Abstract
We cloned a gene, bexA, that codes for a multidrug efflux transporter from the chromosomal DNA of Bacteroides thetaiotaomicron ATCC 29741 by using an Escherichia coli ΔacrAB ΔacrEF mutant as a host. Although the initial recombinant construct contained other open reading frames, the presence of bexA alone was sufficient to confer to the E. coli host elevated levels of resistance to norfloxacin, ciprofloxacin, and ethidium bromide. Disruption of bexA in B. thetaiotaomicron made the strain more susceptible to norfloxacin, ciprofloxacin, and ethidium bromide, showing that this gene is expressed in this organism and functions as a multidrug efflux pump. The deduced BexA protein sequence was homologous to the protein sequence of Vibrio parahaemolyticus NorM, a multidrug efflux transporter, and thus, BexA belongs to the multidrug and toxic compound extrusion (MATE) family.
Members of the Bacteroides fragilis group are anaerobic bacteria of the highest clinical relevance, and B. fragilis and Bacteroides thetaiotaomicron are often isolated from patients with suppurative anaerobic infections. They are gram-negative, obligately anaerobic organisms with a broad spectrum of recognized resistance to antimicrobial agents (23), including aminoglycosides, most of the penicillins and cephalosporins, and fluoroquinolones (except for a few recently developed compounds such as trovafloxacin and clinafloxacin) (2, 3).
Active multidrug efflux processes, usually involving secondary transporters belonging to the major facilitator superfamily, small multidrug resistance family, and resistance-nodulation-division (RND) superfamily, are now known to be important, especially in the baseline or intrinsic resistance of many bacteria to antimicrobial agents (16, 21). More recently, a new family, the multidrug and toxic compound extrusion (MATE) family, has been discovered (4), but its contribution to drug resistance has been known only for a few isolated cases (12, 13).
We found previously that at least a portion of the remarkable norfloxacin resistance (MICs, 16 to 32 μg/ml) of B. fragilis was attributed to active efflux of this agent (12). To elucidate the efflux mechanism, we attempted to clone the genes responsible for norfloxacin efflux from the chromosomal DNAs of B. fragilis and B. thetaiotaomicron using as the host an Escherichia coli mutant strain with defects in multidrug efflux pumps. Here we report on the cloning and sequencing of a gene, bexA, that is involved in the efflux of norfloxacin, ciprofloxacin, and ethidium bromide from B. thetaiotaomicron. Interestingly, the BexA transporter is a member of the MATE family.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. fragilis ATCC 25285 and B. thetaiotaomicron ATCC 29741 5482 and were grown in general anaerobic GAM broth (Nissui, Tokyo, Japan) and supplemented Trypticase soy broth (sTSB) (12) in an anaerobic chamber.
For cloning, two host strains were used. They were E. coli DH5α [K-12 supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1] (26) and AG102AX [K-12 argE3 thi-1 rpsL xyl mtl Δ(gal-uvrB) marR1 ΔacrAB::kan ΔacrEF::spc]. The latter strain was constructed by transducing into AG102A (18) an ΔacrEF::spc allele (made in strain JC7623 [9]) by deleting a 1,133-bp fragment between the Eco47III site about 43% of the way into acrE gene and the EcoRV site about 15% of the way into the acrF gene and by introducing the Ω interposon containing the spectinomycin and streptomycin resistance marker (22) in its place. E. coli strains were grown at 37°C in Luria-Bertani (LB) broth or LB agar supplemented with ampicillin (20 μg/ml) or norfloxacin (50 ng/ml) when necessary.
Susceptibility testing.
The MICs of drugs for E. coli and Bacteroides strains were determined by a broth microdilution assay in LB broth and sTSB, respectively, as described previously (12). For Bacteroides strains, gradient plate assays for MICs were also used when necessary (6). Cultures were incubated at 37°C overnight or for 2 days before the growth was assessed.
Gene cloning and sequencing.
Chromosomal DNAs were prepared from cells of B. fragilis, B. thetaiotaomicron, and their mutants by the method of Smith et al. (30) and later with a MasterPure Genomic DNA purification kit (Epicentre Technologies, Madison, Wis.). The DNA was partially digested with Sau3AI, and digested fragments with sizes of 2 to 10 kb were separated by agarose gel electrophoresis. The DNA fragments extracted from the gels were ligated into vector pBR322 that was cut with BamHI and were then dephosphorylated with calf alkaline phosphatase. Cells of E. coli AG102AX were transformed with the ligated recombinant plasmids by electroporation and were spread on LB agar plates containing 50 ng of norfloxacin and 20 μg of ampicillin per ml. This led to the isolation of pBRBT20 containing 5.3 kb of B. thetaiotaomicron DNA. Other DNA manipulations were carried out by standard procedures as described elsewhere (26).
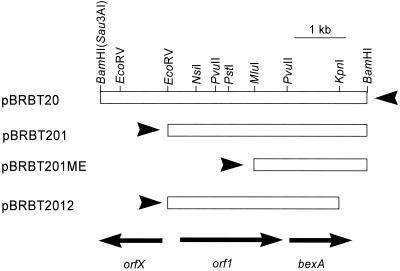
Subcloning of the insert in pBRBT20 were first performed by removing the 5′ end of the insert by double digestion with EcoRV and BamHI and then inserting the shortened fragments into pBR322 digested with both EcoRV and BamHI, generating pBRBT201 (Fig. 1). The same fragment was also inserted into the multiple cloning site of pUC18, generating pUC18BT201. Digestion of pBRBT201 with MluI and EcoRV, followed by conversion of the MluI-digested terminus into a blunt end by filling in with T4 DNA polymerase (Takara DNA Blunting kit; Takara Biotechnology, Kyoto, Japan) and finally religation, generated pBRBT201ME (Fig. 1). Similarly, digestion of pBRBT201 with KpnI and BamHI, followed by conversion of the ends into blunt ends by digestion with mung bean nuclease and religation, gave pBR2012 (Fig. 1). In addition, subcloning of orf1 and bexA (Fig. 1) was performed by PCR amplification of these genes. Primers contained 5′ extensions corresponding to EcoRV and BamHI target sequences for directed insertion into the pBR322 vector. The forward primers for orf1 and bexA started at residues 1,536 and 3,417 of the insert sequence in pBRBT20, respectively (or 85 and 147 bp upstream from the putative translation initiation codons of orf1 and bexA, respectively). The backward primers started at nucleotides 3,511 and 4,998, respectively, in the insert DNA of pBRBT20. The amplified fragments were inserted into pBR322 digested with EcoRV and BamHI, to yield plasmids pYEB (containing orf1) and pNEB (containing bexA); in these plasmids the cloned genes are inserted in the orientation in which the tet promoter of the vector could initiate their transcription.
FIG. 1.
Physical map of a cloned fragment containing the orfX, orf1, and bexA (orf2) genes from B. thetaiotaomicron. Although a 5.3-kb DNA fragment partially digested with Sau3A was cloned into the BamHI site of pBR322, the right side of the insert-vector junction remained cleavable with BamHI. The arrows at the bottom indicate putative open reading frames and their directions of transcription. Arrowheads indicate the approximate positions and the directions of transcription of the tet promoter in the vector pBR322.
In addition, another set of primers starting at nucleotides 3,418 and 4,992, respectively, were used to amplify bexA for insertion in the reverse direction. The fragments were amplified by using pBRBT20 as the template, purified, and cut with EcoRV and BamHI. These fragments were inserted into the pBR322 vector that had been cut with EcoRV and BamHI to yield plasmid pNBE, in which the bexA gene was inserted in a reverse orientation in relation to the tet promoter of the vector. The recombinant plasmids were confirmed to contain the correct genes in the expected direction by PCR amplification as well as digestion with several restriction enzymes.
Preparation of a gene disruption mutant in B. thetaiotaomicron.
pGERM, a suicide vector for Bacteroides spp. (24), was used for preparation of a gene disruption mutant in B. thetaiotaomicron. This vector contains markers selectable in E. coli (bla) as well as in Bacteroides (ermG), replicates in E. coli, and can be mobilized for transfer into other bacteria because of the presence of a transfer origin (oriT) from plasmid RK2. It also carries the multiple cloning sites from pUC19. An approximately 1-kb internal fragment of bexA was cut out from pBRBT201 by digestion with PvuII and KpnI (Fig. 1) and was inserted into pGERM cut with SmaI and KpnI. E. coli S17-1 was transformed by this recombinant construct of pGERM containing an internal fragment of bexA, and a transformant was used as the donor in a mating with B. thetaiotaomicron ATCC 29741. In Bacteroides, pGERM derivatives cannot replicate, so a single homologous recombination with the chromosome within bexA, resulting in the insertion of pGERM sequence into this gene, is the most likely way to make the recipient cell erythromycin resistant (25, 29). Several erythromycin-resistant colonies recovered on plates with erythromycin (10 μg/ml) and gentamicin (200 μg/ml) (added in order to counterselect the donor strain, as Bacteroides spp. are intrinsically resistant to aminoglycosides [23]) were picked up for further analysis. These possible disruption mutants were tested for drug susceptibility and were then tested by genomic Southern analysis to confirm the occurrence of a disruption within bexA. A disruption mutant of bexA in B. thetaiotaomicron, OUT4, was isolated in this manner.
Genomic Southern blot analysis.
Whole-plasmid DNAs of pBRBT20 (carrying the full insert DNA) and of pGERM recombinants pFY13A (containing the 0.65-kbp PstI-NsiI fragment, corresponding to an internal fragment of orf1) and pFY28 (containing the 1-kb KpnI-PvuII fragment, corresponding to an internal fragment of bexA) were biotinylated by nick translation with the BioNick Labeling System (Life Technologies, Frederick, Md.) and were then used as probes. Chromosomal DNAs were extracted as described above, digested with various restriction enzymes, and separated by gel electrophoresis. The Southern hybridization was detected by using the PhotoGene nucleic acid detection system (version 2.0; Life Technologies) according to the manufacturer's instructions.
Norfloxacin efflux assay.
Norfloxacin accumulation in the cells was assayed by filtration of cells incubated with [14C]norfloxacin (12). Energy-dependent efflux was assessed by measurement of an increase in the level of accumulation upon the addition of a proton conductor, carbonyl cyanide m-chlorophenylhydrazone (CCCP), at 100 μM.
Nucleotide sequence accession number.
The nucleotide sequence data have been deposited in the DDBJ database under accession no. AB067769.
RESULTS AND DISCUSSION
Cloning of norfloxacin resistance gene from B. thetaiotaomicron.
Since norfloxacin is actively pumped out from B. fragilis (12) and B. thetaiotaomicron (unpublished data) and since the efflux is catalyzed by a multidrug efflux pump (12), we tried to clone a gene(s) responsible for the efflux activity from these two species. We successfully obtained from B. thetaiotaomicron but not from B. fragilis several recombinant plasmids that decreased the norfloxacin susceptibility of the hypersusceptible E. coli host strain lacking major multidrug efflux pumps (strain AG102AX). One of the recombinants carrying a 5.3-kb insert, pBRBT20, was used for further experiments (Fig. 1). As shown in Table 1, the norfloxacin and ethidium bromide MICs were eight times higher and the ciprofloxacin MIC was four times higher for AG102AX carrying pBRBT20 in comparison with the MICs for the host strain or the strain containing the vector (pBR322) alone. pBRBT20 did not increase the MIC of sparfloxacin, a lipophilic fluoroquinolone, or the MICs of chlorampenicol and puromycin (Table 1).
TABLE 1.
Antibiotic susceptibilities of various strainsa
| Strain | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| Norfloxacin | Ciprofloxacin | Sparfloxacin | Ethidium bromide | Chloramphenicol | Puromycin | |
| E. coli | ||||||
| AG102AXb | 0.03 | 0.004 | 0.001 | 8 | 1 | 4 |
| AG102AX/pBRBT20 | 0.25 | 0.016 | 0.001 | 64 | 1 | 4 |
| AG102AX/pBRBT201 | 0.25 | 0.016 | 0.001 | 64 | 1 | 4 |
| AG102AX/pBRBT201ME | 0.25 | 0.016 | 0.001 | 64 | 1 | 4 |
| AG102AX/pBRBT2012 | 0.03 | 0.004 | 0.001 | 8 | 1 | 4 |
| AG102AX/pYEB | 0.03 | 0.004 | 0.001 | 8 | 1 | 4 |
| AG102AX/pNEB | 0.25 | 0.016 | 0.001 | 64 | 1 | 4 |
| AG102AX/pNBE | 0.03 | 0.004 | 0.001 | 8 | 1 | 4 |
| DH5αb | 0.25 | 0.06 | 0.03 | 64 | 4 | 64 |
| DH5α/pUC18BT201 | 1.0 | 0.25 | 0.03 | 512 | 4 | 64 |
| B. thetaiotaomicron | ||||||
| ATCC 29741 | 128 | 16 | 2 | 128 | 14 | 4 |
| OUT4 (bexA mutant) | 32 | 8 | 2 | 32 | 14 | 4 |
MIC determination by the broth microdilution assay was repeated at least three times in each case, and the consistencies of the MICs were confirmed.
No change in the MIC occurred for strain AG102AX or DH5α when the host was transformed with the vector plasmid alone.
Sequence and characteristics of the putative gene products.
Sequencing of the whole insert of pBRBT20 revealed three open reading frames shown as orfX, orf1, and orf2 (bexA) in Fig. 1. The orf2 product is predicted to contain 443 amino acids. BLASTP analysis of the sequence showed that it had strong similarity to the Vibrio parahaemolyticus NorM sequence (13) and that of its E. coli homolog, VdhE (14), as well as to those of many other proteins discussed below (see “Homology among members of the MATE family” below). Because of its homology with NorM, a known multidrug efflux transporter (13, 14), we propose that orf2 be named bexA (bacteroides exporter A). The family containing NorM was earlier defined as the MATE family (5).
The orf1 sequence is predicted to code for a protein of 618 amino acid residues. The BLASTP program suggested that this protein was a homolog of YidC, the E. coli protein recently shown to be involved in the insertion of membrane proteins (27, 28). Although the similarity was 39%, this involved the creation of many gaps corresponding to 19% of the aligned sequence, and it is not clear if this protein performs a similar function in Bacteroides. In any case, partial deletion of the insert sequence (see below) showed that this gene was unlikely to be involved in drug efflux.
The orfX sequence, transcribed in the opposite direction, was predicted to code for a 403-residue protein, although its C terminus appears to be truncated in pBRBT20. This polypeptide showed strong similarity to the C-terminal halves of dozens of bacterial helicases, with the strongest similarity to those of Staphylococcus aureus (M63176_2) and Neisseria meningitidis (AJ391262-1). The function of this open reading frame is not clear, and its deletion did not affect the drug efflux phenotype, as described below.
Subcloning of insert sequence.
Several subclones were made from pBRRT20 in order to identify the gene essential for the norfloxacin resistance (Fig. 1). pBRBT201, in which an approximately 1.3-kb BamHI-EcoRV fragment (containing orfX) was deleted from the orfX-proximal end of the insert in pBRBT20, still exhibited the same level of resistance as the initial clone (Table 1). When the insert in pBRBT201 was further shortened on the 5′ side by removing the EcoRV-MluI fragment that contains most of orf1, the resultant construct, pBRBT201ME, still maintained the high level of resistance (Table 1), showing that orf1 is not required for resistance. In contrast, pBRBT2012, with a deletion of the 0.6-kb KpnI-BamHI fragment at the bexA-proximal end of the insert, produced no increase in the level of resistance (Table 1), suggesting that the bexA-containing region is essential for resistance.
A promoter-like sequence (TATAAT and TGACAA) in front of orf1 shows strong similarity to the E. coli consensus −10 and −35 sequences, and no rho-independent terminator is found downstream of orf1. Thus, in pBRBT201 and pBRBT2012, which contain orf1 and its upstream sequence, bexA could have been transcribed at least in part by “readthrough” from orf1. The same explanation also applies to pBRBT20, in which the tetracycline promoter of the vector is located at the 3′ end (the right end in Fig. 1) of the insert. In pBRBT201ME, which lacks this putative orf1 promoter, however, the tetracycline promoter of the vector pBR322, located on the 5′ side of the insert (the left side in Fig. 1), was likely to have been responsible for transcription of bexA in E. coli, as the sequence between orf1 and bexA does not seem to contain a promoter sequence typical for E. coli. This hypothesis was supported by the observation that the level of norfloxacin resistance was drastically reduced when the tetracycline promoter was removed by cutting pBRBT201 with AatII and EcoRV and then the upstream region and the 5′-terminal portion of orf1 were removed by exonuclease III digestion (data not shown).
In order to confirm the conclusion that only bexA is needed for antibiotic resistance, we amplified the orf1 and bexA genes (with their upstream sequences) separately through PCR and inserted these genes into pBR322 digested with EcoRV and BamHI so that the tetracycline promoter was located upstream from these genes. The results with these constructs, pYEB and pNEB (Table 1), showed clearly that only bexA is necessary for resistance. However, when the bexA gene was inserted in the reverse orientation in plasmid pNBE, there was no increase in the level of resistance, suggesting that the immediate upstream sequence of bexA indeed did not function as an efficient promoter, at least in E. coli, and that the successful expression of bexA in pNEB was due to the presence of the tetracycline promoter of the vector.
The insert sequence in pBRBT201 was recloned in expression vector pUC18 in a direction that allowed the Plac promoter of the vector to drive the transcription of both orf1 and bexA. The resultant plasmid, pUC18BT201 increased the norfloxacin MIC for strain DH5α, in which the ΔlacU169 deletion covers lacI and allows the constitutive expression of the Plac promoter (Table 1). Since, in contrast to strain AG100AX, DH5α has no known defects in its multidrug efflux systems and its OmpF porin level is not reduced by the marR mutation (15), these features of AG100AX are not needed to see the effect of the BexA pump in the increased level of resistance. Since NorM also pumps out aminoglycosides (14), we tested the MICs of kanamycin and streptomycin using DH5α/pUC18BT201. No detectable increase in the MICs of these compounds for DH5α/pUC18BT201 in comparison with those for the parent strain, DH5α, was found (data not shown).
Homology among members of MATE family.
The MATE family was reported to contain three branches: the NorM branch, a branch containing several plant proteins (such as T51035 of Arabidopsis thaliana; see below), and a branch containing E. coli DinF (5). Among these three clusters, BexA had a high degree of similarity to members of the NorM branch. Thus, the Expect (E) value in BLASTP was 9 × 10−17 with V. parahaemolyticus NorM and 4 × 10−20 with the Haemophilus influenzae homolog of NorM (HI1612). The closest homologs of BexA in terms of E values, however, were MATE family members that have not been tested for their efflux functions and that have often been described as DinF homologs in databases, such as Thermotoga maritima T0815 (AAD35897.1) and Pyrococcus abysii PAB0243 (CAB49288.1), with E values of 4 × 10−30 and 2 × 10−29, respectively. Nevertheless, BexA does not appear to belong to the DinF branch or the plant protein branch, because a two-sequence BLAST search between BexA and representatives of these clusters (DinF of E. coli and T05135 of A. thaliana [F7H19.220, listed as H19.22 in Fig. 3 of reference 5]) produced rather high E values (4 × 10−4 and 0.047, respectively).
Multiple alignment (with the Clustal W program [31]) also confirmed this conclusion. A previous report (5) concluded that a region corresponding to putative transmembrane helices 5 and 6, as well as the segment containing helix 8 and the following loop, are most characteristic in the proteins of MATE family. However, many more MATE family members have been sequenced since then, and in particular, inclusion of proteins from extremophiles produces a somewhat different picture. In the regions noted earlier (5), BexA and its most closely related homologs, from Thermotoga and Pyrococcus, show only low degrees of similarity to “classical” MATE family members such as NorM. However, other regions of the protein turn out to be more instructive. In Fig. 2 we show the alignment of the segment corresponding to the putative transmembrane helix 6 and the segment that follows it. All members of the putative NorM branch show a remarkable conservation of the sequence GKFGXP. In contrast, this sequence was not conserved at all in the plant protein branch (A. thaliana proteins) or the DinF branch. This confirms that BexA indeed belongs to the NorM cluster, although the group that includes BexA and the Thermotoga and Pyrococcus proteins seems to be somewhat more distantly related to the close relatives of NorM from the facultatively anaerobic gram-negative bacteria. Thus, the sequences that are remarkably conserved in the latter group (TKPXMVIGFIGLLXNIPLN or LGGVGCGVAT) are not well conserved in the former group. Apparently, this distance was responsible for the misclassification of some of the extremophile proteins, such as the proteins from the two Pyrococcus species, as DNA damage-induced protein or homologs of DinF in the databases.
FIG. 2.
Multiple alignment of the new signature region of MATE family members.
Norfloxacin efflux activity.
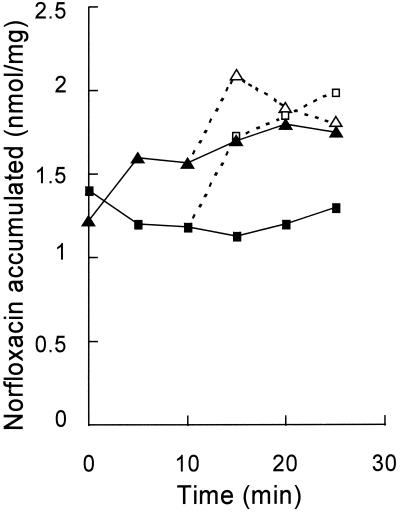
As shown in Fig. 3, the addition of CCCP to E. coli AG102AX carrying pBRBT201 produced a large increase in the level of norfloxacin accumulation. In addition, in the absence of CCCP the steady-state level of norfloxacin accumulation in AG102AX/pBRBT201 was lower than that in the same host carrying the pBR322 vector alone. AG102AX with no plasmid showed a similar, higher level of accumulation of norfoxacin (data not shown). These results suggest that this lower steady-state level of accumulation in AG102AX/pBRBT201 was achieved by an active efflux driven by the gene product expressed from the recombinant plasmid in E. coli.
FIG. 3.
Accumulation of [14C]norfloxacin by E. coli AG102AX cells with either the recombinant plasmid, pBRBT201 (squares), or the vector alone (triangles). At 10 min a proton conductor, CCCP, was added to one-half of the bacterial cell suspension. Closed and open symbols, norfloxacin accumulation in cells in the absence and presence of CCCP, respectively.
Effects of inhibitors.
We examined the effects of various inhibitors of bacterial multidrug efflux pumps. As shown in Table 2, several compounds appeared to inhibit the increase in the norfloxacin MIC, presumably by inhibiting drug efflux. These compounds included reserpine and verapamil, both of which are known to be inhibitors of major facilitator superfamily pumps (1, 15), and MC-207,110, which is known to inhibit RND pumps in Pseudomonas aeruginosa (10). We note that reserpine and verapamil were earlier shown to decrease the norfloxacin MICs for B. fragilis strains (12).
TABLE 2.
Susceptibility of BexA multidrug resistance pump to potential inhibitors
| Inhibitora | Norfloxacin MIC (μg/ml)
|
||
|---|---|---|---|
| AG102AX/pBR322 | AG102AX/pBRBT201 | AG102AX/pBRBT201ME | |
| None | 0.03 | 0.25 | 0.25 |
| Reserpine (20 μg/ml) | 0.015 | 0.06 | 0.06 |
| Reserpine (100 μg/ml) | 0.015 | 0.06 | 0.06 |
| Verapamil (20 μg/ml) | 0.015 | 0.06 | 0.06 |
| Verapamil (100 μg/ml) | 0.015 | 0.015 | 0.015 |
| MC 207,110 (10 μg/ml) | 0.015 | 0.125 | 0.125 |
| MC 207,110 (15 μg/ml) | 0.015 | 0.015 | 0.06 |
These inhibitors inhibited the growth of the E. coli strains at high concentrations; and the MICs of reserpine, verapamil, and MC 207, 110 for AG102AX/pBR322 were >512, 512, and 64 μg/ml, respectively.
Disruption mutant of bexA.
A disruption mutant of B. thetaiotaomicron bexA, OUT4, was isolated by using a suicide vector, pGERM (see Materials and Methods). Genomic Southern analysis confirmed that the bexA gene was interrupted in the mutant (data not shown). It was more susceptible than the parent strain, B. thetaiotaomicron ATCC 29741, to norfloxacin, ciprofloxacin, and ethidium bromide (Table 1), confirming that the gene is expressed in Bacteroides and contributes to its intrinsic resistance to fluoroquinolones.
Homologs in B. fragilis.
Southern blot analysis with pGERM recombinants (pFY28) carrying an internal fragment of bexA as a probe did not reveal any homologous DNA fragments from B. fragilis ATCC 25285. Two clinical isolates of B. fragilis used previously (12) had no positive bands either, but B. thetaiotaomicron ATCC 29741 and 5482 contained similar positive bands carrying the genes (data not shown).
Since the genome sequencing of B. fragilis ATCC 25285 is in progress at the Sanger Centre and the raw sequences of the fragments are available at its website (www.sanger.ac.uk), we looked for homologs of the BexA protein by using the translated products of these sequences with the program TBLASTN. Indeed, the product of three sequences spliced together (Bf221 h02.q1c + Bf236a12 [reverse complement] + Bf142c11.qlc) had the strongest similarity (84% identity at the protein level), and at least three other sequences had strong similarity. However, this degree of similarity would not have been detected by Southern blotting under high-stringency conditions. We are trying to disrupt this and other homologs in B. fragilis in an attempt to evaluate their contribution to the drug resistance of this organism.
Other possible mechanisms contributing to intrinsic fluoroquinolone resistance of Bacteroides.
When we inactivated the bexA gene in B. thetaiotaomicron, the norfloxacin MIC decreased from 128 to 32 μg/ml (Table 1), yet the MIC for the mutant was still much higher than those for the wild-type strains of E. coli, for example. The presence of at least four homologs of bexA in B. fragilis suggests the possibility that there may also be other homologs of bexA in B. thetaiotaomicron which could contribute to the fluoroquinolone resistance. In addition, the target genes in various Bacteroides species might already contain “mutations” that make these targets resistant to fluoroquinolones, as has been demonstrated in several species of Mycobacterium (7). In fact, a partial sequence of B. thetaiotaomicron GyrA has recently been reported to contain an Asp86-to-Tyr change (17), corresponding to a frequent mutation (Asp87Tyr) found in fluoroquinolone-resistant mutants of E. coli (8, 32). The gyrA and gyrB genes of B. fragilis have been sequenced (19), and interestingly, Asp86 of the GyrA enzyme is again changed into phenylalanine. As the mutation of this residue to various neutral amino acids increases the level of fluoroquinolone resistance (8, 20, 32), this change may also make the target less susceptible in B. fragilis. Mutations of the target enzymes, however, usually produce only moderate levels of resistance, and the additional increase in efflux activity is needed for the production of very high levels of resistance, at least in E. coli (see reference 11 and references cited therein). These considerations suggest that active efflux plays a major role in the intrinsic fluoroquinolone resistance in Bacteroides. (However, in B. fragilis it has been reported that mutations in gyrA are usually introduced at later stages in the creation of strains with high levels of fluoroquinolone resistance [4].)
ACKNOWLEDGMENTS
This work was supported by a grant-in-aid for Science Promoted Research (grant 1006) from the Ministry of Education, Science, Sports and Culture of Japan (to F.Y.) and a grant from the U.S. Public Health Service (grant AI-09644) (to H.N.).
We thank N. Shoemaker for sending bacterial strains and plasmids for determination of the genetics of Bacteroides spp. and the Sanger Centre for making the B. fragilis genomic sequence freely available to the public.
REFERENCES
- 1.Ahmed M, Borsch C M, Neyfakh A A, Schuldiner S. Mutants of Bacillus subtilis multidrug transporter Mmr with altered sensitivity to the antihypertensive alkaloid reserpine. J Biol Chem. 1993;268:11086–11089. [PubMed] [Google Scholar]
- 2.Alekshun M N, Levy S B. Bacterial drug response to survival threats. In: Storz G, Hengge-Aronis R, editors. Bacteria stress responses. Washington, D.C.: ASM Press; 2000. pp. 323–366. [Google Scholar]
- 3.Appelbaum P A. Quinolone activity against anaerobes. Drugs. 1999;58:60–64. doi: 10.2165/00003495-199958002-00012. [DOI] [PubMed] [Google Scholar]
- 4.Bachoual R, Dubreuil L, Soussy C-J, Tankovic J. Roles of gyrA mutations in resistance of clinical isolates and in vitro mutants of Bacteroides fragilis to the new fluoroquinolone trovafloxacin. Antimicrob Agents Chemother. 2000;44:1842–1845. doi: 10.1128/aac.44.7.1842-1845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown M H, Paulsen I T, Skurray R A. The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol Microbiol. 1999;31:394–395. doi: 10.1046/j.1365-2958.1999.01162.x. [DOI] [PubMed] [Google Scholar]
- 6.Curiale M S, Levy S B. Two complementation groups mediate tetracycline resistance determined by Tn10. J Bacteriol. 1982;151:209–215. doi: 10.1128/jb.151.1.209-215.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guillemin I, Cambau E, Jarlier V. Sequences of conserved region in the A subunit of DNA gyrase from nine species of the genus Mycobacterium: phylogenetic analysis and implications for intrinsic susceptibility to quinolones. Antimicrob Agents Chemother. 1995;39:2145–2149. doi: 10.1128/aac.39.9.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heisig P. Genetic evidence for a role of parC mutations in development of high-level fluoroquinolone resistance in Escherichia coli. Antimicrob Agents Chemother. 1996;40:879–885. doi: 10.1128/aac.40.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kushner S R, Templin A, Nagaishi H, Clark A J. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc Natl Acad Sci USA. 1971;68:824–827. doi: 10.1073/pnas.68.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lomovskaya O, Warren M S, Lee A, Galazzo J, Fronko R, Lee M, Blais J, Cho D, Chamberland S, Renau T, Leger R, Hecker S, Watkins W, Hoshino K, Ishida H, Lee V J. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob Agents Chemother. 2001;45:105–116. doi: 10.1128/AAC.45.1.105-116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzariol A, Tokue Y, Kanegawa T, Cornaglia G, Nikaido H. High-level fluoroquinolone-resistant clinical isolates of Escherichia coli overproduce multidrug efflux protein AcrA. Antimicrob Agents Chemother. 2000;44:3441–3443. doi: 10.1128/aac.44.12.3441-3443.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyamae S, Nikaido H, Tanaka Y, Yoshimura F. Active efflux of norfloxacin by Bacteroides fragilis. Antimicrob Agents Chemother. 1998;42:2119–2121. doi: 10.1128/aac.42.8.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morita Y, Kataoka A, Shiota S, Mizushima T, Tsuchiya T. NorM of Vibrio parahaemolyticus is an Na+-driven multidrug efflux pump. J Bacteriol. 2000;182:6694–6697. doi: 10.1128/jb.182.23.6694-6697.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morita Y, Kodama K, Shiota S, Mine T, Kataoka A, Mizushima T, Tsuchiya T. NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob Agents Chemother. 1998;42:1778–1782. doi: 10.1128/aac.42.7.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neyfakh A A. The multidrug efflux transporter of Bacillus subtilis is a structural and functional homolog of the Staphylococcus NorA protein. Antimicrob Agents Chemother. 1992;36:484–485. doi: 10.1128/aac.36.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikaido H. Antibiotic resistance caused by gram-negative multidrug efflux pumps. Clin Infect Dis. 1998;27(Suppl. 1):S32–S41. doi: 10.1086/514920. [DOI] [PubMed] [Google Scholar]
- 17.Oh H, El Amin N, Davies T, Appelbaum P C, Edlund C. gyrA mutations associated with quinolone resistance in Bacteroides fragilis group strains. Antimicrob Agents Chemother. 2001;45:1977–1981. doi: 10.1128/AAC.45.7.1977-1981.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okusu H, Ma D, Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol. 1996;178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onodera Y, Sato K. Molecular cloning of the gyrA and gyrB genes of Bacteroides fragilis encoding DNA gyrase. Antimicrob Agents Chemother. 1999;43:2423–2429. doi: 10.1128/aac.43.10.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oram M, Fisher L M. 4-Quinolone resistance mutations in the DNA gyrase of Escherichia coli clinical isolates identified by using the polymerase chain reaction. Antimicrob Agents Chemother. 1991;35:387–389. doi: 10.1128/aac.35.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen B A, Bush K, Tally F P. Antimicrobial resistance in anaerobes. Clin Infect Dis. 1997;24(Suppl. 1):S110–S120. doi: 10.1093/clinids/24.supplement_1.s110. [DOI] [PubMed] [Google Scholar]
- 24.Salyers A A, Bonheyo G, Shoemaker N B. Starting a new genetic system: lessons from Bacteroides. Methods. 2000;20:35–46. doi: 10.1006/meth.1999.0903. [DOI] [PubMed] [Google Scholar]
- 25.Salyers A A, Shoemaker N B, Cooper A, D'Elia J, Shipman J A. Genetic methods for Bacteroides species. Methods Microbiol. 1999;29:229–276. [Google Scholar]
- 26.Sambrook J, Russell D W. Molecular cloning: a laboratory manual. 3rd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 27.Samuelson J C, Minyong M, Jiang F, Moller I, Wiedmann M, Kuhn A, Phillips G J, Dalbey R E. YidC mediates membrane protein insertion in bacteria. Nature. 2000;406:637–641. doi: 10.1038/35020586. [DOI] [PubMed] [Google Scholar]
- 28.Scotti P A, Urbanus M L, Brunner J, de Gier J-W L, von Heijne G, van der Does C, Driessen A J M, Oudega B, Luirink J. YidC, the Escherichia coli homologue of mitochondrial Oxa1p, is a component of the Sec translocase. EMBO J. 2000;19:542–549. doi: 10.1093/emboj/19.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shoemaker N B, Getty C, Guthrie P E, Salyers A A. Two Bacteroides plasmids, pBFTM10 and pB8–51, contain transfer regions that are recognized by broad host range IncP plasmids and a conjugative Bacteroides tetracycline element. J Bacteriol. 1986;166:959–965. doi: 10.1128/jb.166.3.959-965.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith G L E, Socranski S S, Smith C M. Rapid method for the purification of DNA from subgingival microorganisms. Oral Microbiol Immunol. 1989;4:47–51. doi: 10.1111/j.1399-302x.1989.tb00406.x. [DOI] [PubMed] [Google Scholar]
- 31.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vila J, Ruiz J, Goni P, Jimenez de Anta M T. Detection of mutations in parC in quinolone-resistant clinical isolates of Escherichia coli. Antimicrob Agents Chemother. 1996;40:491–493. doi: 10.1128/aac.40.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]