Supplemental Digital Content is Available in the Text.
Key Words: tacrolimus intrapatient variability, donor-reactive cells, donor-specific anti-Human Leukocyte Antigen antibodies, graft failure
Background:
Kidney transplant recipients with high intrapatient variability (IPV) in tacrolimus (Tac) exposure experience more rejection and reduced graft survival. To understand the underlying pathophysiology of this association, the authors investigated whether patients with high tacrolimus IPV have a more activated immune system than patients with low IPV. In addition, exposure to tacrolimus and mycophenolic acid (MPA) was studied in relation to rejection and graft survival.
Methods:
At the time of patient inclusion (5–7 years post-transplantation), the frequency of donor-reactive cells was determined by enzyme-linked immunosorbent assay, and the development of donor-specific anti-Human Leukocyte Antigen antibodies (DSA) was measured by Luminex Single Antigen assay. Tacrolimus IPV was retrospectively calculated between 6 and 12 months and the exposure to tacrolimus and MPA was determined between 1 and 5 years post-transplantation.
Results:
A total of 371 kidney transplant recipients were included in this study, of whom 56 developed a rejection episode after 12 months and 60 experienced graft failure after 5–7 years. No correlations were found between tacrolimus IPV or immunosuppression exposure and the number of donor-reactive cells after 5 years of transplantation. DSA were detected more often in patients with low exposure to both tacrolimus and MMF [4/21 (19%) versus 17/350 (4.9%), P = 0.04]. In this cohort, neither tacrolimus IPV nor low overall immunosuppression exposure was associated with a higher incidence of rejection. However, regression analysis showed that a higher tacrolimus IPV was associated with an increased incidence of graft failure (odds ratio = 1.03, P = 0.02).
Conclusions:
This study verifies the relationship between high tacrolimus IPV and impaired kidney allograft survival in long-term follow-up. DSA was also found to be more prevalent in patients with subtherapeutic concentrations of tacrolimus and MPA. An increased prevalence of donor-specific alloreactivity is yet to be demonstrated in patients with high IPV.
INTRODUCTION
Following the publication of Borra et al 2010,1 several groups have confirmed the relationship between high intrapatient variability (IPV) in tacrolimus concentrations and impaired clinical outcomes after solid organ transplantation. In both pediatric and adult patients, a higher variability in tacrolimus exposure has been associated with more acute rejection, doubling of serum creatinine, development of donor-specific anti-Human Leukocyte Antigen (HLA) antibodies (DSA), chronic histological lesions, reduced graft survival, or a combination of several of these outcome parameters.2–6 After solid organ transplantation, therapeutic drug monitoring of tacrolimus is routinely performed, and calculating the coefficient of variation or alternative methods to quantify IPV of tacrolimus predose concentrations do not require complicated software.7 In a recent study, Kuypers 2020 recommended aiming for an IPV with tacrolimus concentrations below 20%.8
Although the association is strong, it is unclear how a higher tacrolimus IPV leads to poorer outcomes. The most likely explanation is that fluctuations in tacrolimus concentrations lead to prolonged periods of under-immunosuppression, during which immune activation occurs.9 Accordingly, patients may develop cellular or antibody-mediated rejection (ABMR) episodes, causing loss of renal function and graft loss. Another explanation may be that graft deterioration is the result of intermittent periods of over-exposure to tacrolimus, causing calcineurin inhibitor-mediated nephrotoxicity.10 If the latter mechanism is the most important contributor to graft loss, immune activation would not be a frequently observed phenomenon in patients with higher IPV.
In this study, to investigate the pathophysiology of this association, the authors investigated whether patients with high tacrolimus IPV have signs of immune activation, such as higher numbers of donor-reactive memory interferon-gamma (IFN-γ) and interleukin (IL)-21 producing cells, or the development of DSA.11 IFN-γ and IL-21 are pro-inflammatory cytokines that play an active role in the process of transplant rejection.12,13 IFN-γ has been indicated to promote leukocyte migration to the allograft, enhance natural killer (NK) cell activity, and regulate immunoglobulin production and class switching in B cells.14 IL-21 plays a central role in the formation of germinal centers, and stimulates the differentiation of B cells into memory B cells and antibody-producing plasmablasts.15 Furthermore, IL-21 stimulates the expansion of CD8+ T cells and enhances the cytotoxicity of CD8+ T and NK cells.16,17 The enzyme-linked immunosorbent spot (ELISPOT) assay is a validated immune-monitoring tool in kidney transplantation, and its outcomes are associated with cellular and humoral rejection processes. The ELISPOT assay is a sensitive quantification assay that enables the detection of low-frequency cell numbers (as low as 10 cells/1 × 105 cells) or low-frequency donor-reactive memory T cells, in the case of transplantation.18,19 Because patients typically lose their grafts several years post-transplantation, a population with long-term follow-up (5–7 years after transplantation) was included in this study. In addition to tacrolimus IPV, the absolute exposure to tacrolimus and mycophenolic acid (MPA) was investigated as well.
MATERIALS AND METHODS
Patients
Blood from renal transplant recipients transplanted between 2010 and 2014 was sampled cross-sectionally during their routine yearly outpatient clinic visits between April 2017 and November 2019. The patients were between 5.0 and 7.7 years (median 5.9) post-transplant at the time of inclusion, and were required to have a functioning graft at that time. All patients had to be treated with a tacrolimus/MMF-based immunosuppressive regimen. Kidney function was assessed by an estimated glomerular filtration rate (eGFR, mL/min per 1.73 m2) as calculated by the CKD-EPI equation (see Table 1, Supplemental Digital Content 1, http://links.lww.com/TDM/A541).20 After collecting the blood samples, patients were followed up until March 2020 to determine which of them experienced graft loss (Fig. 1). All patients provided a written informed consent, and the study was approved by the Medical Ethics Committee of the Erasmus MC Rotterdam, the Netherlands (biobank protocol MEC-2010-022, MEC-2016-718). All transplantations were performed in accordance with the Declaration of Istanbul, and all the experiments were performed in accordance with the relevant guidelines and regulations of our institution and the ethical standards of the Declaration of Helsinki. The maintenance postoperative immunosuppressive regimen after transplantation comprised tacrolimus (Prograf or Advagraf; Astellas Pharma, Tokyo, Japan; aiming for predose concentrations of 5–8 ng/mL after 6 months) and mycophenolate mofetil (MMF; Cellcept; Roche, Basel, Switzerland; starting dose of 1 g twice daily, aiming for predose concentrations of 1.5–3.0 mg/L).
FIGURE 1.
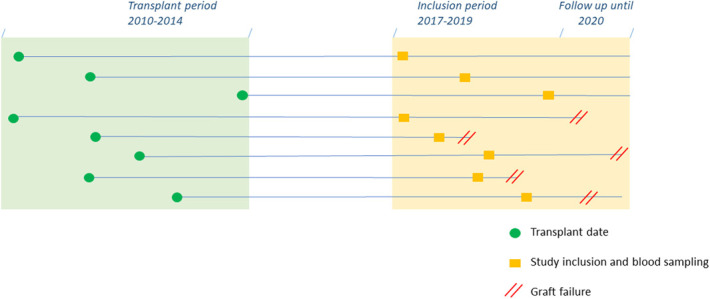
Schematic drawing of the transplant period, patient inclusion, and blood sampling up to the patient follow-up in March 2020.
IPV and Overall Immunosuppression
For the calculation of tacrolimus IPV, at least 3 predose tacrolimus concentrations for an individual patient had to be available. For this goal, solely data on tacrolimus concentrations measured during outpatient clinic visits in the period of 6–12 months post-transplantation were collected. Tacrolimus measurements obtained during hospitalization were not considered because transplant recipients would often use interacting drugs, such as antibiotics and pulse glucocorticoids, during this period. The IPV in tacrolimus exposure was calculated as described by Shuker et al 2016,21 with the sole difference being that tacrolimus concentrations were not corrected for the daily tacrolimus dose. MPA IPV was calculated in a manner similar to that of tacrolimus IPV. The overall immunosuppressive load during long-term follow-up was calculated using the average tacrolimus and MPA concentrations. Solely data on tacrolimus and MPA concentrations measured during follow-up outpatient clinic visits (for each patient, one measurement per year) in the period of 1—5 years post-transplantation were collected. The 25th percentile of tacrolimus and MPA concentrations was adopted as a cut-off to define the occurrence of low immunosuppression.22,23 This resulted in 4 groups based on overall immunosuppression concentrations: (1) Moderate-to-high tacrolimus, moderate-to-high MPA concentrations; (2) Moderate-to-high tacrolimus, low MPA concentrations; (3) Low MPA, moderate-to-high tacrolimus concentrations; and (4) Low tacrolimus and MPA concentrations.
Immune Activation Markers
Serum samples taken 5–7 years after transplantation were screened for the presence of anti-HLA antibodies using the Lifecodes Lifescreen Deluxe (LMX) kit (Immucor Transplant Diagnostics Inc. software, Stamford, CT).24 Furthermore, anti-HLA class I (HLA-A, HLA-B, and/or HLA-C) or anti-HLA class II (HLA-DR and/or HLA-DQ) antibodies were analyzed with a Luminex Single Antigen assay using LABscreen HLA class I and II antigen beads (One Lambda, Canoga Park, GA).24 The data were analyzed using the MATCHIT! antibody software version 1.3.1 (Immucor), respectively. The results were expressed as raw or background-corrected mean fluorescence intensity (MFI). A bead-specific cut-off based on raw MFI/lowest ranked antigen (LRA) (MFI/LRA), in combination with raw MFI >750 was used to assign positive beads. The presence of DSA was determined by comparing the measured HLA specificities with donor HLA typing. As described in a previous study,25 polyvinylidene fluoride (PVDF) plates (Millipore, Darmstadt, Germany) were coated with antihuman IFN-γ or IL-21 mAb (U-CyTech Biosciences, Utrecht, Netherlands). Patient's PBMCs were incubated for 5–7 years after transplantation (IFN-γ assay: 20 hours incubation after resting all samples overnight, and IL-21: 44 hours incubation) with irradiated (40 Gy) PBMCs or spleen cells derived from the donor or irradiated third-party cells, which were completely HLA-mismatched with donor and recipient, in a culture medium [RPMI-1640 with GlutaMAX (Life Technologies/Gibco) + 10% heat-inactivated FBS (Biowest, Haarlem, The Netherlands) + penicillin + streptomycin (100 IU/mL penicillin, 100 IU/mL streptomycin; Lonza)]. The numbers of cells per sample seeded in each well were 1 × 105 cells/well and 3 × 105 cells/well for the IFN-γ and IL-21 assays, respectively. Cells were incubated in the ELISPOT plate at 37°C, 5% CO2, and 95% humidity to allow spot formation. Thereafter, the wells were washed with PBS, and biotinylated antihuman IFN- γ or IL-21 detection antibody (U-CyTech Biosciences) was added. After washing, the wells were incubated with streptavidin–HRP conjugate (U-CyTech Biosciences) followed by AEC substrate (U-CyTech Biosciences) until distinct spots were formed; color development was stopped by washing with water, and spots were counted automatically using a Bioreader 6000 ELISPOT-reader (Bio-Sys GmbH, Karben, Germany).
Statistical Analysis
Statistical analyses were performed using SPSS (version 21.0; SPSS, Chicago, IL) and figures were illustrated using GraphPad Prism version 6.01 (GraphPad, La Jolla, CA). Univariate regressions were performed to determine whether tacrolimus IPV and MPA IPV were associated with the presence of DSA, incidence of rejection after a year, and incidence of graft failure. Pearson correlations were performed to determine the strength between the tacrolimus IPV and MPA IPV, including the frequency of IFN-γ and IL-21 donor-reactive cells. Furthermore, Pearson χ2 test was used to analyze whether overall immunosuppression after a year was associated with differences in the frequency of DSA and graft failure rates. The Kruskal–Wallis test was performed to determine whether patients in the distinct overall immunosuppression groups had different frequencies of IFN-γ and IL-21 donor-reactive cells. Finally, multivariable binary logistic regression was performed to assess the odds ratio (OR) and 95% confidence interval (CI) for developing graft failure. Regression analysis was performed using a stepwise backward selection method. Statistical significance was set at a 2-sided P-value of ≤0.05.
RESULTS
Patients
Table 1 presents the characteristics of the 371 transplant recipients included in this study. The median age at the time of transplantation was 55 years, and more than half of the patients were men (57.1%) and received their first kidney transplant (83.6%) from a living donor (75.7%). A total of 127 patients experienced at least one rejection event after transplantation. A total of 56 patients developed a rejection episode after 12 months, and 60 patients experienced long-term graft failure after blood sampling [mean time to graft failure and range in years: 7.5 (5.2–10.4)]. The reasons for graft failure were as follows: 41 patients died with a functioning graft, 17 grafts were lost to an active rejection (n = 4 chronic-active ABMR, n = 4 chronic/recurrent cellular rejections, n = 6 late acute rejections, and n = 3 mixed ABMR and acute cellular rejections). One graft was lost to infection and another was lost because of the recurrence of primary kidney disease (diabetic nephropathy). The death of the patients was because of malignancies (n = 13), cardiovascular disease/complications (n = 10), and infections (n = 7). The exact cause of death was unclear in 11 patients who died at home or in another hospital.
TABLE 1.
Patient Characteristics
| Kidney transplant recipients | 371 |
| Age,* yrs, median (range) | 55 (18–80) |
| Male gender, N (%) | 212 (57.1) |
| Donor | |
| Living donor, N (%) | 281 (75.7) |
| Age,* yrs, median (range) | 54.2 (21–82) |
| Delayed graft function, N (%) | 47 (12.7) |
| HLA mismatch, N (%) | |
| 0–2 | 112 (30.2) |
| 3–4 | 161 (43.4) |
| 5–6 | 98 (26.4) |
| Previous KTX, N (%) | |
| Second/third | 49 (13.2)/12 (3.2) |
| PRA %, mean (range) | |
| Current | 15.0 (0–83) |
| Historical | 11.3 (0–100) |
Age at transplantation.
IPV and Overall Immunosuppression
The median IPV of tacrolimus concentrations measured between 6 and 12 months was 19.4% (range: 1.0%—60.0%), of which 55/371 (14.8%) patients had a tacrolimus IPV ≥30% (Fig. 2). In addition, the median IPV of MPA measured between 6 and 12 months was 26.7% (range: 5.0%—62.0%, see Fig. 1, Supplemental Digital Content 2, http://links.lww.com/TDM/A542). The average tacrolimus and MPA concentrations between 1 and 5 years was 6.0 ng/mL and 1.75 mg/L, respectively. Thresholds (25th percentile) of 5.3 ng/mL and 1.24 mg/L were adopted for tacrolimus and MPA, respectively, to determine which patients had a low immunosuppressive load. The patients were divided into 4 groups as follows: 210 patients in group 1: moderate-to-high tacrolimus–moderate-to-high MPA concentrations, 72 patients in group 2: moderate-to-high tacrolimus–low MPA concentrations, 68 patients in group 3: low MPA–moderate-to-high tacrolimus concentrations, and 21 patients in group 4: low tacrolimus–low MPA concentrations.
FIGURE 2.
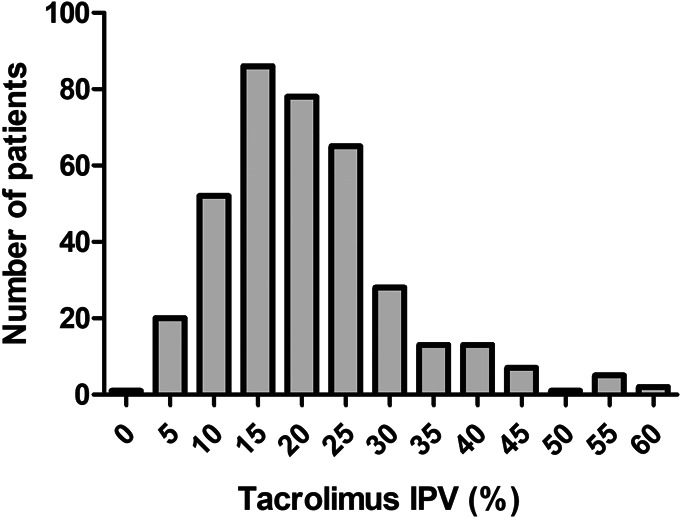
Distribution of tacrolimus intrapatient variability.
Associations Between IPV and/or Overall Immunosuppression and Immune Activation Markers
Anti-HLA antibodies were measured within 5–7 years after transplantation. A total of 63 (17%) patients had anti-HLA antibodies at the time of inclusion, of which 21 (5.7%) patients had detectable DSA. Univariate regression analysis indicated that tacrolimus IPV was not associated with the presence of anti-HLA or DSA (P = 0.7 and P = 0.4, respectively; Figs. 3A, B). Donor-reactive IFN-γ and IL-21 producing cells were measured in 74 patients, within 5—7 years post-transplantation. No correlations were found between tacrolimus IPV and the frequency of donor-reactive IFN-γ and IL-21 after transplantation (Figs. 3C, D). Similarly, no correlations were found between tacrolimus IPV and third-party reactive IFN-γ and IL-21 within 5–7 years post-transplantation. There was also no association between MPA IPV, the presence of anti-HLA or DSA and frequency of donor-reactive IFN-γ and IL-21 cells. There was no difference in the prevalence of anti-HLA in the 4 groups of patients with overall immunosuppression (P = 0.3). However, DSA was more prevalent in patients with low tacrolimus and MPA average concentrations than in patients in the other 3 groups of overall immunosuppression load [4/21 (19%) versus 17/350 (4.9%), P = 0.04]. No differences in donor-reactive (or third-party reactive) cells were found between the groups (donor-reactive IFN-, P = 0.77; donor-reactive IL-21, P = 0.27; Fig. 4).
FIGURE 3.
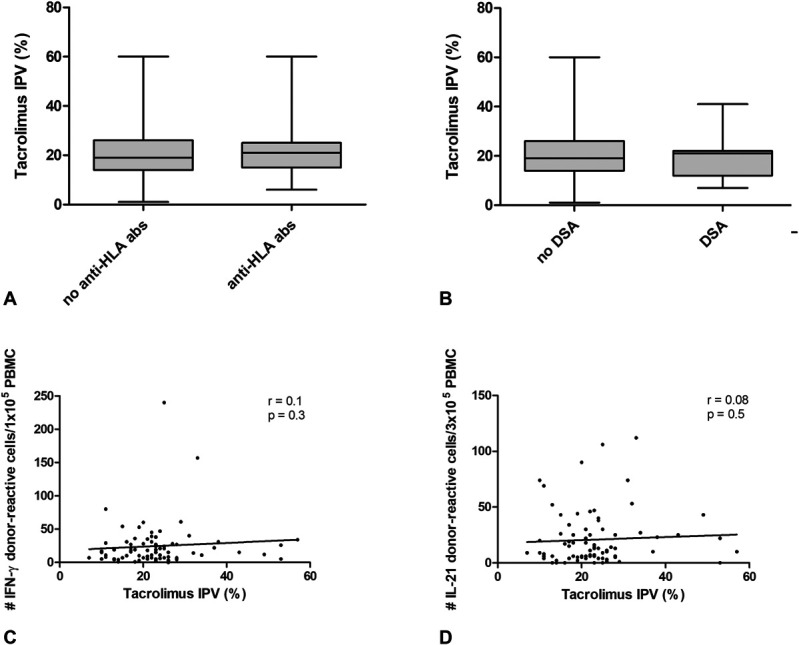
Association between tacrolimus IPV and immune markers at 5—7 years post-transplantation. A, anti-HLA antibodies, (B) donor-specific anti-HLA antibodies, (C) donor-reactive IFN-g producing cells and (D) donor-reactive IL-21 producing cells.
FIGURE 4.
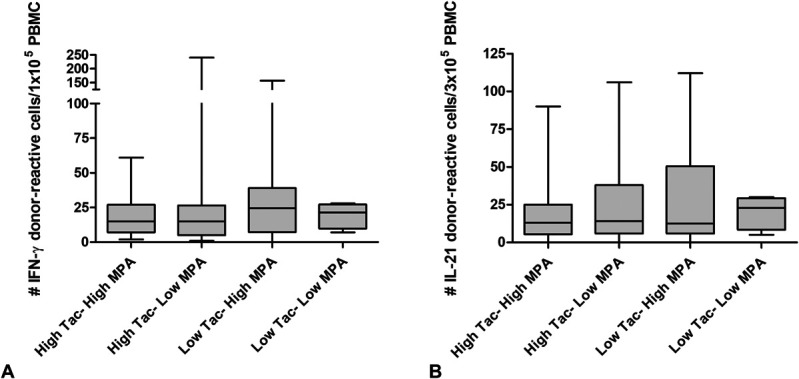
Donor-reactive IFN-γ and IL-21 producing cells at 5–7 years post-transplantation and overall immunosuppression from years 1—5 post-transplantation.
Risk Factors for the Development of Rejection Beyond 12 Months
Regression analysis revealed that tacrolimus IPV between months 6 and 12 post-transplantation was not associated with the incidence of rejection after a year (OR = 1.01, 95% CI 0.98–1.04; P = 0.4). The IPV of MPA was also analyzed in relation to the incidence of rejection after a year. No significant associations were found between MPA IPV and the incidence of rejection (OR = 0.99, 95% CI 0.97–1.03; P = 0.8). In addition, the incidence of rejection beyond 12 months post-transplantation was not different among the 4 overall immunosuppression groups (P = 0.84).
The total number of HLA mismatches was not correlated with a higher incidence of rejection a year post-transplantation (OR = 1.008, 95% CI 0.85–1.20; P = 0.9). However, higher current pretransplant and historic PRA scores were associated with graft rejection after a year (OR = 1.02, 95% CI 1.008–1.04; P = 0.003 and OR = 1.02, 95% CI 1.009–1.03; P < 0.0001, respectively).
Risk Factors for the Development of Graft Failure
A high tacrolimus IPV was associated with an increased incidence of graft failure (OR = 1.03, 95% CI 1.006–1.06; P = 0.02). Patients with graft failure had an average tacrolimus IPV of 23.5% (range 1–57), compared with a tacrolimus IPV of 20.0% (range 7–60) in patients who did not experience graft failure. Furthermore, a high tacrolimus IPV was associated with an increased incidence of death-censored graft failure (OR = 1.05, 95% CI 1.003–1.09; P = 0.04). No significant associations were found between MPA IPV and the incidence of graft or death-censored graft failure. The incidence of graft failure was not different between the 4 overall immunosuppression groups (P = 0.3), and there was no difference in the incidence of death-censored graft failure (P = 0.3).
In addition to the associations between maintenance immunosuppression and graft failure, the authors examined other variables that may also have an influence on death-censored graft failure. The degree of HLA mismatches was not correlated with death-censored graft loss (OR = 0.90, 95% CI 0.68–1.19; P = 0.4). Higher current and historic PRAs were associated with death-censored graft failure (OR = 1.02, 95% CI 1.0–1.04; P = 0.05 and OR = 1.02, 95% CI 1.007–1.03; P = 0.003, respectively). The percentage of death-censored graft failure in the group of patients with DSA (1/18, 5.6%) was the same as that in patients without DSA (18/312, 5.8%). In addition, neither donor-reactive IFN-γ or IL-21 producing cells were associated with death-censored graft failure (OR = 0.99, 95% CI 0.96–1.03; P = 0.7; OR = 1.01, 95% CI 0.98–1.04; P = 0.5, respectively).
To account for the different variables that contribute to death-censored graft failure, a multivariate regression was performed, which included current PRA, historic PRA, and tacrolimus IPV. A higher historic PRA and tacrolimus IPV were indicated to be independent risk factors for the incidence of death-censored graft failure (OR = 1.02, 95% CI 1.006–1.03; P = 0.005 and OR = 1.04, 95% CI 1.00–1.09; P = 0.05, respectively).
Associations Between IPV and/or Overall Immunosuppression and Kidney Function
When kidney function at 5 years was assessed in relation to tacrolimus IPV, no correlations were found between tacrolimus IPV and eGFR, serum creatinine, and urine protein/creatinine (see Fig. 2, Supplemental Digital Content 3, http://links.lww.com/TDM/A543). In addition, kidney function at 5 years was assessed (see Fig. 3, Supplemental Digital Content 4, http://links.lww.com/TDM/A544), and no significant differences in kidney function were found in the 4 overall immunosuppression groups.
DISCUSSION
The aim of this study was to investigate whether patients with high tacrolimus IPV in their first year post-transplant had signs of immune activation in the form of higher numbers of donor-reactive IFN-γ and IL-21 producing cells, or the development of DSA after 5–7 years of follow-up. No studies have explored the underlying biological mechanisms of high tacrolimus IPV and poor transplant outcomes. The authors hypothesized that patients with a high tacrolimus IPV (calculated based on therapeutic drug monitoring results obtained between 6- and 12-months post-transplantation) experience prolonged periods of under-immunosuppression, which leads to an expansion in donor-reactive cells. A meta-analysis of donor-specific ELISPOT demonstrated that recipients with rejection had significantly higher frequencies of pretransplant donor-reactive cytokines (IFN-γ or IL-21)-producing cells.19 No correlation was found between tacrolimus IPV and the number of donor-reactive IFN-γ and IL-21 producing cells after 5–7 years of transplantation. One possible explanation for this finding is that patient samples were acquired and measured long after transplantation, and it may have been too late to detect the markers of immune activation. Another explanation is that memory T cells are present at much higher frequencies in other human tissues (including lymphoid tissues) than in blood.26 Therefore, measuring donor-reactive IFN-γ and IL-21 producing cells in blood may lead to an underestimation of actual donor-reactive cell frequencies, or subtle differences between patient subpopulations. The presence of DSA at this time point was not associated with high tacrolimus IPV. This finding contrasts other studies where a higher frequency of DSA and/or de novo DSA was found in patients with high tacrolimus IPV.27–30 One notable difference between these studies and ours is that most of the other studies had shorter follow-up periods and possibly included patients who lost their grafts within the first 5 years. All patients in this study had a functioning graft 5–7 years post-transplantation. This study design may have led to the selection of a relatively low-risk patient population that experienced no major adverse events such as graft failure or loss within the first 5—7 years post-transplantation. In addition, the authors assessed whether tacrolimus IPV was associated with the incidence of rejection and graft failure after a year. Although no association was found between tacrolimus IPV and the incidence of rejection, a high tacrolimus IPV was associated with an increased incidence of graft failure.
There are 2 general mechanisms believed to cause damage to the allograft in patients with a high tacrolimus IPV, which include immune-mediated damages to the allograft because of under-immunosuppression caused by tacrolimus underexposure and/or non–immune-mediated damage such as tacrolimus nephrotoxicity caused by tacrolimus overexposure. Graft failure in this patient cohort was mainly caused by non–immune-mediated processes, such as malignancies and infections. Thus, the relationship between the high tacrolimus IPV and graft failure described in this manuscript is more likely to be the result of tacrolimus overexposure rather than tacrolimus underexposure. In addition to tacrolimus IPV, absolute exposure to tacrolimus and MPA between 1 and 5 years post-transplantation was investigated. The authors identified no differences in the number of donor-reactive IFN-γ and IL-21 producing cells in patients with high and/or low tacrolimus and MPA exposure. Patients with an overall lower exposure to both tacrolimus and MPA had an approximate 4-fold higher frequency of DSA 5 years post-transplantation. Reduced exposure to tacrolimus has been previously associated with the formation of de novo DSA31,32 even in a population with a low-immunological risk population.33 Although relatively few studies have revealed a relationship between low MPA and DSA development,34 several studies have suggested a protective effect of MPA in preventing DSA formation.35,36 Therefore, it is not surprising that patients who present with overall lower exposure to both tacrolimus and MPA have more DSA at 5–7 years post-transplantation. However, this risk was mitigated in patients who had adequate exposure to tacrolimus or MPA. Furthermore, the group of patients with low exposure to both tacrolimus and MPA did not have a higher incidence of rejection or graft failure. This suggests that in patients with a tacrolimus/MPA-based immunosuppression regimen and a stable graft function up to 5 years post-transplantation, the overall immunosuppression load after the first year of transplantation had negligible detrimental effects on the outcomes of the kidney allograft. It may be interesting to conduct a study with a longer follow-up period to determine whether the presence of DSA in combination with a low overall immunosuppression load can lead to adverse outcomes that develop over a longer period of time.
To the best of our knowledge, this is the first study to explore the possible underlying mechanism for the increased incidence of graft rejection and failure in patients with a high tacrolimus IPV. The lack of data on this subject makes it challenging to compare our current findings with those of others. However, to mitigate the adverse effects in patients with a high tacrolimus IPV, a better understanding of the changes to the immune system in response to this variability in tacrolimus exposure is required. One of the limitations of this study is the lack of consecutive data on immune markers. It may be better to measure donor-reactive cells and DSA yearly to research on the early rise of immune reactivity. In addition, the inclusion of patients should preferably occur early after transplantation to include patients who experience graft failure before 5 years post-transplantation. Furthermore, medication nonadherence is recognized as one of the most important contributors to the development of a high-tacrolimus IPV. In this study, the authors acquired data on tacrolimus and MPA concentrations; however, there are very limited data available about medication adherence in this patient cohort. The calculated MPA area under the concentration–time curve (AUC) was indicated to be associated with acute rejection.37 Frequent measurement of the MPA AUC is the recommended method for monitoring MPA.38,39 However, in our dataset, solely MPA predose concentrations were available. For a comprehensive understanding of the impact of overall immunosuppression, future studies are required to examine the combined effect of MPA-AUC with tacrolimus IPV on kidney allograft outcomes.
In conclusion, this study confirmed the relationship between high tacrolimus IPV and an increased risk of graft failure. The authors also determined that patients with subtherapeutic concentrations of tacrolimus and MPA had a higher incidence of DSA at 5–7 years post-transplantation. In conclusion, the authors could not demonstrate an increased prevalence of donor-specific alloreactivity in patients with a high IPV. Despite the lack of an underlying biological mechanism for the adverse outcomes in patients with a high tacrolimus IPV, there was still a positive association between a high tacrolimus IPV and graft failure 5—7 years post-transplantation. Monitoring tacrolimus IPV could be a valuable tool in assessing the risk of late graft failure, even in patients with stable graft function up to 5 years post-transplantation.
Supplementary Material
Footnotes
Supported by the Erasmus MC (mRACE grant 2016).
A. Mendoza Rojas, N. M. van Besouw, C. C. Baan, and T. van Gelder: conceptualization. A. Mendoza Rojas and T. van Gelder: methodology. A. Mendoza Rojas, M. Dieterich, RK: investigation. A. Mendoza Rojas: formal analysis. A. Mendoza Rojas and T. van Gelder: writing the original draft. D. A. Hesselink, N. van Besouw, and C. C. Baan: editing. N. van Besouw, C. C. Baan, and T. van Gelder: supervision. All authors contributed to the manuscript and approved it for submission.
D. A. Hesselink has received lecture and consulting fees from Astellas Pharma and Chiesi Farmaceutici SpA, including grant support from Astellas Pharma, Chiesi Farmaceutici SpA, and Bristol Myers-Squibb. T. van Gelder reports study grants from Astellas Pharma and Chiesi Farmaceutici SpA, including honoraria for lectures or consultations from Astellas Pharma and Novartis. The other authors declare no conflict of interest.
The studies involving human participants were reviewed and approved by METC Erasmus Medisch Centrum Rotterdam. The patients/participants provided their written informed consent to participate in this study.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.drug-monitoring.com).
REFERENCES
- 1.Borra LC, Roodnat JI, Kal JA, et al. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transpl. 2010;25:2757–2763. [DOI] [PubMed] [Google Scholar]
- 2.Shuker N, van Gelder T, Hesselink DA. Intra-patient variability in tacrolimus exposure: causes, consequences for clinical management. Transplant Rev. 2015;29:78–84. [DOI] [PubMed] [Google Scholar]
- 3.Süsal C, Döhler B. Late intra-patient tacrolimus trough level variability as a major problem in kidney transplantation: a Collaborative Transplant Study Report. Am J Transpl. 2019;19:2805–2813. [DOI] [PubMed] [Google Scholar]
- 4.Defrancq C, De Wilde N, Raes A, et al. Intra-patient variability in tacrolimus exposure in pediatric liver transplant recipients: evolution, risk factors, and impact on patient outcomes. Pediatr Transpl. 2019;23:e13388. [DOI] [PubMed] [Google Scholar]
- 5.Gold A, Tönshoff B, Döhler B, et al. Association of graft survival with tacrolimus exposure and late intra-patient tacrolimus variability in pediatric and young adult renal transplant recipients-an international CTS registry analysis. Transpl Int. 2020;33:1681–1692. [DOI] [PubMed] [Google Scholar]
- 6.Vanhove T, Vermeulen T, Annaert P, et al. High intrapatient variability of tacrolimus concentrations predicts accelerated progression of chronic histologic lesions in renal recipients. Am J Transpl. 2016;16:2954–2963. [DOI] [PubMed] [Google Scholar]
- 7.van Gelder T. A new method to calculate intra-patient variability in tacrolimus concentrations. Br J Clin Pharmacol. 2021;29:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuypers DR. From nonadherence to adherence. Transplantation . 2020;104:1330–1340. [DOI] [PubMed] [Google Scholar]
- 9.Neuberger JM, Bechstein WO, Kuypers DR, et al. Practical recommendations for long-term management of modifiable risks in kidney and liver transplant recipients: a guidance report and clinical checklist by the consensus on managing modifiable risk in transplantation (COMMIT) group. Transplantation . 2017;101:S1–S56. [DOI] [PubMed] [Google Scholar]
- 10.Naesens M, Kuypers DRJ, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481–508. [DOI] [PubMed] [Google Scholar]
- 11.Brunet M, Shipkova M, van Gelder T, et al. Barcelona consensus on biomarker-based immunosuppressive drugs management in solid organ transplantation. Ther Drug Monit. 2016;38(suppl 1):S1–S20. [DOI] [PubMed] [Google Scholar]
- 12.Hidalgo LG, Halloran PF. Role of IFN-gamma in allograft rejection. Crit Rev Immunol. 2002;22:317–349. [PubMed] [Google Scholar]
- 13.Baan CC, Balk AH, Dijke IE, et al. Interleukin-21: an interleukin-2 dependent player in rejection processes. Transplantation . 2007;83:1485–1492. [DOI] [PubMed] [Google Scholar]
- 14.Schroder K, Hertzog PJ, Ravasi T, et al. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, van Besouw NM, Shi Y, et al. The biological effects of IL-21 signaling on B-cell-mediated responses in organ transplantation. Front Immunol. 2016;7:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parmigiani A, Pallin MF, Schmidtmayerova H. Interleukin-21 and cellular activation concurrently induce potent cytotoxic function and promote antiviral activity in human CD8 T cells. Hum Immunol. 2011;72:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi X, Que R, Liu B, et al. Role of IL-21 signaling pathway in transplant-related biology Transpl Rev (Orlando) . 2016;30:27–30. [DOI] [PubMed] [Google Scholar]
- 18.van Besouw NM, Yan L, de Kuiper R, et al. The number of donor-specific IL-21 producing cells before and after transplantation predicts kidney graft rejection. Front Immunol. 2019;10:748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Udomkarnjananun S, Kerr SJ, Townamchai N, et al. Donor-specific ELISPOT assay for predicting acute rejection and allograft function after kidney transplantation: a systematic review and meta-analysis. Clin Biochem. 2021;94:1–11. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shuker N, Shuker L, van Rosmalen J, et al. A high intrapatient variability in tacrolimus exposure is associated with poor long-term outcome of kidney transplantation. Transpl Int. 2016;29:1158–1167. [DOI] [PubMed] [Google Scholar]
- 22.Brunet M, van Gelder T, Asberg A, et al. Therapeutic drug monitoring of tacrolimus-personalized therapy: second consensus report. Ther Drug Monit. 2019;41:261–307. [DOI] [PubMed] [Google Scholar]
- 23.Bergan S, Brunet M, Hesselink DA, et al. Personalized therapy for mycophenolate: consensus report by the international association of therapeutic drug monitoring and clinical toxicology. Ther Drug Monit. 2021;43:150–200. [DOI] [PubMed] [Google Scholar]
- 24.Niu Q, Mendoza Rojas A, Dieterich M, et al. Immunosuppression has long-lasting effects on circulating follicular regulatory T cells in kidney transplant recipients. Front Immunol. 2020;11:1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendoza Rojas A, van Gelder T, de Kuiper R, et al. Pre-transplant donor-reactive IL-21 producing T cells as a tool to identify an increased risk for acute rejection. Sci Rep. 2021;11:12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendoza Rojas A, Hesselink DA, van Besouw NM, et al. Impact of low tacrolimus exposure and high tacrolimus intra-patient variability on the development of de novo anti-HLA donor-specific antibodies in kidney transplant recipients. Expert Rev Clin Immunol. 2019;15:1323–1331. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigo E, Segundo DS, Fernandez-Fresnedo G, et al. Within-patient variability in tacrolimus blood levels predicts kidney graft loss and donor-specific antibody development Transplantation . 2016;100:2479–2485. [DOI] [PubMed] [Google Scholar]
- 29.Wiebe C, Rush DN, Nevins TE, et al. Class II eplet mismatch modulates tacrolimus trough levels required to prevent donor-specific antibody development. J Am Soc Nephrol . 2017;28:3353–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis S, Gralla J, Klem P, et al. Lower tacrolimus exposure and time in therapeutic range increase the risk of de novo donor-specific antibodies in the first year of kidney transplantation. Am J Transpl. 2018;18:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gatault P, Kamar N, Buchler M, et al. Reduction of extended-release tacrolimus dose in low-immunological-risk kidney transplant recipients increases risk of rejection and appearance of donor-specific antibodies: a randomized study. Am J Transpl. 2017;17:1370–1379. [DOI] [PubMed] [Google Scholar]
- 32.Jung HY, Kim SH, Seo MY, et al. Characteristics and clinical significance of de novo donor-specific anti-HLA antibodies after kidney transplantation. J Korean Med Sci. 2018;33:e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Girerd S, Schikowski J, Girerd N, et al. Impact of reduced exposure to calcineurin inhibitors on the development of de novo DSA: a cohort of non-immunized first kidney graft recipients between 2007 and 2014. BMC Nephrol. 2018;19:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Filler G, Todorova EK, Bax K, et al. MC Minimum mycophenolic acid levels are associated with donor-specific antibody formation. Pediatr Transpl. 2016;20:34–38. [DOI] [PubMed] [Google Scholar]
- 35.Lederer SR, Friedrich N, Banas B, et al. Effects of mycophenolate mofetil on donor-specific antibody formation in renal transplantation. Clin Transpl. 2005;19:168–174. [DOI] [PubMed] [Google Scholar]
- 36.O'Leary JG, Samaniego M, Barrio MC, et al. The influence of immunosuppressive agents on the risk of de novo donor-specific HLA antibody production in solid organ transplant recipients. Transplantation . 2016;100:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barraclough KA, Staatz CE, Johnson DW, et al. Kidney transplant outcomes are related to tacrolimus, mycophenolic acid and prednisolone exposure in the first week. Transpl Int. 2012;25:1182–1193. [DOI] [PubMed] [Google Scholar]
- 38.Le Meur Y, Borrows R, Pescovitz MD, et al. Therapeutic drug monitoring of mycophenolates in kidney transplantation: report of the Transplantation Society consensus meeting. Transpl Rev (Orlando) . 2011;25:58–64. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Sun Z, Zhu Z, et al. Pharmacokinetics of Mycophenolate Mofetil and Development of Limited Sampling Strategy in Early Kidney Transplant Recipients. Front Pharmacol . 2018;9:908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


