Abstract
Objective
To prospectively evaluate the feasibility and safety of a novel, second-generation telementoring interface (Connect™; Intuitive Surgical Inc., Sunnyvale, CA, USA) for the da Vinci robot.
Materials and Methods
Robotic surgery trainees were mentored during portions of robot-assisted prostatectomy and renal surgery cases. Cases were assigned as traditional in-room mentoring or remote mentoring using Connect. While viewing two-dimensional, real-time video of the surgical field, remote mentors delivered verbal and visual counsel, using two-way audio and telestration (drawing) capabilities. Perioperative and technical data were recorded. Trainee robotic performance was rated using a validated assessment tool by both mentors and trainees. The mentoring interface was rated using a multifactorial Likert-based survey. The Mann–Whitney and t-tests were used to determine statistical differences.
Results
We enrolled 55 mentored surgical cases (29 in-room, 26 remote). Perioperative variables of operative time and blood loss were similar between in-room and remote mentored cases. Robotic skills assessment showed no significant difference (P > 0.05). Mentors preferred remote over in-room telestration (P = 0.05); otherwise no significant difference existed in evaluation of the interfaces. Remote cases using wired (vs wireless) connections had lower latency and better data transfer (P = 0.005). Three of 18 (17%) wireless sessions were disrupted; one was converted to wired, one continued after restarting Connect, and the third was aborted. A bipolar injury to the colon occurred during one (3%) in-room mentored case; no intraoperative injuries were reported during remote sessions.
Conclusion
In a tightly controlled environment, the Connect interface allows trainee robotic surgeons to be telementored in a safe and effective manner while performing basic surgical techniques. Significant steps remain prior to widespread use of this technology.
Keywords: robotics, telemedicine, mentors, training techniques, minimally invasive
Introduction
For many years, tele-medicine has proven useful in extending the reach of specialised medical knowledge, from familiar applications, such as tele-radiology, to more novel applications, such as remote management of Intensive Care Unit or international patients [1–4]. Tele-medicine as applied to the field of surgical instruction is termed ‘telementoring’, and is remote surgical mentoring via transmission of video and/or audio data.
Since the advent of minimally invasive surgery, telementoring has been discussed as a method of bringing specialised surgical mentoring to locations that lack surgical subspecialists or where hazardous conditions (i.e. battlefield) make in-room mentoring impossible [5,6]. Investigations into various methods have thus far failed to identify an ideal telementoring platform [7]. In the more specialised field of robot-assisted laparoscopic surgery, there have been even fewer identified options for telementoring, and as yet there are no widely adopted interfaces [8,9].
The need for improved surgical mentoring has been highlighted by recent media scrutiny over surgical complications allegedly related to insufficient training and oversight with robot-assisted surgery [10,11]. Remote telementoring is one potential solution to this complex problem, as it allows an experienced surgeon to mentor trainees at their home institutions however geographically remote.
In the present study, we evaluate a second-generation telementoring interface developed by Intuitive Surgical Inc. (Sunnyvale, CA, USA), called Connect™. This is a software feature that fully integrates remote telementoring capability into the da Vinci surgical system (Intuitive Surgical). It is the first interface to merge the mentor’s ‘telestrations’, or drawings, onto the operating surgeon’s view of the surgical field via the robot console. It also uses existing, secure hospital Internet networks for connectivity and a simple desktop or laptop computer for mentoring. Based upon the da Vinci Si system, this new mentoring interface requires no additional hardware installation. Only software installation onto a personal computer and Internet connectivity are required [12].
In the present study, we report the first pilot study to evaluate the feasibility and clinical safety of the Connect remote-mentoring interface during robotic prostate and kidney cases. Endpoints included comparison between in-room and remote mentored cases in terms of connectivity data, interface rating by participants, and surgical skills rating by expert surgeons.
Materials and Methods
Over an 8-month period from October 2013 to May 2014, following Institutional Ethics Review Board approval, robot-assisted prostate and kidney cases were prospectively enrolled. Cases were designated to have the trainee surgeon mentored in either a remote or in-room fashion. Portions of each type of surgery were included for mentoring. For robotic radical prostatectomy, sigmoid mobilisation and dropping of the bladder were included. For robotic partial or radical nephrectomy, we included mobilisation of the colon.
Operating as the console surgeon on the pre-selected steps, robotic surgery trainees were recruited from urology residents rotating through the minimally invasive surgical service. All trainees were of intermediate robotic experience level and previously had completed a virtual reality robotic simulation course as part of residency training. Live robotic experience varied, but none had independently completed any console cases. All residents were eligible for inclusion. Mentors were either robotic fellows or attending physicians from the Urology Department who had completed at least 150 console cases as primary surgeon. In previous validations studies, 100 robotic cases was set as the ‘expert’ threshold, but with limited published evidence of the true qualifications of mentorship, the authors felt that ≥150 robotic cases would be a more appropriate ‘expert’ threshold [13]. In-room mentors had no restrictions, and were permitted to teach via any method, including telestration or dual console demonstration. For all remote sessions, a ‘backup’ experienced surgeon was present in the operating room as a safety precaution. The ‘backup’ surgeon was told to intervene only if clinically necessary.
Telementoring Interface
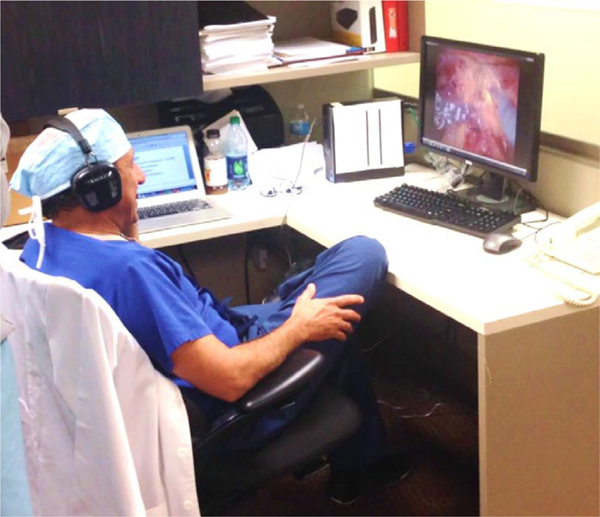
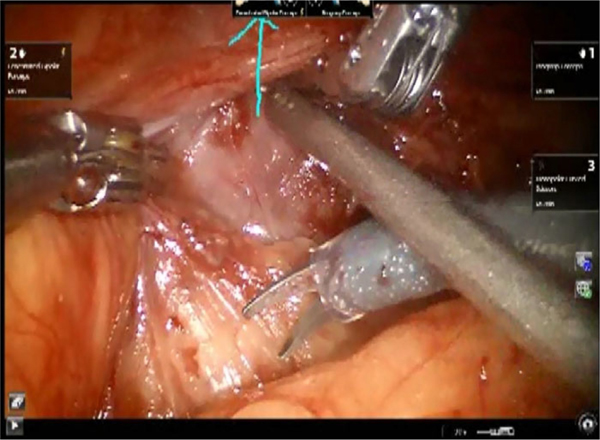
Connect is a software program that adds remote mentoring capability to the da Vinci Si robot, allowing surgical mentoring from a location outside of the operating room. It uses Internet connectivity to add three main features: one-way video, two-way audio, and telestration. The video feed from the robot camera, visible to the training surgeon on the surgeon console, is also viewable in two-dimensions by the mentor on a desktop or laptop computer in real-time (Fig. 1). The audio feed allows the mentor to speak with the trainee, and vice versa. Finally, the interface allows the mentor to telestrate. Using a mouse or trackpad, the mentor may draw on his screen, and these line drawings are viewable to the training surgeon as an overlay onto the surgical field in the surgeon console (Fig. 2). With these three features, the Connect interface allows the mentor to counsel the trainee on surgical technique and anatomy. The surgical mentor may provide oversight from any computer with high-speed Internet connectivity.
Fig. 1.
Illustration of the mentor side interface.
Fig. 2.
Illustration of telestration overlaid onto the surgeon console view of the surgical field.
Location and Network Equipment
This study was conducted at the University of Southern California (USC) Health Science Campus, with the maximum distance between the remote mentor and trainee being 0.35 km. Secure network systems maintained by USC were used through wired (Ethernet) or wireless (802.11 g network standard) connections. Remote mentors used USC-maintained, password-protected computers. For wireless connections, a dedicated laptop computer was used. A desktop computer was used for wired connections. All surgeries were performed using the da Vinci Si surgical system.
Inclusion and Exclusion Criteria
Prostatectomy and kidney cases (radical or partial nephrectomy) that were planned to be performed in a robot-assisted laparoscopic fashion were included. Cases were excluded by attending surgeon discretion for reasons including complex anatomy or time constraints.
Data Collection
Before each case, surgical mentors and trainees completed pre-study questionnaires, which inquired about robotic surgical experience and postgraduate year. Additional objective data included: connection setup time for the Connect interface, estimated case duration, estimated blood loss (EBL), and intraoperative complications.
After each mentored case, both mentors and trainees completed a Global Evaluative Assessment of Robotic Skill (GEARS) form and an evaluation of the mentoring interface. GEARS is a robotic skills assessment tool, which has been validated in numerous studies [14–16]. All participants (trainees and mentors) underwent orientation to GEARS, and our previous work with GEARS showed good interobserver reliability amongst expert surgeons [15]. In addition, we created a Likert-scale-based survey to evaluate both the traditional in-room mentoring interface (i.e. telestration feature on the da Vinci Vision Cart) and the remote mentoring (Connect) interface. This survey addressed various aspects of the mentoring experience, including ability to show anatomy, mentor with confidence, teach surgical skill, guide surgery safely, smoothness of interface, ease of use, and helpfulness of the telestration feature.
From a technical aspect, we recorded the mode of Internet connection for the remote mentoring cases (wired vs wireless), the connection speed (latency), and the reliability of the data transfer (percentage signal loss). The latter two parameters were collected from a software program created by Intuitive Surgical’s development team.
Statistical Analysis
Statistical analysis was conducted using two-tailed Mann–Whitney and t-tests to determine statistical significance. Significance level was designated as 0.05. Because an equivalence study for perioperative parameters would require the recruitment of thousands of cases per arm of the study, statistical analysis was only reported for connection-related technical data, surgical skills, and interface ratings data.
Results
In all, 55 robot-assisted cases were enrolled, involving 11 trainees and 10 mentors (Table 1). Traditional in-room mentored cases totalled 29, of which 12 were prostatectomies and 17 were kidney cases, either partial or radical nephrectomies (Table 2). Remote mentored cases using the Connect interface totalled 26, of which 15 were prostatectomies and 11 were kidney cases.
Table 1.
Trainee/mentor demographic data.
| Median (range) | Postgraduate year | Full console cases completed, n |
|---|---|---|
|
| ||
| Trainee (n = 11) | 4.5 (3–6) | 0 |
| Mentor (n = 10) | - | 500 (150–3 000) |
Table 2.
Clinical perioperative data.
| In-room | Remote | |
|---|---|---|
|
| ||
| Robotic radical prostatectomy (mobilise sigmoid + drop bladder), n | 12 | 15 |
| Median (range) | ||
| Estimated duration, min | 15.0 (7.5–30) | 20.0 (12.5–30) |
| EBL, mL | 2.5 (0–17.5) | 2.5 (0–10) |
| Robotic partial nephrectomy (mobilise colon), n | 17 | 11 |
| Median (range) | ||
| Estimated duration, min | 15.0 (5–25) | 15.0 (5–35) |
| EBL, mL | 2.5 (0–7) | 2.5 (0–7) |
| Intraoperative complications, n/N (%) | 1/29 (3) | 0/26 |
Duration for both prostatectomy and kidney sessions appeared to be similar. EBL for prostatectomy and kidney cases in the included segments also appeared comparable.
When trainees rated their own robotic skill using the GEARS tool, there was no significant difference between in-room and remote cases (P > 0.05; Table 3). When mentors rated the trainees using GEARS, there also was no significant difference between the aggregate scores for in-room and remote cases (P > 0.05). Of note, after in-room mentored cases, trainees rated their robotic skill lower than did their mentors (P = 0.07).
Table 3.
GEARS robotic skills assessment (maximum = 30 points).
| Median (range) | In-room | Remote | P |
|---|---|---|---|
|
| |||
| Trainee self-rating | 20.5 (15–28) | 21 (16–24) | 0.5 |
| Mentor rating of trainee | 22 (13–30) | 21 (14–28) | 0.8 |
| P | 0.07 | 0.3 | |
When trainees rated the mentoring interface, there was no significant difference between the in-room interface and the Connect interface, for all aspects queried (P > 0.05; Table 4). Mentors trended towards a preference of remote telestration over in-room telestration (P = 0.05); otherwise they displayed no significant difference in their evaluation of the interface.
Table 4.
Rating of the mentoring interface: in-room vs remote mentoring (Connect).
| In-room | Remote | P | |
|---|---|---|---|
|
| |||
| Trainee rating of interface | |||
| Median (range) | |||
| Anatomical recognition | 4.5 (1–5) | 5 (2–5) | 0.3 |
| Improvement of surgical skills | 5 (2–5) | 5 (2–5) | 0.4 |
| Surgical confidence | 5 (3–5) | 5 (2–5) | 0.1 |
| Safety | 5 (2–5) | 5 (3–5) | 0.4 |
| ‘Worked well’ | 5 (4–5) | 5 (2–5) | 0.7 |
| Ease of use | 5 (3–5) | 5 (2–5) | 0.7 |
| Telestration helpfulness | 5 (5–5) | 5 (1–5) | 0.9 |
| Mentor rating of interface | |||
| Median (range) | |||
| Anatomical recognition | 4 (2–5) | 4 (3–5) | 1.0 |
| Improvement of surgical skills | 4 (3–5) | 4 (3–5) | 0.2 |
| Surgical confidence | 5 (4–5) | 4 (4–5) | 0.3 |
| Safety | 4 (4–5) | 4 (4–5) | 0.4 |
| ‘Worked well’ | 4.5 (3–5) | 4 (2–5) | 0.1 |
| Ease of use | 4 (3–5) | 4 (4–5) | 0.8 |
| Telestration helpfulness | 4 (2–4) | 4 (3–5) | 0.05 |
Of the 26 remote mentoring cases, eight (31%) were conducted using a wired Internet connection, and 18 (69%) used a wireless Internet connection (Table 5). There was no significant difference in the data connection setup time regardless of type of connection used [mean (SD) for wired 5.5 (3.8) min and wireless 4.6 (2.2) min, P > 0.05]. Remote mentoring cases using the wired connection were significantly faster, with mean (SD) latency, or one-way connection time of 7.4 (1.4) ms for the wired connection and 13.2 (7.4) ms for the wireless connection (P = 0.005). Despite this difference in data transfer speed, mentor and trainee perception of the mentoring experience did not statistically differ between wired and wireless connections (P > 0.05). The reliability of data transmission was estimated by rate of ‘lost data packets’. Data loss was noted in two of the 26 remote mentoring cases (8%); both were wireless connections and 1–2% of data loss was reported. In one of the remote cases with data loss, the Connect connection was disrupted and the session was converted from wireless to wired to proceed with mentoring; no further disruptions occurred in that remote mentored session. The other wireless remote mentoring session experiencing data loss was completed after re-establishing the data connection. There was one additional session in which the video froze and the mentoring session was aborted entirely by the attending surgeon. The remote sessions took place at a mean (range) of 202 (30–350) m separation between the trainee and mentor.
Table 5.
Setup and connection data.
| Remote cases | P | ||
|---|---|---|---|
|
|
|||
| Wired | Wireless | ||
|
| |||
| Number of cases | 8 | 18 | |
| Mean (SD) | |||
| Connect setup time, min | 5.5 (3.8) | 4.6 (2.2) | 0.5 |
| Connection latency, ms | 7.4 (1.4) | 13.2 (7.4) | 0.005 |
| n/N (%) | |||
| Sessions with data loss | 0/8 | 2/18 (11) | n/a |
| Dropped sessions | 0/8 | 3/18 (17) | n/a |
There were no instances in which ‘backup’ surgeons intervened during remote cases by taking over as console surgeon. All interactions were limited to verbal guidance only.
There was one intraoperative complication reported. During an in-room mentored robotic partial nephrectomy, a colon serosal injury occurred from bipolar energy of a fenestrated bipolar forcep. This was immediately recognised by the mentor and over-sutured, resulting in no postoperative sequelae. No intraoperative complications were noted in remote mentored cases.
Discussion
The rapid proliferation of robot-assisted surgery has created intense scrutiny focused on appropriate training and oversight for training robotic surgeons. Telementoring has the potential to extend the capabilities of surgeons with a more general skill set and efficiently share exceptionally specialised surgical expertise. However, currently there is little experience in telementoring for robotic surgery. The present pilot study is the first to report on the clinical safety and feasibility of robotic cases using the telementoring interface Connect.
We prospectively assessed pre-selected steps of robotic prostate and kidney cases mentored in the traditional in-room vs remote fashion. We found that in select portions of prostate and kidney surgeries, telementoring with the Connect interface can be achieved safely, with similar perioperative parameters noted between in-room and remotely mentored cases. This was true for objective measures such as EBL and case duration, and GEARS assessments of the training surgeons’ robotic performance. There were no intraoperative complications during remote mentored cases; there was one minor colon serosal injury during an in-room mentored case. We also found that unlike traditional in-room mentoring, successful telementoring is heavily reliant on a fast, reliable data network. Compromised network connection, particularly with the wireless remote method, can significantly derail the mentoring session, as we experienced in three of our present study cases. Interestingly, despite the technical problems encountered with remote mentoring, there were certain aspects of telementoring that were viewed favourably. Mentor feedback suggested that telestration was more helpful in the remote interface, perhaps reflecting the more prominent role telestration played during telementoring compared with in-room mentoring.
While various platforms exist for remote mentoring of open or laparascopic surgery, to our knowledge only one other interface has been applied specifically for robot-assisted surgery [8,17,18]. Hinata et al. [8] recently described a robot telementoring system created by their institution, which used a dedicated optical fibre link-based network, audio-visual transmission, three-dimensional (3D) monitors, and the TilePro function of the da Vinci robot. They successfully used this system to telementor 30 robot-assisted laparascopic prostatectomies. In terms of technical differences, the Connect interface requires only the pre-existing Internet network. Also, the Connect telestration is overlaid directly onto the training surgeon’s view of the operative field via the console (vs use of separate displays via TilePro). While the Connect system currently offers substantial flexibility for mentors in its compatibility with any Windows-based desktop or laptop computer, some remote mentors did express a desire for a 3D display and 3D telestration function. Demonstration of instrument articulation and 3D tissue retraction direction are not possible in the current generation of Connect.
There were limitations to the present study. Due to the logistical complexity of the study, we were able to enroll a relatively small number of cases. Also, our study was not randomised, with cases assigned to be remote mentored when an additional ‘backup’ experienced surgeon was available to be present in the operating room as a safety precaution; otherwise, the case was assigned to be mentored in-room. Further limitations include our inclusion of only the beginning segments of the prostate and kidney surgeries. These portions, arguably the ‘easier’ steps of the operation, were intentionally selected to preserve patient safety as this interface was being initially tested. Thus, our EBL and duration data reflect a minority of the total operative case. Finally, ‘complex’ cases, e.g. cases with a history of abdominal surgery or advanced malignancy, were excluded by the discretion of the attending surgeon. Overall, these factors limit the ability to generalise our present findings.
Recent reports suggest that current training regimens for robot-assisted surgery may be falling short [10,11]. While virtual simulation is emerging as an alternative form of instruction that allows training surgeons to refine their technique in a safe environment, there remains the need for real-time mentoring during live surgeries [19]. Traditional mentoring typically involves a surgical mentor providing guidance via direct verbal communication or demonstration on the robotic console. Dual console systems offer the ability for both mentor and trainee to share instrument control, potentially leading to more efficient transfer of robotic skills [20]. However, this type of mentoring is limited by the availability of a dual console and necessity of the mentor to be physically present in the operating room.
Telementoring has been presented as an option to bring specialised mentoring to situations where in-room mentoring may be unavailable or impossible [21,22]. For example, if specialised surgical instruction is needed in a remote/rural or hazardous geographic location, the option of ‘calling in’ an expert for recommendations and instruction can be very valuable. Specific cases have been reported where telementoring has been used for these indications, using interfaces of varying sophistication [23–26]. Situations have also been described in which community general surgeons have benefited from tertiary level expert advice via telementoring to successfully perform advanced laparoscopic surgeries [27].
The Connect interface has the potential to bring telementoring to robotic surgery in an efficient manner without the need to purchase additional equipment. While our present pilot study has notable limitations, it lays the necessary groundwork for further testing and refinement of this interface. Next steps will include mentoring of more complex segments of procedures and the evaluation of inter-hospital telementoring (i.e. remote mentorship over greater geographical distance). Next iterations of the program must address significant concerns over connection reliability, especially over wireless internet, and the desire of mentors for 3D viewing and telestration.
Telesurgery might be considered a natural extension of telementoring, and would allow the remote mentor to assume remote control of the trainee’s robot for the purposes of demonstration or assistance. Examples of this have been described in the literature. For example, a telerobotic system was used to assist in a laparoscopic renal cyst ablation between Baltimore and Munich in 2002 [28].
Legal barriers exist to the full implementation of telementoring and telesurgery. As summarised by Lendvay et al. [29] in 2013, the current inability to practice medicine across state lines in the USA without having multiple medical licenses is a significant hurdle. Furthermore, reimbursement of telemedicine has only recently begun to be addressed by the legislature. Complex issues of liability also exist for the future of telesurgery, and between the mentor and mentee during telementoring. However, it is likely that these legal barriers will be overcome as the technology of telementoring continues to be proven.
In conclusion, in the present pilot study of Connect, a novel interface for the telementoring of robotic surgery, we concluded that in a tightly controlled environment, basic surgical steps may be safely mentored in a remote fashion. Significant challenges still remain before telementoring may be implemented on a larger scale.
Acknowledgments
Conflict of Interests
M.D. reports Boston Scientific: Scientific Study/Trial - principal investigator of ‘Backstop’ study. M.M.D. reports ‘Other’ from Hansen Medical, outside the submitted work. M.A. reports ‘Other’ from Intuitive Surgical, outside the submitted work. I.S.G. reports ‘Other’ from Hansen Medical, personal fees from Mimic Technologies, outside the submitted work; and EDAP: Consultant/Advisor - consultant for product development. A.J.H. reports grants from Intuitive Surgical, during the conduct of the study; and personal fees from Mimic Technologies, outside the submitted work.
Abbreviations:
- 3D
three-dimensional
- EBL
estimated blood loss
- GEARS
Global Evaluative Assessment of Robotic Skill
- USC
University of Southern California
References
- 1.Guljas R, Ahmed A, Chang K, Whitlock A. Impact of telemedicine in managing type 1 diabetes among school-age children and adolescents: an integrative review. J Pediatr Nurs 2013; 29: 198–204 [DOI] [PubMed] [Google Scholar]
- 2.Eadie LH, Seifalian AM, Davidson BR. Telemedicine in surgery. Br J Surg 2003; 90: 647–58 [DOI] [PubMed] [Google Scholar]
- 3.Weinstein RS, Lopez AM, Joseph BA et al. Telemedicine, telehealth, and mobile health applications that work: opportunities and barriers. Am J Med 2014; 127: 183–7 [DOI] [PubMed] [Google Scholar]
- 4.Mars M. Telemedicine and advances in urban and rural healthcare delivery in Africa. Prog Cardiovasc Dis 2013; 56: 326–35 [DOI] [PubMed] [Google Scholar]
- 5.Rentschler ME, Platt SR, Berg K, Dumpert J, Oleynikov D, Farritor SM. Miniature in vivo robots for remote and harsh environments. IEEE Trans Inf Technol Biomed 2008; 12: 66–75 [DOI] [PubMed] [Google Scholar]
- 6.Haidegger T, Sandor J, Benyó Z. Surgery in space: the future of robotic telesurgery. Surg Endosc 2011; 25: 681–90 [DOI] [PubMed] [Google Scholar]
- 7.Ereso AQ, Garcia P, Tseng E, Dua MM, Victorino GP, Guy LT. Usability of robotic platforms for remote surgical teleproctoring. Telemed J E Health 2009; 15: 445–53 [DOI] [PubMed] [Google Scholar]
- 8.Hinata N, Miyake H, Kurahashi T et al. Novel telementoring system for robot-assisted radical prostatectomy: impact on the learning curve. Urology 2014; 83: 1088–92 [DOI] [PubMed] [Google Scholar]
- 9.Havranek EG, Sharfi AR, Nour S, Motiwala H, Karim O. Low-cost telemedicine. BJU Int 2011; 107: 1701–2 [DOI] [PubMed] [Google Scholar]
- 10.Carreyou J. Surgical Robot Examined in Injuries. The Wall Street Journal, 2010. Available at: http://www.wsj.com/articles/SB10001424052702304703104575173952145907526. Accessed February 2015 [Google Scholar]
- 11.Langreth R. Robot Surgical Incidents May Pressure Hospital Training. Bloomberg, 2013. Available at: http://www.bloomberg.com/news/2013-10-08/robot-surgerydamaging-patientsrises-with-marketing.html. Accessed February 2015 [Google Scholar]
- 12.Lenihan J, Brower M. Web-connected surgery: using the internet for teaching and proctoring of live robotic surgeries. J Robot Surg 2011; 6:47–52 [DOI] [PubMed] [Google Scholar]
- 13.Hung AJ, Zehnder P, Patil MB et al. Face, content and construct validity of a novel robotic surgery simulator. J Urol 2011; 186: 1019–24 [DOI] [PubMed] [Google Scholar]
- 14.Goh AC, Goldfarb DW, Sander JC, Miles BJ, Dunkin BJ. Global evaluative assessment of robotic skills: validation of a clinical assessment tool to measure robotic surgical skills. J Urol 2012; 187: 247–52 [DOI] [PubMed] [Google Scholar]
- 15.Hung A, Patil M, Zehnder P et al. Concurrent and predictive validation of a novel robotic surgery simulator: a prospective, randomized study. J Urol 2012; 187: 630–7 [DOI] [PubMed] [Google Scholar]
- 16.Hung AJ, Jayaratna IS, Teruya K, Desai MM, Gill IS, Goh AC. Comparative assessment of three standardized robotic surgery training methods. BJU Int 2013; 112: 864–71 [DOI] [PubMed] [Google Scholar]
- 17.Sawyer MA, Lim RB, Wong SY, Cirangle PT, Birkmire-Peters D. Telementored laparoscopic cholecystectomy: a pilot study. Stud Health Technol Inform 2000; 70: 302–8 [PubMed] [Google Scholar]
- 18.Rosser JC Jr, Gabriel N, Herman B, Murayama M. Telementoring and teleproctoring. World J Surg 2001; 25: 1438–48 [DOI] [PubMed] [Google Scholar]
- 19.Schreuder HW, Wolswijk R, Zweemer RP, Schijven MP, Verheijen RH. Training and learning robotic surgery, time for a more structured approach: a systematic review. BJOG 2012; 119: 137–49 [DOI] [PubMed] [Google Scholar]
- 20.Smith AL, Krivak TC, Scott EM et al. Dual-console robotic surgery compared to laparoscopic surgery with respect to surgical outcomes in a gynecologic oncology fellowship program. Gynecol Oncol 2012; 126: 432–6 [DOI] [PubMed] [Google Scholar]
- 21.Ereso AQ, Garcia P, Tseng E et al. Live transference of surgical subspecialty skills using telerobotic proctoring to remote general surgeons. J Am Coll Surg 2010; 211: 400–11 [DOI] [PubMed] [Google Scholar]
- 22.Santomauro M, Reina GA, Stroup SP, L’Esperance JO. Telementoring in robotic surgery. Curr Opin Urol 2013; 23: 141–5 [DOI] [PubMed] [Google Scholar]
- 23.Campana BA, Jarvis-Selinger S, Ho K, Evans WL, Zwimpfer TJ. Use of telemedicine for an emergency craniotomy in a pediatric trauma. CMAJ 2004; 171: 444–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricci MA, Caputo M, Amour J et al. Telemedicine reduces discrepancies in rural trauma care. Telemed J E Health 2003; 9: 3–11 [DOI] [PubMed] [Google Scholar]
- 25.Mendez I, Hill R, Clarke D, Kolyvas G, Walling S. Robotic long-distance telementoring in neurosurgery. Neurosurgery 2005; 56: 434–40 [DOI] [PubMed] [Google Scholar]
- 26.Challacombe B, Kandaswamy R, Dasgupta P, Mamode N. Telementoring facilitates independent hand-assisted laparoscopic living donor nephrectomy. Transplant Proc 2005; 37: 613–6 [DOI] [PubMed] [Google Scholar]
- 27.Sebajang H, Trudeau P, Dougall A, Hegge S, McKinley C, Anvari M. Telementoring: an important enabling tool for the community surgeon. Surg Innov 2005; 12: 327–31 [DOI] [PubMed] [Google Scholar]
- 28.Frimberger D, Kavoussi L, Stoianovici D et al. [Telerobotic surgery between Baltimore and Munich]. Urologe A 2002; 41: 489–92 [DOI] [PubMed] [Google Scholar]
- 29.Lendvay TS, Hannaford B, Satava RM. Future of robotic surgery. Cancer J 2013; 19: 109–19 [DOI] [PubMed] [Google Scholar]