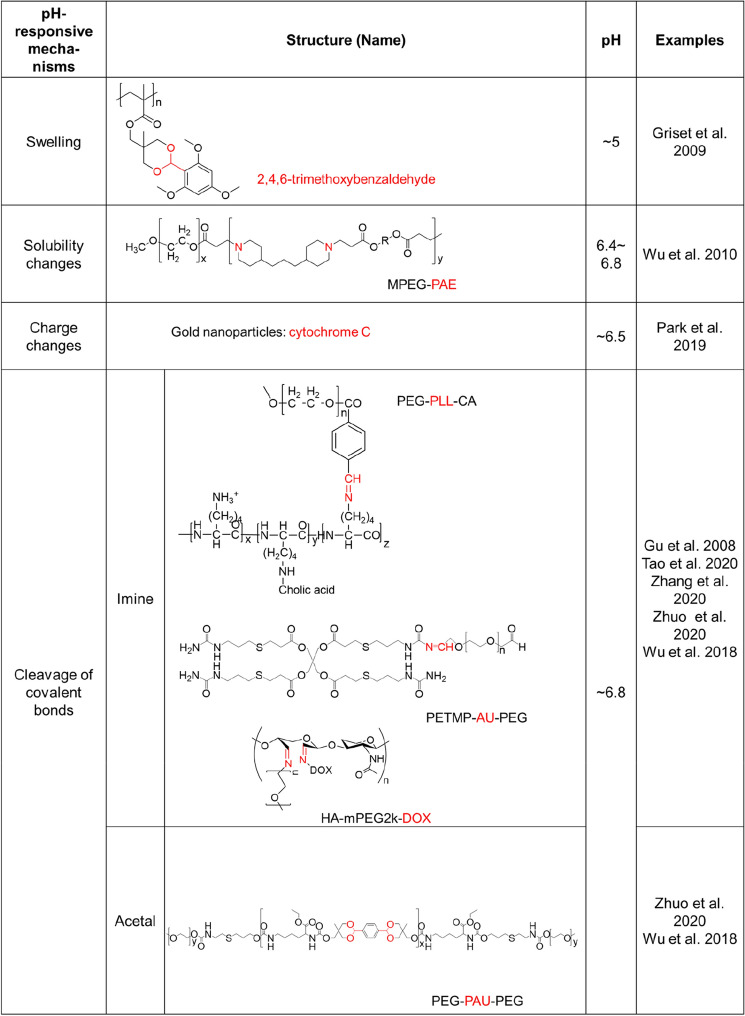
Many researchers have demonstrated that the acidic condition could be utilized as a promising target for tumor-specific imaging and therapy based on nanoparticles, offering advantages over conventional receptor-ligand-based tumor-targeting strategies (Gao et al. 2010; Deirram et al. 2019; He et al. 2020; Thomas et al. 2020). Furthermore, in many cases, nanoparticles are formulated to load drugs via stable interactions, such as hydrophobic interactions, charge-charge interactions, and covalent conjugation, for the prevention of free-drug release. However, the stable interactions may cause poor drug release at the desired target sites. To achieve target-specific drug release from the nanoparticles while maintaining a stable drug load in the nanoparticles at non-target sites such as normal tissue, the acidic condition of the TME could be utilized (Gao et al. 2010; Deirram et al. 2019; He et al. 2020; Thomas et al. 2020; Ward et al. 2020). Nanoparticles have been formulated for pH-dependent drug release by using polymers that change their physicochemical properties based on local pH levels (Gao et al. 2010; Wu et al. 2018; Deirram et al. 2019; Yan et al. 2020), such as acid-responsive swelling, acid-responsive solubility changes, acid-mediated charge changes, and acid-triggered cleavage of covalent bonds (Fig. 1, Table 1).
Table 1.
Nanoparticle responsiveness to tissue pH: tumor targeting
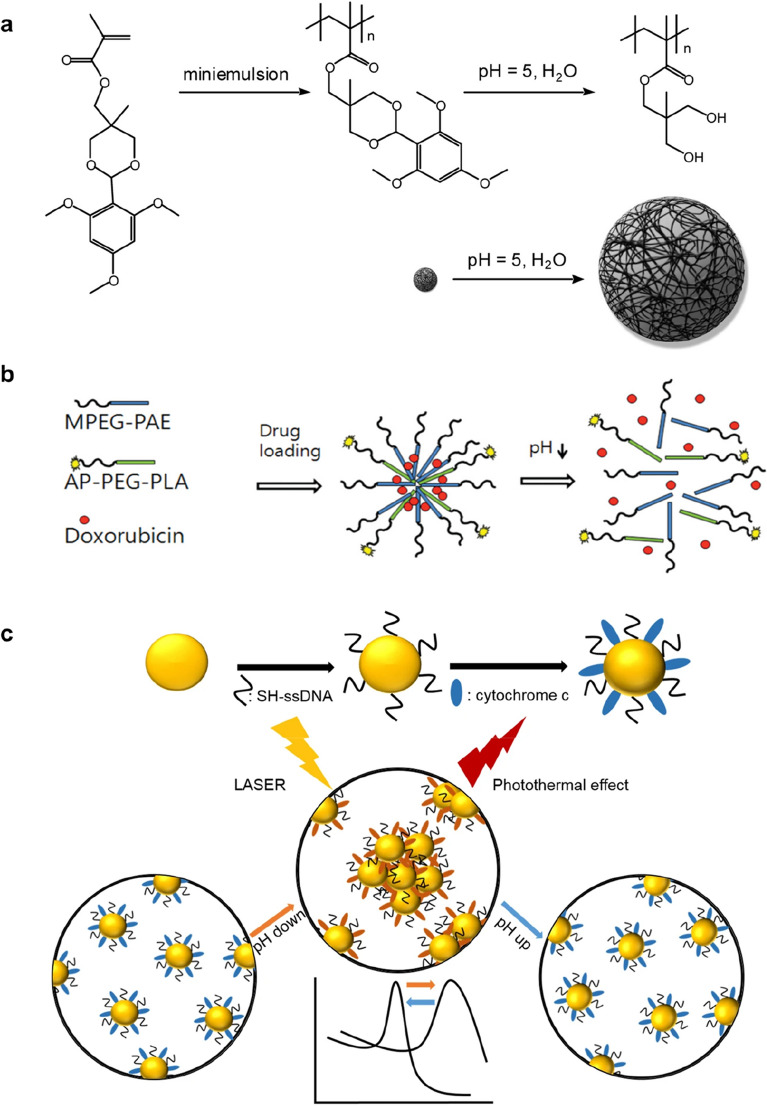
As an example of nanoparticles having an acid-responsive swelling property, engineered polymeric nanoparticles using acrylate-based cross-linked hydrophobic polymers with hydroxyl groups that were masked by pH-labile protecting groups (e.g., 2,4,6-trimethoxybenzaldehyde) have been reported (Griset et al. 2009, Fig. 2a). The nanoparticles undergo a hydrophobic-to-hydrophilic transition at a mildly acidic pH due to pH-specific cleavage of the protecting group in mildly acidic conditions, which leads to swelling and rapid drug release, showing effective in vitro and in vivo anticancer activity.
Fig. 2.
Strategies for tumor microenvironment acidic pH-responsive nanoparticles. (I). a Swelling: The protecting group of nanoparticle derived from 2,4,6-trimethoxybenzaldehyde is cleaved at a pH of ~ 5. This transformation reveals the hydrophilic hydroxyl groups and formation of nanoparticle with resulting expansion of the hydrogel nanoparticle in water. b Solubility changes: Schematic diagram depicting tumor-targeting and pH-responsive polymeric micelles of AP-PEG-PLA/MPEG-PAE (AP-pH-PM), which triggered doxorubicin (DOX) release by the sharp pH-dependent micellization/demicellizaton transition at the tumoral acidic pH. c, Charge changes: Schematic diagram of surface modification process of AuNPs with ssDNA and cytochrome c modification process (upper) and pH responsive behavior of CytC/ssDNA-AuNPs (lower). Panels a, b and c are reproduced with permission from Griset et al. (2009), Wu et al. (2010), and Park et al. (2019), respectively
pH-dependent hydrophobic-to-hydrophilic transitions were also used to control polymer dissolution, in which the polymer matrix collapses to drive drug release. For example, nanoparticles based on poly(ethylene glycol) (PEG)-poly(β-amino ester) polymers with a pKb of ∼6.5 were formulated (Griset et al. 2009). At neutral pH, the nanoparticles were stable, but mildly acidic pH (6.4–6.8) conditions induced a sharp micellization-demicellization transition leading to efficient drug release due to an amine protonation-mediated increase of the polymer solubility. In another study, spontaneous self-assembly of poly(amidoamine) dendrimers into nanoparticles occurred at physiological pH levels, while drug release due to nanoparticle disassembly took place at mildly acidic pH levels (pH ~ 6) (Wu et al. 2010, Fig. 2b). On the other hand, pH-dependent hydrophilic to hydrophobic transitions could be utilized for TME-activated in situ self-assembly of biopolymers for targeted cancer therapy. (Cong et al. 2019; Wang et al. 2020). For example, tumor penetrating polymer-peptide conjugates (poly(β-thioester) backbones conjugated with a cytotoxic peptide KLAK decorated with a pH-sensitive moiety, cis-aconitic anhydride (CAA), and a cell-penetrating peptide TAT; PT-K-CAA) was developed to attack solid tumor deeply (Cong et al. 2019). Upon the acidic conditions (pH 6.5) of TME in the deep tumor mass, the hydrophilic biopolymer underwent CAA hydrolysis-mediated hydrophobicity increase, leading to in situ self-assembly and restoration of anti-cancer activity. In another study, PEG-conjugated the photosensitizer zinc phthalocyanine (ZnPc) with pH-labile maleic acid amide linker was developed for tumor-specific photodynamic therapy (Wang et al. 2020). In the acidic conditions of TME, the biopolymer could readily form large clusters in situ to improve tumor retention by PEG-shedding-mediated hydrophobicity increases.
In another report, pH-responsive gold nanoparticles that were designed to aggregate in an acidic condition similar to the TME were prepared by introducing a mixed layer of single-stranded DNA and cytochrome c on the surface of the gold nanoparticles (Park et al. 2019, Fig. 2c). In a normal physiological pH environment (pH ~ 7.4), the surface charge of the gold nanoparticles was negative and the electrostatic repulsion between negative charges of the gold nanoparticles kept them separated. However, in the acidic conditions (pH < 6.5) of the TME, the charge of cytochrome c on the surface of the gold nanoparticles gradually became positive, leading to the formation of large clusters of gold nanoparticles through electrostatic attraction induced by the opposite charge of the cytochrome c and the DNA strands. The low pH-specific aggregation of gold nanoparticles resulted in a red shift of the spectroscopic absorption peak, showing a low pH-specific high photothermal efficacy of near-infrared radiation for cancer-specific photothermal therapy.
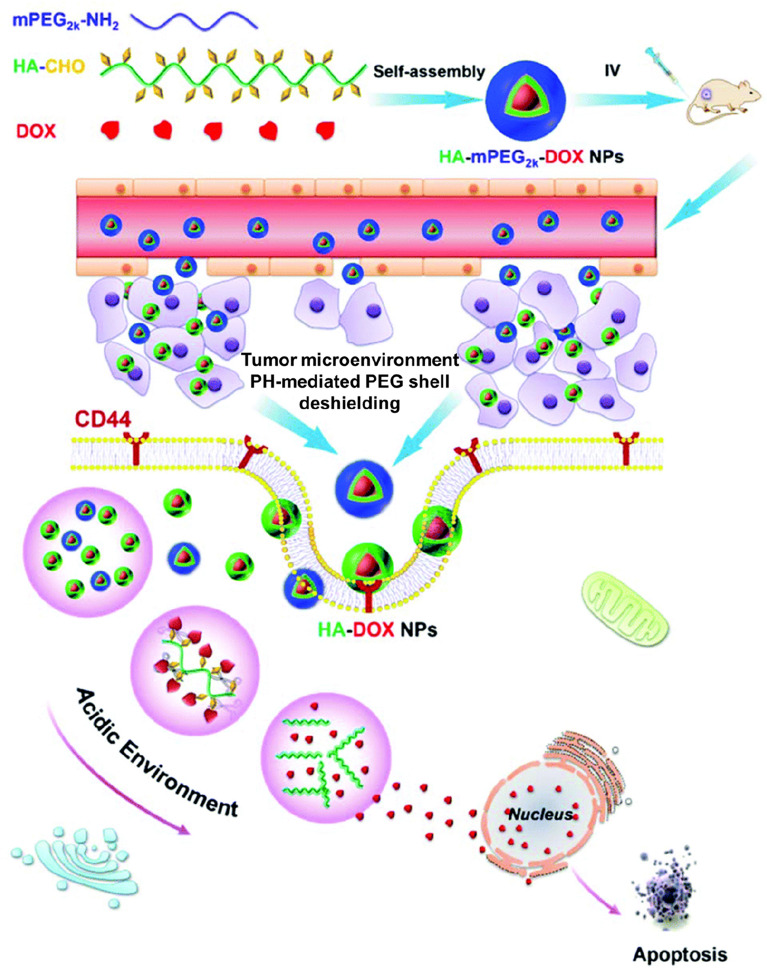
For the decomposition of acidic pH-specific nanoparticles, nanoformulations can be designed with a pH-labile linker such as an imine, ester, hydrazine, carboxydimethylmaleic anhydride, orthoester, beta-thiopropionate, vinylether, or phosphoramide (Wu et al. 2018; Deirram et al. 2019). Notably, the imine bond, which is formed by conjugation of a primary amine with ketones or aldehydes, can be hydrolyzed in a weakly acidic environment (pH 6.8), and thus, the imine bond can be used as a linker responsive to the pH of the TME (Gu et al. 2008; Wu et al. 2018; Cheng et al. 2019; Tao et al. 2020; Zhuo et al. 2020). To prepare nanoparticles that are responsive to the pH of the TME, an amphiphilic polycation composed of cholic acid grafted to poly-L-lysine (PLL-CA) has been conjugated with PEG via a pH-responsive benzoic imine linker (Gu et al. 2008). The PEG layer improves the stability and the circulation time of the nanoparticles. The PEG shell of polycationic micelles formed by self-assembly of PEG-PLL-CA is detached by the acidic pH of the TME (pH < 6.8). This leads to efficient intracellular uptake of polycation nanoparticles due to the attraction between the positive charges of the nanoparticles and the negative charges of the cancer cell membrane. Thus, the system has a high potential for tumor-specific anti-cancer activity. In another study, a pH-responsive nanopolymer, pentaerythritol tetra(3-mercaptopropionate)-allylurea-poly(ethylene glycol) (PETMP-AU-PEG) has been prepared by the Schiff-base imine formation between terminal-aldehyde PEG and PETMP-AU (Tao et al. 2020). While PETMP-AU-PEG loaded with doxorubicin (DOX) is highly stable in the neutral environment, the formation is responsive to weakly acidic conditions (pH 6.8 and 6.0) for the release of DOX for cancer-specific therapy. A cleavable PEGylated hyaluronic acid nanoparticle-based drug delivery system (HA–mPEG2k–DOX) based on a imine bond responsive to the pH of the TME has also been reported (Zhang et al. 2020, Fig. 3). The nanoparticles can efficiently target CD44-positive CT26 cells and the pH-responsive cleavable PEG shell can be detached in weakly acidic environments to effectively promote the cellular uptake of HA-DOX nanoparticles, leading to effective in vitro and in vivo anticancer activity.
Fig. 3.
Strategies for tumor microenvironment acidic pH-responsive nanoparticles (II). Cleavage of covalent bond: schematic diagram of the HA–mPEG2k–DOX conjugate from fabrication, self-assembly and the pH responsive DOX release for active targeting colorectal cancer therapy in vivo.
Reproduced with permission from Zhang et al. (2020)
The acetal group is another example of a functional group that is responsive to the pH of the TME (Wu et al. 2018; Zhuo et al. 2020). Cancer-associated fibroblast cells in the TME can target valsartan-loaded pH-sensitive nanoparticles (TME-activated angiotensin receptor blockers) for enhanced immune checkpoint blockade antibody therapeutics by reversing immune suppression (Chauhan et al. 2019). While ~ 30% of the drugs are released from the nanoparticles in 74 h in a normal physiological (pH ~ 7.4) environment, about 70% of the drugs release in 74 h in a mildly acidic (pH ~ 6.7) environment, highlighting the responsiveness of the nanoparticles to the acidic conditions of the TME.
The pH-responsive mechanisms described here draw upon a general phenomenon of the acidity of TMEs. Here, nanoparticles maintain stability in circulation and undergo physicochemical changes that favor localized drug release.
Nanoparticle responsiveness to organelle pH: intracellular delivery
In many cases, target sites and target molecules for drugs are usually located in the intracellular regions (Pack et al. 2005; Torchilin 2008; Kanasty et al. 2013; Zhao et al. 2014; Qin et al. 2019; Chaudhary et al. 2021; Damase et al. 2021). Most small molecule drugs can easily reach the target site and show their activity. In contrast, to achieve intracellular target site-specific therapeutic efficacy, some drugs, including DNA drugs, RNA drugs, protein and peptides drugs, and some acid/enzymes-labile small molecule drugs, must penetrate cellular membranes and should be protected from intracellular harsh conditions (e.g., hydrolysis, enzyme-mediated degradation, and low pH), especially in endosomes or lysosomes (Pack et al. 2005; Torchilin 2008; Kanasty et al. 2013; Zhao et al. 2014; Qin et al. 2019; Chaudhary et al. 2021; Damase et al. 2021). To be efficiently delivered into the desired intracellular regions, such as the nucleus and cytoplasm, while overcoming the limitations, various nanotechnologies that are capable of protecting drugs from harsh conditions and facilitating the entry and escape of the drugs into the cytoplasm have been explored (Fig. 4) (Pack et al. 2005; Torchilin 2008; Kanasty et al. 2013; Zhao et al. 2014; Qin et al. 2019; Chaudhary et al. 2021; Damase et al. 2021).
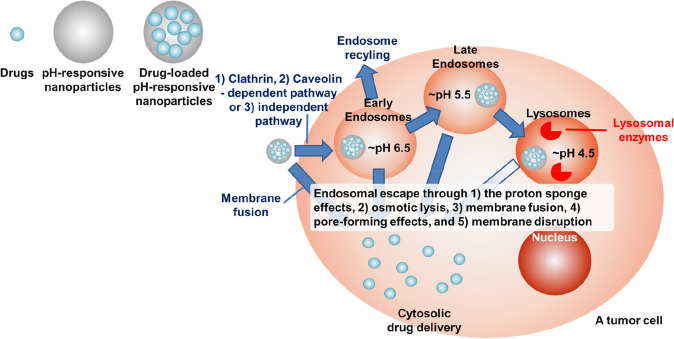
Fig. 4.
Nanoparticles that are responsive to the pH of acidic organelles: intracellular delivery. pH-responsive nanoparticles can be accumulated in the tumor tissue by passive targeting (via enhanced permeability and retention) and active targeting. Nanoparticles are typically taken up into cancer cells via endocytosis into endosomes (early: ~ pH 6.5; late: ~ pH 4.5) and are then subsequently fused with acidic lysosomal compartments (~ pH 4.5, containing hydrolytic lysosomal enzymes). For drugs that are susceptible to the harsh conditions of endosomes and lysosomes, such as DNA drugs, RNA drugs, protein and peptides drugs, and some enzyme and acid labile-small molecule drugs, delivery with nanomedicines necessitates nanoparticles that can escape from the endosome before fusing with lysosomes. Endosomal escape usually occurs by one or a combination of the following mechanisms: (1) proton sponge effects, (2) osmotic lysis, (3) membrane fusion, (4) pore-forming effects, and (5) membrane disruption. The efficient endosomal escape by pH-responsive nanoparticles leads to cytosolic drug delivery. On the other hand, nanoparticles themselves could be directly fused with cellular membranes, resulting in direct drug release into cytosol without joining in the endosomes
Nanoparticles are typically taken into cells via endocytosis into endosomes and are then subsequently fused with acidic lysosomal compartments that are typically considered as a final destination for the degradation of macromolecules and serve as organelles for the storage of hydrolytic enzymes (Canton and Battaglia 2012). Usually, in endosomes and lysosomes, rapid endosomal acidification (~ 2–3 min) occurs, which is optimal for many hydrolases and other endo/lysosomal enzymes (Murphy et al. 1984; Deirram et al. 2019). Because of the activity of the ATP-dependent proton influx pumps present in the membrane of both endosomes and lysosomes (Diering and Numata 2014), a stable pH gradient is established in different compartments during the maturation process (early endosomes: pH 6.5, late endosomes: pH 5.5, and lysosomes: pH 4.5) compared with a cytoplasmic pH (~ 7.0) and a normal physiologic pH (~ 7.4) (Hu et al. 2015). The lower acidic conditions (pH ~ 4.5) and different enzymatic activities in lysosomes compared to endosomes can be harmful to the therapeutic molecules being delivered, especially for macromolecules such as DNA drugs, RNA drugs, peptides, proteins, and other enzyme or acid-labile small molecule drugs (Pack et al. 2005; Torchilin 2008; Kanasty et al. 2013; Zhao et al. 2014; Qin et al. 2019; Chaudhary et al. 2021; Damase et al. 2021). Therefore, nanoparticles carrying therapeutics that are vulnerable to these conditions need to escape from the endosomes before they fuse with lysosomes (Pack et al. 2005; Torchilin 2008; Kanasty et al. 2013; Zhao et al. 2014; Qin et al. 2019; Chaudhary et al. 2021; Damase et al. 2021). Endosomal acidification can be used as a trigger for endosomal escape and payload release. The mechanism underlying nanoparticle escape from endosomes has not been fully explained. Several hypotheses have been formulated, including the proton sponge effect and osmotic lysis, membrane fusion, a pore-forming effect, and membrane disruption (Fig. 4, Table 2) (Selby et al. 2017).
Table 2.
Nanoparticle responsiveness to organelle pH: intracellular delivery
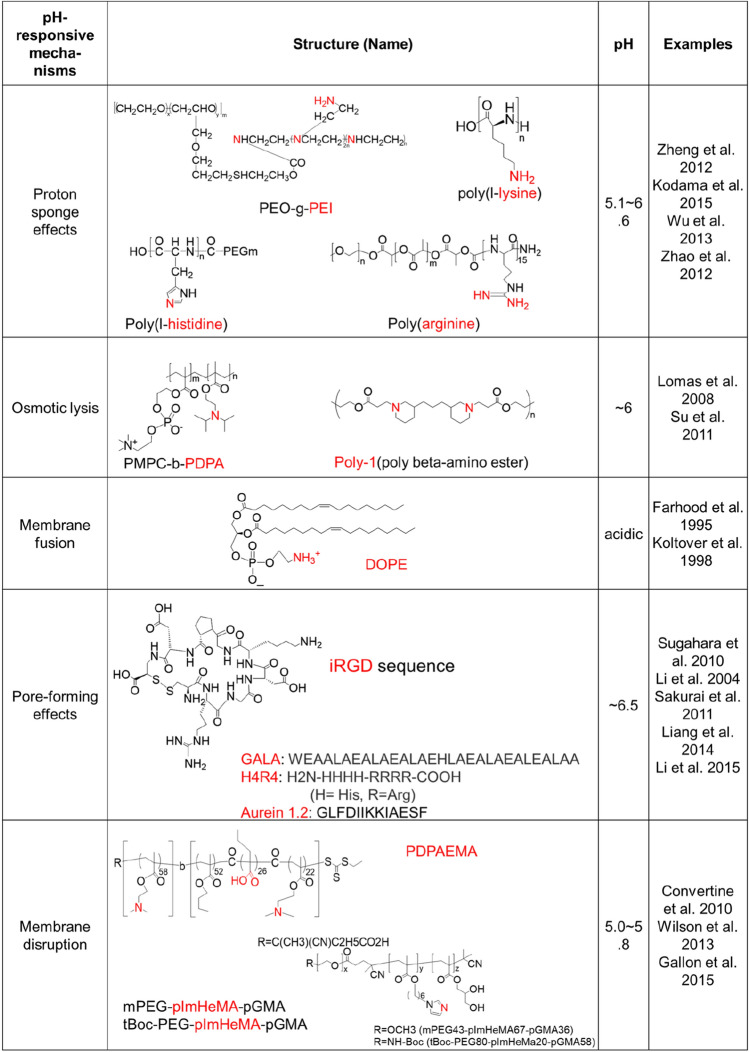
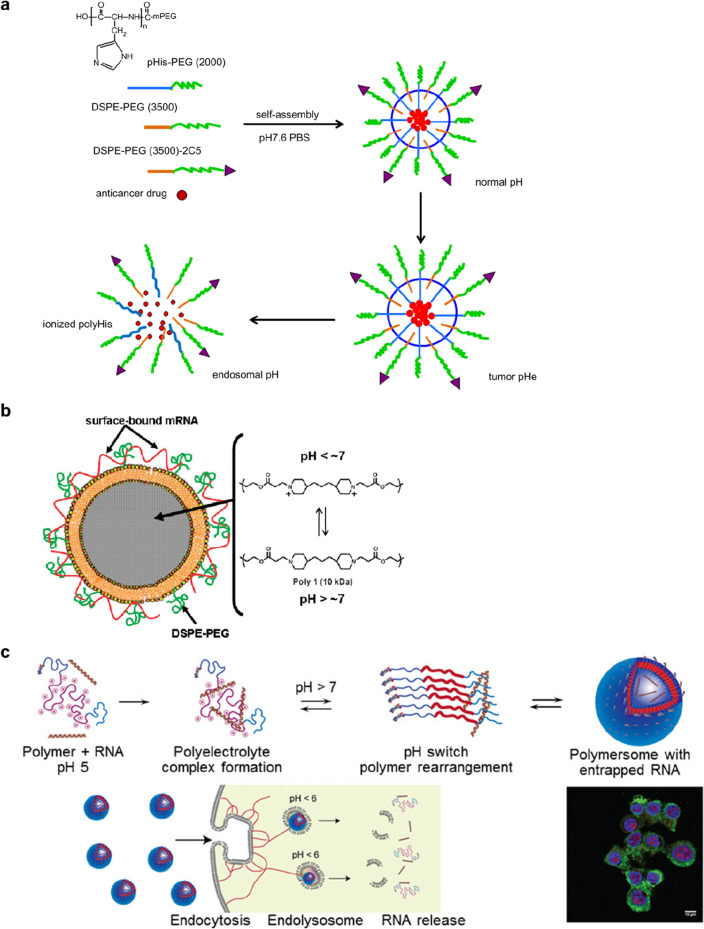
A common strategy for inducing endosomal escape is via the proton sponge effect, which is based on the buffering effect of polymers with a pKa in a physiologically relevant range. As the components of nanoparticles become protonated, protons are continuously pumped into the endosomes or lysosomes, leading to the transport of chloride counterions to maintain a charge balance. This results in a high osmotic pressure that eventually induces rupturing of the endosome or lysosome. A well-known example of a polymer having a buffering effects is polyethyleneimine, which is commonly used to load DNA and RNA drugs by forming polyion complexes (Zheng et al. 2012). The polypeptides poly(l-lysine) (PLys) (Kodama et al. 2015), poly(l-histidine) (PHis) (Wu et al. 2013, Fig. 5a) and poly(arginine) (PArg) (Zhao et al. 2012) are other examples for cytosolic delivery of DNA and RNA drugs by the endosomal buffering mechanism.
Fig. 5.
Strategies for organelle acidic pH-responsive nanoparticles. a Proton sponge effects: The acid-triggered drug release from the pH-sensitive mixed micelles. c Osmotic lysis: Schematic of structure and composition of lipid-coated PBAE particles and mRNA cargo association. c Membrane disruption: Schematic diagram of endosomal pH-responsive triblock copolymer nanovesicles which contain poly-ImHeMA. Panels a, b, and c are reproduced with permission from Wu et al. (2013), Su et al. (2011), and (Gallon et al. 2015), respectively
Another endosomal escape mechanism is the disassembly of a cationic polymer particle into its component monomers, leading to an increase in osmotic pressure that is associated with rupturing of the endosome (Lomas et al. 2008; Massignani et al. 2009). An initial study of a responsive polymersome prepared from poly(2-methacryloyloxy)ethylphosphorylchlorine-block-poly(2-(diisopropylamino)ethyl methacrylate) (PMPC-b-PDPAEMA) block copolymer that could disassemble within the endosomal pH range supports the mechanism (Lomas et al. 2008). While the vesicles are stable at physiological pH, they disassemble due to a change in hydrophobicity of the PDPAEMA component at endosomal acidic conditions (pH ~ 6), leading to endosomal escape by endosomal rupture caused by an increase in osmotic pressure. A rapidly degradable, pH-dependent nanoparticle composed of 10 kDa molecular weight poly(β-amino ester) (poly-1), a lipid bilayer, and a PEG-lipid has also been reported (Su et al. 2011, Fig. 5b). Poly-1 is a weak polyelectrolyte that is water-insoluble at elevated pH but dissolves in aqueous solutions below pH 7. A higher transfection efficiency has been observed in poly-1-based nanoparticles with electrostatically associated mRNA encoding for GFP compared to a control consisting of a nanoparticle based on non-responsive polylactic-co-glycolic acid, indicating evidence of endosomal escape. In addition to osmotic pressure increase and endosomal rupture associated with disassembly of the nanoparticles, the authors suggest that the proton sponge effect may also influence the endosomal escape of the poly-1 particles.
In nature, enveloped viruses can induce endosomal escape by fusion of the viral capsid to envelop with the endosomal membrane, allowing the viral capsid to enter the cytoplasm (White and Whittaker 2016). Likewise, particles (especially liposomes) assembled from lipids or amphiphilic materials may also allow the loaded drug to enter the cytoplasm by fusion of the nanoparticle membrane (e.g., the phospholipid bilayer of a liposome) with the endosomal membrane (Zelphati and Szoka 1996). For example, liposomal formulations based on the cationic lipids (N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride, N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride, or dioleoyldimethylammonium chloride) have demonstrated potential for cytoplasmic gene delivery (Balazs and Godbey 2011). Usually, incorporating neutral or helper lipids such as 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) or 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) into the lipid formation mixture to prepare cationic liposomes provides stabilization of the lipid bilayer. Whereas cationic liposomes prepared with DOPC, which promotes stable lamellar structures, do not exhibit improved endosomal escape, cationic liposomes prepared with DOPE have been shown to facilitate escape due to structural transitions from a multilamellar structure to an inverted hexagonal liquid crystalline phase when exposed to the low pH of endocytic vesicles (Cullis et al. 1986; Farhood et al. 1995; Koltover et al. 1998). Electron microscopy results demonstrate that DOPE-containing cationic liposomes show an endosomal destabilizing effect but DOPC formulations do not have an effect, supporting the mechanism (Zhou and Huang 1994). The inverted hexagonal structure interacts more favorably with anionic membranes, resulting in membrane fusion and DNA release (Koltover et al. 1998). However, conventional liposomes have limitations, such as short circulation time due to removal by immune cells including macrophages. Thus, modification of liposomes with PEG is a conventional method to improve in vivo circulation time due to the reduction of interactions with macrophages. However, the steric barrier provided by PEG is thought to interfere with a fusion of fusogenic lipids-based nanoparticles and polymer-based nanoparticles with the endosomal membrane (Song et al. 2002; Mishra et al. 2004). To overcome the adverse effect of PEGylation on endosomal escape, some groups have developed liposomes prepared with PEG analogues that are sensitive to either pH or the reduction condition.
Mechanical stress was used to induce endosomal disruption by designing nanoparticles that change size (e.g., by swelling) upon a change in pH. Cross-linked polymer nanoparticles that were assembled from pH-responsive polymers could swell due to repulsion between the polymer chains when they become ionized (Mura et al. 2013). Nanoparticles that were prepared with a 2-diethylamino ethyl methacrylate (DEAEMA) core cross-linked with poly(ethylene glycol) dimethacrylate and a 2-aminoethyl methacrylate shell underwent endosomal acidic pH (~ 5)-specific swelling behavior (a 2.8-fold change in diameter) (Hu et al. 2007). Approximately 90% of cells incubated with the nanoparticles loaded with a fluorescence molecule, calcein, had cytosol fluorescence signals, but cytosol fluorescence signals were observed in fewer than 5% of the cells incubated with calcein or calcein with non-responsive particles in a control group, indicative of endosomal escape. The endosome escape was also confirmed by loading the nanomedicine with the model antigen ovalbumin (OVA) and measuring interferon-γ (IFN-γ) secretion by primary T cells in response to antigen presentation by dendritic cells (Hu et al. 2009). However, while 40% of the cells had diffuse siRNA fluorescence when incubated for 1 h, the knockdown by this system was significantly lower than by a commercially available lipid reagent. This may be due to strong electrostatic interactions between the cargo and the carrier when in the cytosol. A nanoparticle formulated by polymerization of DEAEMA in the presence of heterobifunctional PEG (CH2 = CH-Ph-PEG-COOH) and using ethylene glycol dimethacrylate as a cross-linker also showed swelling behavior (5.1- to 6.8-fold increases) when the PDEAEMA was protonated in an endosomal acidic condition (Oishi and Nagasaki 2007). The nanoparticles combined with a PEG-b-poly(l-Lysine)/pDNA polyplex showed significant increases in transfection efficiency.
Endosomal disruption-mediated escape can also occur through the direct interaction of polymers with the endosomal membrane (Nakase et al. 2010). A large body of evidence suggests that polymer-based systems induce escape by interacting with cellular membranes. While the major escape mechanism of polycationic polymer-based nanomedicines is the proton sponge effect, the escape mechanism for polyanionic polymer-based nanomedicines is attributed to an increase in hydrophobicity due to protonated carboxylate groups, which results in membrane insertion and subsequent membrane disruption (Nakase et al. 2010). Some useful polymers to achieve such interactions are poly(propylacrylic acid), poly(butyl acrylate), and polyimidazole-hexyl methacrylate (poly-ImHeMA). Incorporating one of the polymers into polymer-based nanoparticles has been shown to induce a pH-mediated increase in hydrophobicity due to the protonated carboxylate, leading to escape caused by the disruption of the endosomal membrane. For example, a micelle carrier prepared with cationic poly(2-dimethylamino ethyl methacrylate) (PDMAEMA) and a pH-responsive membrane disruption block comprised of DMAEMA, propylacrylic acid, and butyl methacrylate, showed low pH (pH 5.8)-specific disassembly and endosomal escape behaviors (Convertine et al. 2010). The micelles loaded with siRNA that targeted mRNA for the housekeeping gene GAPDH by interacting their negatively charged cargo with the PDMAEMA shell induced significant knockdown of the GAPDH. A PDMAEMA polymer micelle has also been used as a vaccine delivery system by including pyridyl disulfide methacrylate in the PDMAEMA block to load OVA (Wilson et al. 2013). Polymersomes assembled from a triblock copolymer consisting of PEG and poly(glycerol methacrylate) ends, and a central weakly basic block, poly-ImHeMA, showed similar siRNA delivery efficiency (Gallon et al. 2015, Fig. 5c). Another novel pH-responsive nanomedicine for cancer immunotherapy was also reported. The particles were self-assembled from a peptide–drug conjugate consisting of a hydrophilic targeting motif (arginyl-glycyl-aspartic acid; RGD), two protonatable histidines (the pH-responsive moiety), and an ester bond-linked hydrophobic IDO inhibitor, which exhibits pH-responsive disassembly and esterase-catalyzed drug release, augmenting antitumor immunity of checkpoint inhibitors. (Han et al. 2020).
Escape mediated by endosomal disruption can also occur via peptides that self-assemble across the membrane to create defined pores (Li et al. 2004; Nakase et al. 2010; Danial et al. 2015). Nanoparticles can be designed to contain protein transduction domains (PTDs), which are cationic, 10–30 amino acid sequences that hypothetically disrupt endosomal membranes upon endosomal acidification (Lundberg et al. 2003). Various viral or bacterial protein-derived polypeptides have been shown to facilitate endosomal escape. For example, the co-administration of a free tumor-penetrating peptide (e.g., iRGD sequence) exerted enhancement of the efficacy of DOX (DOX liposomes), paclitaxel (nab-paclitaxel), and monoclonal antibody (Anti-HER-2 antibody, trastuzumab) treatments (Sugahara et al. 2010). Generally, the dimensions of transmembrane pores, thought to be 1–2 nm and therefore limiting the usefulness of PTDs, induce the efficient release of therapeutic cargo. The engineered peptide GALA can induce the release of molecules up to ~ 5000 Da (Li et al. 2004). GALA is negatively charged at pH 7 and comprises a repeated EALA (glu-ala-leu-ala) motif, which forms an alpha helix when the glutamic acid residues become protonated at the endosomal acidic pH level. This protonation causes them to span the lipid bilayer of the endosome. shGALA-modified and PEGylated lipid-based particles called multifunctional nanodevices (PEG-MEND) loaded with siRNA showed 82% knockdown of the target gene through enhanced endosomal escape (Sakurai et al. 2011). Using a screen of antimicrobial peptides from the antimicrobial peptide database, the aurein 1.2 peptide sequence and a H4R4 peptide consisting of four arginine units and four histidine units have also been reported to have endosomolytic properties (Liang et al. 2014; Li et al. 2015). These sequences have been demonstrated to induce endosomal escape both in vitro and in vivo.
In the cases of drugs (e.g., small molecular anti-cancer drugs) that are stable in endo/lysosomes, nanoparticles are designed to conjugate with the drugs via an acidic pH-liable linker so that the drugs can be released from the nanoparticles by the endo/lysosomal acidic pH and escape the endo/lysosomes via passive diffusion. The linkers include esters, hydrazine, carboxydimethylmaleic anhydride, orthoesters, beta-thiopropionate, vinylether, and phosphoramide (Gao et al. 2010; Wu et al. 2018; Deirram et al. 2019; Zhuo et al. 2020). For example, drug release at low pH (< 6.0) has been reported with bi(PEG-PLA)-Pt(IV)[cisplatin] conjugate-based nanoparticles having hydrazone cross-linkers (Aryal et al. 2010). The pH-responsive nanoparticles exhibit endosomal intracellular drug release, leading to enhanced cellular cytotoxicity over free cisplatin in vitro. DOX conjugated with polymer backbones via a hydrazine bond showed endosomal acidic pH-mediated specific cleavage, and thus DOX loading into nanoparticles and acidic pH-mediated drug release profiles have been widely applied when designing nanoparticles with polymeric backbones, including poly(hexyl methacrylate) (Filippov et al. 2013; Zhang et al. 2018), metallic cores (Fe3O4 (Zhang et al. 2007) and Au (Hudlikar et al. 2016)), polyphosphoester (Sun et al. 2014), poly (methacryloyloxyethyl phosphorylcholine) (Chen et al. 2012), hyaluronan (Xu et al. 2013), sugars (pullulan (Wang et al. 2016) and dextran (Zhang et al. 2015)), and poly(amino acid). As an example, a DOX conjugated polymer has been prepared via thiol-maleimide click chemistry of thiolated poly(methacrylic acid) and a maleimide-functionalized DOX derivative having a hydrazone bond (Cui et al. 2012). pH-responsive nanoparticles were prepared via mesoporous silica-templated assembly. While there was a limited release at a physiological pH of 7.2 (~ 20%), ~ 80% of the drug was released at pH 5.5 in 24 h due to endosomal/lysosomal acidic pH-mediated hydrazone bond cleavage, leading to enhancement of anti-cancer activity.
Ester groups have also been used for acidic pH-mediated drug release. Liposome-like nanoparticles have been prepared by conjugating camptothecin with PEG via an ester bond, followed by loading with DOX (Bruyere et al. 2010; Shen et al. 2010). The rapid release of both DOX and camptothecin at endolysosomal acidic conditions (pH < 5) has been described (Bruyere et al. 2010; Shen et al. 2010). Similarly, other researchers have reported on nanoparticles based on a series of orthoester model compounds that have different hydrolysis rates at pH values of 4.5–7.4 (Bruyere et al. 2010). However, when pH-responsive drug-cleavage strategies are applied, the released drugs in the lysosomes must be stable in the harsh acidic and enzymatic conditions of lysosomes. If not, other pH-responsive systems for endosomal escapes mentioned above should be considered.
Recently, the success of lipid nanoparticle (LNP)-based mRNA vaccines have highlighted the importance of pH-responsive nanocarriers to enhance endosomal escape of therapeutics into the cytosol. LNPs are typically taken up into cells via a variety of endocytosis routes, such as clathrin- and caveolae-mediated endocytosis, into endosomes (Li et al. 2019; Wu and Li 2021). The lipids composition, degree of PEGylation, and surface charge, as well as the size of the nanoparticles affect the efficiency of the internalization. The ionizable cationic lipids in the compositions of LNPs play a pivotal role in promoting the endosomal escape of mRNA. The ionizable lipids become protonated within the acidic environment of the endosome (pH < ~ 6.5), and thus, those positively charged lipids form ion pairs with the negatively charged phospholipids of the endosome membrane, disrupting the bilayer structure (Reichmuth et al. 2016). The cholesterol component of the LNP structure may also affect the fusion of the LNPs with the endosome (Aldosari et al. 2021). These two interactions result in the endosomal escape of mRNA cargo. In addition to the membrane disruption activity, the proton sponge effect may also contribute to endosomal escape (Reichmuth et al. 2016).
Perspectives, challenges, and future research directions
A number of elegant strategies have been employed to design pH-responsive nanoparticles for therapeutic delivery. The pH-responsiveness of nanoparticles can be created by pH-degradable linkages, pH-cleavable cross-links, or charge-shifting polymers. These strategies enable the nanoparticles to deliver their therapeutic agent site-specifically in response to pH changes found in the acidic organelle compartments within the cell (endosomes, lysosomes) or in acidic tumor tissue. This pH-responsive site specificity is especially necessary when applied with therapeutics, such as DNA or RNA drugs, peptide or proteins drugs, and acid- and enzyme-labile small molecular drugs, that are very susceptible to high levels of enzymes and acidic conditions in lysosomes, to which the majority of nanomedicines are delivered.
Even though some pH-responsive nanoparticles, such as LNPs for mRNA delivery, showed promising results in the clinic and in the market, there are still challenges remaining. First, issues such as biodistribution and the non-specific accumulation of nanoparticles in organs like the liver still limit the in vivo use of many nanoparticles. This is especially true in the case of LNPs, most of which usually accumulate in the liver due to specific interactions of ApoE on the surface of nanoparticles with LDL receptors on the hepatocytes after forming a protein corona with ApoE protein (Sato et al. 2020; Sebastiani et al. 2021). Second, while some materials presented in this review show some degree of endosomal escape, the efficiency of escape generally remains low, and thus further work is required to significantly improve the efficiency. For example, current LNPs generally have a low degree of endosomal escape (< 2%), and thus, there is still room for the improvement of mRNA delivery in pH-responsive LNPs research (Wu and Li 2021). Third, even though it is challenging to compare different delivery systems across studies because there is wide variation in cell or tumor types and experimental protocols used, understanding the mechanisms of escape with thorough studies is absolutely necessary to improve the amount of endosomal escape. More than one escape mechanism are likely required for efficient cytoplasmic delivery of therapeutics. The physical and chemical properties of nanoparticles, such as size, shape, or charge, may affect escape from the endosome. Fourth and last, many researchers have questioned the exact roles of targeting ligands on the surface of the nanoparticles in endosomal escape. Caution must be taken when a targeting ligand is used to improve intracellular delivery, because the extent of endosomal acidification could be affected by the choice of targeting ligand used and thus the endocytic pathway taken. For example, folic acid functionalization on the surface of nanoparticles was demonstrated to lead to endocytosis through recycling centers characterized by a near-neutral pH of 6–7, which may make it less suitable for pH-based mechanisms (Yang et al. 2007).
In conclusion, nanoparticle formulations that can respond to pH gradients within the microenvironments of tissues and cell organelles may be a powerful tool for therapeutic drug delivery. Because cytosolic delivery and TME delivery mediated by pH-responsive nanoparticles have gained a great deal of attention from a multitude of researchers and clinicians, massive efforts will be put into additional research and thus more pH-responsive nanoparticles will be designed, developed, and clinically tested. After the challenges indicated above are addressed, the systems will become ideal tools for the development of vaccines as well as for the treatment of a variety of diseases.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1F1A106212711, 2022R1C1C1003141).
Declarations
Conflict of interest
All authors (J. Shinn, N. Kwon, S.A. Lee, and Y. Lee) declare that they have no conflict of interest.
Research involving human and animal rights
This article does not contain any studies with human and animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jongyoon Shinn and Nuri Kwon have contributed equally to this work.
References
- Aldosari BN, Alfagih IM, Almurshedi AS. Lipid nanoparticles as delivery systems for RNA-based vaccines. Pharmaceutics. 2021;13(2):206. doi: 10.3390/pharmaceutics13020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal S, Hu CM, Zhang L. Polymer–cisplatin conjugate nanoparticles for acid-responsive drug delivery. ACS Nano. 2010;4:251–258. doi: 10.1021/nn9014032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs DA, Godbey W. Liposomes for use in gene delivery. J Drug Deliv. 2011;2011:326497. doi: 10.1155/2011/326497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyere H, Westwell AD, Jones AT. Tuning the pH sensitivities of orthoester based compounds for drug delivery applications by simple chemical modification. Bioorg Med Chem Lett. 2010;20:2200–2203. doi: 10.1016/j.bmcl.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Canton I, Battaglia G. Endocytosis at the nanoscale. Chem Soc Rev. 2012;41:2718–2739. doi: 10.1039/c2cs15309b. [DOI] [PubMed] [Google Scholar]
- Chaudhary N, Weissman D, Whitehead KA. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat Rev Drug Discov. 2021;20:817–838. doi: 10.1038/s41573-021-00283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan VP, Chen IX, Tong R, Ng MR, Martin JD, Naxerova K, Wu MW, Huang P, Boucher Y, Kohane DS, Langer R, Jain RK. Reprogramming the microenvironment with tumor-selective angiotensin blockers enhances cancer immunotherapy. Proc Natl Acad Sci USA. 2019;116:10674–10680. doi: 10.1073/pnas.1819889116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Parelkar SS, Henchey E, Schneider S, Emrick T. PolyMPC-doxorubicin prodrugs. Bioconjug Chem. 2012;23:1753–1763. doi: 10.1021/bc200667s. [DOI] [PubMed] [Google Scholar]
- Cheng C, Meng Y, Zhang Z, Chen J, Zhang Q. Imine bond- and coordinate bond-linked pH-sensitive cisplatin complex nanoparticles for active targeting to tumor cells. J Nanosci Nanotechnol. 2019;19:3277–3287. doi: 10.1166/jnn.2019.16314. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Cong Y, Ji L, Gao YJ, Liu FH, Cheng DB, Hu ZY, Qiao ZY, Wang H. Microenvironment-induced in situ self-assembly of polymer-peptide conjugates that attack solid tumors deeply. Angew Chem Int Ed. 2019;58:4632–4637. doi: 10.1002/anie.201900135. [DOI] [PubMed] [Google Scholar]
- Convertine AJ, Diab C, Prieve M, Paschal A, Hoffman AS, Johnson PH, Stayton PS. pH-responsive polymeric micelle carriers for siRNA drugs. Biomacromolecules. 2010;11:2904–2911. doi: 10.1021/bm100652w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui JW, Yan Y, Wang YJ, Caruso F. Templated Assembly of pH-Labile Polymer-Drug Particles for Intracellular Drug Delivery. Adv Funct Mater. 2012;22:4718–4723. [Google Scholar]
- Cullis PR, Hope MJ, Tilcock CP. Lipid polymorphism and the roles of lipids in membranes. Chem Phys Lipids. 1986;40:127–144. doi: 10.1016/0009-3084(86)90067-8. [DOI] [PubMed] [Google Scholar]
- Damase TR, Sukhovershin R, Boada C, Taraballi F, Pettigrew RI, Cooke JP. The limitless future of RNA therapeutics. Front Bioeng Biotechnol. 2021;9:628137. doi: 10.3389/fbioe.2021.628137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial M, Perrier S, Jolliffe KA. Effect of the amino acid composition of cyclic peptides on their self-assembly in lipid bilayers. Org Biomol Chem. 2015;13:2464–2473. doi: 10.1039/c4ob02041c. [DOI] [PubMed] [Google Scholar]
- Deirram N, Zhang C, Kermaniyan SS, Johnston APR, Such GK. pH-Responsive polymer nanoparticles for drug delivery. Macromol Rapid Commun. 2019;40:e1800917. doi: 10.1002/marc.201800917. [DOI] [PubMed] [Google Scholar]
- Diering GH, Numata M. Endosomal pH in neuronal signaling and synaptic transmission: role of Na(+)/H(+) exchanger NHE5. Front Physiol. 2014;4:412. doi: 10.3389/fphys.2013.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhood H, Serbina N, Huang L. The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochim Biophys Acta. 1995;1235:289–295. doi: 10.1016/0005-2736(95)80016-9. [DOI] [PubMed] [Google Scholar]
- Filippov SK, Franklin JM, Konarev PV, Chytil P, Etrych T, Bogomolova A, Dyakonova M, Papadakis CM, Radulescu A, Ulbrich K, Stepanek P, Svergun DI. Hydrolytically degradable polymer micelles for drug delivery: a SAXS/SANS kinetic study. Biomacromolecules. 2013;14:4061–4070. doi: 10.1021/bm401186z. [DOI] [PubMed] [Google Scholar]
- Gallon E, Matini T, Sasso L, Mantovani G, Arminan De Benito A, Sanchis J, Caliceti P, Alexander C, Vicent MJ, Salmaso S. Triblock copolymer nanovesicles for pH-responsive targeted delivery and controlled release of siRNA to cancer cells. Biomacromolecules. 2015;16:1924–1937. doi: 10.1021/acs.biomac.5b00286. [DOI] [PubMed] [Google Scholar]
- Gao W, Chan JM, Farokhzad OC. pH-Responsive nanoparticles for drug delivery. Mol Pharm. 2010;7:1913–1920. doi: 10.1021/mp100253e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Yang K, Shelling AN, Wu Z. Nanotechnology-enabled COVID-19 mRNA vaccines. Encyclopedia. 2021;1:773–780. [Google Scholar]
- Griset AP, Walpole J, Liu R, Gaffey A, Colson YL, Grinstaff MW. Expansile nanoparticles: synthesis, characterization, and in vivo efficacy of an acid-responsive polymeric drug delivery system. J Am Chem Soc. 2009;131:2469–2471. doi: 10.1021/ja807416t. [DOI] [PubMed] [Google Scholar]
- Gu J, Cheng WP, Liu J, Lo SY, Smith D, Qu X, Yang Z. pH-triggered reversible "stealth" polycationic micelles. Biomacromol. 2008;9:255–262. doi: 10.1021/bm701084w. [DOI] [PubMed] [Google Scholar]
- Han X, Cheng K, Xu Y, Wang Y, Min H, Zhang Y, Zhao X, Zhao R, Anderson GJ, Ren L, Nie G, Li Y. Modularly designed peptide nanoprodrug augments antitumor immunity of PD-L1 checkpoint blockade by targeting indoleamine 2,3-dioxygenase. J Am Chem Soc. 2020;142:2490–2496. doi: 10.1021/jacs.9b12232. [DOI] [PubMed] [Google Scholar]
- He Q, Chen J, Yan J, Cai S, Xiong H, Liu Y, Peng D, Mo M, Liu Z. Tumor microenvironment responsive drug delivery systems. Asian J Pharm Sci. 2020;15:416–448. doi: 10.1016/j.ajps.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Litwin T, Nagaraja AR, Kwong B, Katz J, Watson N, Irvine DJ. Cytosolic delivery of membrane-impermeable molecules in dendritic cells using pH-responsive core-shell nanoparticles. Nano Lett. 2007;7:3056–3064. doi: 10.1021/nl071542i. [DOI] [PubMed] [Google Scholar]
- Hu Y, Atukorale PU, Lu JJ, Moon JJ, Um SH, Cho EC, Wang Y, Chen J, Irvine DJ. Cytosolic delivery mediated via electrostatic surface binding of protein, virus, or siRNA cargos to pH-responsive core-shell gel particles. Biomacromolecules. 2009;10:756–765. doi: 10.1021/bm801199z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YB, Dammer EB, Ren RJ, Wang G. The endosomal-lysosomal system: from acidification and cargo sorting to neurodegeneration. Transl Neurodegener. 2015;4:18. doi: 10.1186/s40035-015-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudlikar MS, Li X, Gagarinov IA, Kolishetti N, Wolfert MA, Boons GJ. Controlled multi-functionalization facilitates targeted delivery of nanoparticles to cancer cells. Chemistry. 2016;22:1415–1423. doi: 10.1002/chem.201503999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12:967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- Kim JW, Dang CV. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- Kodama Y, Yatsugi Y, Kitahara T, Kurosaki T, Egashira K, Nakashima M, Muro T, Nakagawa H, Higuchi N, Nakamura T, Sasaki H. Quaternary complexes modified from pDNA and poly-l-lysine complexes to enhance pH-buffering effect and suppress cytotoxicity. J Pharm Sci. 2015;104:1470–1477. doi: 10.1002/jps.24364. [DOI] [PubMed] [Google Scholar]
- Koltover I, Salditt T, Radler JO, Safinya CR. An inverted hexagonal phase of cationic liposome-DNA complexes related to DNA release and delivery. Science. 1998;281:78–81. doi: 10.1126/science.281.5373.78. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kamada N, Moon JJ. Oral nanomedicine for modulating immunity, intestinal barrier functions, and gut microbiome. Adv Drug Deliv Rev. 2021;179:114021. doi: 10.1016/j.addr.2021.114021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Zhang X, Dong Y. Nanoscale platforms for messenger RNA delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11:e1530. doi: 10.1002/wnan.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Tao Y, Shu Y, Larochelle JR, Steinauer A, Thompson D, Schepartz A, Chen ZY, Liu DR. Discovery and characterization of a peptide that enhances endosomal escape of delivered proteins in vitro and in vivo. J Am Chem Soc. 2015;137:14084–14093. doi: 10.1021/jacs.5b05694. [DOI] [PubMed] [Google Scholar]
- Li W, Nicol F, Szoka FC., Jr GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv Drug Deliv Rev. 2004;56:967–985. doi: 10.1016/j.addr.2003.10.041. [DOI] [PubMed] [Google Scholar]
- Liang K, Richardson JJ, Ejima H, Such GK, Cui J, Caruso F. Peptide-tunable drug cytotoxicity via one-step assembled polymer nanoparticles. Adv Mater. 2014;26:2398–2402. doi: 10.1002/adma.201305002. [DOI] [PubMed] [Google Scholar]
- Lomas H, Massignani M, Abdullah KA, Canton I, Lo Presti C, Macneil S, Du J, Blanazs A, Madsen J, Armes SP, Lewis AL, Battaglia G. Non-cytotoxic polymer vesicles for rapid and efficient intracellular delivery. Faraday Discuss. 2008;139:143–159. doi: 10.1039/b717431d. [DOI] [PubMed] [Google Scholar]
- Lundberg M, Wikstrom S, Johansson M. Cell surface adherence and endocytosis of protein transduction domains. Mol Ther. 2003;8:143–150. doi: 10.1016/s1525-0016(03)00135-7. [DOI] [PubMed] [Google Scholar]
- Massignani M, Lopresti C, Blanazs A, Madsen J, Armes SP, Lewis AL, Battaglia G. Controlling cellular uptake by surface chemistry, size, and surface topology at the nanoscale. Small. 2009;5:2424–2432. doi: 10.1002/smll.200900578. [DOI] [PubMed] [Google Scholar]
- Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol. 2004;83:97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
- Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- Murphy RF, Powers S, Cantor CR. Endosome pH measured in single cells by dual fluorescence flow cytometry: rapid acidification of insulin to pH 6. J Cell Biol. 1984;98:1757–1762. doi: 10.1083/jcb.98.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase I, Kobayashi S, Futaki S. Endosome-disruptive peptides for improving cytosolic delivery of bioactive macromolecules. Biopolymers. 2010;94:763–770. doi: 10.1002/bip.21487. [DOI] [PubMed] [Google Scholar]
- Oishi M, Nagasaki Y. Synthesis, characterization, and biomedical applications of core-shell-type stimuli-responsive nanogels - Nanogel composed of poly[2-(N, N-diethylamino)ethyl methacrylate] core and PEG tethered chains. React Funct Polym. 2007;67:1311–1329. [Google Scholar]
- Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- Park S, Lee WJ, Park S, Choi D, Kim S, Park N. Reversibly pH-responsive gold nanoparticles and their applications for photothermal cancer therapy. Sci Rep. 2019;9:20180. doi: 10.1038/s41598-019-56754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Yu C, Wei J, Li L, Zhang C, Wu Q, Liu J, Yao SQ, Huang W. Rational design of nanocarriers for intracellular protein delivery. Adv Mater. 2019;31:e1902791. doi: 10.1002/adma.201902791. [DOI] [PubMed] [Google Scholar]
- Reichmuth AM, Oberli MA, Jaklenec A, Langer R, Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther Deliv. 2016;7:319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y, Hatakeyama H, Sato Y, Akita H, Takayama K, Kobayashi S, Futaki S, Harashima H. Endosomal escape and the knockdown efficiency of liposomal-siRNA by the fusogenic peptide shGALA. Biomaterials. 2011;32:5733–5742. doi: 10.1016/j.biomaterials.2011.04.047. [DOI] [PubMed] [Google Scholar]
- Sato Y, Kinami Y, Hashiba K, Harashima H. Different kinetics for the hepatic uptake of lipid nanoparticles between the apolipoprotein E/low density lipoprotein receptor and the N-acetyl-D-galactosamine/asialoglycoprotein receptor pathway. J Control Release. 2020;322:217–226. doi: 10.1016/j.jconrel.2020.03.006. [DOI] [PubMed] [Google Scholar]
- Sebastiani F, Arteta MY, Lerche M, Porcar L, Lang C, Bragg RA, Elmore CS, Krishnamurthy VR, Russell RA, Darwish T, Pichler H, Waldie S, Moulin M, Haertlein M, Forsyth VT, Lindfors L, Cardenas M. Apolipoprotein E binding drives structural and compositional rearrangement of mRNA-containing lipid nanoparticles. ACS Nano. 2021;15:6709–6722. doi: 10.1021/acsnano.0c10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby LI, Cortez-Jugo CM, Such GK, Johnston APR. Nanoescapology: progress toward understanding the endosomal escape of polymeric nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017 doi: 10.1002/wnan.1452. [DOI] [PubMed] [Google Scholar]
- Shen Y, Jin E, Zhang B, Murphy CJ, Sui M, Zhao J, Wang J, Tang J, Fan M, Van Kirk E, Murdoch WJ. Prodrugs forming high drug loading multifunctional nanocapsules for intracellular cancer drug delivery. J Am Chem Soc. 2010;132:4259–4265. doi: 10.1021/ja909475m. [DOI] [PubMed] [Google Scholar]
- Song LY, Ahkong QF, Rong Q, Wang Z, Ansell S, Hope MJ, Mui B. Characterization of the inhibitory effect of PEG-lipid conjugates on the intracellular delivery of plasmid and antisense DNA mediated by cationic lipid liposomes. Biochim Biophys Acta. 2002;1558:1–13. doi: 10.1016/s0005-2736(01)00399-6. [DOI] [PubMed] [Google Scholar]
- Su X, Fricke J, Kavanagh DG, Irvine DJ. In vitro and in vivo mRNA delivery using lipid-enveloped pH-responsive polymer nanoparticles. Mol Pharm. 2011;8:774–787. doi: 10.1021/mp100390w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science. 2010;328:1031–1035. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CY, Dou S, Du JZ, Yang XZ, Li YP, Wang J. Doxorubicin conjugate of poly(ethylene glycol)-block-polyphosphoester for cancer therapy. Adv Healthc Mater. 2014;3:261–272. doi: 10.1002/adhm.201300091. [DOI] [PubMed] [Google Scholar]
- Tao Y, Cai K, Liu S, Zhang Y, Chi Z, Xu J. Pseudo target release behavior of simvastatin through pH-responsive polymer based on dynamic imine bonds: promotes rapid proliferation of osteoblasts. Mater Sci Eng C. 2020;113:110979. doi: 10.1016/j.msec.2020.110979. [DOI] [PubMed] [Google Scholar]
- Thews O, Riemann A. Tumor pH and metastasis: a malignant process beyond hypoxia. Cancer Metastasis Rev. 2019;38:113–129. doi: 10.1007/s10555-018-09777-y. [DOI] [PubMed] [Google Scholar]
- Thomas RG, Surendran SP, Jeong YY. Tumor microenvironment-stimuli responsive nanoparticles for anticancer therapy. Front Mol Biosci. 2020;7:610533. doi: 10.3389/fmolb.2020.610533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchilin V. Intracellular delivery of protein and peptide therapeutics. Drug Discov Today Technol. 2008;5:e95–e103. doi: 10.1016/j.ddtec.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Wang HM, Wan GY, Liu YY, Chen BW, Chen HL, Zhang SP, Wang D, Xiong QQ, Zhang N, Wang YS. Dual-responsive nanoparticles based on oxidized pullulan and a disulfide-containing poly(beta-amino) ester for efficient delivery of genes and chemotherapeutic agents targeting hepatoma. Polym Chem. 2016;7:6340–6353. [Google Scholar]
- Wang X, Li MH, Hou YH, Li YN, Yao XM, Xue CC, Fei Y, Xiang Y, Cai KY, Zhao YL, Luo Z. Tumor-microenvironment-activated in situ self-assembly of sequentially responsive biopolymer for targeted photodynamic therapy. Adv Funct Mater. 2020 doi: 10.1002/adfm.202000229. [DOI] [Google Scholar]
- Ward C, Meehan J, Gray ME, Murray AF, Argyle DJ, Kunkler IH, Langdon SP. The impact of tumour pH on cancer progression: strategies for clinical intervention. Explor Targeted Anti-Tumor Ther. 2020;1:71–100. doi: 10.37349/etat.2020.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM, Whittaker GR. Fusion of enveloped viruses in endosomes. Traffic. 2016;17:593–614. doi: 10.1111/tra.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JT, Keller S, Manganiello MJ, Cheng C, Lee CC, Opara C, Convertine A, Stayton PS. pH-Responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano. 2013;7:3912–3925. doi: 10.1021/nn305466z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Zhu L, Torchilin VP. pH-sensitive poly(histidine)-PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery. Biomaterials. 2013;34:1213–1222. doi: 10.1016/j.biomaterials.2012.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Luo L, Wang Y, Wu Q, Dai HB, Li JS, Durkan C, Wang N, Wang GX. Endogenous pH-responsive nanoparticles with programmable size changes for targeted tumor therapy and imaging applications. Theranostics. 2018;8:3038–3058. doi: 10.7150/thno.23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XL, Kim JH, Koo H, Bae SM, Shin H, Kim MS, Lee BH, Park RW, Kim IS, Choi K, Kwon IC, Kim K, Lee DS. Tumor-targeting peptide conjugated pH-responsive micelles as a potential drug carrier for cancer therapy. Bioconjug Chem. 2010;21:208–213. doi: 10.1021/bc9005283. [DOI] [PubMed] [Google Scholar]
- Wu Z, Li T. Nanoparticle-mediated cytoplasmic delivery of messenger RNA vaccines: challenges and future perspectives. Pharm Res. 2021;38:473–478. doi: 10.1007/s11095-021-03015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Qian J, Suo A, Wang H, Yong X, Liu X, Liu R. Reduction/pH dual-sensitive PEGylated hyaluronan nanoparticles for targeted doxorubicin delivery. Carbohydr Polym. 2013;98:181–188. doi: 10.1016/j.carbpol.2013.05.077. [DOI] [PubMed] [Google Scholar]
- Yan Y, Ding H. pH-Responsive nanoparticles for cancer immunotherapy: a brief review. Nanomaterials (Basel) 2020;10:1613. doi: 10.3390/nano10081613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Chen HT, Vlahov IR, Cheng JX, Low PS. Characterization of the pH of folate receptor-containing endosomes and the rate of hydrolysis of internalized acid-labile folate-drug conjugates. J Pharmacol Exp Ther. 2007;321:462–468. doi: 10.1124/jpet.106.117648. [DOI] [PubMed] [Google Scholar]
- Zelphati O, Szoka FC., Jr Mechanism of oligonucleotide release from cationic liposomes. Proc Natl Acad Sci USA. 1996;93:11493–11498. doi: 10.1073/pnas.93.21.11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Misra RD. Magnetic drug-targeting carrier encapsulated with thermosensitive smart polymer: core-shell nanoparticle carrier and drug release response. Acta Biomater. 2007;3:838–850. doi: 10.1016/j.actbio.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Zhang X, Niebuur BJ, Chytil P, Etrych T, Filippov SK, Kikhney A, Wieland DCF, Svergun DI, Papadakis CM. Macromolecular pHPMA-based nanoparticles with cholesterol for solid tumor targeting: behavior in HSA protein environment. Biomacromolecules. 2018;19:470–480. doi: 10.1021/acs.biomac.7b01579. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhao M, Cao N, Qin W, Zhao M, Wu J, Lin D. Construction of a tumor microenvironment pH-responsive cleavable PEGylated hyaluronic acid nano-drug delivery system for colorectal cancer treatment. Biomater Sci. 2020;8:1885–1896. doi: 10.1039/c9bm01927h. [DOI] [PubMed] [Google Scholar]
- Zhang YN, Wang HL, Mukerabigwi JF, Liu M, Luo SY, Lei SJ, Cao Y, Huang XY, He HX. Self-organized nanoparticle drug delivery systems from a folate-targeted dextran-doxorubicin conjugate loaded with doxorubicin against multidrug resistance. Rsc Adv. 2015;5:71164–71173. [Google Scholar]
- Zhao L, Seth A, Wibowo N, Zhao CX, Mitter N, Yu C, Middelberg AP. Nanoparticle vaccines. Vaccine. 2014;32:327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- Zhao ZX, Gao SY, Wang JC, Chen CJ, Zhao EY, Hou WJ, Feng Q, Gao LY, Liu XY, Zhang LR, Zhang Q. Self-assembly nanomicelles based on cationic mPEG-PLA-b-Polyarginine(R15) triblock copolymer for siRNA delivery. Biomaterials. 2012;33:6793–6807. doi: 10.1016/j.biomaterials.2012.05.067. [DOI] [PubMed] [Google Scholar]
- Zheng M, Zhong Z, Zhou L, Meng F, Peng R, Zhong Z. Poly(ethylene oxide) grafted with short polyethylenimine gives DNA polyplexes with superior colloidal stability, low cytotoxicity, and potent in vitro gene transfection under serum conditions. Biomacromol. 2012;13:881–888. doi: 10.1021/bm2017965. [DOI] [PubMed] [Google Scholar]
- Zhou X, Huang L. DNA transfection mediated by cationic liposomes containing lipopolylysine: characterization and mechanism of action. Biochim Biophys Acta. 1994;1189:195–203. doi: 10.1016/0005-2736(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Zhuo S, Zhang F, Yu J, Zhang X, Yang G, Liu X. pH-sensitive biomaterials for drug delivery. Molecules. 2020;25:5696. doi: 10.3390/molecules25235649. [DOI] [PMC free article] [PubMed] [Google Scholar]